Patent application title: Integrated Solvent Deasphalting and Slurry Hydrocracking Process
Inventors:
Robert S. Haizmann (Rolling Meadows, IL, US)
Robert S. Haizmann (Rolling Meadows, IL, US)
Lorenz J. Bauer (Schaumburg, IL, US)
Lorenz J. Bauer (Schaumburg, IL, US)
Manuela Serban (Glenview, IL, US)
James F. Mcgehee (Mt. Prospect, IL, US)
IPC8 Class: AC10C300FI
USPC Class:
208 86
Class name: Chemical conversion of hydrocarbons with preliminary treatment of feed deasphalting
Publication date: 2010-05-20
Patent application number: 20100122934
acking (SHC) and coking methods for making slurry
hydrocracking (SHC) distillates are disclosed. Representative methods
involve passing a slurry comprising a deasphalted oil (DAO) produced in a
solvent deasphalting (SDA) process, optionally with recycled SHC gas oil
and recycled SHC pitch, and a solid particulate through an SHC reaction
zone in the presence of hydrogen to obtain the SHC distillate. Recovery
and recycle of SHC gas oil and pitch from the SHC effluent improves the
overall conversion to naphtha and distillate products and decreases
catalyst requirements.Claims:
1. An integrated process for preparing a slurry hydrocracking (SHC)
distillate, the process comprising:(a) passing a heavy hydrocarbon
feedstock comprising a deasphalted oil (DAO) through an SHC reaction zone
in the presence of hydrogen to provide an SHC effluent; and(b) recovering
said SHC distillate from said SHC effluent.
2. The process of claim 1, further comprising, prior to step (a), subjecting a vacuum distillation column residue to solvent deasphalting (SDA) in the presence of a solvent to obtain said DAO and an SDA pitch.
3. The process of claim 1, wherein the heavy hydrocarbon feedstock is present as a slurry, in combination with a solid particulate, in said SHC reaction zone.
4. The process of claim 3, wherein said solid particulate comprises a compound of a metal of Group IVB, Group VB, Group VIB, Group VIIB, or Group VIII.
5. The process of claim 1, wherein said heavy hydrocarbon feedstock further comprises at least one liquid product recovered from said SHC effluent and recycled to said SHC reaction zone.
6. The process of claim 5, wherein said liquid product is selected from the group consisting of an SHC high pressure separator bottoms liquid, an SHC gas oil, an SHC pitch, recycled portions thereof, and combinations thereof.
7. The process of claim 6, wherein said heavy hydrocarbon feedstock further comprises both a recycled portion of an SHC gas oil and a recycled portion of an SHC pitch obtained from vacuum column distillation of an SHC high pressure separator bottoms liquid.
8. The process of claim 6, wherein said liquid product comprises (i) a recycled portion of an SHC high pressure separator bottoms liquid, (ii) a recycled portion of an SHC gas oil, and (iii) a recycled portion of an SHC pitch, wherein (ii) and (iii) are obtained from fractionation of a non-recycled portion of said SHC high pressure separator bottoms liquid.
9. The process of claim 6, wherein, said SHC distillate is separated, as a lower boiling component, from an SHC high pressure separator bottoms liquid by flash separation or fractionation of said SHC effluent.
10. The process of claim 6, wherein said SHC gas oil has a distillation end point temperature from about 427.degree. C. (800.degree. F.) to about 538.degree. C. (1000.degree. F.).
11. The process of claim 6, wherein said heavy hydrocarbon feedstock further comprises an SHC pitch obtained from vacuum column distillation of an SHC high pressure separator bottoms, and wherein a non-recycled portion of said SHC pitch is removed as a solid particulate drag stream.
12. The process of claim 11, wherein said non-recycled portion of said SHC pitch removed as a solid particulate drag stream represents less than about 70% of said SHC pitch obtained from vacuum column distillation of said SHC high pressure separator bottoms.
13. The process of claim 11, wherein said SHC pitch comprises hydrocarbons boiling at a temperature of greater than about 538.degree. C. (1000.degree. F.).
14. The process of claim 1, wherein said SHC reaction zone is maintained at a temperature from about 343.degree. C. (650.degree. F.) to about 593.degree. C. (1100.degree. F.), a pressure from about 3.5 MPa (500 psig) to about 21 MPa (3000 psig), and a space velocity from about 1 to about 30 volumes of heavy hydrocarbon feedstock per hour per volume of said SHC zone.
15. The process of claim 1, wherein a conversion of said heavy hydrocarbon feedstock to hydrocarbons having a boiling point temperature of 538.degree. C. (1000.degree. F.) or less in said SHC reaction zone is at least about 90% per pass.
16. The process of claim 1, further comprising hydrotreating said SHC distillate in a hydrotreating zone to obtain a hydrotreated distillate.
17. The process of claim 1, wherein said DAO has a metals content of at least about 500 ppm by weight.
18. The process of claim 1, wherein said DAO has a Conradson carbon residue of at least about 10% by weight.
19. A method for making a distillate hydrocarbon by integrating solvent deasphalting and slurry hydrocracking (SHC), the method comprising:(a) passing a slurry comprising a recycled portion of an SHC gas oil, a recycled portion of an SHC pitch, a deasphalted oil obtained from solvent deasphalting, and a solid particulate through an SHC reaction zone in the presence of hydrogen to provide an SHC effluent,(b) recovering said distillate hydrocarbon and an SHC high pressure separator bottoms liquid from flash separation of said SHC effluent,(c) recycling a first portion of said SHC high pressure separator bottoms liquid to said SHC reaction zone,(d) fractionating a second portion of said SHC high pressure separator bottoms liquid in an SHC vacuum distillation column to provide said SHC pitch and said SHC gas oil, as a heavy fraction and a light fraction, respectively,(e) recycling said recycled portion of said SHC gas oil and said recycled portion of said SHC pitch to said SHC reaction zone, and(f) removing a non-recycled portion of said SHC pitch as a solid particulate drag stream.
20. The method of claim 19, further comprising hydrotreating said distillate hydrocarbon to obtain a hydrotreated distillate.Description:
FIELD OF THE INVENTION
[0001]The present invention relates to methods for preparing distillate hydrocarbons using slurry hydrocracking (SHC) to upgrade high-boiling or heavy hydrocarbons obtained from refinery operations and particularly solvent deasphalting (SDA). The integration of SHC with SDA and optionally other processes such as crude oil fractionation and/or hydrotreating may be used to obtain a high quality (e.g., high API gravity and/or low sulfur) distillate.
DESCRIPTION OF RELATED ART
[0002]Solvent deasphalting (SDA) generally refers to refinery processes that upgrade hydrocarbon fractions using an extraction process in the presence of a solvent. The hydrocarbon fractions are often obtained from the distillation of crude oil, and include hydrocarbon residues (or resids) or gas oils from atmospheric column or vacuum column distillation. Solvents used in SDA are typically lower-boiling paraffinic hydrocarbons such as propane, butane, and their mixtures, having the ability to extract a deasphalted oil (DAO) with relatively lower levels of contaminants such as sulfur- and nitrogen-containing compounds, metals, and Conradson carbon residue. The extraction usually occurs in a countercurrent extractor, with the solvent phase and its extracted components flowing in an upward direction. In addition to DAO, the other major product of SDA is pitch, a highly viscous hydrocarbon that contains significant portions of the (non-extracted) contaminants present in crude oil.
[0003]The yields and quality of both the DAO and pitch depend on the composition of the SDA feed, the type and amount of solvent, and the extraction conditions. The DAO produced from SDA is a generally nondistillable product requiring further upgrading with fluid catalytic cracking (FCC), hydrocracking, and/or hydrotreating. Additionally, the significant quantities of pitch from SDA make this process less economically attractive compared to alternative heavy oil conversion processes.
[0004]Further refinery process streams normally sent to conventional conversion processes such as FCC, in order to yield more salable products, include gas oils and particularly vacuum gas oil (VGO). VGO is produced in a number of refinery operations, including slurry hydrocracking, coking, crude oil fractionation, and visbreaking, which process heavy hydrocarbon feedstocks. Because of their significant levels of contaminants (e.g., metals and sulfur compounds) that deactivate supported metal catalysts, in addition to coke precursors in these streams, gas oils are unfortunately not easily processed according to conventional catalytic conversion methods. The conversion of coker gas oils to more salable distillate and naphtha blending components for transportation fuels is therefore associated with a number of drawbacks.
[0005]Like SDA, slurry hydrocracking (SHC) is also used for the upgrading of heavy hydrocarbon feedstocks including those mentioned above. In SHC, these feedstocks are converted in the presence of hydrogen and solid catalyst particles (e.g., as a particulate metallic compound such as a metal sulfide) in a slurry phase. Representative slurry hydrocracking processes are described, for example, in U.S. Pat. No. 5,755,955 and U.S. Pat. No. 5,474,977. In addition to the VGO normally present in the reactor effluent, SHC (like SDA) produces a low-value, refractory pitch stream that normally cannot be economically upgraded or even blended into other products such as fuel oil or synthetic crude oil, due to its high viscosity and solids content. Moreover, SHC has disadvantages relative to other heavy hydrocarbon conversion processes, including a significant catalyst consumption requirement and a high capital cost due to the comparatively larger reactor size and high operating pressures.
[0006]Particular sources of synthetic crude oil of increasing interest, and for which blending components are sought to improve their flow characteristics, are bitumen and oil sands. Bitumen refers to the low-quality hydrocarbonaceous material recovered from oil sand deposits, such as those found in the vast Athabasca region of Alberta, Canada, as well as in Venezuela and the United States. Bitumen and oil sands are recognized as a valuable sources of "semi-solid" petroleum or synthetic crude oil, which can be refined into many valuable end products including transportation fuels such as gasoline or even petrochemicals.
[0007]There is an ongoing need in the art for process in which heavy hydrocarbons (e.g., atmospheric column and vacuum column resids as well as gas oils) are converted or upgraded with improved efficiency. There is also a need for such processes in which the net production of low-value end products, including gas oils and pitch, is minimized. There is further a need for overall crude oil refining processes that include the upgrading of crude oil residues and particularly those obtained in significant proportions from heavy crude oil feedstocks. Ideally, the products of such refining processes should be suitable as transportation fuel (e.g., diesel and/or naphtha) blending components or even for blending into synthetic crude oils to improve their properties (e.g., viscosity and/or specific gravity).
SUMMARY OF THE INVENTION
[0008]Aspects of the invention relate to the finding that slurry hydrocracking (SHC) can be effectively integrated with other refining processes and particularly solvent deasphalting (SDA), hydrotreating, and/or crude oil fractionation to produce a high value distillate stream while recycling low-value gas oils, preferably to extinction, as well as at least a portion of the SHC pitch. SHC is generally known in the art for its ability to convert vacuum column residues to lighter products. However, it has now been discovered that the use of a deasphalted oil (DAO) from SDA as a heavy hydrocarbon feedstock component or incremental feed to SHC results in operating synergies having commercially important advantages.
[0009]The DAO products from SDA processes, and particularly those processes that are operated with relatively high recoveries of DAO and low recoveries of pitch, have high levels of metal contaminants and Conradson carbon residue, making such DAO products difficult to upgrade with conventional FCC or hydrocracking, using either a fixed bed or ebullating bed of catalyst. Embodiments of the invention are associated with the discovery that DAO is effectively processed as a hydrocarbon feedstock component of SHC, with complete or essentially complete overall conversion to an SHC distillate. This distillate may be fractionated to provide, for example, lower- and higher-boiling products such as naphtha and diesel fuel.
[0010]The use of a heavy hydrocarbon feedstock comprising DAO may be combined with other SHC process features to obtain additional benefits. For example, recycling (e.g., to extinction) a portion of an SHC pitch and/or an SHC gas oil recovered from the SHC effluent allows for the complete or essentially complete overall conversion of these recycle streams (as well as the DAO) to the higher value SHC distillate. Moreover, the high content of polar aromatic compounds (e.g., mono- and multi-ring aromatics) in the recycle SHC gas oil, such as vacuum gas oil (VGO) obtained from vacuum column fractionation of a liquid product recovered from the SHC effluent, reduces the catalyst requirement of SHC. Without being bound by theory, it is believed that these recycled aromatics help solubilize asphaltenes in SHC recycle streams and thereby prevent the formation of precipitated agglomerates of asphaltenes and the solid particulate catalyst, leading to coking in the reactor or increased catalyst requirements to limit this coking. The recycling of SHC pitch can also help minimize make-up requirements, as this pitch contains catalyst with activity comparable to that of fresh catalyst.
[0011]Overall, the use of DAO as a component of the heavy hydrocarbon feedstock in SHC results in a surprisingly high conversion of this component (both on a per-pass and overall basis). Compared to conventional SHC, processing a vacuum column residue from crude oil fractionation in the absence of DAO, integrated processes according to the present invention have significantly reduced catalyst requirements, smaller reactor sizes, and/or lower reactor operating pressures. Relative to fixed bed or ebullating bed hydrocracking processes, the catalyst make-up rates of SHC processes described herein are surprisingly less adversely impacted by the high metals content of the DAO feed components, known to deactivate catalysts used in these conventional conversion processes. In fact, the types and amounts of contaminants in DAO products renders these streams difficult, in general, to further upgrade using FCC, hydrocracking, or hydrotreating.
[0012]In a representative integrated process, DAO is utilized in combination with recycled SHC gas oil, recovered from downstream fractionation/separation of the SHC effluent, in the overall heavy hydrocarbon feedstock to SHC. While portions of this feedstock may also generally include conventional components such as vacuum column resid, the presence of DAO results in the improvements discussed above. Moreover, DAO from solvent deasphalting (e.g., of a crude oil vacuum column distillation residue) is often readily available in large quantities, particularly in the case of refineries processing heavy crude oils.
[0013]The present invention is therefore associated with the effective utilization of DAO as an attractive incremental feedstock (e.g., in combination with a vacuum column residue), which is efficiently upgraded (e.g., cracked) using SHC to yield lighter and more valuable distillate and optionally naphtha products. According to some embodiments, the integration of SDA with SHC is carried out such that a portion of the SHC pitch and the SHC gas oil products, obtained from vacuum column fractionation of a high pressure separator bottoms liquid recovered from the SHC effluent, are recycled back to the SHC reactor or reaction zone.
[0014]In other embodiments, an integrated SDA/SHC process is combined with hydrotreating of the SHC distillate. As a result of the low (or non-existent) net yield of gas oil products such as VGO, due to recycling of heavy-boiling fractions back to the SHC reaction zone, the hydrotreated distillate has a sufficiently high API gravity (e.g., at least about 20°), making it attractive for blending into a synthetic crude oil that is transported via a pipeline. Thus, the hydrotreated distillate, or even the SHC distillate without hydrotreating, may be obtained as a high quality transportation fuel blending component with only a minor amount or essentially no hydrocarbons boiling at a temperature representative of gas oils (e.g., greater than about 343° C. (650° F.)). The integration of SHC with an existing refinery hydrotreating process, conventionally used for sulfur- and nitrogen-containing compound removal from distillates, may involve hydrotreating a recovered SHC distillate product in conjunction with a straight-run distillate obtained from crude oil fractionation and/or other refinery streams. This integration may advantageously reduce overall capital costs of the complex. As discussed above, the integration of SDA with SHC, optionally hydrotreating, and optionally other conventional refinery operations has the potential to provide significant benefits in terms of improved processing efficiency and product yields, reduction or elimination of low-value refractory byproducts, and/or the associated capital cost reduction.
[0015]In other representative embodiments of the invention, a crude oil vacuum column bottoms residue stream is a feedstock to SDA, used to generate the DAO as a component of the heavy hydrocarbon feedstock to SHC. The DAO is then combined at the inlet of the SHC reactor with one or more recycle SHC liquid product streams, which may include a recycled portion of SHC pitch and/or SHC gas oil, as discussed above, as well as a recycled portion of an SHC high pressure separator bottoms liquid. In addition to DAO and recycled liquid products, other components of the heavy hydrocarbon feedstock to SHC include straight-run hydrocarbon fractions from crude oil distillation, such as straight-run gas oils (e.g., straight-run VGO) and vacuum column residues or portions of these streams that are not sent to SDA.
[0016]These and other aspects and embodiments relating to the present invention are apparent from the following Detailed Description.
BRIEF DESCRIPTION OF THE DRAWING
[0017]FIG. 1 depicts a representative, integrated solvent deasphalting and slurry hydrocracking process, which is incorporated into a typical refinery flowscheme.
DETAILED DESCRIPTION
[0018]Embodiments of the invention relate to the use of solvent deasphalting (SDA) in combination with slurry hydrocracking (SHC) to upgrade a heavy hydrocarbon feedstock. A representative heavy hydrocarbon feedstock to the SHC is a mixture of deasphalted oil (DAO), as a fresh feed component, and at least one liquid product recovered from the SHC effluent and recycled to an SHC reactor (or reaction zone). The DAO is often obtained from subjecting a crude oil vacuum distillation column residue to solvent deasphalting in the presence of a solvent. The heavy hydrocarbon feedstock to SHC may therefore comprise all or a portion of DAO produced from SDA, either directly from this process or following one or more pretreatment and/or fractionation steps. To reduce the overall costs and complexity of the integrated process, the DAO is usually not subjected to an additional extraction (e.g., involving phase separation), prior to passing the DAO to SHC for use as a heavy hydrocarbon feedstock component in this process.
[0019]In addition to DAO, the SDA process also generates an SDA pitch stream containing a large proportion of the metals, Conradson carbon residue, and other impurities present in the vacuum resid and/or other feed(s) to SDA. The SDA pitch may be used as a fuel oil blending component or in the production of asphalts or cement. Advantageously, the SDA upstream of SHC may be operated under conditions that favor a relatively high recovery/low purity of DAO, compared to conventional SDA processes that produce DAO for other conversion processes (e.g., hydrocracking) that require higher quality feeds.
[0020]According to one embodiment, for example, the heavy hydrocarbon feedstock comprises DAO together with one or more liquid products recovered from the effluent of the SHC reactor or reaction zone (e.g., by separation and/or fractionation) and recycled. Representative liquid products include the high pressure separator bottoms liquid, as well as gas oil and pitch products, which may be partially or entirely recycled. Combinations of these liquid products and/or portions of these products, may also be recycled. For example, in a representative embodiment, the hydrocarbon feedstock comprises, as recycled liquid products, both an SHC gas oil (or a recycled portion thereof) and a recycled portion of an SHC pitch obtained from vacuum column distillation of an SHC high pressure separator bottoms liquid. The integration of SDA with SHC in this manner provides important benefits as discussed above.
[0021]The heavy hydrocarbon feedstock, in addition to DAO and recycled liquid products as discussed above, may contain further components that can benefit from conversion in the SHC reaction zone to decrease the overall molecular weight of the heavy hydrocarbon feedstock, and/or remove organic sulfur and nitrogen compounds and metals. According to various embodiments, SHC is improved (e.g., by the suppression of coke formation) when a significant portion of the heavy hydrocarbon feedstock boils in a representative gas oil range (e.g., from about 343° C. (650° F.) to about 566° C. (1050° F.)) and only at most about 60% by weight, and often at most about 40% by weight, of the heavy hydrocarbon feedstock are compounds boiling above 566° C. (1050° F.), which generally originate from a recycled portion of SHC pitch.
[0022]Representative further components of the heavy hydrocarbon feedstock include residual oils such as a crude oil atmospheric distillation column residuum boiling above about 343° C. (650° F.), a crude oil vacuum distillation column residuum boiling above 566° C. (1050° F.), tars, bitumen, coal oils, and shale oils. Other asphaltene-containing materials such as whole or topped petroleum crude oils including heavy crude oils may also be used as components processed by SHC. In addition to asphaltenes, these further possible components of the heavy hydrocarbon feedstock, as well as others, generally also contain significant metallic contaminants (e.g., nickel, iron and vanadium), a high content of organic sulfur and nitrogen compounds, and a high Conradson carbon residue. The metals content of such components, for example, may be 100 ppm to 1,000 ppm by weight, the total sulfur content may range from 1% to 7% by weight, and the API gravity may range from about -5° to about 35°. The Conradson carbon residue of such components is generally at least about 5%, and is often from about 10% to about 30% by weight. Overall, many of the heavy hydrocarbon feedstock components of the SHC process, including DAO, have properties that render them detrimental to other types of catalytic conversion processes such as hydrocracking (both fixed bed and ebullating bed) and fluid catalytic cracking. A representative DAO, for example, has a metals content of at least about 500 ppm (e.g., from about 500 ppm to about 2000 ppm) and a Conradson carbon residue of at least about 10% (e.g., from about 10% to about 30%) by weight.
[0023]Integrated methods or processes for preparing SHC distillates generally involve passing a heavy hydrocarbon feedstock comprising DAO through an SHC reaction zone in the presence of hydrogen to provide an SHC effluent. The heavy hydrocarbon feedstock may be, but is not necessarily, present in a heterogeneous slurry catalyst system in the SHC reactor, in which the catalyst is in the form of a solid particulate. For purposes of the present disclosure, however, homogeneous catalyst systems, in which the catalytically active metal is present in the liquid phase and is dissolved in the heavy hydrocarbon feedstock (e.g., as an oil-soluble metal compound such as a metal sulfide), also fall within the definition of an SHC process, since homogeneous processes are equally applicable for upgrading the same types of heavy hydrocarbon feedstocks with the same advantageous results associated with the embodiments discussed herein.
[0024]The SHC reaction is normally carried out in the presence of a combined recycle gas containing hydrogen and under conditions sufficient to crack at least a portion of the heavy hydrocarbon feedstock to a lighter-boiling SHC distillate fraction that is recovered from the effluent of the SHC reactor. The combined recycle gas is a mixture of a hydrogen-rich gas stream, recovered from the SHC effluent (e.g., as an overhead gas stream from a high pressure separator) and fresh make-up hydrogen that is used to replace hydrogen consumed in the SHC reactor or reaction zone and lost through dissolution as well as in purge or vent streams. The recovery of SHC distillate typically involves the use of flash separation and/or distillation of the SHC effluent, or a lower boiling fraction or cut thereof (e.g., a fraction having a lower distillation endpoint), to separate the SHC distillate as a lower boiling component from the co-produced (or unconverted) liquid products, including SHC gas oil and SHC pitch, in the SHC effluent. As discussed above, portions or all of these recovered liquid products may be recycled to the SHC reactor or reaction zone.
[0025]A slurry formed with the heavy hydrocarbon feedstock is normally passed upwardly through the SHC reaction zone, with the slurry generally having a solid particulate content in the range from about 0.01% to about 10% by weight. The solid particulate is generally a compound of a catalytically active metal, or a metal in elemental form, either alone or supported on a refractory material such as an inorganic metal oxide (e.g., alumina, silica, titania, zirconia, and mixtures thereof). Other suitable refractory materials include carbon, coal, and clays. Zeolites and non-zeolitic molecular sieves are also useful as solid supports. One advantage of using a support is its ability to act as a "coke getter" or adsorbent of asphaltene precursors that might otherwise lead to fouling of process equipment.
[0026]Catalytically active metals for use in SHC include those from Group IVB, Group VB, Group VIB, Group VIIB, or Group VIII of the Periodic Table, which are incorporated in the heavy hydrocarbon feedstock in amounts effective for catalyzing desired hydrotreating and/or hydrocracking reactions to provide, for example, lower boiling hydrocarbons that may be fractionated from the SHC effluent as naphtha and/or distillate products in the substantial absence of the solid particulate. Representative metals include iron, nickel, molybdenum, vanadium, tungsten, cobalt, ruthenium, and mixtures thereof. The catalytically active metal may be present as a solid particulate in elemental form or as an organic compound or an inorganic compound such as a sulfide (e.g., iron sulfide) or other ionic compound. Metal or metal compound nanoaggregates may also be used to form the solid particulates.
[0027]Often, it is desired to form such metal compounds, as solid particulates, in situ from a catalyst precursor such as a metal sulfate (e.g., iron sulfate monohydrate) that decomposes or reacts in the SHC reaction zone environment, or in a pretreatment step, to form a desired, well-dispersed and catalytically active solid particulate (e.g., as iron sulfide). Precursors also include oil-soluble organometallic compounds containing the catalytically active metal of interest that thermally decompose to form the solid particulate (e.g., iron sulfide) having catalytic activity. Such compounds are generally highly dispersible in the heavy hydrocarbon feedstock and normally convert under pretreatment or SHC reaction zone conditions to the solid particulate that is contained in the slurry effluent. An exemplary in situ solid particulate preparation, involving pretreating the heavy hydrocarbon feedstock and precursors of the ultimately desired metal compound, is described, for example, in U.S. Pat. No. 5,474,977.
[0028]Other suitable precursors include metal oxides that may be converted to catalytically active (or more catalytically active) compounds such as metal sulfides. In a particular embodiment, a metal oxide containing mineral may be used as a precursor of a solid particulate comprising the catalytically active metal (e.g., iron sulfide) on an inorganic refractory metal oxide support (e.g., alumina). Bauxite represents a particular precursor in which conversion of iron oxide crystals contained in this mineral provides an iron sulfide catalyst as a solid particulate, where the iron sulfide after conversion is supported on the alumina that is predominantly present in the bauxite precursor.
[0029]Conditions in the SHC reactor or reaction zone generally include a temperature from about 343° C. (650° F.) to about 538° C. (1000° F.), a pressure from about 3.5 MPa (500 psig) to about 21 MPa (3000 psig), and a space velocity from about 1 to about 30 volumes of heavy hydrocarbon feedstock per hour per volume of said SHC zone. Advantageously, the SHC conditions generally include a relatively high space velocity and low reactor pressure (or hydrogen partial pressure), relative to conventional fixed or ebullating bed hydrocracking processes. The catalyst and conditions used in the SHC reaction zone are suitable for upgrading the heavy hydrocarbon feedstock to provide a lower boiling component, namely an SHC distillate fraction, in the SHC effluent exiting the SHC reaction zone. The SHC distillate is generally recovered from the total SHC effluent (optionally after the removal of a hydrogen-rich gas stream for recycle to the SHC reactor, as discussed above) as a fraction having a distillation end point which is normally above that of naphtha. The SHC distillate, for example, may be recovered as a fraction having a distillation end point temperature typically in the range from about 204° C. (400° F.) to about 399° C. (750° F.), and often from about 260° C. (500° F.) to about 343° C. (650° F.), with heavier boiling compounds being recovered as liquid products, discussed above, that are completely or partially recycled to the SHC reactor or reaction zone.
[0030]According to a particular embodiment, the SHC distillate and a higher-boiling SHC fraction may be recovered as an overhead and a bottoms stream, respectively, exiting a hot high pressure separator to which the SHC effluent is fed (optionally after the removal of the hydrogen-rich gas stream). Fractionation (e.g., in a vacuum distillation column) of all or a portion of the higher-boiling SHC fraction, namely the SHC high pressure separator bottoms liquid, can then provide the SHC gas oil and SHC pitch, all or portions of which may be recycled. According to representative embodiments of the invention, the yield of SHC distillate (having a distillation end point in these ranges), is generally at least 30% by weight (e.g., from about 30% to about 65% by weight), normally at least about 35% by weight (e.g., from about 35% to about 55% by weight), and often at least about 40% by weight (e.g., from about 40% to about 50% by weight), of the combined SHC effluent weight (e.g., the combined weight of the SHC distillate and SHC gas oil), excluding the solid particulate.
[0031]Depending on the desired end products, the SHC distillate may itself be fractionated to yield, for example, naphtha and diesel fuel having varying distillation end point temperatures. For example, a relatively light naphtha may be separated from the SHC distillate, having a distillation end point temperature from about 175° C. (347° F.) to about 193° C. (380° F.). According to other embodiments, a relatively heavy naphtha may be separated, having a distillation end point temperature from about 193° C. (380° F.) to about 204° C. (400° F.). The naphtha may be fractionated into one or more naphtha fractions, for example light naphtha, gasoline, and heavy naphtha, with representative distillation end points being in the ranges from about 138° C. (280° F.) to about 160° C. (320° F.), from about 168° C. (335° F.) to about 191° C. (375° F.), and from about 193° C. (380° F.) to about 216° C. (420° F.), respectively.
[0032]Depending on the particular separation/fractionation conditions used to recover the SHC distillate, this stream will normally contain quantities of organic nitrogen compounds and organic sulfur compounds. For example, the amount of total sulfur, substantially present in the form of organic sulfur compounds such as alkylbenzothiophenes, in this stream is generally from about 0.1% to about 4%, normally from about 0.2% to about 2.5%, and often from about 0.5% to about 2%. The amount of total nitrogen in the SHC distillate, substantially present in the form of organic nitrogen compounds such as non-basic aromatic compounds including cabazoles, will normally be from about 100 ppm to about 2%, and often from about 100 ppm to about 750 ppm. The SHC distillate will also generally contain a significant fraction of polyaromatics such as 2-ring aromatic compounds (e.g., fused aromatic rings such as naphthalene and naphthalene derivatives) as well as multi-ring aromatic compounds. According to some representative embodiments, the combined amount of 2-ring aromatic compounds and multi-ring aromatic compounds is at least about 50% by weight of the SHC distillate, whereas the amount of mono-ring aromatic compounds (e.g., benzene and benzene derivatives such as alkylaromatic compounds) typically represents only at most about 20% by weight.
[0033]The heavy hydrocarbon feedstock to the SHC reactor or reaction zone, as discussed above, comprises, in addition to the SHC gas oil, DAO produced from subjecting a vacuum column resid to SDA in the presence of a solvent. In addition to the DAO and recycled liquid components as discussed above, other representative gas oil components that may be present in the heavy hydrocarbon feedstock include straight-run gas oils such as vacuum gas oil, recovered by fractional distillation of crude petroleum. Other gas oils produced in refineries include deasphalted gas oil and visbreaker gas oil. Gas oils, as well as the combined heavy hydrocarbon feedstock to the SHC reaction zone that comprises these gas oils, can therefore be a mixture of hydrocarbons boiling in range from about 343° C. (650° F.) to an end point of about 593° C. (1100° F.), with other representative distillation end points being about 566° C. (1050° F.), about 538° C. (1000° F.), and about 482° C. (900° F.). A representative SHC gas oil has a distillation end point temperature from about 427° C. (800° F.) to about 538° C. (1000° F.). In the case of a straight-run vacuum gas oil, the distillation end point is governed by the crude oil vacuum fractionation column and particularly the fractionation temperature cutoff between the vacuum gas oil and vacuum column bottoms split. Thus, refinery gas oil components suitable in heavy hydrocarbon feedstocks to the SHC reactor, such as straight-run fractions, often result from crude oil fractionation or distillation operations, while other gas oil components are obtained following one or more hydrocarbon conversion reactions.
[0034]The SHC may be beneficially combined with hydrotreating, such that the recovered SHC distillate or a fraction thereof, (e.g., a naphtha fraction or a diesel fuel fraction) is catalytically hydrotreated in a hydrotreating zone to reduce the content of total sulfur and/or total nitrogen. According to specific embodiments, for example, a hydrotreated naphtha fraction may be obtained having a sulfur content of less than about 30 ppm by weight, often less than about 10 ppm by weight, and even less than about 5 ppm by weight. A hydrotreated diesel fuel may be obtained having a sulfur content of less than about 50 ppm by weight, often less than about 20 ppm by weight, and even less than about 10 ppm by weight. Hydrotreating of SHC distillates to provide a hydrotreated distillate, or hydrotreating of fractions of the SHC distillates, may therefore provide low-sulfur products and even ultra low sulfur naphtha and diesel fractions in compliance with applicable tolerances. According to a preferred embodiment, the SHC distillate has a sufficient API gravity for incorporation into a crude oil or synthetic crude oil obtained, for example, from tar sands. Representative API gravity values are greater than about 20° (e.g., from about 25° to about 40°) and greater than about 35° (e.g., from about 40° to about 55°).
[0035]In other embodiments, integration of the SHC process with hydrotreating can involve, for example, passing an additional refinery distillate stream, such as a straight-run distillate, to the hydrotreating zone or reactor. Whether or not one or more additional streams are hydrotreated in combination with the SHC distillate, the hydrotreating is normally carried out in the presence of a fixed bed of hydrotreating catalyst and a combined recycle gas stream containing hydrogen. Typical hydrotreating conditions include a temperature from about 260° C. (500° F.) to about 426° C. (800° F.), a pressure from about 7.0 MPa (1000 psig) to about 21 MPa (3000 psig), and a liquid hourly space velocity (LHSV) from about 0.1 hr-1 to about 10 hr-1. As is understood in the art, the Liquid Hourly Space Velocity (LHSV, expressed in units of hr-1) is the volumetric liquid flow rate over the catalyst bed divided by the bed volume and represents the equivalent number of catalyst bed volumes of liquid processed per hour. The LHSV is closely related to the inverse of the reactor residence time. Suitable hydrotreating catalysts comprise a metal selected from the group consisting of nickel, cobalt, tungsten, molybdenum, and mixtures thereof, on a refractory inorganic oxide support.
[0036]As discussed above, the SHC process is advantageously integrated with SDA, wherein a DAO produced from this process is passed to the SHC reaction zone for upgrading. Recycled liquid products, such as a combination of both a recycled portion of an SHC gas oil and a recycled portion of an SHC pitch, beneficially reduce SHC catalyst consumption requirements while providing a significant level of conversion the DAO, and the heavy hydrocarbon feedstock in general, to higher-value products. According to representative embodiments, the heavy hydrocarbon feedstock is converted completely or substantially completely (e.g., with at least about 95% overall conversion) to hydrocarbons having a boiling point temperature of 538° C. (1000° F.) or less in the SHC reaction zone. Conversion levels to these hydrocarbons on a "per pass" basis are generally at least about 75%, typically at least about 85%, and often at least about 90%.
[0037]A typical SHC pitch stream, all or a portion of which may be recycled to the SHC reaction zone as a component of the heavy hydrocarbon feedstock, may be obtained, for example, from vacuum column distillation of an SHC high pressure separator bottoms liquid. In particular, this vacuum column distillation results in, relative to the separator bottoms liquid fed to this column, a lower-boiling SHC gas oil (e.g., SHC VGO) and a higher-boiling SHC pitch, normally obtained as a slurry in combination with the solid particulate. Accordingly, in a representative embodiment, a higher-boiling SHC effluent fraction is recovered as a bottoms liquid, itself in the form of a slurry with the solid particulate, exiting a hot high pressure separator. A portion of this bottoms stream may be recycled directly to the SHC reactor or reaction zone, while a non-recycled portion may be fractionated using vacuum fractionation to yield the SHC gas oil, all or some of which may be recycled to the SHC reactor, and the heavier SHC pitch, as described above. As with the SHC gas oil, all or a portion of the SHC pitch may also be recycled to the SHC reactor or reaction zone. In a specific embodiment, the heavy hydrocarbon feedstock to the SHC comprises, as recycle components, (i) a recycled portion of the SHC high pressure separator bottoms liquid, (ii) a recycled portion of an SHC gas oil, and (iii) a recycled portion of an SHC pitch, with (ii) and (iii) being obtained from fractionation of a non-recycled portion of the SHC high pressure separator bottoms liquid.
[0038]A typical SHC pitch, recovered as a higher boiling component, by flash separation or fractionation of said SHC effluent, will comprise or consist essentially of hydrocarbons boiling at temperatures greater than about 482° C. (900° F.), usually greater than about 538° C. (1000° F.), and often greater than about 593° C. (1100° F.). While aspects of the invention are associated with the advantages obtained, particularly with respect to catalyst make-up requirements, from recycling the SHC pitch to the SHC reactor or reaction zone, it is often desired to remove a non-recycled portion of the SHC pitch as a drag stream containing solid particulate. This prevents the accumulation of unwanted solids and other contaminants to unacceptably high levels. In an exemplary process, the particulate drag stream represents less than about 70%, normally less than about 50%, and often less than about 25%, of the total SHC pitch stream obtained from vacuum column distillation of the SHC high pressure separator bottoms. These purge rates of the solid particulate correspond to associated catalyst make-up rates that are considerably less than those required in the conventional processing of vacuum distillation column resids using SHC, but without DAO as a heavy hydrocarbon feedstock component, optionally in combination with the other recycled liquid products described above.
[0039]The present invention therefore relates to overall refinery flowschemes or processes for upgrading heavy hydrocarbon feedstocks discussed above, and especially those comprising DAO. Due to the recycle of liquid products such as SHC gas oil and SHC pitch, or portions of these streams, substantially all of the net products are either distillates or coke, with a relatively minor production of low-value SHC gas oil and SHC pitch. According to representative embodiments of the invention, the yield of distillate products (e.g., a hydrotreated distillate as discussed above) accounts for at least 80% of the SHC process yield (e.g., from about 80% to about 99%), and often accounts for at least 85% of this yield (e.g., from about 85% to about 95%).
[0040]Further aspects of the invention relate to utilizing the SHC processes discussed above for making a synthetic crude oil or synthetic crude oil blending component. The processes involve passing a DAO derived from solvent deasphalting, with optional integration of the process with a hydrotreater as discussed above. Depending on the fractionation conditions used for downstream processing of the SHC effluent, an SHC distillate may be obtained having hydrocarbons essentially all boiling in the distillate range or lower. In representative embodiments, less than about 10% by weight, and often less than about 5% by weight, of the SHC distillate are hydrocarbons boiling at a temperature of greater than 343° C. (650° F.).
[0041]The integrated SDA/SHC processes described herein may be further integrated with crude oil fractionation, such that a straight-run distillate from a crude oil atmospheric distillation column is hydrotreated together the SHC distillate. Fractionation of the bottoms product from this distillation column in a separate vacuum distillation column may then be carried out to yield VGO and/or vacuum residuum (or resid) that is/are passed to the SDA extractor, as discussed above, to provide DAO used in the heavy hydrocarbon feedstock to SHC.
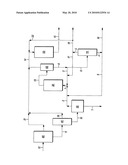
[0042]A representative process flowscheme illustrating a particular embodiment for carrying out the methods described above is depicted in FIG. 1. FIG. 1 is to be understood to present an illustration of the invention and/or principles involved. As is readily apparent to one of skill in the art having knowledge of the present disclosure, methods according to various other embodiments of the invention will have configurations, components, and operating parameters determined, in part, by the specific feedstocks, products, and product quality specifications.
[0043]According to the embodiment illustrated in FIG. 1, a slurry hydrocracking (SHC) reactor or reaction zone 20 is integrated into a refinery flowscheme. The heavy hydrocarbon feedstock 1 to this reaction zone is a combination of (i) a deasphalted oil (DAO) 2 produced from solvent deasphalting process 30, (ii) a recycled portion 3 of the liquid (in combination with solid particulate) obtained as a bottoms liquid product 8 from hot high pressure separator (HHPS) 60, (iii) a recycled portion 9 of an SHC pitch stream 4 that is obtained as a heavier-boiling fraction or bottoms product of SHC vacuum distillation column 70, and (iv) a recycled portion 17 of an SHC VGO stream 5 obtained as a lighter-boiling fraction or overhead product from this column. Both (iii) and (iv) are therefore obtained from fractionation, in vacuum distillation column 70, of non-recycled portion 6 of bottoms liquid product 8 from HHPS 60.
[0044]As a component of heavy hydrocarbon feedstock 1 to SHC reactor or reaction zone 20, DAO stream 2 is obtained as the solvent-extracted product from upstream solvent deasphalting (SDA) process 30 which also generates SDA pitch stream 7. The SDA/SHC process illustrated in FIG. 1 is further integrated in an overall refinery process flowscheme, with the feed stream to SDA process 30 comprising vacuum column residue stream (or resid) 10 from crude vacuum column or tower 40, typically containing hydrocarbons boiling above (i.e., having a cutpoint temperature) of about 566° C. (1050° F.). As shown in FIG. 1, atmospheric column 80 generates atmospheric residue or reduced crude stream 11, with a typical cutpoint temperature of about 343° C. (650° F.) that is fractionated in vacuum column 40.
[0045]The SHC process is therefore utilized in an integrated manner to upgrade DAO stream 2 from solvent deasphalting process 30, which, as discussed above, is efficiently processed in the SHC reactor or reaction zone, relative to other conversion processes. The total SHC effluent stream 13 is then subjected to downstream separation/fractionation operations to recover upgraded products, remove pitch, and recycle intermediates. According to the embodiment illustrated in FIG. 1, total SHC effluent stream 13 is separated using hot high pressure separator (HHPS) 60 to recover SHC distillate 14, generally boiling in a range above that of naphtha. A non-recycled portion 6 of higher-boiling fraction 8 recovered from SHC effluent stream 13, and in particular from the bottoms of HHPS 60, is then fractionated in SHC fractionator 70, typically operating as a vacuum column. SHC fractionator separates SHC VGO stream 5 from SHC pitch stream 4, with portions 17 and 9 of streams 5 and 4, respectively, being recycled back to SHC reaction zone 20. Both of recycled streams 17,9 benefit the SHC process, in terms of minimizing catalyst requirements, as discussed above. A non-recycled portion 16 of SHC pitch stream 4 is removed as a solid particulate-containing drag stream to prevent the accumulation of excessive amounts of impurities. The removed solid particulate (e.g., catalyst) is replaced with fresh makeup catalyst (not shown), where the makeup (and removal) rates of solid particulate typically represent less than about 25% of the solid particulate flow rate in SHC pitch stream 4. A non-recycled portion 18 of SHC VGO stream 5 is also removed.
[0046]Optionally, the heavy hydrocarbon feedstock 1 can further include a VGO fraction 19 from vacuum column 40, which, for example, contains hydrocarbons boiling in the range from about 343° C. (650° F.) to about 566° C. (1050° F.). This VGO fraction 19 may optionally be fed directly to distillate hydrotreating process 50. Another stream optionally used as an incremental feedstock to hydrotreating process 50 is a straight-run distillate 21 obtained as a distillate fraction of crude oil stream 12, fractionated in crude atmospheric column or tower 80. A further stream which may be hydrotreated is a portion of the SHC VGO stream 5 or 18, namely SHC VGO hydrotreater feed stream 22, from SHC vacuum distillation column 70. Thus, SHC distillate 14, optionally with any combination or all of these streams 19, 21, or 22 is used to obtain hydrotreated distillate 15 as a product of the overall process having reduced nitrogen compound and sulfur compound impurities and/or an API gravity as discussed above that may be utilized as a blending component for synthetic crude oil.
[0047]In the overall integrated SDA/SHC process illustrated in FIG. 1, the SDA process 30 is advantageously operated with a high recovery of DAO that cannot be economically processed using conventional fixed bed or ebullating bed hydroprocessing. Also, the integration of SDA process 30 with SHC produces essentially the net products of pitch streams 7,16 and hydrotreated distillate 15. As is apparent from this description, overall aspects of the invention are directed to the integration of solvent deasphalting and slurry hydrocracking to optimize refinery operations. In view of the present disclosure, it will be seen that several advantages may be achieved and other advantageous results may be obtained. In view of the present disclosure, those having skill in the art will recognize the applicability of the methods disclosed herein to any of a number of integrated SHC processes. It will also be appreciated that various changes could be made in the above processes without departing from the scope of the present disclosure.
Claims:
1. An integrated process for preparing a slurry hydrocracking (SHC)
distillate, the process comprising:(a) passing a heavy hydrocarbon
feedstock comprising a deasphalted oil (DAO) through an SHC reaction zone
in the presence of hydrogen to provide an SHC effluent; and(b) recovering
said SHC distillate from said SHC effluent.
2. The process of claim 1, further comprising, prior to step (a), subjecting a vacuum distillation column residue to solvent deasphalting (SDA) in the presence of a solvent to obtain said DAO and an SDA pitch.
3. The process of claim 1, wherein the heavy hydrocarbon feedstock is present as a slurry, in combination with a solid particulate, in said SHC reaction zone.
4. The process of claim 3, wherein said solid particulate comprises a compound of a metal of Group IVB, Group VB, Group VIB, Group VIIB, or Group VIII.
5. The process of claim 1, wherein said heavy hydrocarbon feedstock further comprises at least one liquid product recovered from said SHC effluent and recycled to said SHC reaction zone.
6. The process of claim 5, wherein said liquid product is selected from the group consisting of an SHC high pressure separator bottoms liquid, an SHC gas oil, an SHC pitch, recycled portions thereof, and combinations thereof.
7. The process of claim 6, wherein said heavy hydrocarbon feedstock further comprises both a recycled portion of an SHC gas oil and a recycled portion of an SHC pitch obtained from vacuum column distillation of an SHC high pressure separator bottoms liquid.
8. The process of claim 6, wherein said liquid product comprises (i) a recycled portion of an SHC high pressure separator bottoms liquid, (ii) a recycled portion of an SHC gas oil, and (iii) a recycled portion of an SHC pitch, wherein (ii) and (iii) are obtained from fractionation of a non-recycled portion of said SHC high pressure separator bottoms liquid.
9. The process of claim 6, wherein, said SHC distillate is separated, as a lower boiling component, from an SHC high pressure separator bottoms liquid by flash separation or fractionation of said SHC effluent.
10. The process of claim 6, wherein said SHC gas oil has a distillation end point temperature from about 427.degree. C. (800.degree. F.) to about 538.degree. C. (1000.degree. F.).
11. The process of claim 6, wherein said heavy hydrocarbon feedstock further comprises an SHC pitch obtained from vacuum column distillation of an SHC high pressure separator bottoms, and wherein a non-recycled portion of said SHC pitch is removed as a solid particulate drag stream.
12. The process of claim 11, wherein said non-recycled portion of said SHC pitch removed as a solid particulate drag stream represents less than about 70% of said SHC pitch obtained from vacuum column distillation of said SHC high pressure separator bottoms.
13. The process of claim 11, wherein said SHC pitch comprises hydrocarbons boiling at a temperature of greater than about 538.degree. C. (1000.degree. F.).
14. The process of claim 1, wherein said SHC reaction zone is maintained at a temperature from about 343.degree. C. (650.degree. F.) to about 593.degree. C. (1100.degree. F.), a pressure from about 3.5 MPa (500 psig) to about 21 MPa (3000 psig), and a space velocity from about 1 to about 30 volumes of heavy hydrocarbon feedstock per hour per volume of said SHC zone.
15. The process of claim 1, wherein a conversion of said heavy hydrocarbon feedstock to hydrocarbons having a boiling point temperature of 538.degree. C. (1000.degree. F.) or less in said SHC reaction zone is at least about 90% per pass.
16. The process of claim 1, further comprising hydrotreating said SHC distillate in a hydrotreating zone to obtain a hydrotreated distillate.
17. The process of claim 1, wherein said DAO has a metals content of at least about 500 ppm by weight.
18. The process of claim 1, wherein said DAO has a Conradson carbon residue of at least about 10% by weight.
19. A method for making a distillate hydrocarbon by integrating solvent deasphalting and slurry hydrocracking (SHC), the method comprising:(a) passing a slurry comprising a recycled portion of an SHC gas oil, a recycled portion of an SHC pitch, a deasphalted oil obtained from solvent deasphalting, and a solid particulate through an SHC reaction zone in the presence of hydrogen to provide an SHC effluent,(b) recovering said distillate hydrocarbon and an SHC high pressure separator bottoms liquid from flash separation of said SHC effluent,(c) recycling a first portion of said SHC high pressure separator bottoms liquid to said SHC reaction zone,(d) fractionating a second portion of said SHC high pressure separator bottoms liquid in an SHC vacuum distillation column to provide said SHC pitch and said SHC gas oil, as a heavy fraction and a light fraction, respectively,(e) recycling said recycled portion of said SHC gas oil and said recycled portion of said SHC pitch to said SHC reaction zone, and(f) removing a non-recycled portion of said SHC pitch as a solid particulate drag stream.
20. The method of claim 19, further comprising hydrotreating said distillate hydrocarbon to obtain a hydrotreated distillate.
Description:
FIELD OF THE INVENTION
[0001]The present invention relates to methods for preparing distillate hydrocarbons using slurry hydrocracking (SHC) to upgrade high-boiling or heavy hydrocarbons obtained from refinery operations and particularly solvent deasphalting (SDA). The integration of SHC with SDA and optionally other processes such as crude oil fractionation and/or hydrotreating may be used to obtain a high quality (e.g., high API gravity and/or low sulfur) distillate.
DESCRIPTION OF RELATED ART
[0002]Solvent deasphalting (SDA) generally refers to refinery processes that upgrade hydrocarbon fractions using an extraction process in the presence of a solvent. The hydrocarbon fractions are often obtained from the distillation of crude oil, and include hydrocarbon residues (or resids) or gas oils from atmospheric column or vacuum column distillation. Solvents used in SDA are typically lower-boiling paraffinic hydrocarbons such as propane, butane, and their mixtures, having the ability to extract a deasphalted oil (DAO) with relatively lower levels of contaminants such as sulfur- and nitrogen-containing compounds, metals, and Conradson carbon residue. The extraction usually occurs in a countercurrent extractor, with the solvent phase and its extracted components flowing in an upward direction. In addition to DAO, the other major product of SDA is pitch, a highly viscous hydrocarbon that contains significant portions of the (non-extracted) contaminants present in crude oil.
[0003]The yields and quality of both the DAO and pitch depend on the composition of the SDA feed, the type and amount of solvent, and the extraction conditions. The DAO produced from SDA is a generally nondistillable product requiring further upgrading with fluid catalytic cracking (FCC), hydrocracking, and/or hydrotreating. Additionally, the significant quantities of pitch from SDA make this process less economically attractive compared to alternative heavy oil conversion processes.
[0004]Further refinery process streams normally sent to conventional conversion processes such as FCC, in order to yield more salable products, include gas oils and particularly vacuum gas oil (VGO). VGO is produced in a number of refinery operations, including slurry hydrocracking, coking, crude oil fractionation, and visbreaking, which process heavy hydrocarbon feedstocks. Because of their significant levels of contaminants (e.g., metals and sulfur compounds) that deactivate supported metal catalysts, in addition to coke precursors in these streams, gas oils are unfortunately not easily processed according to conventional catalytic conversion methods. The conversion of coker gas oils to more salable distillate and naphtha blending components for transportation fuels is therefore associated with a number of drawbacks.
[0005]Like SDA, slurry hydrocracking (SHC) is also used for the upgrading of heavy hydrocarbon feedstocks including those mentioned above. In SHC, these feedstocks are converted in the presence of hydrogen and solid catalyst particles (e.g., as a particulate metallic compound such as a metal sulfide) in a slurry phase. Representative slurry hydrocracking processes are described, for example, in U.S. Pat. No. 5,755,955 and U.S. Pat. No. 5,474,977. In addition to the VGO normally present in the reactor effluent, SHC (like SDA) produces a low-value, refractory pitch stream that normally cannot be economically upgraded or even blended into other products such as fuel oil or synthetic crude oil, due to its high viscosity and solids content. Moreover, SHC has disadvantages relative to other heavy hydrocarbon conversion processes, including a significant catalyst consumption requirement and a high capital cost due to the comparatively larger reactor size and high operating pressures.
[0006]Particular sources of synthetic crude oil of increasing interest, and for which blending components are sought to improve their flow characteristics, are bitumen and oil sands. Bitumen refers to the low-quality hydrocarbonaceous material recovered from oil sand deposits, such as those found in the vast Athabasca region of Alberta, Canada, as well as in Venezuela and the United States. Bitumen and oil sands are recognized as a valuable sources of "semi-solid" petroleum or synthetic crude oil, which can be refined into many valuable end products including transportation fuels such as gasoline or even petrochemicals.
[0007]There is an ongoing need in the art for process in which heavy hydrocarbons (e.g., atmospheric column and vacuum column resids as well as gas oils) are converted or upgraded with improved efficiency. There is also a need for such processes in which the net production of low-value end products, including gas oils and pitch, is minimized. There is further a need for overall crude oil refining processes that include the upgrading of crude oil residues and particularly those obtained in significant proportions from heavy crude oil feedstocks. Ideally, the products of such refining processes should be suitable as transportation fuel (e.g., diesel and/or naphtha) blending components or even for blending into synthetic crude oils to improve their properties (e.g., viscosity and/or specific gravity).
SUMMARY OF THE INVENTION
[0008]Aspects of the invention relate to the finding that slurry hydrocracking (SHC) can be effectively integrated with other refining processes and particularly solvent deasphalting (SDA), hydrotreating, and/or crude oil fractionation to produce a high value distillate stream while recycling low-value gas oils, preferably to extinction, as well as at least a portion of the SHC pitch. SHC is generally known in the art for its ability to convert vacuum column residues to lighter products. However, it has now been discovered that the use of a deasphalted oil (DAO) from SDA as a heavy hydrocarbon feedstock component or incremental feed to SHC results in operating synergies having commercially important advantages.
[0009]The DAO products from SDA processes, and particularly those processes that are operated with relatively high recoveries of DAO and low recoveries of pitch, have high levels of metal contaminants and Conradson carbon residue, making such DAO products difficult to upgrade with conventional FCC or hydrocracking, using either a fixed bed or ebullating bed of catalyst. Embodiments of the invention are associated with the discovery that DAO is effectively processed as a hydrocarbon feedstock component of SHC, with complete or essentially complete overall conversion to an SHC distillate. This distillate may be fractionated to provide, for example, lower- and higher-boiling products such as naphtha and diesel fuel.
[0010]The use of a heavy hydrocarbon feedstock comprising DAO may be combined with other SHC process features to obtain additional benefits. For example, recycling (e.g., to extinction) a portion of an SHC pitch and/or an SHC gas oil recovered from the SHC effluent allows for the complete or essentially complete overall conversion of these recycle streams (as well as the DAO) to the higher value SHC distillate. Moreover, the high content of polar aromatic compounds (e.g., mono- and multi-ring aromatics) in the recycle SHC gas oil, such as vacuum gas oil (VGO) obtained from vacuum column fractionation of a liquid product recovered from the SHC effluent, reduces the catalyst requirement of SHC. Without being bound by theory, it is believed that these recycled aromatics help solubilize asphaltenes in SHC recycle streams and thereby prevent the formation of precipitated agglomerates of asphaltenes and the solid particulate catalyst, leading to coking in the reactor or increased catalyst requirements to limit this coking. The recycling of SHC pitch can also help minimize make-up requirements, as this pitch contains catalyst with activity comparable to that of fresh catalyst.
[0011]Overall, the use of DAO as a component of the heavy hydrocarbon feedstock in SHC results in a surprisingly high conversion of this component (both on a per-pass and overall basis). Compared to conventional SHC, processing a vacuum column residue from crude oil fractionation in the absence of DAO, integrated processes according to the present invention have significantly reduced catalyst requirements, smaller reactor sizes, and/or lower reactor operating pressures. Relative to fixed bed or ebullating bed hydrocracking processes, the catalyst make-up rates of SHC processes described herein are surprisingly less adversely impacted by the high metals content of the DAO feed components, known to deactivate catalysts used in these conventional conversion processes. In fact, the types and amounts of contaminants in DAO products renders these streams difficult, in general, to further upgrade using FCC, hydrocracking, or hydrotreating.
[0012]In a representative integrated process, DAO is utilized in combination with recycled SHC gas oil, recovered from downstream fractionation/separation of the SHC effluent, in the overall heavy hydrocarbon feedstock to SHC. While portions of this feedstock may also generally include conventional components such as vacuum column resid, the presence of DAO results in the improvements discussed above. Moreover, DAO from solvent deasphalting (e.g., of a crude oil vacuum column distillation residue) is often readily available in large quantities, particularly in the case of refineries processing heavy crude oils.
[0013]The present invention is therefore associated with the effective utilization of DAO as an attractive incremental feedstock (e.g., in combination with a vacuum column residue), which is efficiently upgraded (e.g., cracked) using SHC to yield lighter and more valuable distillate and optionally naphtha products. According to some embodiments, the integration of SDA with SHC is carried out such that a portion of the SHC pitch and the SHC gas oil products, obtained from vacuum column fractionation of a high pressure separator bottoms liquid recovered from the SHC effluent, are recycled back to the SHC reactor or reaction zone.
[0014]In other embodiments, an integrated SDA/SHC process is combined with hydrotreating of the SHC distillate. As a result of the low (or non-existent) net yield of gas oil products such as VGO, due to recycling of heavy-boiling fractions back to the SHC reaction zone, the hydrotreated distillate has a sufficiently high API gravity (e.g., at least about 20°), making it attractive for blending into a synthetic crude oil that is transported via a pipeline. Thus, the hydrotreated distillate, or even the SHC distillate without hydrotreating, may be obtained as a high quality transportation fuel blending component with only a minor amount or essentially no hydrocarbons boiling at a temperature representative of gas oils (e.g., greater than about 343° C. (650° F.)). The integration of SHC with an existing refinery hydrotreating process, conventionally used for sulfur- and nitrogen-containing compound removal from distillates, may involve hydrotreating a recovered SHC distillate product in conjunction with a straight-run distillate obtained from crude oil fractionation and/or other refinery streams. This integration may advantageously reduce overall capital costs of the complex. As discussed above, the integration of SDA with SHC, optionally hydrotreating, and optionally other conventional refinery operations has the potential to provide significant benefits in terms of improved processing efficiency and product yields, reduction or elimination of low-value refractory byproducts, and/or the associated capital cost reduction.
[0015]In other representative embodiments of the invention, a crude oil vacuum column bottoms residue stream is a feedstock to SDA, used to generate the DAO as a component of the heavy hydrocarbon feedstock to SHC. The DAO is then combined at the inlet of the SHC reactor with one or more recycle SHC liquid product streams, which may include a recycled portion of SHC pitch and/or SHC gas oil, as discussed above, as well as a recycled portion of an SHC high pressure separator bottoms liquid. In addition to DAO and recycled liquid products, other components of the heavy hydrocarbon feedstock to SHC include straight-run hydrocarbon fractions from crude oil distillation, such as straight-run gas oils (e.g., straight-run VGO) and vacuum column residues or portions of these streams that are not sent to SDA.
[0016]These and other aspects and embodiments relating to the present invention are apparent from the following Detailed Description.
BRIEF DESCRIPTION OF THE DRAWING
[0017]FIG. 1 depicts a representative, integrated solvent deasphalting and slurry hydrocracking process, which is incorporated into a typical refinery flowscheme.
DETAILED DESCRIPTION
[0018]Embodiments of the invention relate to the use of solvent deasphalting (SDA) in combination with slurry hydrocracking (SHC) to upgrade a heavy hydrocarbon feedstock. A representative heavy hydrocarbon feedstock to the SHC is a mixture of deasphalted oil (DAO), as a fresh feed component, and at least one liquid product recovered from the SHC effluent and recycled to an SHC reactor (or reaction zone). The DAO is often obtained from subjecting a crude oil vacuum distillation column residue to solvent deasphalting in the presence of a solvent. The heavy hydrocarbon feedstock to SHC may therefore comprise all or a portion of DAO produced from SDA, either directly from this process or following one or more pretreatment and/or fractionation steps. To reduce the overall costs and complexity of the integrated process, the DAO is usually not subjected to an additional extraction (e.g., involving phase separation), prior to passing the DAO to SHC for use as a heavy hydrocarbon feedstock component in this process.
[0019]In addition to DAO, the SDA process also generates an SDA pitch stream containing a large proportion of the metals, Conradson carbon residue, and other impurities present in the vacuum resid and/or other feed(s) to SDA. The SDA pitch may be used as a fuel oil blending component or in the production of asphalts or cement. Advantageously, the SDA upstream of SHC may be operated under conditions that favor a relatively high recovery/low purity of DAO, compared to conventional SDA processes that produce DAO for other conversion processes (e.g., hydrocracking) that require higher quality feeds.
[0020]According to one embodiment, for example, the heavy hydrocarbon feedstock comprises DAO together with one or more liquid products recovered from the effluent of the SHC reactor or reaction zone (e.g., by separation and/or fractionation) and recycled. Representative liquid products include the high pressure separator bottoms liquid, as well as gas oil and pitch products, which may be partially or entirely recycled. Combinations of these liquid products and/or portions of these products, may also be recycled. For example, in a representative embodiment, the hydrocarbon feedstock comprises, as recycled liquid products, both an SHC gas oil (or a recycled portion thereof) and a recycled portion of an SHC pitch obtained from vacuum column distillation of an SHC high pressure separator bottoms liquid. The integration of SDA with SHC in this manner provides important benefits as discussed above.
[0021]The heavy hydrocarbon feedstock, in addition to DAO and recycled liquid products as discussed above, may contain further components that can benefit from conversion in the SHC reaction zone to decrease the overall molecular weight of the heavy hydrocarbon feedstock, and/or remove organic sulfur and nitrogen compounds and metals. According to various embodiments, SHC is improved (e.g., by the suppression of coke formation) when a significant portion of the heavy hydrocarbon feedstock boils in a representative gas oil range (e.g., from about 343° C. (650° F.) to about 566° C. (1050° F.)) and only at most about 60% by weight, and often at most about 40% by weight, of the heavy hydrocarbon feedstock are compounds boiling above 566° C. (1050° F.), which generally originate from a recycled portion of SHC pitch.
[0022]Representative further components of the heavy hydrocarbon feedstock include residual oils such as a crude oil atmospheric distillation column residuum boiling above about 343° C. (650° F.), a crude oil vacuum distillation column residuum boiling above 566° C. (1050° F.), tars, bitumen, coal oils, and shale oils. Other asphaltene-containing materials such as whole or topped petroleum crude oils including heavy crude oils may also be used as components processed by SHC. In addition to asphaltenes, these further possible components of the heavy hydrocarbon feedstock, as well as others, generally also contain significant metallic contaminants (e.g., nickel, iron and vanadium), a high content of organic sulfur and nitrogen compounds, and a high Conradson carbon residue. The metals content of such components, for example, may be 100 ppm to 1,000 ppm by weight, the total sulfur content may range from 1% to 7% by weight, and the API gravity may range from about -5° to about 35°. The Conradson carbon residue of such components is generally at least about 5%, and is often from about 10% to about 30% by weight. Overall, many of the heavy hydrocarbon feedstock components of the SHC process, including DAO, have properties that render them detrimental to other types of catalytic conversion processes such as hydrocracking (both fixed bed and ebullating bed) and fluid catalytic cracking. A representative DAO, for example, has a metals content of at least about 500 ppm (e.g., from about 500 ppm to about 2000 ppm) and a Conradson carbon residue of at least about 10% (e.g., from about 10% to about 30%) by weight.
[0023]Integrated methods or processes for preparing SHC distillates generally involve passing a heavy hydrocarbon feedstock comprising DAO through an SHC reaction zone in the presence of hydrogen to provide an SHC effluent. The heavy hydrocarbon feedstock may be, but is not necessarily, present in a heterogeneous slurry catalyst system in the SHC reactor, in which the catalyst is in the form of a solid particulate. For purposes of the present disclosure, however, homogeneous catalyst systems, in which the catalytically active metal is present in the liquid phase and is dissolved in the heavy hydrocarbon feedstock (e.g., as an oil-soluble metal compound such as a metal sulfide), also fall within the definition of an SHC process, since homogeneous processes are equally applicable for upgrading the same types of heavy hydrocarbon feedstocks with the same advantageous results associated with the embodiments discussed herein.
[0024]The SHC reaction is normally carried out in the presence of a combined recycle gas containing hydrogen and under conditions sufficient to crack at least a portion of the heavy hydrocarbon feedstock to a lighter-boiling SHC distillate fraction that is recovered from the effluent of the SHC reactor. The combined recycle gas is a mixture of a hydrogen-rich gas stream, recovered from the SHC effluent (e.g., as an overhead gas stream from a high pressure separator) and fresh make-up hydrogen that is used to replace hydrogen consumed in the SHC reactor or reaction zone and lost through dissolution as well as in purge or vent streams. The recovery of SHC distillate typically involves the use of flash separation and/or distillation of the SHC effluent, or a lower boiling fraction or cut thereof (e.g., a fraction having a lower distillation endpoint), to separate the SHC distillate as a lower boiling component from the co-produced (or unconverted) liquid products, including SHC gas oil and SHC pitch, in the SHC effluent. As discussed above, portions or all of these recovered liquid products may be recycled to the SHC reactor or reaction zone.
[0025]A slurry formed with the heavy hydrocarbon feedstock is normally passed upwardly through the SHC reaction zone, with the slurry generally having a solid particulate content in the range from about 0.01% to about 10% by weight. The solid particulate is generally a compound of a catalytically active metal, or a metal in elemental form, either alone or supported on a refractory material such as an inorganic metal oxide (e.g., alumina, silica, titania, zirconia, and mixtures thereof). Other suitable refractory materials include carbon, coal, and clays. Zeolites and non-zeolitic molecular sieves are also useful as solid supports. One advantage of using a support is its ability to act as a "coke getter" or adsorbent of asphaltene precursors that might otherwise lead to fouling of process equipment.
[0026]Catalytically active metals for use in SHC include those from Group IVB, Group VB, Group VIB, Group VIIB, or Group VIII of the Periodic Table, which are incorporated in the heavy hydrocarbon feedstock in amounts effective for catalyzing desired hydrotreating and/or hydrocracking reactions to provide, for example, lower boiling hydrocarbons that may be fractionated from the SHC effluent as naphtha and/or distillate products in the substantial absence of the solid particulate. Representative metals include iron, nickel, molybdenum, vanadium, tungsten, cobalt, ruthenium, and mixtures thereof. The catalytically active metal may be present as a solid particulate in elemental form or as an organic compound or an inorganic compound such as a sulfide (e.g., iron sulfide) or other ionic compound. Metal or metal compound nanoaggregates may also be used to form the solid particulates.
[0027]Often, it is desired to form such metal compounds, as solid particulates, in situ from a catalyst precursor such as a metal sulfate (e.g., iron sulfate monohydrate) that decomposes or reacts in the SHC reaction zone environment, or in a pretreatment step, to form a desired, well-dispersed and catalytically active solid particulate (e.g., as iron sulfide). Precursors also include oil-soluble organometallic compounds containing the catalytically active metal of interest that thermally decompose to form the solid particulate (e.g., iron sulfide) having catalytic activity. Such compounds are generally highly dispersible in the heavy hydrocarbon feedstock and normally convert under pretreatment or SHC reaction zone conditions to the solid particulate that is contained in the slurry effluent. An exemplary in situ solid particulate preparation, involving pretreating the heavy hydrocarbon feedstock and precursors of the ultimately desired metal compound, is described, for example, in U.S. Pat. No. 5,474,977.
[0028]Other suitable precursors include metal oxides that may be converted to catalytically active (or more catalytically active) compounds such as metal sulfides. In a particular embodiment, a metal oxide containing mineral may be used as a precursor of a solid particulate comprising the catalytically active metal (e.g., iron sulfide) on an inorganic refractory metal oxide support (e.g., alumina). Bauxite represents a particular precursor in which conversion of iron oxide crystals contained in this mineral provides an iron sulfide catalyst as a solid particulate, where the iron sulfide after conversion is supported on the alumina that is predominantly present in the bauxite precursor.
[0029]Conditions in the SHC reactor or reaction zone generally include a temperature from about 343° C. (650° F.) to about 538° C. (1000° F.), a pressure from about 3.5 MPa (500 psig) to about 21 MPa (3000 psig), and a space velocity from about 1 to about 30 volumes of heavy hydrocarbon feedstock per hour per volume of said SHC zone. Advantageously, the SHC conditions generally include a relatively high space velocity and low reactor pressure (or hydrogen partial pressure), relative to conventional fixed or ebullating bed hydrocracking processes. The catalyst and conditions used in the SHC reaction zone are suitable for upgrading the heavy hydrocarbon feedstock to provide a lower boiling component, namely an SHC distillate fraction, in the SHC effluent exiting the SHC reaction zone. The SHC distillate is generally recovered from the total SHC effluent (optionally after the removal of a hydrogen-rich gas stream for recycle to the SHC reactor, as discussed above) as a fraction having a distillation end point which is normally above that of naphtha. The SHC distillate, for example, may be recovered as a fraction having a distillation end point temperature typically in the range from about 204° C. (400° F.) to about 399° C. (750° F.), and often from about 260° C. (500° F.) to about 343° C. (650° F.), with heavier boiling compounds being recovered as liquid products, discussed above, that are completely or partially recycled to the SHC reactor or reaction zone.
[0030]According to a particular embodiment, the SHC distillate and a higher-boiling SHC fraction may be recovered as an overhead and a bottoms stream, respectively, exiting a hot high pressure separator to which the SHC effluent is fed (optionally after the removal of the hydrogen-rich gas stream). Fractionation (e.g., in a vacuum distillation column) of all or a portion of the higher-boiling SHC fraction, namely the SHC high pressure separator bottoms liquid, can then provide the SHC gas oil and SHC pitch, all or portions of which may be recycled. According to representative embodiments of the invention, the yield of SHC distillate (having a distillation end point in these ranges), is generally at least 30% by weight (e.g., from about 30% to about 65% by weight), normally at least about 35% by weight (e.g., from about 35% to about 55% by weight), and often at least about 40% by weight (e.g., from about 40% to about 50% by weight), of the combined SHC effluent weight (e.g., the combined weight of the SHC distillate and SHC gas oil), excluding the solid particulate.
[0031]Depending on the desired end products, the SHC distillate may itself be fractionated to yield, for example, naphtha and diesel fuel having varying distillation end point temperatures. For example, a relatively light naphtha may be separated from the SHC distillate, having a distillation end point temperature from about 175° C. (347° F.) to about 193° C. (380° F.). According to other embodiments, a relatively heavy naphtha may be separated, having a distillation end point temperature from about 193° C. (380° F.) to about 204° C. (400° F.). The naphtha may be fractionated into one or more naphtha fractions, for example light naphtha, gasoline, and heavy naphtha, with representative distillation end points being in the ranges from about 138° C. (280° F.) to about 160° C. (320° F.), from about 168° C. (335° F.) to about 191° C. (375° F.), and from about 193° C. (380° F.) to about 216° C. (420° F.), respectively.
[0032]Depending on the particular separation/fractionation conditions used to recover the SHC distillate, this stream will normally contain quantities of organic nitrogen compounds and organic sulfur compounds. For example, the amount of total sulfur, substantially present in the form of organic sulfur compounds such as alkylbenzothiophenes, in this stream is generally from about 0.1% to about 4%, normally from about 0.2% to about 2.5%, and often from about 0.5% to about 2%. The amount of total nitrogen in the SHC distillate, substantially present in the form of organic nitrogen compounds such as non-basic aromatic compounds including cabazoles, will normally be from about 100 ppm to about 2%, and often from about 100 ppm to about 750 ppm. The SHC distillate will also generally contain a significant fraction of polyaromatics such as 2-ring aromatic compounds (e.g., fused aromatic rings such as naphthalene and naphthalene derivatives) as well as multi-ring aromatic compounds. According to some representative embodiments, the combined amount of 2-ring aromatic compounds and multi-ring aromatic compounds is at least about 50% by weight of the SHC distillate, whereas the amount of mono-ring aromatic compounds (e.g., benzene and benzene derivatives such as alkylaromatic compounds) typically represents only at most about 20% by weight.
[0033]The heavy hydrocarbon feedstock to the SHC reactor or reaction zone, as discussed above, comprises, in addition to the SHC gas oil, DAO produced from subjecting a vacuum column resid to SDA in the presence of a solvent. In addition to the DAO and recycled liquid components as discussed above, other representative gas oil components that may be present in the heavy hydrocarbon feedstock include straight-run gas oils such as vacuum gas oil, recovered by fractional distillation of crude petroleum. Other gas oils produced in refineries include deasphalted gas oil and visbreaker gas oil. Gas oils, as well as the combined heavy hydrocarbon feedstock to the SHC reaction zone that comprises these gas oils, can therefore be a mixture of hydrocarbons boiling in range from about 343° C. (650° F.) to an end point of about 593° C. (1100° F.), with other representative distillation end points being about 566° C. (1050° F.), about 538° C. (1000° F.), and about 482° C. (900° F.). A representative SHC gas oil has a distillation end point temperature from about 427° C. (800° F.) to about 538° C. (1000° F.). In the case of a straight-run vacuum gas oil, the distillation end point is governed by the crude oil vacuum fractionation column and particularly the fractionation temperature cutoff between the vacuum gas oil and vacuum column bottoms split. Thus, refinery gas oil components suitable in heavy hydrocarbon feedstocks to the SHC reactor, such as straight-run fractions, often result from crude oil fractionation or distillation operations, while other gas oil components are obtained following one or more hydrocarbon conversion reactions.
[0034]The SHC may be beneficially combined with hydrotreating, such that the recovered SHC distillate or a fraction thereof, (e.g., a naphtha fraction or a diesel fuel fraction) is catalytically hydrotreated in a hydrotreating zone to reduce the content of total sulfur and/or total nitrogen. According to specific embodiments, for example, a hydrotreated naphtha fraction may be obtained having a sulfur content of less than about 30 ppm by weight, often less than about 10 ppm by weight, and even less than about 5 ppm by weight. A hydrotreated diesel fuel may be obtained having a sulfur content of less than about 50 ppm by weight, often less than about 20 ppm by weight, and even less than about 10 ppm by weight. Hydrotreating of SHC distillates to provide a hydrotreated distillate, or hydrotreating of fractions of the SHC distillates, may therefore provide low-sulfur products and even ultra low sulfur naphtha and diesel fractions in compliance with applicable tolerances. According to a preferred embodiment, the SHC distillate has a sufficient API gravity for incorporation into a crude oil or synthetic crude oil obtained, for example, from tar sands. Representative API gravity values are greater than about 20° (e.g., from about 25° to about 40°) and greater than about 35° (e.g., from about 40° to about 55°).
[0035]In other embodiments, integration of the SHC process with hydrotreating can involve, for example, passing an additional refinery distillate stream, such as a straight-run distillate, to the hydrotreating zone or reactor. Whether or not one or more additional streams are hydrotreated in combination with the SHC distillate, the hydrotreating is normally carried out in the presence of a fixed bed of hydrotreating catalyst and a combined recycle gas stream containing hydrogen. Typical hydrotreating conditions include a temperature from about 260° C. (500° F.) to about 426° C. (800° F.), a pressure from about 7.0 MPa (1000 psig) to about 21 MPa (3000 psig), and a liquid hourly space velocity (LHSV) from about 0.1 hr-1 to about 10 hr-1. As is understood in the art, the Liquid Hourly Space Velocity (LHSV, expressed in units of hr-1) is the volumetric liquid flow rate over the catalyst bed divided by the bed volume and represents the equivalent number of catalyst bed volumes of liquid processed per hour. The LHSV is closely related to the inverse of the reactor residence time. Suitable hydrotreating catalysts comprise a metal selected from the group consisting of nickel, cobalt, tungsten, molybdenum, and mixtures thereof, on a refractory inorganic oxide support.
[0036]As discussed above, the SHC process is advantageously integrated with SDA, wherein a DAO produced from this process is passed to the SHC reaction zone for upgrading. Recycled liquid products, such as a combination of both a recycled portion of an SHC gas oil and a recycled portion of an SHC pitch, beneficially reduce SHC catalyst consumption requirements while providing a significant level of conversion the DAO, and the heavy hydrocarbon feedstock in general, to higher-value products. According to representative embodiments, the heavy hydrocarbon feedstock is converted completely or substantially completely (e.g., with at least about 95% overall conversion) to hydrocarbons having a boiling point temperature of 538° C. (1000° F.) or less in the SHC reaction zone. Conversion levels to these hydrocarbons on a "per pass" basis are generally at least about 75%, typically at least about 85%, and often at least about 90%.
[0037]A typical SHC pitch stream, all or a portion of which may be recycled to the SHC reaction zone as a component of the heavy hydrocarbon feedstock, may be obtained, for example, from vacuum column distillation of an SHC high pressure separator bottoms liquid. In particular, this vacuum column distillation results in, relative to the separator bottoms liquid fed to this column, a lower-boiling SHC gas oil (e.g., SHC VGO) and a higher-boiling SHC pitch, normally obtained as a slurry in combination with the solid particulate. Accordingly, in a representative embodiment, a higher-boiling SHC effluent fraction is recovered as a bottoms liquid, itself in the form of a slurry with the solid particulate, exiting a hot high pressure separator. A portion of this bottoms stream may be recycled directly to the SHC reactor or reaction zone, while a non-recycled portion may be fractionated using vacuum fractionation to yield the SHC gas oil, all or some of which may be recycled to the SHC reactor, and the heavier SHC pitch, as described above. As with the SHC gas oil, all or a portion of the SHC pitch may also be recycled to the SHC reactor or reaction zone. In a specific embodiment, the heavy hydrocarbon feedstock to the SHC comprises, as recycle components, (i) a recycled portion of the SHC high pressure separator bottoms liquid, (ii) a recycled portion of an SHC gas oil, and (iii) a recycled portion of an SHC pitch, with (ii) and (iii) being obtained from fractionation of a non-recycled portion of the SHC high pressure separator bottoms liquid.
[0038]A typical SHC pitch, recovered as a higher boiling component, by flash separation or fractionation of said SHC effluent, will comprise or consist essentially of hydrocarbons boiling at temperatures greater than about 482° C. (900° F.), usually greater than about 538° C. (1000° F.), and often greater than about 593° C. (1100° F.). While aspects of the invention are associated with the advantages obtained, particularly with respect to catalyst make-up requirements, from recycling the SHC pitch to the SHC reactor or reaction zone, it is often desired to remove a non-recycled portion of the SHC pitch as a drag stream containing solid particulate. This prevents the accumulation of unwanted solids and other contaminants to unacceptably high levels. In an exemplary process, the particulate drag stream represents less than about 70%, normally less than about 50%, and often less than about 25%, of the total SHC pitch stream obtained from vacuum column distillation of the SHC high pressure separator bottoms. These purge rates of the solid particulate correspond to associated catalyst make-up rates that are considerably less than those required in the conventional processing of vacuum distillation column resids using SHC, but without DAO as a heavy hydrocarbon feedstock component, optionally in combination with the other recycled liquid products described above.
[0039]The present invention therefore relates to overall refinery flowschemes or processes for upgrading heavy hydrocarbon feedstocks discussed above, and especially those comprising DAO. Due to the recycle of liquid products such as SHC gas oil and SHC pitch, or portions of these streams, substantially all of the net products are either distillates or coke, with a relatively minor production of low-value SHC gas oil and SHC pitch. According to representative embodiments of the invention, the yield of distillate products (e.g., a hydrotreated distillate as discussed above) accounts for at least 80% of the SHC process yield (e.g., from about 80% to about 99%), and often accounts for at least 85% of this yield (e.g., from about 85% to about 95%).
[0040]Further aspects of the invention relate to utilizing the SHC processes discussed above for making a synthetic crude oil or synthetic crude oil blending component. The processes involve passing a DAO derived from solvent deasphalting, with optional integration of the process with a hydrotreater as discussed above. Depending on the fractionation conditions used for downstream processing of the SHC effluent, an SHC distillate may be obtained having hydrocarbons essentially all boiling in the distillate range or lower. In representative embodiments, less than about 10% by weight, and often less than about 5% by weight, of the SHC distillate are hydrocarbons boiling at a temperature of greater than 343° C. (650° F.).
[0041]The integrated SDA/SHC processes described herein may be further integrated with crude oil fractionation, such that a straight-run distillate from a crude oil atmospheric distillation column is hydrotreated together the SHC distillate. Fractionation of the bottoms product from this distillation column in a separate vacuum distillation column may then be carried out to yield VGO and/or vacuum residuum (or resid) that is/are passed to the SDA extractor, as discussed above, to provide DAO used in the heavy hydrocarbon feedstock to SHC.
[0042]A representative process flowscheme illustrating a particular embodiment for carrying out the methods described above is depicted in FIG. 1. FIG. 1 is to be understood to present an illustration of the invention and/or principles involved. As is readily apparent to one of skill in the art having knowledge of the present disclosure, methods according to various other embodiments of the invention will have configurations, components, and operating parameters determined, in part, by the specific feedstocks, products, and product quality specifications.
[0043]According to the embodiment illustrated in FIG. 1, a slurry hydrocracking (SHC) reactor or reaction zone 20 is integrated into a refinery flowscheme. The heavy hydrocarbon feedstock 1 to this reaction zone is a combination of (i) a deasphalted oil (DAO) 2 produced from solvent deasphalting process 30, (ii) a recycled portion 3 of the liquid (in combination with solid particulate) obtained as a bottoms liquid product 8 from hot high pressure separator (HHPS) 60, (iii) a recycled portion 9 of an SHC pitch stream 4 that is obtained as a heavier-boiling fraction or bottoms product of SHC vacuum distillation column 70, and (iv) a recycled portion 17 of an SHC VGO stream 5 obtained as a lighter-boiling fraction or overhead product from this column. Both (iii) and (iv) are therefore obtained from fractionation, in vacuum distillation column 70, of non-recycled portion 6 of bottoms liquid product 8 from HHPS 60.
[0044]As a component of heavy hydrocarbon feedstock 1 to SHC reactor or reaction zone 20, DAO stream 2 is obtained as the solvent-extracted product from upstream solvent deasphalting (SDA) process 30 which also generates SDA pitch stream 7. The SDA/SHC process illustrated in FIG. 1 is further integrated in an overall refinery process flowscheme, with the feed stream to SDA process 30 comprising vacuum column residue stream (or resid) 10 from crude vacuum column or tower 40, typically containing hydrocarbons boiling above (i.e., having a cutpoint temperature) of about 566° C. (1050° F.). As shown in FIG. 1, atmospheric column 80 generates atmospheric residue or reduced crude stream 11, with a typical cutpoint temperature of about 343° C. (650° F.) that is fractionated in vacuum column 40.
[0045]The SHC process is therefore utilized in an integrated manner to upgrade DAO stream 2 from solvent deasphalting process 30, which, as discussed above, is efficiently processed in the SHC reactor or reaction zone, relative to other conversion processes. The total SHC effluent stream 13 is then subjected to downstream separation/fractionation operations to recover upgraded products, remove pitch, and recycle intermediates. According to the embodiment illustrated in FIG. 1, total SHC effluent stream 13 is separated using hot high pressure separator (HHPS) 60 to recover SHC distillate 14, generally boiling in a range above that of naphtha. A non-recycled portion 6 of higher-boiling fraction 8 recovered from SHC effluent stream 13, and in particular from the bottoms of HHPS 60, is then fractionated in SHC fractionator 70, typically operating as a vacuum column. SHC fractionator separates SHC VGO stream 5 from SHC pitch stream 4, with portions 17 and 9 of streams 5 and 4, respectively, being recycled back to SHC reaction zone 20. Both of recycled streams 17,9 benefit the SHC process, in terms of minimizing catalyst requirements, as discussed above. A non-recycled portion 16 of SHC pitch stream 4 is removed as a solid particulate-containing drag stream to prevent the accumulation of excessive amounts of impurities. The removed solid particulate (e.g., catalyst) is replaced with fresh makeup catalyst (not shown), where the makeup (and removal) rates of solid particulate typically represent less than about 25% of the solid particulate flow rate in SHC pitch stream 4. A non-recycled portion 18 of SHC VGO stream 5 is also removed.
[0046]Optionally, the heavy hydrocarbon feedstock 1 can further include a VGO fraction 19 from vacuum column 40, which, for example, contains hydrocarbons boiling in the range from about 343° C. (650° F.) to about 566° C. (1050° F.). This VGO fraction 19 may optionally be fed directly to distillate hydrotreating process 50. Another stream optionally used as an incremental feedstock to hydrotreating process 50 is a straight-run distillate 21 obtained as a distillate fraction of crude oil stream 12, fractionated in crude atmospheric column or tower 80. A further stream which may be hydrotreated is a portion of the SHC VGO stream 5 or 18, namely SHC VGO hydrotreater feed stream 22, from SHC vacuum distillation column 70. Thus, SHC distillate 14, optionally with any combination or all of these streams 19, 21, or 22 is used to obtain hydrotreated distillate 15 as a product of the overall process having reduced nitrogen compound and sulfur compound impurities and/or an API gravity as discussed above that may be utilized as a blending component for synthetic crude oil.
[0047]In the overall integrated SDA/SHC process illustrated in FIG. 1, the SDA process 30 is advantageously operated with a high recovery of DAO that cannot be economically processed using conventional fixed bed or ebullating bed hydroprocessing. Also, the integration of SDA process 30 with SHC produces essentially the net products of pitch streams 7,16 and hydrotreated distillate 15. As is apparent from this description, overall aspects of the invention are directed to the integration of solvent deasphalting and slurry hydrocracking to optimize refinery operations. In view of the present disclosure, it will be seen that several advantages may be achieved and other advantageous results may be obtained. In view of the present disclosure, those having skill in the art will recognize the applicability of the methods disclosed herein to any of a number of integrated SHC processes. It will also be appreciated that various changes could be made in the above processes without departing from the scope of the present disclosure.
User Contributions:
Comment about this patent or add new information about this topic: