Patent application title: NOVEL NUCLEIC ACID SEQUENCES AND THEIR USE IN METHODS FOR ACHIEVING PATHOGEN RESISTANCE IN PLANTS
Inventors:
Karl-Heinz Kogel (Lollar, DE)
Ralph Hueckelhoven (Giessen, DE)
Holger Schultheiss (Friedberg, DE)
Markus Frank (Neustadt, DE)
Assignees:
BASF Plant Science GmbH
IPC8 Class: AC12N1582FI
USPC Class:
800279
Class name: Multicellular living organisms and unmodified parts thereof and related processes method of introducing a polynucleotide molecule into or rearrangement of genetic material within a plant or plant part the polynucleotide confers pathogen or pest resistance
Publication date: 2009-06-25
Patent application number: 20090165173
Claims:
1-31. (canceled)
32. A method of generating or increasing a resistance to at least one pathogen in a plant, which comprises:reducing an amount, activity or function of an RacB protein in a plant or a tissue, organ, part or cell thereof,wherein the RacB protein is a polypeptide comprising the sequence of SEQ ID NO: 4 or a sequence having at least 90% homology to the sequence of SEQ ID NO: 4;wherein the reducing comprises introducing into the plant, tissue, organ, part or cell thereof:a nucleic acid encoding a double-stranded RacB RNA or an expression cassette comprising said nucleic acid;and wherein the pathogen is selected from the group consisting of Plasmodiophoramycota, Oomycota, Ascomycota, Chytridiomycetes, Zygomycetes, Basidiomycota, Deuteromycetes, and combinations thereof.
33. The method of claim 32, comprising:stably transforming a plant cell with a recombinant expression cassette comprising a nucleic acid encoding a double-stranded RacB RNA;regenerating a plant from the plant cell; andexpressing said nucleic acid in an amount and over a period of time sufficient to generate or increase said resistance.
34. The method of claim 32, wherein the plant is a monocot or dicot.
35. The method of claim 34, wherein the monocot is selected from the group consisting of wheat oats, millet, barley, rye, maize, rice, buckwheat, sorghum, triticale, spelt, linseed, sugar cane, and combinations thereof.
36. An isolated nucleic acid comprising a nucleic acid sequence encoding a rice RacB protein, wherein the RacB protein is a polypeptide comprising the sequence of SEQ ID NO: 4.
37. An isolated nucleic acid comprising the sequence of SEQ ID NO: 3 or the complement thereof.
38. A recombinant double-stranded RNA molecule for reducing expression of an RacB protein,wherein one of the two RNA strands comprises the sequence of SEQ ID NO: 3 or a sequence having at least 90% homology to the sequence of SEQ ID NO: 3.
39. A recombinant double-stranded RNA molecule for reducing expression of an RacB protein, comprising:a sense strand and an antisense strand, which is essentially complementary to the sense strand;wherein one of the strands comprises the sequence of SEQ ID NO: 3 or a sequence having at least 90% homology to the sequence of SEQ ID NO. 3.
40. The recombinant double-stranded RNA molecule of claim 38, wherein the two RNA strands are linked covalently to each other.
41. An expression cassette comprising a nucleic acid encoding the recombinant double-stranded RNA molecule of claim 38 in operable linkage with a promoter.
42. The expression cassette of claim 41, wherein the promoter is functional in a plant.
43. The expression cassette of claim 42, wherein the promoter is a pathogen-inducible promoter.
44. A vector comprising the expression cassette of claim 41.
45. A transgenic organism comprising the expression cassette of claim 41, wherein the organism is selected from the group consisting of bacteria, yeasts, and plants.
46. The transgenic organism of claim 45, wherein the plants are selected from the group consisting of wheat, oats, millet, barley, rye, maize, rice, buckwheat, sorghum, triticale, spelt, linseed, sugar cane, oil seed rape, canola, cress, Arabidopsis, cabbages, soya, alfalfa, pea, beans, peanut, potato, tobacco, tomato, eggplant, bell pepper, sunflower, Tagetes, lettuce, Calendula, melon, pumpkin, squash and zucchini.
47. A transgenic plant or a tissue, organ, part or cell produced by the method of claim 32.
48. A stably transformed plant produced by the method of claim 33.
49. The method of claim 32, wherein activity comprises GTPase activity of said RacB protein.
50. The method of claim 32, wherein function comprises a substrate-binding capacity of said RacB protein.
51. A method of selecting a plant cell with an increased resistance to a pathogen comprising:introducing into a plant cell a nucleic acid encoding a double stranded RacB RNA or an expression cassette comprising said nucleic acid; said double stranded RNA reduces an amount, activity or function of an RacB protein of the plant cell; andselecting at least one plant cell wherein resistance to a pathogen is increased as compared to an untransformed plant cell;wherein the RacB protein comprises the sequence of SEQ ID NO: 4 or a sequence having at least 90% homology to the sequence of SEQ ID NO: 4; andwherein the pathogen is selected from the group consisting of Plasmodiophoramycota, Oomycota, Ascomycota, Chytridiomycetes, Zygomycetes, Basidiomycota, Deuteromycetes, and combinations thereof.
52. The method of claim 51, wherein the activity comprises GTPase activity of the RacB protein.
53. The method of claim 51, wherein the function comprises a substrate-binding capacity of the RacB protein.
54. An expression cassette comprising a nucleic acid encoding the double-stranded RNA molecule of claim 39 in operable linkage with a promoter.
55. A transgenic organism comprising the expression cassette of claim 54, wherein the organism is selected from the group of organisms consisting of bacteria, yeasts, plants.
56. A transgenic organism comprising the vector of claim 44, wherein the organism is selected from the group of organisms consisting of bacteria, yeasts, and plants.
57. A recombinant plant cell wherein an expressed amount, activity or function of an endogenous RacB protein is reduced by stable transformation with a nucleic acid or an expression cassette comprising the nucleic acid as compared to an untransformed plant cell, wherein the nucleic acid comprises a double-stranded RacB RNA; and wherein the nucleic acid comprises the sequence of SEQ ID NO: 3 or a sequence having at least 90% homology to the sequence of SEQ ID NO: 3.
58. The cell of claim 57, wherein the activity comprises GTPase activity of said RacB protein.
59. The cell of claim 57, wherein the function comprises a substrate-binding capacity of said RacB protein.
60. The plant cell of claim 57, wherein the endogenous RacB protein is reduced by at least 60%.
61. The plant cell of claim 57, wherein the endogenous RacB protein is reduced by at least 90%.
62. The plant cell of claim 57, wherein the double-stranded RNA molecule comprises a sense strand and an antisense strand which is essentially complementary to the sense strand.
63. The plant cell of claim 57, wherein the reduction provides the plant cell with an increased resistance to a pathogen as compared to the untransformed plant cell.
64. The cell of claim 63, wherein the pathogen is selected from the group consisting of Plasmodiophoramycota, Oomycota, Ascomycota, Chytridiomycetes, Zygomycetes, Basidiomycota, Deuteromycetes, and combinations thereof.
65. The recombinant double-stranded RNA molecule of claim 39, wherein the two RNA strands are linked covalently to each other.
66. A transgenic organism comprising the recombinant double-stranded RNA of claim 39, wherein the organism is selected from the group of organisms consisting of bacteria, yeasts, and plants.
67. A transgenic organism comprising a nucleic acid encoding the double-stranded RNA molecule of claim 38, wherein the organism is selected from the group consisting of bacteria, yeasts, and plants.
68. A plant comprising the transformed plant cell of claim 48.
69. A plant comprising the recombinant plant cell of claim 57.
70. A transformed plant cell obtained by the method of claim 51.
71. A plant comprising the transformed plant cell of claim 70.
72. The method of claim 32, wherein the plant tissue, organ, or part thereof is from a monocot or dicot.
Description:
RELATED APPLICATIONS
[0001]This application is a divisional of U.S. application Ser. No. 10/488,222, filed Mar. 2, 2004 which is a 35 U.S.C. 371 National stage filing of International Application No. PCT/EP2002/009719, filed Aug. 3, 2002, which claims priority to German Application No. 10142579.1, filed Sep. 3, 2001 and German Application No. 10229729.0, filed Jul. 2, 2002. The entire contents of each of these applications are hereby incorporated by reference herein.
SUBMISSION OF SEQUENCE LISTING
[0002]The Sequence Listing associated with this application is filed in electronic format via EFS-Web and hereby incorporated by reference into the specification in its entirety. The name of the text file containing the Sequence Listing is Revised_Sequence_List--12810--00810_US. The size of the text file is 171 KB, and the text file was created on Feb. 18, 2009.
FIELD OF THE INVENTION
[0003]The invention relates to novel RacB cDNA sequences from barley and to expression cassettes and vectors comprising these sequences. The invention furthermore relates to transgenic plants transformed with these expression cassettes or vectors, to cultures, parts or transgenic propagation material derived from them, and to their use for the production of foodstuffs, feeding stuffs, seed, pharmaceuticals or fine chemicals. The invention furthermore relates to methods of generating or increasing a pathogen resistance in plants by reducing the expression of an RacB protein or of a functional equivalent thereof.
BACKGROUND OF THE INVENTION
[0004]The aim of plant biotechnology work is the generation of plants with advantageous novel properties, for example for increasing agricultural productivity, increasing the quality in the case of foodstuffs, or for producing specific chemicals or pharmaceuticals (Dunwell J M (2000) J Exp Bot 51 Spec No: 487-96). The plant's natural defense mechanisms against pathogens are frequently insufficient. Fungal diseases alone result in annual yield losses of many billions of US$. The introduction of foreign genes from plants, animals or microbial sources can increase the defenses. Examples are the protection of tobacco against feeding damage by insects by expressing Bacillus thuringiensis endotoxins under the control of the 35S CaMV promoter (Vaeck et al. (1987) Nature 328:33-37) or the protection of tobacco against fungal infection by expressing a bean chitinase under the control of the CaMV promoter (Broglie et al. (1991) Science 254:1194-1197). However, most of the approaches described only offer resistance to a single pathogen or a narrow spectrum of pathogens.
[0005]Only a few approaches exist which impart a resistance to a broader spectrum of pathogens, in particular fungal pathogens, to plants. Systemic acquired resistance (SAR)--a defense mechanism in a variety of plant/pathogen interactions--can be mediated by the application of endogenous messenger substances such as jasmonate (JA) or salicylic acid (SA) (Ward, et al. (1991) Plant Cell 3:1085-1094; Uknes, et al. (1992) Plant Cell 4(6):645-656). Similar effects can also be achieved by synthetic compounds such as 2,6-dichloroisonicotinic acid (INA) or S-methyl benzo(1,2,3)thiadiazole-7-thiocarboxylate (BTH; Bion®) (Friedrich et al. (1996) Plant J 10(1):61-70; Lawton et al. (1996) Plant J. 10:71-82). The expression of pathogenesis-related (PR) proteins, which are highly regulated in the case of an SAR, may also cause pathogen resistance in some cases.
[0006]In barley, the Mlo locus has been described for some time as a negative regulator of plant defense. The loss, or loss of function, of the Mlo gene causes an increased and, above all, race-unspecific resistance for example against a large number of mildews (Buschges R et al. (1997) Cell 88:695-705; Jorgensen J H (1977) Euphytica 26:55-62; Lyngkjaer M F et al. (1995) Plant Pathol 44:786-790). The Mlo phenotype is inherited recessively, which also suggests a function as a susceptibility gene. Mlo-deficient barley varieties obtained by traditional breeding are already being widely used in agriculture. Although these varieties are being grown intensively, this resistance has proved to be extraordinarily durable, probably owing to the recessivity. Resistance breakdown has not been observed as yet. Mlo-like resistances in other plants, especially in cereal species, have not been described even though wheat, rye and other cereals are also attacked by comparable mildew pathogens. The reason in the case of wheat may be, for example, the existence of a hexaploid genome, which makes the identification of mutants in which each of the six copies of the gene has been inactivated extremely difficult.
[0007]The Mlo gene has only recently been cloned (Buschges R et al. (1997) Cell 88:695-705; WO 98/04586; Schulze-Lefert P, Vogel J (2000) Trends Plant Sci. 5:343-348). As a consequence, various homologs have been isolated from other cereal species. Various methods for obtaining pathogen resistance using these genes have been described (WO 98/04586; WO 00/01722; WO 99/47552).
[0008]Mlo resistance of a plant to mildew pathogens manifests itself in two important events, both of which bring about resistance to penetration: cell wall apposition (CWA) underneath the penetration site of the pathogen in the epidermal cell wall. Spreading of this fungal pathogen is almost exclusively restricted to this subcellular structure (Jorgensen J H and Mortensen K (1977) Phytopathology 67:678-685; Freialdenhoven A et al. (1996) Plant Cell 8:5-14). This reaction is caused by the genes Ror1 and Ror2, which are required for the effect of Mlo (Peterhansel C et al. (1997) 9:1397-1409).
[0009]The disadvantage in Mlo pathogen resistance is that Mlo-deficient plants--even in the absence of a pathogen--initiate a defense mechanism which manifests itself for example in the spontaneous death of leaf cells (Wolter M et al. (1993) Mol Gen Genet 239:122-128). A further disadvantage is that the Mlo-deficient genotypes are hypersusceptible to hemibiotrophic pathogens such as Magnaporte grisea (M. grisea) and Cochliobolus sativus (Bipolaris sorokiniana) (Jarosch B et al. (1999) Mol Plant Microbe Interact 12:508-514; Kumar J et al. (2001) Phytopathology 91:127-133). The Mlo gene therefore appears to be a negative regulator of cell death. Again, the cause is probably the induction of cell death in the absence of the Mlo gene, which increases the susceptibility to these fairly necrotrophic pathogens. This ambivalent effect, which limits the biotechnological use of Mlo, is probably due to the fact that necrotrophic fungi are capable of exploiting the more pronounced HR of the Mlo-deficient host plant for their infection process. A resistance comparable to Mlo deficiency, but without the characteristic of inducing cell death, would be desirable.
[0010]The proteins Rho, Rac and Cdc42 are members of the small GTP (guanosine triphosphate) binding protein family and regulate a large number of intracellular processes as "molecular switches", both in plant and animal organisms. As elements of signal transduction, they play an important role in the conversion of extracellular stimuli. For example, they regulate NADPH oxidase and thus the release of reactive oxygen molecules ("oxidative burst"). Animal or human Rac1 is essential for the formation of the active NADPH oxidase complex which, in turn, is important for the formation of superoxide, thus contributing to plant defense (Irani K and Goldschmidt-Clermont P J (1998) Biochem Pharmacol 55: 1339-1346). The function in plant defense in plants and animals is largely analogous (Kwong et al. (1995) J Biol Chem 270(34): 19868-19872; Dusi et al. (1996) Biochem J 314:409-412; Diekmann et al. (1994) Science 265:531-533; Purgin et al. (1997) The Plant Cell 9:2077-2091; Kleinberg et al. (1994) Biochemistry 33:2490-2495; Prigmore et al. (1995) Journal of Biol Chem 27(18): 10717-10722; Irani et al. (1997) Science 275:1649-1652; Low et al. (1994) Advances in Molecular Genetics of Plant-Microbe Interactions 3:361-369 (1994) eds. M J Daniels, Kluwer Acadmic Publishers, Netherlands; Mehdy et al. (1994) Plant Physiol 105: 467-472; Sundaresan et al. (1996) Biochem J 318:379-382). Moreover, GTP binding proteins function in restructuring the cytoskeleton and in cell transformation (Symon M. (1996) TIBS 21: 178-181), and also in the activation of transcription (Hill et al. (1995) Cell 81:1159-1170; Chandra et al. (1996) Proc Natl Acad Sci USA 93:13393-13397).
[0011]In plants, there exists a substantial family of Rac-like proteins (Winge et al. (1997) Plant Mol Biol 35:483-495), which is also termed Rop family (Lin et al. (1997) The Plant Cell 9:1647-1659). In plants, the Rac proteins appear to have a function in the release of reactive oxygen molecules as the consequence of pathogen infection (Groom Q J et al. (1996) Plant J 10: 515-522; Hassanain H H et al. (2000) Biochem Biophys Res Commun 272(3):783-788; Ono E et al. (2001) Proc Natl Acad Sci USA 98: 759-764). Rac modulates, inter alia, cell wall architecture, signal transduction in the meristem and the defense against pathogens (Valster A H et al. (2000) Trends Cell Biol 10(4):141-146). When the constitutively active form is overexpressed, Rac1 from rice is capable of inducing a hypersensitive response (HR) at the sites of M. grisea attack, thus causing pathogen resistance. Analogously, the expression of a negative dominant form of Rac1 brings about an increased susceptibility to M. grisea (Kawasaki T et al. (1999) Proc Natl Acad Sci USA 96:10922-10926; Ono E et al. (2001) Proc Natl Acad Sci USA 98: 759-764). These findings suggest that an overexpression of Rac proteins in the plant can bring about advantageous effects with regard to plant defense.
[0012]WO 00/15815 describes five Rac genes from maize. Although methods for both an up regulation and a down regulation of Rac proteins are described and speculatively discussed in connection with obtaining a resistance to pathogens (p. 55/line 25 et seq.), the only technical teaching, which describes this use in real terms, concerns merely an overexpression of the claimed Rac genes for obtaining pathogen resistance (p. 60/line 21 et seq.). The author postulates quite unambiguously and in agreement with the situation described in the prior art (p. 60/line 31 et seq.): "Thus the present invention is useful in protecting plants from pathogens. Once a plant is transformed with a polynucleotide sequence encoding an Rac polypeptide, expression of the polypeptide in the plant confers resistance to infection by plant pathogens." The rationale behind this hypothesis (plants defense via reactive oxygen molecules) is explained hereinbelow and supported by a large number of references. Beyond this, no differentiation is being made between the five claimed Rac genes.
[0013]It is an object of the present invention to provide novel methods of plant defense against pathogens which bring about an effective defense against as broad a spectrum of pathogens as possible in as large a number of plant species as possible, preferably the crop plants used in agriculture. We have found that this object is achieved by the method according to the invention.
BRIEF SUMMARY OF THE INVENTION
[0014]The invention firstly comprises a method of generating or increasing the resistance to at least one pathogen in plants, which comprises the following steps
[0015]a) reducing the amount, activity or function of an RacB protein in a plant or a tissue, organ, part or cell thereof, and
[0016]b) selecting those plants in which, as opposed or as compared to the original plant, the resistance to at least one pathogen exists or is increased.
[0017]Surprisingly, the Rac homolog RacB from barley (Hordeum vulgare) (SEQ ID NO: 1) (hereinbelow: hvRacB), despite a great similarity with rice Rac1, has a negative control function upon attack by powdery mildew of barley Blumeria (syn. Erysiphe) graminis f.sp. hordei (Bgh), as opposed to the former: reducing the hvRacB expression in the epidermal cell by a sequence-specific RNA interference approach using double-stranded hvRacB dsRNA ("gene silencing") significantly prevented the development of haustoria owing to Bgh infection. Further experiments demonstrated (cf. Example 7) that this phenotype cannot be observed in an mlo5-ror1-mutant genotype, namely barley A89. This suggests that RacB is linked operably to Mlo or Ror1 or both, that is to say they probably act within a signal cascade.
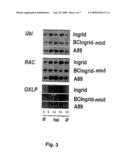
[0018]Similarly to the loss of function of Mlo, that of HvRacB confers broad resistance to various Blumeria graminis f.sp. hordei isolates. In transient gene silencing experiments, HvRacB reduced the penetration efficiency (development of haustoria) of Bgh by 44% (cf. Example 7), an effect whose magnitude corresponds to the effect achieved by Mlo dsRNA (Schweizer P et al. (2000) Plant J 24:895-903). In the wild-type barley variety Ingrid, approximately 60% of the fungal penetrations resulted in the development of haustoria, while the penetration rate in BCIngrid-mlo5 is virtually 0%. The barley variety A89 (mlo-ror1 dual mutant) shows a penetration efficiency of approximately 20 to 35%. An altered RacB expression owing to Bgh inoculation was observed in none of these variants (cf. Example 7; FIG. 3). The fact that only a penetration of approximately 50% can be observed even in pathogen-sensitive wild-type varieties, such as Pallas or Ingrid, can be attributed to the basal resistance which is always present.
[0019]Interestingly, the gene silencing of hvRacB only enhances cell wall apposition, but apparently not the spontaneous cell death of the plant, which is in contrast to Mlo. Thus, HvRacB differs from OsRac1, a rice homolog of Rac1 (Ono E et al. (2001) Proc Natl Acad Sci USA 98: 759-764). HvRacB acts predominantly as negative regulator of cell wall apposition. This difference is of outstanding importance for its use for obtaining pathogen resistance in plants. As already described above, Mlo resistance to biotrophic fungi (for example mildew fungi) is indeed caused, inter alia, by increased cell wall apposition, but the trade-off is a higher susceptibility to necrotrophic fungi (Jarosch B et al. (1999) Mol Plant Microbe Interact 12:508-514; Kumar J et al. (2001) Phytopathology 91:127-133). Since HvRacB only affects cell all apposition, this problem of ambivalence can be circumvented.
[0020]Owing to the above findings, RacB must be considered as a key element for the successful penetration of a pathogen such as Bgh into the plant cell. Accordingly, the method according to the invention has all the advantages of Mlo deficiency without simultaneously showing its biggest shortcoming, namely increased spontaneous cell death.
[0021]Moreover, the method outperforms all those methods in which a pathogen-resistant phenotype is realized by overexpressing a resistance-conferring protein. Switching off a gene can be realized without expressing a (foreign) protein. In an ideal case, all that needs doing is to deactivate the endogenous gene. This has not inconsiderable advantages for approval and acceptance by the consumer, who is frequently apprehensive toward plants with foreign proteins. Very especially advantageous in this context is the use of inducible promoters for reducing the amount, activity or function of RacB protein, which, for example when using pathogen-inducible promoters, allows expression only when required (i.e. pathogen infection).
BRIEF DESCRIPTION OF THE FIGURES
[0022]FIG. 1: Alignment of the amino acid sequences of barley RacB (RACB H.vul.; SEQ ID NO: 2), rice RacB (RAB--O.sat.; SEQ ID NO: 4), maize RacB (RACB--Z.mays; SEQ ID NO: 6), and human Rac1 (RAC1--H.sap.; SEQ ID NO: 78) and Rac2 proteins (RAC2--H.sap.; SEQ ID NO: 77).
[0023]FIG. 2: Expression of RacB in epidermal tissue.
[0024]FIG. 3: RacB is expressed constitutively in various resistant barley lines.
[0025]FIG. 4: "RNA interference" with RacB-dsRNA reduces the penetration efficacy of barley powdery mildew BghA6 in barley.
[0026]FIG. 5: Effect of the genetic background on RacB function.
[0027]FIG. 6: Overexpression of a constitutively active RacB mutant in barley cv. Pallas.
[0028]FIG. 7. Plasmid map for expression vector pGY-1 (Schweizer P et al. (1999) Mol Plant Microbe Interact 12: 647-54; Shinshi H et al. (1990) Plant Mol Biol 14:357-368).
DETAILED DESCRIPTION OF THE INVENTION
[0029]A partial sequence of the barley RacB cDNA (HvRacB-cDNA) (GenBank Acc. No.: AJ290240), which is highly conserved relative to rice RacB (GenBank Acc. No.: AF250327) and maize RacB (GenBank Ace. No.: AF126053) and very similar to rice Rac1 has been described. Maize RacB is also one of the five Rac genes in the abovementioned application WO 00/15815 (Sequence No. 3). The complete coding sequence of the HvRacB protein has not been described as yet (see Example 1). Barley RacB has a homology of 95% identity with rice RacB and maize RacB and is over 55% identical to human RAC1 or RAC2 (Hassanain et al. 2000, FIG. 1). HvRacB is expressed constitutively in primary leaves of barley (epidermis-specifically) and its expression level is not affected substantially by Bgh infection. Expression thus takes place in the tissue which interacts directly with the Bgh pathogen.
[0030]In principle, the method according to the invention can be applied to all plant species, preferably to those in which an RacB protein or a functional equivalent thereof is expressed naturally. Since the function of RacB is closely connected functionally to the Mlo gene and the latter has been identified in a large number of plants, including dicots (Devoto A et al. (1999) J Biol Chem 274(49):34993-5004), it can be assumed that RacB and its homologs are similarly widely distributed. The sequences from other plants (for example Arabidopsis thaliana) which are homologous to the RacB sequences disclosed within the scope of the present invention can be found readily for example by database searches or by screening genetic libraries using the RacB sequences as search sequence or probe.
[0031]The term "plant" as used herein refers to all genera and species of higher and lower plants of the Plant Kingdom. The term includes the mature plants, seed, shoots and seedlings and their derived parts, propagation material, plant organs, tissue, protoplasts, callus and other cultures, for example cell cultures, and any other type of plant cell grouping to give functional or structural units. Mature plants refers to plants at any desired developmental stage beyond that of the seedling. Seedling refers to a young immature plant at an early developmental stage. "Plant" comprises all annual and perennial monocotyledonous and dicotyledonous plants and includes by way of example but not by limitation those of the genera Cucurbita, Rosa, Vitis, Juglans, Fragaria, Lotus, Medicago, Onobrychis, Trifolium, Trigonella, Vigna, Citrus, Linum, Geranium, Manihot, Daucus, Arabidopsis, Brassica, Raphanus, Sinapis, Atropa, Capsicum, Datura, Hyoscyamus, Lycopersicon, Nicotiana, Solarium, Petunia, Digitalis, Majorana, Ciahorium, Helianthus, Lactuca, Bromus, Asparagus, Antirrhinum, Heterocallis, Nemesis, Pelargonium, Panieum, Pennisetum, Ranunculus, Senecio, Salpiglossis, Cucumis, Browoalia, Glycine, Pisum, Phaseolus, Lolium, Oryza, Zea, Avena, Hordeum, Secale, Triticum, Sorghum, Picea and Populus.
[0032]The term "plant" preferably comprises monocotyledonous crop plants such as, for example, cereal species such as wheat, barley, millet rye, triticale, maize, rice, sorghum or oats and also sugar cane.
[0033]The term furthermore comprises dicotyledonous crop plants such as, for example,
[0034]Brassicacae such as oilseed rape, canola, cress, Arabidopsis, cabbages or canola, Leguminosae such as soybean, alfalfa, pea, beans or peanut
[0035]Solanaceae such as potato, tobacco, tomato, egg plant or pepper, Asteraceae such as sunflower, Tagetes, lettuce or Calendula,
[0036]Cucurbitaceae such as melon, pumpkin/squash or zucchini,
[0037]and also linseed, cotton, hemp, clover, spinach, flax, red pepper, carrot, beet, radish, sugar beet, sweet potato, cucumber, chicory, cauliflower, broccoli, asparagus, onion, garlic, celery/celeriac, strawberry, raspberry, blackberry, pineapple, avocado and the various tree, bush, nut and vine species. Tree species preferably comprise plum, cherry, peach, nectarine, apricot, banana, papaya, mango, apple, pear, quince.
[0038]Also comprised are ornamental plants, useful trees, ornamental trees, flowers, cut flowers, shrubs or lawns such as by way of example but not by limitation the families of the Rosaceae such as rose, Ericaceae such as rhododendrons and azaleas, Euphorbiaceae such as poinsettias and croton, Caryophyllaceae such as carnations, Solanaceae such as petunias, Gesneriaceae such as African violet, Balsaminaceae such as touch-me-not, Orchidaceae such as orchids, Iridaceae such as gladioli, iris, freesia and crocus, Compositae such as calendula, Geraniaceae such as geraniums, Liliaceae such as dracaena, Moraceae such as ficus, Araceae such as philodendron and many others.
[0039]Preferred within the scope of the invention are those plants which are employed as foodstuffs or feeding stuffs, very especially preferably monocotyledonous genera and species like the above-described cereal species.
[0040]The method is applied very especially preferably to monocotyledonous plants, most preferably to agriculturally important plants such as wheat, oats, millet, barley, rye, maize, rice, buckwheat, sorghum, triticale, spelt, linseed or sugar cane.
[0041]"Pathogen resistance" denotes the reduction or weakening of disease symptoms of a plant following infection by a pathogen. The symptoms can be manifold, but preferably comprise those which directly or indirectly have an adverse effect on the quality of the plant, the quantity of the yield, the suitability for use as feeding stuff or foodstuff, or else which make sowing, planting, harvesting or processing of the crop difficult.
[0042]"Conferring", "existing", "generating" or "increasing" a pathogen resistance means that the defense mechanisms of a specific plant species or variety is increasingly resistant to one or more pathogens due to the use of the method according to the invention in comparison with the wild type of the plant ("original plant"), to which the method according to the invention has not been applied, under otherwise identical conditions (such as, for example, climatic conditions, growing conditions, pathogen species and the like). The increased resistance manifests itself preferably in a reduced manifestation of the disease symptoms, disease symptoms comprising--in addition to the above-mentioned adverse effects--for example also the penetration efficiency of a pathogen into the plant or plant cells or the proliferation efficiency in or on the same. In this context, the disease symptoms are preferably reduced by at least 10% or at least 20%, especially preferably by at least 40% or 60%, very especially preferably by at least 70% or 80% and most preferably by at least 90% or 95%.
[0043]"Selection" with regard to plants in which--as opposed or as compared to the original plant--resistance to at least one pathogen exists or is increased means all those methods which a are suitable for recognizing an existing or increased resistance to pathogens. These may be symptoms of pathogen infection (for example the development of haustoria in the case of fungal infection), but may also comprise the above-described symptoms which relate to the quality of the plant, the quantity of the yield, the suitability for use as feeding stuff or foodstuff and the like.
[0044]"Pathogen" within the scope of the invention means by way of example but not by limitation viruses or viroids, bacteria, fungi, animal pests such as, for example, insects or nematodes. Especially preferred are fungi such as, for example, mildew. However, it can be assumed that a reduced expression of an RacB protein, its activity or function also brings about resistance to other pathogens. Changes in the cell wall structure can constitute a prime mechanism of pathogen resistance.
[0045]The following pathogens may be mentioned by way of example but not by limitation:
[0046]1. Fungal Pathogens or Fungus-Like Pathogens:
[0047]Fungal pathogens or fungus-like pathogens (e.g. Chromista) are preferably from the group comprising the Plasmodiophoramycota, Oomycota, Ascomycota, Chytridiomycetes, Zygomycetes, Basidiomycota and Deuteromycetes (Fungi imperfecti). The pathogens mentioned in Tables 1 and 2 and the diseases with which they are associated may be mentioned by way of example but not by limitation.
TABLE-US-00001 TABLE 1 Fungal plant diseases Disease Pathogen Leag rust Puccinia recondita Yellow rust P. striiformis Powdery mildew Erysiphe graminis/Blumeria graminis Glume blotch Septoria nodorum Leaf blotch Septoria tritici Ear fusarioses Fusarium spp. Eyespot Pseudocercosporella herpotrichoides Smut Ustilago spp. Bunt Tilletia caries Take-all Gaeumannomyces graminis Anthrocnose leaf blight Colletotrichum graminicola Anthracnose stalk rot (teleomorph; Glomerella graminicola Politis); Glomerella tucumanensis (anamorph; Glomerella falcatum Went) Aspergillus ear and Aspergillus flavus kernel rot Banded leaf and sheath Rhizoctonia solani Kuhn = Rhizoctonia spot microsclerotia J. Matz (telomorph: Thanatephorus cucumeris) Black bundle disease Acremonium strictum W. Gams = Cephalosporium acremonium Auct. non Corda Black kernel rot Lasiodiplodia theobromae = Botryodiplodia theobromae Borde blanco Marasmiellus sp. Brown spot (black spot, Physoderma maydis stalk rot) Cephalosporium kernel Acremonium strictum = Cephalosporium rot acremonium Charcoal rot Macrophomina phaseolina Corticium ear rot Thanatephorus cucumeris = Corticium sasakii Curvularia leaf spot Curvularia clavata, C. eragrostidis, = C. maculans (teleomorph: Cochliobolus eragrostidis), Curvularia inaequalis, C. intermedia (teleomorph: Cochliobolus intermedius), Curvularia lunata (teleomorph: Cochliobolus lunatus), Curvularia pallescens (teleomorph: Cochliobolus pallescens), Curvularia senegalensis, C. tuberculata (teleomorph: Cochliobolus tuberculatus) Didymella leaf spot Didymella exitalis Diplodia ear rot and Diplodia frumenti (teleomorph: stalk rot Botryosphaeria festucae) Diplodia ear rot, stalk Diplodia maydis = rot, seed rot and Stenocarpella maydis seedling blight Diplodia leaf spot or Stenocarpella macrospora = streak Diplodialeaf macrospora
TABLE-US-00002 TABLE 2 Downy mildew Disease Pathogen Brown stripe downy Sclerophthora rayssiae var. zeae mildew Crazy top downy mildew Sclerophthora macrospora = Sclerospora macrospora Green ear downy mildew Sclerospora graminicola (graminicola downy mildew) Java downy mildew Peronosclerospora maydis = Sclerospora maydis Philippine downy mildew Peronosclerospora philippinensis = Sclerospora philippinensis Sorghum downy mildew Peronosclerospora sorghi = Sclerospora sorghi Spontaneum downy mildew Peronosclerospora spontanea = Sclerospora spontanea Sugarcane downy mildew Peronosclerospora sacchari = Sclerospora sacchari Dry ear rot (cob, Nigrospora oryzae kernel and stalk rot) (teleomorph: Khuskia oryzae) Ear rots, minor Alternaria alternata = A. tenuis, Aspergillus glaucus, A. niger, Aspergillus spp., Botrytis cinerea (teleomorph: Botryotinia fuckeliana), Cunninghamella sp., Curvularia pallescens, Doratomyces stemonitis = Cephalotrichum stemonitis, Fusarium culmorum, Gonatobotrys simplex, Pithomyces maydicus, Rhizopus microsporus Tiegh., R. stolonifer = R. nigricans, Scopulariopsis brumptii Ergot (horse's tooth) Claviceps gigantea (anamorph: Sphacelia sp.) Eyespot Aureobasidium zeae = Kabatiella zeae Fusarium ear and stalk Fusarium subglutinans = rot F. moniliforme var. subglutinans Fusarium kernel, root Fusarium moniliforme and stalk rot, seed rot (teleomorph: Gibberella fujikuroi) and seedling blight Fusarium stalk rot, Fusarium avenaceum seedling root rot (teleomorph: Gibberlla avenacea) Gibberella ear and stalk Gibberella zeae rot (anamorph: Fusarium graminearum) Gray ear rot Botryosphaeria zeae = Physalospora zeae (anamorph: Macrophoma zeae) Gray leaf spot Cercospora sorghi = C. sorghi var. (Cercospora leaf spot) maydis, C. zeae-maydis Helminthosporium root Exserohilum pedicellatum = rot Helminthosporium pedicellatum (teleomorph: Setosphaeria pedicellata) Hormodendrum ear rot Cladosporium cladosporioides = (Cladosporium rot) Hormodendrum cladosporioides, C. herbarum (teleomorph: Mycosphaerella tassiana) Hyalothyridium leaf spot Hyalothyridium maydis Late wilt Cephalosporium maydis Leaf spots, minor Alternaria alternata, Ascochyta maydis, A. tritici, A. zeicola, Bipolaris victoriae = Helminthosporium victoriae (teleomorph: Cochliobolus victoriae), C. sativus (anamorph: Bipolaris sorokiniana = H. sorokinianum = H. sativum), Epicoccum nigrum, Exserohilum prolatum = Drechslera prolata (teleomorph: Setosphaeria prolata) Graphium penicillioides, Leptosphaeria maydis, Leptothyrium zeae, Ophiosphaerella herpotricha, (anamorph: Scolecosporiella sp.) Paraphaeosphaeria michotii, Phoma Sp., Septoria zeae, S. zeicola, S. zeina Northern corn leaf Setosphaeria turcica (anarnorph: blight (white blast, Exserohilum turcicum = crown stalk rot, stripe) Helminthosporim turcicum) Northern corn leaf spot Cochliobolus carbonum (anamorph: Helminthosporium ear rot Bipolaris zeicola = Helminthosporium (race 1) carbonum) Penicillium ear rot Penicillium spp., P. chrysogenum, (blue eye, blue mold) P. expansum, P. oxalicum Phaeocytostroma stalk Phaeocytostroma ambiguum, = rot and root rot Phaeocytosporella zeae Phaeosphaeria leaf spot Phaeosphaeria maydis = Sphaerulina maydis Physalospora ear rot Botryosphaeria festucae = Physalospora (Botryosphaeria ear rot) zeicola (anamorph: Diplodia frumenti) Purple leaf sheath Hemiparasitic bacteria and fungi Pyrenochaeta stalk rot Phoma terrestris = and root rot Pyrenochaeta terrestris Pythium root rot Pythium spp., P. arrhenomanes, P. graminicola Pythium stalk rot Pythium aphanidermatum = P. butleri L. Red kernel disease (ear Epicoccum nigrum mold, leaf and seed rot) Rhizoctonia ear rot Rhizoctonia zeae (teleomorph: Waitea (sclerotial rot) circinata) Rhizoctonia root rot and Rhizoctonia solani, Rhizoctonia zeae stalk rot Root rots, minor Alternaria alternata, Cercospora sorghi, Dictochaeta fertilis, Fusarium acuminatum (teleomorph: Gibberella acuminata), F. equiseti (teleomorph: G. intricans), F. oxysporum, F. pallidoroseum, F. poae, F. roseum, G. cyanogena, (anamorph: F. sulphureum), Microdochium bolleyi,) Mucor sp., Periconia circinata, Phytophthora cactorum, P. drechsleri, P. nicotianae var. parasitica, Rhizopus arrhizus Rostratum leaf spot Setosphaeria rostrata, (anamorph: (Helminthosporium leaf Exserohilum rostratum = disease, ear and stalk He/minthosporium rostratum) rot) Rust, common corn Puccinia sorghi Rust, southern corn Puccinia polysora Rust, tropical corn Physopella pallescens, P. zeae = Angiopsora zeae Sclerotium ear rot Sclerotium rolfsii Sacc. (teleomorph: (southern blight) Athelia rolfsii) Seed rot-seedling blight Bipolaris sorokiniana, P. zeicola = Helminthosporium carbonum, Diplodia maydis, Exserohilum pedicillatum, Exserohilum turcicum = Helminthosporium turcicum, Fusarium avenaceum, F. culmorum, F. moniliforme, Gibberella zeae (anamorph: F. graminearum), Macrophomina phaseolina, Penicillium spp., Phomopsis sp., Pythium spp., Rhizoctonia solani, R. zeae, Sclerotium rolfsii, Spicaria sp. Selenophoma leaf spot Selenophoma sp. Sheath rot Gaeumannomyces graminis Shuck rot Myrothecium gramineum Silage mold Monascus purpureus, M ruber Smut, common Ustilago zeae = U. maydis Smut, false Ustilaginoidea virens Smut, head Sphacelotheca reiliana = Sporisorium holcisorghi Southern corn leaf Cochliobolus heterostrophus (anamorph: blight and stalk rot Bipolaris maydis = Helminthosporium maydis) Southern leaf spot Stenocarpella macrospora = Diplodia macrospora Stalk rots, minor Cercospora sorghi, Fusarium episphaeria, F. merismoides, F. oxysporum Schlechtend, F. poae, F. roseum, F. solani (teleomorph: Nectria haematococca), F. tricinctum, Mariannaea elegans, Mucor sp., Rhopographus zeae, Spicaria sp. Storage rots Aspergillus spp., Penicillium spp. and other fungi Tar spot Phyllachora maydis Trichoderma ear rot and Trichoderma viride = T. lignorum root rot teleomorph: Hypocrea sp. White ear rot, root and Stenocarpella maydis = Diplodia zeae stalk rot Yellow leaf blight Ascochyta ischaemi, Phyllosticta maydis (teleomorph: Mycosphaerella zeae-maydis) Zonate leaf spot Gloeocercospora sorghi
[0048]The following are especially preferred:
[0049]Plasmodiophoromycota such as Plasmodiophora brassicace (clubroot of crucifers), Spongospora subterranea (powdery scab of potato tubers), Polymyxa graminis (root disease of cereals and grasses),
[0050]Oomycota such as Bremia lactucae (downy mildew of lettuce), Peronospora (downy mildew) in snapdragon (P. antirrhini), onion (P. destructor), spinach (P. effusa), soybean (P. manchurica), tobacco ("blue-mold"; P. tabacina) alfalfa and clover (P. trifolium), Pseudoperonospora humuli (downy mildew of hops), Plasmopara (downy mildew in grapevines) (P. viticola) and sunflower (P. halstedii), Sclerophtohra macrospora (downy mildew in cereals and grasses), Pythlum (seed rot, seedling damping-off, and root rot and all types of plants, for example damping-off of Beta beet caused by P. debaryanum), Phytophthora infestans (blight in potato, brown rot in tomato and the like), Albugo spec. (white rust on cruciferous plants).
[0051]Ascomycola such as Microdochium nivale (snow mold of rye and wheat), Fusarium graminearum, Fusarium culmorum (partial ear sterility mainly in wheat), Fusarium oxysporum (Fusarium wilt of tomato), Blumeria graminis powdery mildew of barley (f.sp. hordei) and wheat (f.sp. tritici)), Erysiphe pisi (powdery mildew of pea), Nectria galligena (Nectria canker of fruit trees), Unicnula necator (powdery mildew of grapevine), Pseudopeziza tracheiphila (red fire disease of grapevine), Claviceps purpurea (ergot on, for example, rye and grasses), Gaeumannomyces graminis (take-all on wheat, rye and other grasses), Magnaporthe grisea (rice blast disease), Pyrenophora graminea (leaf stripe of barley), Pyrenophora teres (net blotch of barley), Pyrenophora tritici-repentis (leaf blight of wheat), Venturia inaequalis (apple scab), Sclerotinia sclerotium (stalk break, stem rot), Pseudopeziza medicaginis (leaf spot of alfalfa, white and red clover).
[0052]Basidiomycetes such as Typhula incarnata (typhula blight on barley, rye, wheat), Ustilago maydis (blister smut on maize), Ustilago nuda (loose smut on barley), Ustilago tritici (loose smut on wheat, spelt), Ustilago avenae (loose smut on oats), Rhizoctonia solani (rhizoctonia root rot of potato), Sphacelotheca spp. (head smut of sorghum), Melampsora lini (rust of flax), Puccinia graminis (stem rust of wheat, barley, rye, oats), Puccinia recondita (leaf rust on wheat), Puccinia dispersa (brown rust on rye), Puccinia hordei (leaf rust of barley), Puccinia coronata (crown rust of oats), Puccinia striiformis (yellow rust of wheat, barley, rye and a large number of grasses), Uromyces appendiculatus (brown rust of bean), Sclerotium rolfsii (root and stem rots of many plants).
[0053]Deuteromycetes (Fungi imperfecti) such as Septoria nodorum (glume blotch) of wheat (Septoria tritici), Pseudocercosporella herpotrichoides (eyespot of wheat, barley, rye), Rynchosporium secalis (leaf spot on rye and barley), Alternaria solani (early blight of potato, tomato), Phoma betae (blackleg on Beta beet), Cercospora beticola (leaf spot on Beta beet), Alternaria brassicae (black spot on oilseed rape, cabbage and other crucifers), Verticillium dahliae (verticillium wilt), Colletotrichum lindemuthianum (bean anthracnose), Phoma lingam (blackleg of cabbage and oilseed rape), Botrytis cinerea (grey mold of grapevine, strawberry, tomato, hops and the like).
[0054]Most preferred are Phytophthora infestans (potato blight, brown rot in tomato and the like), Microdochium nivale (previously Fusarium nivale; snow mold of rye and wheat), Fusarium graminearum, Fusarium culmorum (partial car sterility of wheat), Fusarium oxysporum (Fusarium wilt of tomato), Blumeria graminis (powdery mildew of barley (f. sp. hordei) and wheat (f. sp. tritici)), Magnaporthe grisea (rice blast disease), Sclerotinia sclerotium (stalk break, stem rot), Septoria nodorum and Septoria tritici (glume blotch of wheat), Alternaria brassicae (black spot of oilseed rape, cabbage and other crucifers), Phoma lingam (blackleg of cabbage and oilseed rape).
[0055]2. Bacterial Pathogens:
[0056]The pathogens and the diseases associated with them which are mentioned in Table 3 may be mentioned by way of example but not by limitation.
TABLE-US-00003 TABLE 3 Bacterial diseases Disease Pathogen Bacterial leaf blight and Pseudomonas avenae subsp. avenae stalk rot Bacterial leaf spot Xanthomonas campestris pv. holcicola Bacterial stalk rot Enterobacter dissolvens = Erwinia dissolvens Bacterial stalk and top Erwiria carotovora subsp. rot carotovora, Erwinia chrysanthemi pv. zeae Bacterial stripe Pseudomonas andropogonis Chocolate spot Pseudomonas syringae pv. coronafaciens Goss's bacterial wilt and Clavibacter michiganensis subsp. blight (leaf freckles and nebraskensis = Corynebacterium wilt) michiganense pv. andnebraskense Holcus spot Pseudomonas syringae pv. syringae Purple leaf sheath Hemiparasitic bacteria Seed rot-seedling blight Bacillus subtilis Stewart's disease Pantoea stewartii = (bacterial wilt) Erwinia stewartii Corn stunt Spiroplasma kunkelii (achapparramiento, maize stunt, Mesa Central or Rio Grande maize stunt)
[0057]The following pathogenic bacteria are very especially preferred: Corynebacterium sepedonicum (bacterial ring rot of potato), Erwinia carotovora (black leg of potato), Erwinia amylovora (fire blight of pear, apple, quince), Streptomyces scabies (potato scab), Pseudomonas syringae pv. tabaci (wildfire of tobacco), Pseudomonas syringae pv. phaseolicola (grease spot of dwarf bean), Pseudomonas syringae pv. tomato (bacterial speck of tomato), Xanthomonas campestris pv. malvacearum (bacterial blight of cotton) and Xanthomonas campestris pv. oryzae (bacterial leaf blight of rice and other grasses).
[0058]3. Viral Pathogens:
[0059]"Viral pathogens" includes all plant viruses such as, for example, tobacco or cucumber mosaic virus, ringspot virus, necrosis virus, maize dwarf mosaic virus and the like.
[0060]The pathogens and diseases associated with them which are mentioned in Table 4 may be mentioned by way of example, but not by limitation.
TABLE-US-00004 TABLE 4 Viral diseases Disease Pathogen American wheat striate American wheat striate mosaic virus (wheat striate mosaic) (AWSMV) Barley stripe mosaic Barley stripe mosaic virus (BSMV) Barley yellow dwarf Barley yellow dwarf virus (BYDV) Brome mosaic Brome mosaic virus (BMV) Cereal chlorotic mottle Cereal chlorotic mottle virus (CCMV) Corn chlorotic vein Corn chlorotic vein banding virus banding (Brazilian maize (CCVBV) mosaic) Corn lethal necrosis Virus complex of Maize chlorotic mottle virus (MCMV) and Maize dwarf mosaic virus (MDMV) A or B or Wheat streak mosaic virus(WSMV) Cucumber mosaic Cucumber mosaic virus (CMV) Cynodon chlorotic streak Cynodon chlorotic streak virus (CCSV) Johnsongrass mosaic Johnsongrass mosaic virus (JGMV) Maize bushy stunt Mycoplasma-like organism (MLO) associated Maize chlorotic dwarf Maize chlorotic dwarf virus (MCDV) Maize chlorotic mottle Maize chlorotic mottle virus (MCMV) Maize dwarf mosaic Maize dwarf mosaic virus (MDMV) strains A, D, E and F Maize leaf fleck Maize leaf fleck virus (MLFV) Maize line Maize line virus (MLV) Maize mosaic (corn leaf Maize mosaic virus (MMV) stripe, enanismo rayado) Maize mottle and Maize mottle and chlorotic stunt virus chlorotic stunt Maize pellucid ringspot Maize pellucid ringspot virus (MPRV) Maize raya gruesa Maize raya gruesa virus (MRGV) Maize rayado fino (fine Maize rayado fino virus (MRFV) striping disease) Maize red leaf and red Mollicute stripe Maize red stripe Maize red stripe virus (MRSV) Maize ring mottle Maize ring mottle virus (MRMV) Maize rio IV Maize rio cuarto virus (MRCV) Maize rough dwarf Maize rough dwarf virus (MRDV) (Cereal (nanismo ruvido) tillering disease virus) Maize sterile stunt Maize sterile stunt virus (strains of barley yellow striate virus) Maize streak Maize streak virus (MSV) Maize stripe (maize Maize stripe virus chlorotic stripe, maize hoja blanca) Maize stunting Maize stunting virus Maize tassel abortion Maize tassel abortion virus (MTAV) Maize vein enation Maize vein enation virus (MVEV) Maize wallaby ear Maize wallaby ear virus (MWEV) Maize white leaf Maize white leaf virus Maize white line mosaic Maize white line mosaic virus (MWLMV) Millet red leaf Millet red leaf virus (MRLV) Northern cereal mosaic Northern cereal mosaic virus (NCMV) Oat pseudorosette Oat pseudorosette virus (zakuklivanie) Oat sterile dwarf Oat sterile dwarf virus (OSDV) Rice black-streaked Rice black-streaked dwarf virus dwarf (RBSDV) Rice stripe Rice stripe virus (RSV) Sorghum mosaic Sorghum mosaic virus (SrMV) (auch: sugarcane mosaic virus (SCMV) Stamme H, I and M) Sugarcane Fiji disease Sugarcane Fiji disease virus (FDV) Sugarcane mosaic Sugarcane mosaic virus (SCMV) strains A, B, D, E, SC, BC, Sabi and MB (formerly MDMV-B) Wheat spot mosaic Wheat spot mosaic virus (WSMV)
[0061]4. Animal Pests
[0062]4.1 Insect Pathogens:
[0063]The following may be mentioned by way of example, but not by limitation: insects such as, for example, beetles, caterpillars, lice or mites. Preferred insects are those of the genera Coleoptera, Diptera, Hymenoptera, Lepidoptera, Mallophaga, Homoptera, Hemiptera, Orthoptera, Thysanoptera, Dermaptera, Isoptera, Anoplura, Siphonaptera, Trichoptera, etc. Especially preferred are coleopteran and lepidopteran insects such as, for example, the European corn borer (ECB), Diabrotica barberi (northern corn rootworm), Diabrotica undecimpunctata (southern corn rootworm), Diabrotica virgifera (Western corn rootworm), Agrotis ipsilon (black cutworm), Crymodes devastator (glassy cutworm), Feltia ducens (dingy cutworm), Agrotis gladiaria (claybacked cutworm), Melanotus spp., Aeolus mellillus (wireworm), Aeolus mancus (wheat wireworm), Horistonotus uhlerii (sand wireworm), Sphenophorus maidis (maize billbug), Sphenophorus zeae (timothy billbug), Sphenophorus parvulus (bluegrass billbug), Sphenophorus callosus (southern corn billbug), Phyllogphaga spp. (white grubs), Anuraphis maidiradicis (corn root aphid), Delia platura (seedcorn maggot), Colaspis brunnea (grape colaspis), Stenolophus lecontei (seedcorn beetle) and Clivinia impressifrons (lender seedcorn beetle).
[0064]Other examples are: lema (Oulema melanopus), frit fly (Oscinella frit), wireworms (Agrotis lineatus) and aphids (such as, for example, the oat grain aphid Rhopalosiphum padi, the blackberry aphid Sitobion avenae).
[0065]4.2 Nematodes:
[0066]The pathogens and the diseases associated with them mentioned in Table 6 may be mentioned by way of example, but not by limitation.
TABLE-US-00005 TABLE 6 Parasitic nematodes Damage Pathogenic nematode Awl Dolichodorus spp., D. heterocephalus Bulb and stem nematode Ditylenchus dipsaci disease; Europe Burrowing Radopholus similis Cyst nematode disease Heterodera avenae, H. zeae, Punctodera chalcoensis Dagger Xiphinema spp., X. americanum, X. mediterraneum False root-knot Nacobbus dorsalis Lance, Columbia Hoplolaimus columbus Lance Hoplolaimus spp., H. galeatus Lesion Pratylenchus spp., P. brachyurus, P. crenatus, P. hexincisus, P. neglectus, P. penetrans, P. scribneri, P. thornei, P. zeae Needle Longidorus spp., L. breviannulatus Ring Criconemella spp., C. ornata Root-knot disease Meloidogyne spp., M. chitwoodi, M. incognita, M. javanica Spiral Helicotylenchus spp. Sting Belonolaimus spp., B. longicaudatus Stubby-root Paratrichodorus spp., P. christiei, P. minor, Quinisulcius acutus, Trichodorus spp. Stunt Tylenchorhynchus dubius
[0067]Very especially preferred are Globodera rostochiensis and G. pallida (cyst eelworm on potato, tomato and other Solanaceae), Heterodera schachtii (beet eelworm on sugar and fodder beet, oilseed rape, cabbage and the like), Heterodera avenae (oat cyst nematode on oat and other cereal species), Ditylenchus dipsaci (stem or bulb eelworm, stem eelworm of rye, oats, maize, clover, tobacco, beet), Anguina tritici (grain nematode, cockle disease of wheat (spelt, rye), Meloidogyne hapla (root-knot nematode of carrot, cucumber, lettuce, tomato, potato, sugar beet, lucerne).
[0068]Examples of preferred fungal or viral pathogens for the individual varieties are:
[0069]1. Barley:
[0070]Fungal, bacterial and viral pathogens: Puccinia graminis f.sp. hordei (barley stem rust), Blumeria (Erysiphe) graminis f.sp. hordei (barley powdery mildew), barley yellow dwarf virus (BYDV),
[0071]Pathogenic insects/nematodes: Ostrinia nubilalis (European corn borer); Agrotis ipsilon (black cutworm); Schizaphis graminum (greenbug); Blissus leucopterus leucopterus (chinch bug); Acrosternum hilare (green stink bug); Euschistus servus (brown stink bug); Deliaplatura (seedcorn maggot); Mayetiola destructor (Hessian fly); Petrobia latens (brown wheat mite).
[0072]2. Soybean:
[0073]Fungal, bacterial or viral pathogens: Phytophthora megasperma fsp. glycinea, Macrophomina phaseolina, Rhizoctonia solani, Sclerotinia sclerotiorum, Fusarium oxysporum, Diaporthe phaseolorum var. sojae (Phomopsis sojae), Diaporthe phaseolorum var. caulivora, Sclerotium rolfsii, Cercospora kikuchii, Cercospora sojina, Peronospora manshurica, Colletotrichum dematium (Colletotrichum truncatum), Corynespora cassiicola, Septoria glycines, Phyllosticta sojicola, Alternaria alternata, Pseudomonas syringae p.v. glycinea, Xanthomonas campestris p.v. phaseoli, Microsphaera diffussa, Fusarium semitectum, Phialophora gregata, soybean mosaic virus, Glomerella glycines, Tobacco Ring spot virus, Tobacco Streak virus, Phakopsorapachyrhizi, Pythium aphanidermatum, Pythium ultimun, Pythium debaryanum, Tomato spotted wilt virus, Heterodera glycines Fusarium solani.
[0074]Pathogenic insects/nematodes: Pseudoplusia includens (soybean looper); Anticarsia gemmatalis (velvetbean caterpillar); Plathypena scabra (green cloverworm); Ostrinia nubilalis (European corn borer); Agrotis ipsilon (black cutworm); Spodoptera exigua (beet armyworm); Heliothis virescens (cotton budworm); Helicoverpa zea (cotton bollworm); Epilachna varivestis (Mexican bean beetle); Myzus persicae (green peach aphid); Empoasca fabae (potato leaf hopper); Acrosternum hilare (green stink bug); Melanoplus femurrubrum (redlegged grasshopper); Melanoplus differentialis (differential grasshopper); Hylemya platura (seedcom maggot); Sericothrips variabilis (soybean thrips); Thrips tabaci (onion thrips); Tetranychus turkestani (strawberry spider mite); Tetranychus urticae (two-spotted spider mite).
[0075]3. Canola:
[0076]Fungal, bacterial or viral pathogens: Albugo candida, Alternaria brassicae, Leptosphaeria maculans, Rhizoctonia solani, Sclerotinia sclerotiorum, Mycosphaerella brassiccola, Pythium ultimum, Peronospora parasitica, Fusarium roseum, Alternaria alternata.
[0077]4. Alfalfa:
[0078]Fungal, bacterial or viral pathogens: Clavibater michiganese subsp. insidiosum, Pythium ultimum, Pythium irregulare, Pythium splendens, Pythium debaryanum, Pythium aphanidermatum, Phytophthora megasperma, Peronospora trifoliorum, Phoma medicaginis var. medicaginis, Cercospora medicaginis, Pseudopeziza medicaginis, Leptotrochila medicaginis, Fusarium, Xanthomonas campestris p.v. alfalfae, Aphanomyces euteiches, Stemphylium herbarum, Stemphylium alfalfae.
[0079]5. Wheat:
[0080]Fungal, bacterial or viral pathogens: Pseudomonas syringae p.v. atrofaciens, Urocystis agropyri, Xanthomonas campestris p.v. translucens, Pseudomonas syringae p.v. syringae, Alternaria alternata, Cladosporium herbarum, Fusarium graminearum, Fusarium avenaceum, Fusarium culmorum, Ustilago tritici, Ascochyta tritici, Cephalosporium gramineum, Collotetrichum graminicola, Erysiphe graminis f.sp. tritici, Puccinia graminis f.sp. tritici, Puccinia recondita f.sp. tritici, Puccinia striiformis, Pyrenophora tritici-repentis, Septoria nodorum, Septoria tritici, Septoria avenae, Pseudocercosporella herpotrichoides, Rhizoctonia solani, Rhizoctonia cerealis, Gaeumannomyces graminis var. tritici; Pythium aphanidermatum, Pythium arrhenomanes, Pythium ultimum, Bipolaris sorokiniana, Barley Yellow Dwarf Virus, Brome Mosaic Virus, soil borne wheat mosaic virus, wheat streak mosaic virus, wheat spindle streak virus, American wheat striate virus, Claviceps purpurea, Tilletia tritici, Tilletia laevis, Ustilago tritici, Tilletia indica, Rhizoctonia solani, Pythium arrhenomannes, Pythium gramicola, Pythium aphanidermatum, high plains virus, European wheat striate virus, Puccinia graminis f.sp. tritici (wheat stem rust), Blumeria (Erysiphe) graminis f.sp. tritici (wheat powdery mildew).
[0081]Pathogenic insects/nematodes: Pseudaletia unipunctata (armyworm); Spodoptera, frugiperda (fall armyworm); Elasmopalpus lignosellus (lesser cornstalk borer); Agrotis orthogonia (western cutworm); Elasmopalpus Zignosellus (lesser cornstalk borer); Oulema melanopus (cereal leaf beetle); Hypera punctata (clover leaf weevil); Diabrotica undecimpunctata howardi (southern corn rootworm); Russian wheat aphid; Schizaphis graminum (greenbug); Macrosiphum avenae (English grain aphid); Melanoplus femurrubrum (redlegged grasshopper); Melanoplus differentialis (differential grasshopper); Melanoplus sanguinipes (migratory grasshopper); Mayetiola destructor (Hessian fly); Sitodiplosis mosellana (wheat midge); Meromyza americana (wheat stem maggot); Hylemya coarctata (wheat bulb fly); Frankliniella fusca (tobacco thrips); Cephus cinctus (wheat stem sawfly); Aceria rulipae (wheat curl mite).
[0082]6. Sunflower:
[0083]Fungal, bacterial or viral pathogens: Plasmophora halstedii, Sclerotinia sclerotiorum, aster yellows, Septoria helianthi, Phomopsis helianthi, Alternaria helianthi, Alternaria zinniae, Botrytis cinerea, Phoma macdonaldii, Macrophomina phaseolina, Erysiphe cichoracearum, Rhizopus oryzae, Rhizopus arrhizus, Rhizopus stolonifer, Puccinia helianthi, Verticillium dahliae, Erwinia carotovorum p.v. Carotovora, Cephalosporium acremonium, Phytophthora cryptogea, Albugo tragopogonis.
[0084]Pathogenic insects/nematodes: Suleima helianthana (sunflower bud moth); Homoeosoma electellum (sunflower moth); zygogramma exclamationis (sunflower beetle); Bothyrus gibbosus (carrot beetle); Neolasioptera murtfeldtiana (sunflower seed midge).
[0085]7. Maize:
[0086]Fungal, bacterial or viral pathogens: Fusarium moniliforme var. subglutinans, Erwinia stewartii, Fusarium moniliforme, Gibberella zeae (Fusarium graminearum), Stenocarpella maydi (Diplodia maydis), Pythium irregulare, Pythium debaryanum, Pythium graminicola, Pythium splendens, Pythium ultimum, Pythium aphanidermatum, Aspergillus flavus, Bipolaris maydis 0, T (Cochliobolus heterostrophus), Helminthosporium carbonum I, II & III (Cochliobolus carbonum), Exserohilum turcicum I, II & III, Helminthosporium pedicellatum, Physoderma maydis, Phyllosticta maydis, Kabatiella maydis, Cercospora sorghi, Ustilago maydis, Puccinia sorghi, Puccinia polysora, Macrophomina phaseolina, Penicillium oxalicum, Nigrospora oryzae, Cladosporium herbarum, Curvularia lunata, Curvularia inaequalis, Curvularia pallescens, Clavibacter michiganese subsp. nebraskense, Trichoderma viride, maize dwarf mosaic virus A & B, wheat streak mosaic virus, maize chlorotic dwarf virus, Claviceps sorghi, Pseudonomas avenae, Erwinia chrysanthemi p.v. Zea, Erwinia corotovora, cornstunt spiroplasma, Diplodia macrospora, Sclerophthora macrospora, Peronosclerospora sorghi, Peronosclerospora philippinesis, Peronosclerospora maydis, Peronosclerospora sacchari, Spacelotheca reiliana, Physopella zeae, Cephalosporium maydis, Caphalosporium acremonium, maize chlorotic mottle virus, high plains virus, maize mosaic virus, maize rayado fino virus, maize streak virus (MSV), maize stripe virus, maize rough dwarf virus.
[0087]Pathogenic insects/nematodes: Ostrinia nubilalis (European corn borer); Agrotis ipsilon (black cutworm); Helicoverpa zea (corn earworm); Spodoptera frugiperda (fall armyworm); Diatraea grandiosella (Southwestern corn borer); Elasmopalpus lignosellus (lesser cornstalk borer); Diatraea saccharalis (sugarcane borer); Diabrotica virgifera (Western corn rootworm); Diabrotica longicornis barberi (Northern corn rootworm); Diabrotica undecimpunctata howardi (Southern corn rootworm); Melanotus spp. (wireworms); Cyclocephala borealis (Northern masked chafer; white grub); Cyclocephala immaculata (Southern masked chafer; white grub); Popillia japonica (Japanese beetle); Chaetocnema pulicaria (corn flea beetle); Sphenophorus maidis (maize billbug); Rhopalosiphum maidis (corn leaf aphid); Anuraphis maidiradicis (corn root aphid); Blissus leucopterus leucopterus (chinch bug); Melanoplus femur-rubrum (red-legged grasshopper); Melanoplus sanguinipes (migratory grasshopper); Hylemva platura (seedcom maggot); Agromyza parvicornis (corn blot leafminer); Anaphothrips obscrurus (grass thrips); Solenopsis milesta (thief ant); Tetranychus urticae (two-spotted spider mite).
[0088]8. Sorghum:
[0089]Fungal, bacterial or viral pathogens: Exserohilum turcicum, Colletotrichum graminicola (Glomerella graminicola), Cercospora sorghi, Gloeocercospora sorghi, Ascochyta sorghina, Pseudomonas syringae p.v. syringae, Xanthomonas campestris p.v. holcicola, Pseudomonas andropogonis, Puccinia purpurea, Macrophomina phaseolina, Perconia circinata, Fusarium moniliforme, Alternaria alternate, Bipolaris sorghicola, Helminthosporium sorghicola, Curvularia lunata, Phoma insidiasa, Pseudomonas avenae (Pseudomonas alboprecipitans), Ramulispora sorghi, Ramulispora sorghicola, Phyllachara sacchari, Sporisorium reilianum (Sphacelotheca reiliana), Sphacelotheca cruenta, Sporisorium sorghi, sugarcane mosaic H, maize dwarf mosaic virus A & B, Claviceps sorghi, Rhizoctonia solani, Acremonium strictum, Sclerophthona macrospora, Peronosclerospora sorghi, Peronosclerospora philippinensis, Sclerospora graminicola, Fusarium graminearum, Fusarium oxysporum, Pythium arrhenomanes, Pythium graminicola.
[0090]Pathogenic insects/nematodes: Chilo partellus (sorghum borer); Spodoptera frugiperda (fall armyworm); Helicoverpa zea (corn ear-worm); Elasmopalpus lignosellus (lesser cornstalk borer); Feltia subterranea (granulate cutworm); Phvllophaga crinita(white grub); Eleodes, Conoderus and Aceolus spp. (wireworm); Oulema melanopus (cereal leaf beetle); Chaetocnema pulicaria (corn flea beetle); Sphenophorus maidis (maize billbug); Rhopalosiphum maidis (corn leaf aphid); Siphaflava (yellow sugarcane aphid); Blissas leucopterus leucopterus (chinch bug); Contarinia sorghicola (sorghum midge); Tetranychus cinnabarinus (carmine spider mite); Tetranychus urticae (two-spotted spider mite).
[0091]9. Cotton:
[0092]Pathogenic insects/nematodes: Heliothis virescens (cotton budworm); Helicoverpa zea (cotton bollworm); Spodoptera exigua (beet armyworm); Pectinophora gossypiella (pink bollworm); Anthonomus grandis grandis (boll weevil); Aphis gossypii (cotton aphid); Pseudatomoscelis seriatus (cotton fleahopper); Trialeurodes abutilonea (banded-winged whitefly); Lygus lineolaris (tarnished plant bug); Melanoplus femurrubrum (red-legged grasshopper); Melanoplus differentialis (differential grasshopper); Thrips tabaci (onion thrips); Franklinkiella fusca (tobacco thrips); Tetranychus cinnabarinus (carmine spider mite); Tetranychus urticae (two-spotted spider mite).
[0093]10. Rice:
[0094]Pathogenic insects/nematodes: Diatraea saccharalis (sugarcane borer); Spodoptera frugiperda (fall armyworm); Helicoverpa zea (corn earworm); Colaspis brunnea (grape colaspis); Lissorhoptrus oryzophilus (rice water weevil); Sitophilus oryzae (rice weevil); Nephotettix nigropictus (rice leafhopper); Blissus leucopterus leucopterus (chinch bug); Acrosternum hilare (green stink bug).
[0095]11. Oilseed Rape:
[0096]Pathogenic insects/nematodes: Brevicoryne brassicae (cabbage aphid); Phyilotreta cruciferae (flea beetle); Mamestra conjgurata (bertha armyworm); Plutella xylostella (diamond-back moth); Delia ssp. (root maggots).
[0097]For the purposes of the invention, "RacB protein" is understood as meaning the RacB protein from barley as shown in SEQ ID NO: 2, and its homologs from rice (Oryza sative) as shown in SEQ ID NO: 4 and maize (Zea mays) as shown in SEQ ID NO: 6, and functional equivalents of the above-mentioned.
[0098]"Amount of protein" is understood as meaning the amount of an RacB polypeptide in an organism, a tissue, a cell or a cell compartment. "Reduction" of the amount of protein means the quantitative reduction of the amount of an RacB protein in an organism, a tissue, a cell or a cell compartment--for example by one of the methods described hereinbelow--in comparison with the wild type of the same genus and species, to which this method had not been applied, under otherwise identical conditions (such as, for example, culture conditions, plant age and the like). The reduction amounts to at least 10%, preferably at least 10% or at least 20%, especially preferably at least 40% or 60%, very especially preferably at least 70% or 80%, most preferably at least 90% or 95%.
[0099]"Activity" is preferably understood as meaning the GTPase activity of an RacB polypeptide in an organism, a tissue, a cell or a cell compartment. "Reduction" of the activity is understood as meaning the reduction of the total activity of an RacB protein in an organism, a tissue, a cell or a cell compartment for example by one of the methods described hereinbelow--in comparison with the wild type of the same genus and species, to which this method had not been applied, under otherwise identical conditions (such as, for example, culture conditions, plant age and the like). The reduction amounts to at least 10%, preferably at least 10% or at least 20%, especially preferably at least 40% or 60%, very especially preferably at least 70% or 80%, most preferably at least 90% or 95%.
[0100]"Function" is preferably understood as meaning the substrate-binding capacity of an RacB polypeptide in an organism, a tissue, a cell or a cell compartment. Suitable substrates are low-molecular-weight compounds such as GTP, but also the protein interaction partners of an RacB protein. "Reduction" of the function is understood as meaning, for example, the quantitative reduction of the binding capacity or binding strength of an RacB protein to at least one substrate in an organism, a tissue, a cell or a cell compartment--for example by one of the methods described hereinbelow--in comparison with the wild type of the same genus and species, to which this method had not been applied, under otherwise identical conditions (such as, for example, culture conditions, plant age and the like). Reduction is also understood as meaning the change in substrate specificity as can be expressed for example by the kcat/Km value. The reduction amounts to at least 10%, preferably at least 10% or at least 20%, especially preferably at least 40% or 60%, very especially preferably at least 70% or 80%, most preferably at least 90% or 95%. Binding partners for RacB can be identified in a manner with which the skilled worker is familiar, for example by the yeast-2-hybrid system.
[0101]Methods of determining the amount of protein, the activity of GTPases or the substrate binding capacity are known to the skilled worker and have been described on a number of occasions for GTPases and for Rac proteins from a variety of genera and species (see, inter alia, Benard V et al. (11999) J Biol Chem 274(19):13198-204; Burstein E S (1998) Oncogene. 17(12):1617-23).
[0102]"Functional equivalents" of an RacB protein is preferably understood as meaning those sequences which are derived from, or are homologous to, an RacB protein described by SEQ ID NO: 2, 4 or 6 and which have essentially the same properties.
[0103]"Essentially the same properties" of a functional equivalent is above all understood as meaning conferring a pathogen-resistant phenotype or conferring or increasing the resistance to at least one pathogen while reducing the amount of protein, activity or function of said functional RacB equivalent in a plant or in a tissue, part or cells of the same. The absence of a spontaneously induced cell death in combination with said reduction of the amount of protein, activity or function of the functional equivalent is furthermore understood as an essential property.
[0104]In this context, the efficacy of the pathogen resistance can deviate both downward or upward in comparison with a value obtained when reducing one of the RacB proteins as shown in SEQ ID NO: 2, 4 or 6. Preferred functional equivalents are those in which the efficacy of the pathogen resistance--measured, for example, by the penetration efficacy of a pathogen (formation of haustoria)--differs by not more than 50%, preferably 25%, especially preferably 10% from a comparative value obtained by reducing an RacB protein as shown in NO: 2, 4 or 6. Especially preferred are those sequences where the reduction increases the efficacy of pathogen resistance quantitatively by more than 50%, preferably 100%, especially preferably 500%, very especially preferably 1000% based on a comparative value obtained by reducing one of the RacB protein as shown in SEQ ID NO: 2, 4 or 6.
[0105]The comparison is preferably carried out under analogous conditions. "Analogous conditions" means that all conditions such as, for example, culture or growing conditions, assay conditions (such as buffer, temperature, substrates, pathogen concentration and the like) are kept identical between the experiments to be compared and that the set-ups differ only by the sequence of the RacB polypeptides to be compared, their organism of origin and, if appropriate, the pathogen. When choosing the pathogen, each comparison requires that the pathogen be chosen which is most similar to the other equivalent, taking into consideration the species specificity.
[0106]"Functional equivalents" is understood as meaning, in particular, natural or artificial mutations of the RacB polypeptides as shown in SEQ ID NO: 2, 4 or 6 and homologous polypeptides from other plants which continue to have essentially the same properties. Homologous polypeptides from the above-described preferred plants are preferred. The sequences from other plants (for example Arabidopsis thaliana) which are homologous to the RacB sequences disclosed within the scope of the present invention can be found readily for example by database search or by screening genetic libraries using the RacB sequences as search sequence or probe.
[0107]Mutations comprise substitutions, additions, deletions, inversion or insertions of one or more amino acid residues. Thus, the present invention also comprises those polypeptides which are obtained by modification of a polypeptide as shown in SEQ ID NO: 2, 4 or 6.
[0108]Homology between two nucleic acid sequences is understood as meaning the identity of the nucleic acid sequence over in each case the entire sequence length which is calculated by comparison with the aid of the program algorithm GAP (Wisconsin Package Version 10.0, University of Wisconsin, Genetics Computer Group (GCG), Madison, USA; Altschul et al. (1997) Nucleic Acids Res. 25:3389 et seq.), setting the following parameters:
TABLE-US-00006 Gap weight: 50 Length weight: 3 Average match: 10 Average mismatch: 0
[0109]For example a sequence which has at least 80% homology with sequence SEQ ID NO: 1 at the nucleic acid level is understood as meaning a sequence which, upon comparison with the sequence SEQ ID NO: 1 by the above program algorithm with the above parameter set, has at least 80% homology.
[0110]Homology between two polypeptides is understood as meaning the identity of the amino acid sequence over in each case the entire sequence length which is calculated by comparison with the aid of the program algorithm GAP (Wisconsin Package Version 10.0, University of Wisconsin, Genetics Computer Group (GCG), Madison, USA), setting the following parameters:
TABLE-US-00007 Gap weight: 8 Length weight: 2 Average match: 2,912 Average mismatch: -2,003
[0111]For example a sequence which has at least 80% homology with sequence SEQ ID NO: 2 at the protein level is understood as meaning a sequence which, upon comparison with the sequence SEQ ID NO: 2 by the above program algorithm with the above parameter set, has at least 80% homology.
[0112]Functional equivalents derived from one of the polypeptide as shown in SEQ ID NO: 2, 4 or 6 according to the invention by substitution, insertion or deletion have at least 60%, preferably at least 80%, by preference at least 90%, especially preferably at least 95%, very especially preferably at least 98%, homology with one of the polypeptide as shown in SEQ ID NO: 2, 4 or 6 according to the invention and are distinguished by essentially the same properties as the polypeptide as shown in SEQ ID NO: 2, 4 or 6.
[0113]Functional equivalents derived from the nucleic acid sequence as shown in SEQ ID NO: 1, 3 or 5 according to the invention by substitution, insertion or deletion have at least 60%, preferably at least 80%, by preference at least 90%, especially preferably at least 95%, very especially preferably at least 98%, homology with one of the polypeptides as shown in SEQ ID NO: 1, 3 or 5 according to the invention and encode polypeptides having essentially the same properties as the polypeptide as shown in SEQ ID NO: 2, 4 or 6.
[0114]The RacB proteins comprised as functional equivalents preferably have at least one of the following sequence motifs:
[0115]a) A G1 element GXXXXGKS/T preferably in the N-terminal region, very especially preferably an element with the sequence GDGAVGKT, most preferably an element with the sequence KCVTVGDGAVGKTC.
[0116]b) A G2 effector region comprising a sequence motif with PTVFDN, especially preferably NTFPTDYVPTVFDNFSANVV.
[0117]c) A G3 element comprising LWDTAGQ, especially preferably NLGLWDTAGQEDYN.
[0118]d) A G4 element TKXD, especially preferably TKLD, very especially preferably LVGTKLDLRDDKQ.
[0119]e) A G5 element EXS, preferably ECSS, very especially preferably ECSSKTG.
[0120]f) A C-terminal isoprenylation motif (CXXX, Hassanain U H et al. (2000) Biochem Biophys Res Commun. 272(3):783-8.), especially preferably CSIL.
[0121]Especially preferably, at least 2 or 3 of these motifs (a to f) occur in a functionally equivalent RacB protein, very especially preferably at least 4 or 5 of these motifs, most preferably all motifs a to f. Further sequence motifs which are typical for RacB, in particular also motifs which constitute a delimitation against Rac1 proteins, can be deduced readily by the skilled worker from the sequence alignment of the known RacB (or Rac1) proteins, as shown in FIG. 1.
[0122]Examples of the functional equivalents to the RacB proteins as shown in SEQ ID NO: 2, 4 or 6, which equivalents are to be reduced in the method according to the invention, can be identified for example from a variety of organisms whose genomic sequence is known, such as, for example, from Arabidopsis thaliana, Brassica napus, Nicotiana tabacum, Solanum tuberosum, or Helianthinum from databases of homology comparisons.
[0123]The screening of cDNA libraries or genomic libraries of other organisms, preferably of the plant species which are mentioned further below as hosts for the transformation, using the nucleic acid sequences described under SEQ ID NO: 1, 3 or 5 or parts of these as probe is also a method of identifying homologs in other species with which the skilled worker is familiar. In this context, the probes derived from the nucleic acid sequences as shown in SEQ ID NO: 1, 3 or 5 have a length of at least 20 bp, preferably 50 bp, particularly preferably 100 bp, very especially preferably 200 bp, and most preferably 400 bp. A DNA strand which is complementary to the sequences described under SEQ ID NO: 1, 3 or 5 may also be employed for screening the libraries.
[0124]Functional equivalents, accordingly, comprise DNA sequences which hybridize under standard conditions with the RacB nucleic acid sequence described by SEQ ID NO: 1, 3 or 5, with the sequence complementary thereto or parts of the abovementioned and which, as complete sequences, encode proteins which have the same properties as the proteins described under SEQ ID NO: 2, 4 or 6.
[0125]"Standard hybridization conditions" is to be understood in the broad sense and means stringent or else less stringent hybridization conditions. Such hybridization conditions are described, inter alia, by Sambrook J, Fritsch E F, Maniatis T et al., in Molecular Cloning (A Laboratory Manual), 2nd Edition, Cold Spring Harbor Laboratory Press, 1989, pages 9.31-9.57) or in Current Protocols in Molecular Biology, John Wiley & Sons, N.Y. (1989), 6.3.1-6.3.6.
[0126]For example, the conditions during the wash step can be selected from the range of conditions delimited by low-stringency conditions (approximately 2×SSC at 50° C.) and high-stringency conditions (approximately 0.2×SSC at 50° C., preferably at 65° C.): (20×SSC: 0.3M sodium citrate, 3 M NaCl, pH 7.0). In addition, the temperature during the wash step can be raised from low-stringency conditions at room temperature, approximately 22° C., to higher-stringency conditions at approximately 65° C. Both of the parameters salt concentration and temperature can be varied simultaneously, or else one of the two parameters can be kept constant while only the other is varied. Denaturants, for example formamide or SDS, may also be employed during the hybridization.
[0127]In the presence of 50% formamide, hybridization is preferably effected at 42° C. Some examples of conditions for hybridization and wash step are shown hereinbelow:
[0128](1) Hybridization conditions be selected, for example, from the following conditions:
[0129]a) 4×SSC at 65° C.,
[0130]b) 6×SSC at 45° C.,
[0131]c) 6×SSC, 100 μg/ml denatured fragmented fish sperm DNA at 68° C.,
[0132]d) 6×SSC, 0.5% SDS, 100 μg/ml denatured salmon sperm DNA at 68° C.,
[0133]e) 6×SSC, 0.5% SDS, 100 μg/ml denatured fragmented salmon sperm DNA, 50% formamide at 42° C.,
[0134]f) 50% formamide, 4×SSC at 42° C., or
[0135]g) 50% (vol/vol) formamide, 0.1% bovine serum albumin, 0.1% Ficoll, 0.1% polyvinylpyrrolidone, 50 mM sodium phosphate buffer pH 6.5, 750 mM NaCl, 75 mM sodium citrates at 42° C., or
[0136]h) 2× or 4×SSC at 50° C. (low-stringency condition),
[0137]i) 30 to 40% formamide, 2× or 4×SSC at 42° C. (low-stringency condition).
[0138](2) Wash steps can be selected, for example, from the following conditions:
[0139]a) 0.015 M NaCl/0.0015 M sodium citratc/0.1% SDS at 50° C.
[0140]b) 0.1×SSC at 65° C.
[0141]c) 0.1×SSC, 0.5% SDS at 68° C.
[0142]d) 0.1×SSC, 0.5% SDS, 50% formamide, at 42° C.
[0143]e) 0.2×SSC, 0.1% SDS at 42° C.
[0144]f) 2×SSC at 65° C. (low-stringency condition).
[0145]Functional equivalents derived from a polypeptide as shown in SEQ ID NO: 2, 4 or 6 comprises in particular also the proteins having the SEQ ID NO: 35, 37, 39, 41, 43, 45, 47, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68 or 70. "Functional equivalents" is to be understood as meaning, in particular, proteins encoded by a nucleic acid sequence having the SEQ ID NO: 34, 36, 38, 40, 42, 44, 46, 48, 49, 51, 53, 55, 57, 61, 63, 65, 67 or 69.
[0146]The reduction of the expression of an RacB protein, the RacB activity or the RacB function can be realized in many ways.
[0147]"Reduction" or "reducing" in connection with an RacB protein, an RacB activity or an RacB function is to be interpreted in the wide sense and comprises the partial or essentially complete inhibition or blocking of the functionality of an RacB protein in a plant or a part, tissue, organ, cells or seeds thereof, which inhibition or blocking is based on a variety of cytobiological mechanisms.
[0148]For the purposes of the invention, a reduction also comprises a quantitative reduction of an RacB protein down to the essential complete absence of the RacB protein (i.e. lacking detectability of RacB activity or RacB function, or lacking immunological detectability of the RacB protein). In this context, the expression of a particular RacB protein, or the RacB activity or RacB function, in a cell or an organism is preferably reduced by more than 50%, especially preferably by more than 80%, very especially preferably by more than 90%.
[0149]A variety of strategies for reducing the expression of an RacB protein, the RacB activity or RacB function are comprised in accordance with the invention. The skilled worker is aware of a series of different methods being available for influencing the expression of an RacB protein, the RacB activity or the RacB function in the desired manner.
[0150]A reduction of the RacB activity or the RacB function is preferably achieved by reduced expression of an endogenous RacB protein.
[0151]A reduction of the amount of RacB protein, the RacB activity or the RacB function can be effected using the following methods:
[0152]a) Introduction of a double-stranded RacB RNA nucleic acid sequence (RacB dsRNA) or an expression cassette(s) ensuring the expression thereof.
[0153]b) Introduction of an RacB antisense nucleic acid sequences or an expression cassette ensuring expression thereof comprised are those methods in which the antisense nucleic acid sequence is directed against an RacB gene (i.e. genomic DNA sequences) or an RacB gene transcript (i.e. RNA sequences). α-Anomeric nucleic acid sequences are also comprised
[0154]c) Introduction of an RacB antisense nucleic acid sequences in combination with a ribozyme or an expression cassette ensuring expression thereof.
[0155]d) Introduction of RacB sense nucleic acid sequences for inducing cosuppression or an expression cassette ensuring expression thereof.
[0156]e) Introduction of a nucleic acid sequence encoding dominant-negative RacB protein or an expression cassette ensuring expression thereof.
[0157]f) Introduction of DNA- or protein-binding factors against RacB genes, RacB RNAs or RacB proteins or an expression cassette ensuring expression thereof.
[0158]g) Introduction of viral nucleic acid sequences and expression constructs causing RacB RNA degradation or an expression cassette ensuring expression thereof.
[0159]h) Introduction of constructs for inducing a homologous recombination on endogenous RacB genes, for example for generating knock-out mutants
[0160]i) Introduction of mutations into endogenous RacB genes for generating a loss of function (for example generation of stop codons, reading-frame shifts and the like).
[0161]In this context, each and every of these methods may bring about a reduction of the RacB expression, RacB activity or RacB function for the purposes of the invention. A combined use is also feasible. Further methods are known to the skilled worker and can comprise the hindering or prevention of RacB protein processing, of the RacB protein or RacB mRNA transport, inhibition of ribosome attachment, inhibition of RNA splicing, induction of an RacB-RNA-degrading enzyme and/or inhibition of translational elongation or termination.
[0162]The individual processes which are preferred may be described in greater detail hereinbelow:
[0163]a) Introduction of a Double-Stranded RacB RNA Nucleic Acid Sequence (RacB dsRNA)
[0164]The method of regulating genes by means of double-stranded RNA ("double-stranded RNA interference"; dsRNAi) has been described repeatedly for animal and plant organisms (for example Matzke M A et al. (2000) Plant Mol Biol 43:401-415; Fire A. et al (1998) Nature 391:806-811; WO 99/32619; WO 99/53050; WO 00/68374; WO 00/44914; WO 00/44895; WO 00/49035; WO 00/63364). Express reference is made to the processes and methods described in the above references. Effective gene suppression can also be demonstrated upon transient expression or following transient transformation for example as the consequence of biolistic transformation (Schweizer P et al. (2000) Plant J 2000 24: 895-903). dsRNAi methods are based on the phenomenon that the simultaneous introduction of complementary strand and counterstrand of a gene transcript causes the expression of the gene in question to be suppressed in a highly efficient manner. The phenotype caused greatly resembles a corresponding knock-out mutant (Waterhouse P M et al. (1998) Proc Natl Acad Sci USA 95:13959-64).
[0165]The dsRNAi method has proved to be particularly effective and advantageous for reducing the RacB expression. As described, inter alia, in WO 99/32619, dsRNAi approaches are markedly superior to traditional antisense approaches.
[0166]The invention therefore furthermore relates to double-stranded RNA molecules (dsRNA molecules) which, upon introduction into a plant (or a cell, tissue, organ or seed derived therefrom), bring about the reduction of an RacB.
[0167]In the double-stranded RNA molecule for reducing the expression of an RacB protein,
[0168]a) one of the two RNA strands is essentially identical to at least a portion of an RacB nucleic acid sequence, and
[0169]b) the corresponding other RNA strand is essentially identical to at least a portion of the complementary strand of an RacB nucleic acid sequence.
[0170]In a further preferred embodiment, the double-stranded RNA molecule for reducing the expression of an RacB protein comprises
[0171]a) a sense RNA strand comprising at least one ribonucleotide sequence which is essentially identical to at least part of the sense RNA transcript of a nucleic acid sequence encoding an RacB protein, and
[0172]b) an antisense RNA strand which is essentially--preferably completely--complementary to the RNA sense strand under a).
[0173]With respect to the double-stranded RNA molecules, RacB nucleic acid sequence is to be understood as meaning, preferably, a sequence as shown in SEQ ID NO: 1, 3 or 5 or a functional equivalent thereof as shown in SEQ ID NO: 34, 36, 38, 40, 42, 44, 46, 48, 49, 51, 53, 55, 57, 61, 63, 65, 67 or 69.
[0174]"Essentially identical" means that the dsRNA sequence can also show insertions, deletions or individual point mutations compared with the RacB target sequence or a functionally equivalent target sequence while still bringing about an effective reduction of the expression. The homology in accordance with the above definition preferably amounts to at least 75%, preferably at least 80%, very especially preferably at least 90%, most preferably 100%, between the sense strand of an inhibitory dsRNA and at least part of the sense RNA transcript of a nucleic acid sequence encoding an RacB protein or a functional equivalent thereof (or between the antisense strand of the complementary strand of a nucleic acid sequence encoding an RacB protein or a functional equivalent thereof).
[0175]The length of the part-segment amounts to at least 10 bases, preferably at least 25 bases, especially preferably at least 50 bases, very especially preferably at least 100 bases, most preferably at least 200 bases or at least 300 bases.
[0176]As an alternative, an "essentially identical" dsRNA can also be defined as a nucleic acid sequence which is capable of hybridizing with part of a storage protein gene transcript (for example in 400 mM NaCl, 40 mM PIPES pH 6.4, 1 mM EDTA at 50° C. or 70° C. for 12 to 16 h).
[0177]"Essentially complementary" is to be understood as meaning that the antisense RNA strand may also contain insertions, deletions and individual point mutations, compared to the complement of the sense RNA strand. Preferably, the homology between the antisense RNA strand and the complement of the sense RNA strand is at least 80%, preferably at least 90%, very particularly preferably at least 95%, most preferably 100%.
[0178]"Part of the sense RNA transcript" of a nucleic acid sequence encoding an RacB protein or a functional equivalent thereof is to be understood as meaning fragments of an RNA or mRNA transcribed from a nucleic acid sequence, preferably an RacB gene, encoding an RacB protein or a functional equivalent thereof. Here, the fragments preferably have a sequence length of at least 20 bases, preferably at least 50 bases, particularly preferably at least 100 bases, very particularly preferably at least 200 bases, most preferably at least 500 bases. Also comprised is the complete transcribed RNA or mRNA.
[0179]Also comprised is the use of the dsRNA molecules according to the invention in the methods according to the invention for generating a pathogen resistance in plants.
[0180]The dsRNA can be composed of one or more strands of polymerized ribonucleotides. Modifications both of the sugar-phosphate backbone and of the nucleosides may be present. For example, the phosphodiester bonds of the natural RNA can be modified in such a way that they comprise at least one nitrogen or sulfur hetero atom. Bases can be modified in such a way that the activity of, for example, adenosine deaminase is restricted. These and other modifications are described hereinbelow in the methods of stabilizing antisense RNA.
[0181]To achieve the same purpose, it is, of course, also possible to introduce a plurality of individual dsRNA molecules each comprising one of the ribonucleotide sequence sections defined above into the cell or the organism.
[0182]The dsRNA can be generated enzymatically or fully or partially synthesized chemically.
[0183]The double-stranded dsRNA structure can be formed starting from two complementary, separate RNA strands or, preferably, strands starting from an individual self-complementary RNA strand.
[0184]In a single self-complementary strand, sense and antisense sequence may be linked by a linking sequence ("linker") and can form for example a hairpin structure. The linking sequence can preferably be an intron which is spliced out after the dsRNA has been synthesized.
[0185]The nucleic acid sequence encoding a dsRNA can comprise further elements such as, for example, transcription termination signals or polyadenylation signals.
[0186]If the two dsRNA strands are to be combined in a cell or plant, this can be effected in various ways:
[0187]a) transformation of the cell or plant with a vector comprising both expression cassettes,
[0188]b) cotransformation of the cell or plant with two vectors, one of them comprising the expression cassettes with the sense strand and the other comprising the expression cassettes with the antisense strand,
[0189]c) hybridizing two plants, each of which has been transformed with one vector, one of the vectors comprising the expression cassettes with the sense strand and the other comprising the expression cassettes with the antisense strand.
[0190]The formation of the RNA duplex can be initiated either outside or within the cell. Like in WO 99/53050, the dsRNA can also comprise a hairpin structure by linking sense and antisense strand by means of a linker (for example an intron). The self-complementary dsRNA structures are preferred since they only require the expression of one construct and always comprise the complementary strands in an equimolar ratio.
[0191]The expression cassettes encoding the antisense or sense strand of a dsRNA or the self-complementary strand of the dsRNA are preferably inserted into a vector and, using the methods described hereinbelow, stably inserted into the genome of a plant in order to ensure permanent expression of the dsRNA, using selection markers for example.
[0192]The dsRNA can be introduced using a quantity which allows at least one copy per cell, Greater quantities (for example at least 5, 10, 100, 500 or 1000 copies per cell) may bring about a more effective reduction.
[0193]As already described, 100% sequence identity between dsRNA and an RacB gene transcript or the gene transcript of a functionally equivalent gene is not necessarily required in order to bring about an effective reduction of the RacB expression. Accordingly, there is the advantage that the method is tolerant with regard to sequence deviations as may exist as the consequence of genetic mutations, polymorphisms or evolutionary divergence. Thus, for example, it is possible to use the dsRNA generated on the basis of the RacB sequence of one organism to suppress the RacB expression in another organism. The high sequence homology between the RacB sequences from rice, maize and barley allows the conclusion that this protein is conserved to a high degree within plants, so that the expression of a dsRNA derived from one of the disclosed RacB sequences as shown in SEQ ID NO: 1, 3 or 5 appears to have an advantageous effect in other plant species as well.
[0194]Furthermore, owing to the high homology between the individual RacB proteins and their functional equivalents, it is possible using a single dsRNA generated from a certain RacB sequence of an organism to suppress the expression of further homologous RacB proteins and/or their functional equivalents of the same organism or else the expression of RacB proteins in other related species. For this purpose, the dsRNA preferably comprises sequence regions of RacB gene transcripts which correspond to conserved regions. Said conserved regions can easily be found by comparing sequences.
[0195]The dsRNA can be synthesized either in vivo or in vitro. To this end, a DNA sequence encoding a dsRNA can be brought into an expression cassette under the control of at least one genetic control element (such as, for example, promoter, enhancer, silencer, splice donor or splice acceptor or polyadenylation signal). Suitable advantageous constructions are described hereinbelow. Polyadenylation is not required, nor do elements for initiating translation have to be present.
[0196]A dsRNA can be synthesized chemically or enzymatically.
[0197]Cellular RNA polymerases or bacteriophage RNA polymerases (such as, for example, T3, T7 or SP6 RNA polymerase) can be used for this purpose. Suitable methods for expression of RNA in vitro are described (WO 97/32016; U.S. Pat. No. 5,593,874; U.S. Pat. No. 5,698,425, U.S. Pat. No. 5,712,135, U.S. Pat. No. 5,789,214, U.S. Pat. No. 5,804,693). A dsRNA which has been synthesized in vitro chemically or enzymatically can be isolated completely or to some degree from the reaction mixture, for example by extraction, precipitation, electrophoresis, chromatography or combinations of these methods, before being introduced into a cell, tissue or organism. The dsRNA can be introduced directly into the cell or else be applied extracellularly (for example into the interstitial space).
[0198]However, it is preferred to transform the plant stably with an expression construct which brings about the expression of the dsRNA. Suitable methods are described hereinbelow.
[0199]b) Introduction of an RacB Antisense Nucleic Acid Sequence
[0200]Methods for suppressing a specific protein by preventing its mRNA from accumulating by means of antisense technology have been described in many instances, including in the case of plants (Sheehy et al. (1988) Proc Natl Acad Sci USA 85: 8805-8809; U.S. Pat. No. 4,801,340; Mol J N et al. (1990) FEDS Lett 268(2):427-430). The antisense nucleic acid molecule hybridizes, or binds, with the cellular mRNA and/or genomic DNA encoding the RacB target protein to be suppressed. This suppresses the transcription and/or translation of the target protein. Hybridization can originate conventionally by the formation of a stable duplex or--in the case of genomic DNA--by the antisense nucleic acid molecule binding to the duplex of the genomic DNA by specific interaction in the major groove of the DNA helix.
[0201]An antisense nucleic acid sequence suitable for reducing an RacB protein can be deduced using the nucleic acid sequence encoding this protein, for example the nucleic acid sequence as shown in SEQ ID NO: 1, 3 or 5, or the nucleic acid sequence encoding a functional equivalent thereof, for example a sequence as shown in SEQ ID NO: 34, 36, 38, 40, 42, 44, 46, 48, 49, 51, 53, 55, 57, 61, 63, 65, 67 or 69, following Watson and Crick's base pairing rules. The antisense nucleic acid sequence can be complementary to all of the transcribed mRNA of said protein, be limited to the coding region, or else only be composed of a nucleotide, which is partially complementary to the coding or noncoding sequence of the mRNA. Thus, for example, the oligonucleotide can be complementary to the region comprising the translation start for said protein. Antisense nucleic acid sequences can be, for example, 5, 10, 15, 20, 25, 30, 35, 40, 45 or 50 nucleotides in length, but may also be longer and comprise at least 100, 200, 500, 1000, 2000 or 5000 nucleotides. Antisense nucleic acid sequences can be expressed recombinantly or synthesized chemically or enzymatically using methods known to the skilled worker. In the case of chemical synthesis, natural or modified nucleotides may be used. Modified nucleotides can impart an increased biochemical stability to the antisense nucleic acid sequence and lead to an increased physical stability of the duplex formed of antisense nucleic acid sequence and sense target sequence. The following can be used: for example phosphorothioate derivatives and acridine-substituted nucleotides such as 5-fluorouracil., 5-bromouracil, 5-chlorouracil, 5-iodouracil, hypoxanthin, xanthin, 4-acetylcytosine, 5-(carboxyhydroxylmethyl)uracil, 5-carboxymethylaminomethyl-2-thiouridine-, 5-carboxymethylaminomethyluracil, dihydrouracil, β-D-galactosylqueosine, inosine, N6-isopentenyladenine, 1-methylguanine, 1-methylinosine, 2,2-dimethylguanine, 2-methyladenine, 2-methylguanine, 3-methylcytosine, 5-methylcytosine, N6-adenine, 7-methylguanine, 5-methylaminomethyluracil, 5-methoxyaminomethyl-2-thiouracil, β-D-mannosylqueosine, 5'-methoxycarboxymethyluracil, 5-methoxyuracil, 2-methylthio-N-6-isopentenyladenine, uracil-5-oxyacetic acid, pseudouracil, queosine, 2-thiocytosine, 5-methyl-2-thiouracil, 2-thiouracil, 4-thiouracil, 5-methyluracil, uracil-5-oxyacetic acid methyl ester, uracil-5-oxyacetic acid, 5-methyl-2-thiouracil, 3-(3-amino-3-N-2-carboxypropyl)uracil and 2,6-diaminopurine.
[0202]In a further preferred embodiment, the expression of an RacB protein can be inhibited by nucleotide sequences which are complementary to the regulatory region of an RacB gene (for example an RacB promoter and/or enhancer) and which form triple-helical structures with that DNA double helix so that the transcription of the RacB gene is reduced. Such methods have been described (Helene C (1991) Anticancer Drug Res 6(6):569-84; Helene C et al. (1992) Ann NY Acad Sci 660:27-36; Maher L J (1992) Bioassays 14(12):807-815). In a further embodiment, the antisense nucleic acid molecule can be an α-anomeric nucleic acid. Such α-anomeric nucleic acid molecules form specific double-stranded hybrids with complementary RNA in which--as opposed to the conventional β-nucleic acids--the two strands run parallel to one another (Gautier C et al. (1987) Nucleic Acids Res 15:6625-6641). The antisense nucleic acid molecule can furthermore also comprise 2'-O-methylribonucleotides (Inoue et al. (1987) Nucleic Acids Res 15:6131-6148) or chimeric RNA/DNA analogs (Inoue et al. (1987) FEDS Lett 215:327-330).
[0203]c) Introduction of an RacB Antisense Nucleic Acid Sequence in Combination with a Ribozyme
[0204]The above-described antisense strategy can be combined advantageously with a ribozyme method. Catalytic RNA molecules or ribozymes can be adapted to suit any target RNA and cleave the phosphodiester backbone at specific, positions, functionally deactivating the target RNA (Tanner N K (1999) FEMS Microbiol Rev 23(3):257-275). The ribozyme itself is not modified thereby, but is capable of cleaving further target RNA molecules analogously, thereby assuming the qualities of an enzyme. The incorporation of ribozyme sequences into antisense RNAs confers this enzyme-like RNA-cleaving quality to precisely these antisense RNAs, thus increasing their efficacy in inactivating the target RNA. The generation and the use of such ribozyme antisense RNA molecules is described, for example, in Haseloff et al. (1988) Nature 334: 585-591.
[0205]In this manner, ribozymes (for example "hammerhead" ribozymes; Haselhoff and Gerlach (1988) Nature 334:585-591) can be used catalytically to cleave the mRNA of an enzyme to be suppressed, for example RacB, and to prevent translation. The ribozyme technique can increase the efficacy of an antisense strategy. Methods of expressing ribozymes for reducing specific proteins are described in (EP 0 291 533, EP 0 321 201, BP 0 360 257). The expression of ribozyme in plant cells has also been described (Steinecke P et al. (1992) EMBO J. 11(4):1525-1530, due Feyte R et al. (1996) Mol Gen Genet. 250(3):329-338). Suitable target sequences and ribozymes can be determined as described for example by "Steinecke P, Ribozymes, Methods in Cell Biology 50, Galbraith et al. eds, Academic Press, Inc. (1995), pp. 449-460" by calculating the secondary structure of ribozyme RNA and target RNA as well as by their interaction (Bayley C C et al. (1992) Plant Mol. Biol. 18(2):353-361; Lloyd A M and Davis R W et al. (1994) Mol Gen Genet. 242(6):653-657). For example, derivatives of the Tetrahymena L-19 IVS RNA with regions which are complementary to the mRNA of the RacB protein to be suppressed can be constructed (see also U.S. Pat. No. 4,987,071 and U.S. Pat. No. 5,116,742). As an alternative, such ribozymes can also be identified from a library of diverse ribozymes via a selection process (Bartel D and Szostak J W (1993) Science 261:1411-1418).
[0206]d) Introduction of an RacB Sense Nucleic Acid Sequence for Inducing Cosuppression
[0207]The expression of an RacB nucleic acid sequence in sense orientation can lead to cosuppression of the corresponding homologous endogenous gene. The expression of sense RNA with homology with an endogenous gene can reduce or switch off the expression of the former, similarly to what has been described for antisense approaches (Jorgensen et al. (1996) Plant Mol Biol 31(5):957-973; Goring et al. (1991) Proc Natl Acad Sci USA 88:1770-1774; Smith et al. (1990) Mol Gen Genet 224:447-481; Napoli et al. (1990) Plant Cell 2:279-289; Van der Krol et al. (1990) Plant Cell 2:291-99). In this context, the homologous gene to be reduced can be represented either fully or only in part by the construct introduced. The possibility of translation is not required. The application of this technique to plants is described, for example, by Napoli et al. (1990) The Plant Cell 2: 279-289 and in U.S. Pat. No. 5,034,323.
[0208]The cosuppression is preferably realized by using a sequence essentially identical to at least part of the nucleic acid sequence encoding an RacB protein or a functional equivalent thereof, for example the nucleic acid sequence as shown in SEQ ID NO: 1, 3 or 5, or the nucleic acid sequence encoding a functional equivalent thereof, for example a sequence as shown in SEQ ID NO: 34, 36, 38, 40, 42, 44, 46, 48, 49, 51, 53, 55, 57, 61, 63, 65, 67 or 69.
[0209]e) Introduction of Nucleic Acid Sequences Encoding a Dominant-Negative RacB Protein
[0210]The function or activity of an RacB protein can also be brought about efficiently by expressing a dominant-negative variant of this RacB protein. Methods of reducing the function or activity of a protein by coexpressing its dominant-negative form are known to the skilled worker (Lagna G and Hemmati-Brivanlou A (1998) Current Topics in Developmental Biology 36:75-98; Perlmutter R M and Alberola-Ila J (1996) Current Opinion in Immunology 8(2):285-90; Sheppard D (1994) American Journal of Respiratory Cell & Molecular Biology. 11(1):1-6; Herskowitz I (1987) Nature 329(6136):219-22).
[0211]For example, a dominant-negative RacB variant can be brought about by modifying the amino acid threonine at position 20 in the RacB proteins from maize, rice or barley to, preferably, aspartic acid. The threonine which is preferably to be mutated, or, if appropriate, also serine (similarly to threonine at position 20 in RacB from maize, rice or barley) in RacB homologs from other species can be determined for example by computer-aided comparison ("alignment"). These mutations for achieving a dominant-negative RacB variant are preferably carried out at the level of the nucleic acid sequence encoding RacB proteins. A corresponding mutation can be brought about for example by PCR-mediated in-vitro mutagenesis using suitable oligonucleotide primers, by which the desired mutation is introduced. This is done using methods known to the skilled worker; for example, the "LA PCR in vitro Mutagenesis Kit" (Takara Shuzo, Kyoto) may be used for this purpose. A method of generating a dominant-negative variant of the maize RacB protein is also described in WO 00/15815 (Example 4, p. 69).
[0212]Especially preferred are the dominant-negative variants of the RacB proteins from barley, rice or maize described under SEQ ID NO: 7, 8 and 9.
[0213]f) Introduction of DNA--or Protein-Binding Factors Against RacB Genes, RacB RNAs or RacB Proteins
[0214]RacB gene expression may also be reduced using specific DNA-binding factors, for example factors of the zinc finger transcription factor type. These factors attach to the genomic sequence of the endogenous target gene, preferably in the regulatory regions, and bring about repression of the endogenous gene. The use of such a method makes possible the reduction of the expression of an endogenous RacB gene without it being necessary to recombinantly manipulate its sequence. Suitable methods for the preparation of suitable factors have been described (Dreier B et al. (2001) J Biol Chem 276(31):29466-78; Dreier B et al. (2000) J Mol Biol 303(4):489-502; Beerli R R et al. (2000) Proc Natl Acad Sci USA 97 (4):1495-1500; Beerli R R et al. (2000) J Biol Chem 275(42):32617-32627; Segal D J and Barbas C F 3rd. (2000) Curr Opin Chem Biol 4(1):34-39; Kang J S and Kim J S (2000) J Biol Chem 275(12):8742-8748; Beerli R R et al. (1998) Proc Natl Acad Sci USA 95(25):14628-14633; Kim J S et al. (1997) Proc Natl Acad Sci USA 94(8):3616-3620; Klug A (1999) J Mol Biol 293(2):215-218; Tsai S Y et al. (1998) Adv Drug Deliv Rev 30(1-3):23-31; Mapp A K et al. (2000) Proc Natl Acad Sci USA 97(8):3930-3935; Sharrocks A D et al., (1997) Int J Biochem Cell Biol 29(12):1371-1387; Zhang L et al. (2000) J Biol Chem 275(43):33850-33860).
[0215]These factors can be selected using any desired portion of an RacB gene. This segment is preferably located in the promoter region. For gene suppression, however, it may also be in the region of the coding exons or introns. The segments in question can be obtained by the skilled worker from Genbank by database search or, starting from an RacB cDNA whose gene is not present in Genbank, by screening a genomic library for corresponding genomic clones. The skilled worker is familiar with the methods required therefor.
[0216]Furthermore, it is possible to introduce, into cells, factors which inhibit the RacB target protein itself. The protein-binding factors can be, for example, aptamers (Famulok M and Mayer G (1999) Curr Top Microbiol Immunol 243:123-36) or antibodies or antibody fragments or single-chain antibodies. Methods for obtaining these factors have been described and are known to the skilled worker. For example, a cytoplasmic scFv antibody was employed to modulate the activity of the phytochrome A protein in genetically modified tobacco plants (Owen M et al. (1992) Biotechnology (N Y) 10(7):790-794; Franken F et al. (1997) Curr Opin Biotechnol 8(4):411-416; Whitelam (1996) Trend Plant Sci 1:286-272).
[0217]Gene expression may also be suppressed by tailor-made low-molecular-weight synthetic compounds, for example of the polyamide type (Dervan P B and Burli R W (1999) Current Opinion in Chemical Biology 3:688-693; Gottesfeld J M et al. (2000) Gene Expr 9(1-2):77-91). These oligormers are composed of the units 3-(dimethylamino)propylamine, N-methyl-3-hydroxypyrrole, N-methylimidazole and N-methylpyrrole and can be adapted to any piece of double-stranded DNA in such a way that they bind into the major groove in a sequence-specific manner and block the expression of these gene sequences. Suitable methods have been described (see, inter alia, Bremer R E et al. (2001) Bioorg Med Chem. 9(8):2093-103; Ansari A Z et al. (2001) Chem Biol. 8(6):583-92; Gottesfeld J M et al. (2001) J. Mol. Biol. 309(3):615-29; Wurtz N R et al. (2001) Org Lett 3(8):1201-3; Wang C C et al. (2001) Bioorg Med Chem 9(3):653-7; Urbach A R and Dervan P B (2001) Proc Natl Acad Sci USA 98(8):4343-8; Chiang S Y et al. (2000) J. Biol. Chem. 275(32):24246-54).
[0218]g) Introduction of Viral Nucleic Acid Sequences and Expression Constructs which Cause RacB RNA Degradation
[0219]RacB expression can also be brought about efficiently by inducing the specific RacB RNA degradation by the plant with the aid of a viral expression system (amplicon) (Angell, S M et al. (1999) Plant J. 20(3):357-362). These systems--also termed "VIGS" (viral induced gene silencing)--introduce, into the plant, nucleic acid sequences with homology to the transcripts to be suppressed, with the aid of viral vectors. Then, transcription is switched off, probably mediated by plant defense mechanisms against viruses. Suitable techniques and methods have been described (Ratcliff F et al. (2001) Plant J 25(2):237-45; Fagard M and Vaucheret H (2000) Plant Mol Biol 43(2-3):285-93; Anandalakshmi R et al. (1998) Proc Natl Acad Sci USA 95(22):13079-84; Ruiz M T (1998) Plant Cell 10(6): 937-46).
[0220]h) Introduction of Constructs for Inducing a Homologous Recombination on Endogenous RacB Genes, for Example for Generating Knock-Out Mutants
[0221]An example of what is used for generating a homologously recombinant organism with reduced RacB activity is a nucleic acid construct comprising at least part of an endogenous RacB gene which is modified by a deletion, addition or substitution of at least one nucleotide in such a way that its functionality is reduced or fully destroyed. The modification may also relate to the regulatory elements (for example the promoter) of the gene, so that the coding sequence remains unmodified, but expression (transcription and/or translation) does not take place and is reduced.
[0222]In the case of conventional homologous recombination, the modified region is flanked at its 5' and 3' end by further nucleic acid sequences which must be sufficient in length for making possible recombination. They are, as a rule, in the range of several hundred bases to several kilobases in length (Thomas K R and Capecchi M R (1987). Cell 51:503; Strepp et al. (1998) Proc Natl Acad Sci USA 95(8):4368-4373). For homologous recombination, the host organism--for example a plant--is transformed with the recombination construct using the methods described hereinbelow, and clones which have successfully undergone recombination are selected, for example using a resistance to antibiotics or herbicides.
[0223]Homologous recombination is a relatively rare event in higher eukaryotes, especially in plants. Random integrations into the host genome predominate. One possibility of eliminating the randomly integrated sequences and thus increasing the number of cell clones with a correct homologous recombination is the use of a sequence-specific recombination system as described in U.S. Pat. No. 6,110,736, by which unspecifically integrated sequences can be deleted again, which simplifies the selection of events which have integrated successfully via homologous recombination. A large number of sequence-specific recombination systems can be used, examples being the Cre/lox system of bacteriophage P1, the FLP/FRT system of yeast, the Gin recombinase of phage Mu, the Pin recombinase from E. coli, and the R/RS system of the pSR1 plasmid. The bacteriophage P1 Cre/10× and the yeast FLP/FRT system are preferred. The FLP/FRT and cre/lox recombinase system has already been applied to plant systems (Odell et al. (1990) Mol Gen Genet 223: 369-378).
[0224]i) Introduction of Mutations into Endogenous RacB Genes for Generating a Loss of Function (For Example Generation of Stop Codons, Reading-Frame Shifts and the Like)
[0225]Further suitable methods for reducing the RacB activity are the introduction of nonsense mutations into endogenous RacB genes, for example by introducing RNA/DNA oligonucleotides into the plant (Zhu et al. (2000) Nat Biotechnol 18(5):555-558) and the generation of knock-out mutants with the aid of, for example, T-DNA mutagenesis (Koncz et al. (1992) Plant Mol Biol 20(5):963-976), ENU (N-ethyl-N-nitrosourea) mutagenesis or homologous recombination (Hohn B and Puchta (1999) H Proc Natl Acad Sci USA 96:8321-8323.). Point mutations can also be generated by means of DNA-RNA hybrids also known under the name "chimeraplasty" (Cole-Strauss-et al. (1999) Nucl Acids Res. 27(5):1323-1330; Kmiec (1999) Gene therapy American Scientist 87(3):240-247).
[0226]The methods of dsRNAi, cosuppression by means of sense RNA and "VIGS" ("virus induced gene silencing") are also termed "post-transcriptional gene silencing" (PTGS). PTGS methods, like the reduction of the RacB function or activity with dominant-negative RacB variants, are especially advantageous because the demands regarding the homology between the endogenous gene to be suppressed and the sense or dsRNA nucleic acid sequence expressed recombinantly (or between the endogenous gene and its dominant-negative variant) are lower than, for example, in the case of a traditional antisense approach. Such criteria with regard to homology are mentioned in the description of the dsRNAI method and can generally be applied to PTGS methods or dominant-negative approaches. Owing to the high degree of homology between the RacB proteins from maize, rice and barley, a high degree of conservation of this protein in plants can be assumed. Thus, using the RacB nucleic acid sequences from barley, maize or rice, it is presumably also possible efficiently to suppress the expression of homologous RacB proteins in other species without the isolation and structure elucidation of the RacB homologs occurring therein being required. Considerably less labor is therefore required. Similarly, the use of dominant-negative variants of an RacB protein from rice, maize or barley can presumably also efficiently reduce or suppress the function/activity of its homolog in other plant species.
[0227]All of the substances and compounds which directly or indirectly bring about a reduction in protein quantity, RNA quantity, gene activity or protein activity of an RacB protein shall subsequently be combined in the term "anti-RacB" compounds. The term "anti-RacB" compound explicitly includes the nucleic acid sequences, peptides, proteins or other factors employed in the above-described methods.
[0228]For the purposes of the invention, "introduction" comprises all of the methods which are capable of directly or indirectly introducing an "anti-RacB" compound into a plant or a cell, compartment, tissue, organ or seed thereof, or of generating such a compound there. Direct and indirect methods are comprised. The introduction can lead to a transient presence of an "anti-RacB" compound (for example a dsRNA) or else to its stable presence.
[0229]Owing to the different nature of the above-described approaches, the "anti-RacB" compound can exert its function directly (for example by insertion into an endogenous RacB gene). However, its function can also be exerted indirectly following transcription into all RNA (for example in the case of antisense approaches) or following transcription and translation into a protein (for example in the case of binding factors). The invention comprises both directly and indirectly acting "anti-RacB" compounds.
[0230]The term "introducing" comprises for example methods such as transfection, transduction or transformation.
[0231]"Anti-RacB" compounds therefore also comprises recombinant expression constructs which bring about expression (i.e. transcription and, if appropriate, translation), for example of an RacB dsRNA or an RacB "antisense" RNA, preferably in a plant or a part, tissue, organ or seed thereof.
[0232]In said expression constructs, a nucleic acid molecule whose expression (transcription and, if appropriate, translation) generates an "anti-RacB" compound is preferably operably linked to at least one genetic control element (for example a promoter) which ensures expression in an organism, preferably in plants. If the expression construct is to be introduced directly into a plant and the "anti-RacB" compound (for example the RacB dsRNA) is to be generated therein in plantae, plant-specific genetic control elements (for example promoters) are preferred. However, the "anti-RacB" compound may also be generated in other organisms or in vitro and then be introduced into the plant (as described in Examples 6 and 7). Preferred in this context are all of the prokaryotic or eukaryotic genetic control elements (for example promoters) which permit the expression in the organism chosen in each case for the preparation.
[0233]Operable linkage is to be understood as meaning, for example, the sequential arrangement of a promoter with the nucleic acid sequence to be expressed (for example an "anti-RacB" compound) and, if appropriate, further regulatory elements such as, for example, a terminator in such a way that each of the regulatory elements can fulfil its function when the nucleic acid sequence is expressed recombinantly, depending on the arrangement of the nucleic acid sequences in relation to sense or antisense RNA. To this end, direct linkage in the chemical sense is not necessarily required. Genetic control sequences such as, for example, enhancer sequences, can also exert their function on the target sequence from positions which are further away, or indeed from other DNA molecules. Preferred arrangements are those in which the nucleic acid sequence to be expressed recombinantly is positioned behind the sequence acting as promoter, so that the two sequences are linked covalently to each other. The distance between the promoter sequence and the nucleic acid sequence to be expressed recombinantly is preferably less than 200 base pairs, especially preferably less than 100 base pairs, very especially preferably less than 50 base pairs.
[0234]Operable linkage, and an expression cassette, can be generated by means of customary recombination and cloning techniques as are described, for example, in Maniatis T, Fritsch E F and Sambrook J (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor (NY), in Silhavy T J, Berman M L and Enquist L W (1984) Experiments with Gene Fusions, Cold Spring Harbor Laboratory, Cold Spring Harbor N.Y.), in Ausubel F M et al. (1987) Current Protocols in Molecular Biology, Greene Publishing Assoc. and Wiley Interscience and in Gelvin et al. (1990) In: Plant Molecular Biology Manual. However, further sequences which, for example, act as a linker with specific cleavage sites for restriction enzymes, or as a signal peptide, may also be positioned between the two sequences. The insertion of sequences may also lead to the expression of fusion proteins. Preferably, the expression cassette, consisting of a linkage of promoter and nucleic acid sequence to be expressed, can exist in a vector-integrated form and be inserted into a plant genome, for example by transformation.
[0235]However, expression cassette also denotes those constructions in which a promoter is positioned behind an endogenous RacB gene, for example by means of homologous recombination, and the reduction according to the invention of an RacB protein is brought about by the expression of an antisense RacB RNA.
[0236]Analogously, an "anti-RacB" compound (for example a nucleic acid sequence encoding an RacB dsRNA or an RacB antisense RNA) can be positioned behind an endogenous promoter in such a way that the same effect is manifested. Both approaches lead to inventive expression cassettes.
[0237]The term plant-specific promoters is understood as meaning, in principle, any promoter which is capable of governing the expression of genes, in particular foreign genes, in plants or plant parts, plant cells, plant tissues or plant cultures. In this context, expression can be, for example, constitutive, inducible or development-dependent.
[0238]The following are preferred:
[0239]a) Constitutive Promoters
[0240]Preferred vectors are those which make possible constitutive expression in plants (Benfey et al. (1989) EMBO J. 8:2195-2202). "Constitutive" promoter is understood as meaning those promoters which ensure expression in a large number of, preferably all, tissues over a substantial period of plant development, preferably at all stages of plant development. In particular a plant promoter or a promoter derived from a plant virus are preferably used. Particularly preferred is the promoter of the CaMV cauliflower mosaic virus .sup.35S transcript (Franck et al. (1980) Cell 21:285-294; Odell et al. (1985) Nature 313:810-812; Shewmaker et al. (1985) Virology 140:281-288; Gardner et al. (1986) Plant Mol Biol 6:221-228) or the 19S CaMV promoter (U.S. Pat. No. 5,352,605; WO 84/02913; Benfey et al. (1989) EMBO J. 8:2195-2202). Another suitable constitutive promoter is the "Rubisco small subunit (SSU)" promoter (U.S. Pat. No. 4,962,028), the leguminB promoter (GenBank Acc. No. X03677), the Agrobacterium nopaline synthase promoter, the TR dual promoter, the Agrobacterium OCS (octopine synthase) promoter, the ubiquitin promoter (Holtorf S et al. (1995) Plant Mol Biol 29:637-649), the ubiquitin 1 promoter (Christensen et al. (1992) Plant Mol Biol 18:675-689; Bruce et al. (1989) Proc Natl Acad Sci USA 86:9692-9696), the Smas promoter, the cinnamyl alcohol dehydrogenase promoter (U.S. Pat. No. 5,683,439), the promoters of the vacuolar ATPase subunits or the promoter of a proline-rich protein from wheat (WO 91/13991), and further promoters of genes whose constitutive expression in plants is known to the skilled worker. A particularly preferred constitutive promoter is the promoter of the nitrilase-1 (nit1) gene from A. thaliana (GenBank Acc.-No.: Y07648.2, nucleotides 2456-4340, Hillebrand et al. (1996) Gene 170:197-200).
[0241]b) Tissue-Specific Promoters
[0242]Preferred are furthermore promoters with specificity for the anthers, ovaries, flowers, leaves, stems, roots and seeds.
[0243]Seed-Specific Promoters
[0244]such as, for example, the phaseolin promoter (U.S. Pat. No. 5,504,200; Bustos M M et al. (1989) Plant Cell 1(9):839-53), the 2S albumin gene promoter (Joseffson L G et al. (1987) J Biol Chem 262:12196-12201), the legumin promoter (Shirsat A et al. (1989) Mol Gen Genet 215(2): 326-331), the USP (unknown seed protein) promoter (Baumlein H et al. (1991) Mol Gen Genet 225(3):459-67), the napin gene promoter (U.S. Pat. No. 5,608,152; Stalberg K et al. (1996) L Planta 199:515-519), the sucrose binding protein promoter (WO 00/26388) or the legumin B4 promoter (LeB4; Bumlein H et al. (1991) Mol Gen Genet 225: 121-128; Baeumlein et al. (1992) Plant Journal 2(2):233-9; Fiedler U et al. (1995) Biotechnology (NY) 13(10):1090f), the Arabidopsis oleosin promoter (WO 98/45461), the Brassica Bce4 promoter (WO 91/13980). Further suitable seed-specific promoters are those of the genes encoding the high-molecular-weight glutenin (HMWG), gliadin, branching enzyme, ADP glucose pyrophosphatase (AGPase) or starch synthase. Furthermore preferred are promoters which permit seed-specific expression in monocots such as maize, barley, wheat, rye, rice and the like. The following can be employed advantageously: the promoter of the lpt2 or lpt1 gene (WO 95/15389, WO 95/23230) or the promoters described in WO 99/16890 (promoters of the hordein gene, the glutelin gene, the oryzin gene, the prolamin gene, the gliadin gene, the glutelin gene, the zein gene, the kasirin gene or the secalin gene).
[0245]Tuber-, storage-root- or root-specific promoters such as, for example, the patatin promoter class I (B33), the potato cathepsin D inhibitor promoter.
[0246]Leaf-Specific Promoters
[0247]such as the potato cytosolic FBPase promoter (WO 97/05900), the Rubisco (ribulose-1,5-bisphosphate carboxylase) SSU (small subunit) promoter or the ST-LSI promoter from potato (Stockhaus et al. (1989) EMBO J. 8:2445-2451). Very especially preferred are epidermis-specific promoters such as, for example, the OXLP gene (oxalate-oxidase-like protein) promoter (Wei et al. (1998) Plant Mol. Biol. 36:101-112).
[0248]Flower-Specific Promoters
[0249][0245] such as, for example, the phytoene synthase promoter (WO 92/16635) or the promoter of the P-rr gene (WO 98/22593).
[0250]Anther-Specific Promoters
[0251]such as the 5126 promoter (U.S. Pat. No. 5,689,049, U.S. Pat. No. 5,689,051), the glob-1 promoter and the γ-zein promoter.
[0252]c) Chemically Inducible Promoters
[0253]The expression cassettes can also comprise a chemically inducible promoter (review article: Gatz et al. (1997) Annu Rev Plant Physiol Plant Mol Biol 48:89-108), by which the expression of the exogenous gene in the plant at a particular point in time can be controlled. Such promoters such as, for example, the PRP1 promoter (Ward et al. (1993) Plant Mol Biol 22:361-366), a salicylic-acid-inducible promoter (WO 95/19443), a benzenesulfonamide-inducible promoter (EP 0 388.186), a tetracyclin-inducible promoter (Gatz et al. (1992) Plant J 2:397-404)., an abscisic-acid-inducible promoter (EP 0 335 528) or an ethanol- or cyclohexylnone-inducible promoter (WO 93/21334) can likewise be used.
[0254]d) Stress- or Pathogen-Inducible Promoters
[0255]Further preferred promoters are those which are induced by biotic or abiotic stress such as, for example, the pathogen-inducible promoter of the PRP1 gene (or gst1 promoter) e.g. from potato (WO 96/28561; Ward et al. (1993) Plant Mol Biol 22:361-366), the tomato high-temperature-inducible hsp70 or hsp80 promoter (U.S. Pat. No. 5,187,267), the potato low-temperature-inducible alpha-amylase promoter (WO 96/12814), the light-inducible PPDK promoter, or the wounding-induced pinII promoter (EP-A 0 375 091).
[0256]Further pathogen-inducible promoters comprise the Fis1 promoter from flax (WO 96/34949), the Vst1 promoter (Schubert et al., (1997) Plant Mol Biol 34:417-426) and the EAS4 sesquiterpene cyclase promoter from tobacco (U.S. Pat. No. 6,100,451).
[0257]Pathogen-inducible promoters comprise those of genes which are induced as a consequence of infection by pathogens, such as, for example, genes of PR proteins, SAR proteins, β-1,3-glucanase, chitinase and the like (for example Redolfi et al. (1983) Neth J Plant Pathol 89:245-254; Uknes, et al. (1992) The Plant Cell 4:645-656; Van Loon (1985) Plant Mol Viral 4:111-116; Marineau et al. (1987) Plant Mol Biol 9:335-342; Matton et al. (1987) Molecular Plant-Microbe Interactions 2:325-342; Somssich et al. (1986) Proc Natl Acad Sci USA 83:2427-2430; Somssich et al. (1988) Mol Gen Genetics 2:93-98; Chen et al. (1996) Plant J 10:955-966; Zhang and Sing (1994) Proc Natl Acad Sci USA 91:2507-2511; Warner, et al. (1993) Plant J 3:191-201; Siebertz et al. (1989) Plant Cell 1:961-968 (1989).
[0258]Also comprised are wounding-inducible promoters such as that of the pinII gene (Ryan (1990) Ann Rev Phytopath 28:425-449; Duan et al. (1996) Nat Biotech 14:494-498), of the wun1 and wun2 gene (U.S. Pat. No. 5,428,148), of the win1 and win2 gene (Stanford et al. (1989) Mol Gen Genet 215:200-208), of system in (McGurl et al. (1992) Science 225:1570-1573), of the WIP1 gene (Rohmeier et al. (1993) Plant Mol Biol 22:783-792; Eckelkamp et al. (1993), FEBS Letters 323:73-76), of the MPI gene (Corderok et al. (1994) Plant J 6(2):141-150) and the like.
[0259]A source of further pathogen-inducible promoters is the PR gene family. A number of elements in these promoters have been found to be advantageous. Thus, the region -364 to -288 in the promoter of PR-2d provides salicylate specificity (Buchel et al. (1996) Plant Mol Biol 30, 493-504). The sequence 5'-TCATCTTCTT-3' is encountered repeatedly in the promoter of barley β.-1,3-glucanase and more than 30 further stress-induced genes. In tobacco, this region binds a nuclear protein whose abundance is increased by salicylate. The PR-1 promoters from tobacco and Arabidopsis (EP-A 0 332 104, WO 98/03536) are likewise suitable for use as pathogen-inducible promoters. "Acidic PR-5'-(aPR5)-promoters from barley (Schweizer et al. (1997) Plant Physiol 114:79-88) and wheat (Rebmann et al. (1991) Plant Mol Biol 16:329-331) are preferred, since they are particularly specifically pathogen-induced. aPR5 proteins accumulate within about 4 to 6 hours after pathogen attack and have only very limited background expression (WO 99/66057). One approach to achieve higher pathogen-induced specificity is the preparation of synthetic promoters from combinations of known pathogen-responsive elements (Rushton et al. (2002) Plant Cell 14, 749-762; WO 00/01830; WO 99/66057). Further pathogen-inducible promoters from different species are known to the person skilled in the art (EP-A 1 165 794; EP-A 1 062 356; EP-A 1 041 148; EP-A 1 032 684;
[0260]e) Development-Dependent Promoters
[0261]Further suitable promoters are, for example, fruit-maturation-specific promoters such as, for example, the tomato fruit-maturation-specific promoter (WO 94/21794, EP 409 625). Development-dependent promoters comprise partly the tissue-specific promoters, since individual tissues develop by nature in a development-dependent fashion.
[0262]Especially preferred are constitutive promoters and leaf- and/or stem-specific, pathogen-inducible and epidermis-specific promoters, with pathogen-inducible and epidermis-specific promoters being most preferred.
[0263]Furthermore, further promoters may be linked operably to the nucleic acid sequence to be expressed, which promoters make possible the expression in further plant tissues or in other organisms, such as, for example, E. coli bacteria. Suitable plant promoters are, in principle, all of the above-described promoters.
[0264]The nucleic acid sequences present in the expression cassettes or vectors according to the invention can be linked operably to further genetic control sequences in addition to a promoter. The term "genetic control sequences" is to be understood in the broad sense and refers to all those sequences which have an effect on the materialization or the function of the expression cassette according to the invention. For example, genetic control sequences modify the transcription and translation in prokaryotic or eukaryotic organisms. Preferably, the expression cassettes according to the invention comprise the promoter with specificity for the embryonal epidermis and/or the flower 5'-upstream of the nucleic acid sequence in question to be expressed recombinantly, and 3'-downstream a terminator sequence as additional genetic control sequence and, if appropriate, further customary regulatory elements, in each case linked operably to the nucleic acid sequence to be expressed recombinantly.
[0265]Genetic control sequences also comprise further promoters, promoter elements or minimal promoters, all of which can modify the expression-governing properties. Thus, for example, the tissue-specific expression may additionally depend on certain stressors, owing to genetic control sequences. Such elements have been described, for example, for water stress, abscisic acid (Lam E and Chua N H, J Biol Chem 1991; 266(26): 17131-17135) and heat stress (Schoffl F et al., Molecular & General Genetics 217(2-3):246-53, 1989).
[0266]Further advantageous control sequences are, for example, in the Gram-positive promoters amy and SPO2, and in the yeast or fungal promoters ADC1, MFa, AC, P-60, CYC1, GAPDH, TEF, rp28, ADH.
[0267]In principle, all natural promoters with their regulatory sequences like those mentioned above may be used for the method according to the invention. In addition, synthetic promoters may also be used advantageously.
[0268]Genetic control sequences furthermore also comprise the 5'-untranslated regions, introns or noncoding 3'-region of genes, such as, for example, the actin-1 intron, or the Adh1-S introns 1, 2 and 6 (general reference: The Maize Handbook, Chapter 116, Freeling and Walbot, Eds., Springer, New York (1994)). It has been demonstrated that they may play a significant roles in the regulation of gene expression. Thus, it has been demonstrated that 5'-untranslated sequences can enhance the transient expression of heterologous genes. Examples of translation enhancers which may be mentioned are the tobacco mosaic virus 5' leader sequence (Gallie et al. (1987) Nucl Acids Res 15:8693-8711) and the like. Furthermore, they may promote tissue specificity (Rouster J et al. (1998) Plant J 15:435-440).
[0269]The expression cassette may advantageously comprise one or more of what are known as enhancer sequences, linked operably to the promoter, which make possible an increased recombinant expression of the nucleic acid sequence. Additional advantageous sequences, such as further regulatory elements or terminators, may also be inserted at the 3' end of the nucleic acid sequences to be expressed recombinantly. One or more copies of the nucleic acid sequences to be expressed recombinantly may be present in the gene construct.
[0270]Polyadenylation signals which are suitable as control sequences are plant polyadenylation signals, preferably those which essentially correspond to T-DNA polyadenylation signals from Agrobacterium tumefaciens, in particular gene 3 of the T-DNA (octopin synthase) of the Ti plasmid pTiACHS (Gielen et al. (1984) EMBO J. 3:835 et seq.) or functional equivalents thereof. Examples of terminator sequences which are especially suitable are the OCS (octopin synthase) terminator and the NOS (nopalin synthase) terminator.
[0271]Control sequences are furthermore to be understood as those which make possible homologous recombination or insertion into the genome of a host organism or which permit removal from the genome. In the case of homologous recombination, for example the natural promoter of a particular gene may be exchanged for a promoter with specificity for the embryonal epidermis and/or the flower. Methods such as the cre/lox technology permit a tissue specific, if appropriate inducible, removal of the expression cassette from the genome of the host organism (Sauer B (1998) Methods. 14(4):381-92). In this method, specific flanking sequences (lox sequences), which later allow removal by means of cre recombinase, are attached to the target gene.
[0272]An expression cassettes and the vectors derived from it may comprise further functional elements. The term functional element is to be understood in the broad sense and refers to all those elements which have an effect on the generation, amplification or function of the expression cassettes, vectors or transgenic organisms according to the invention. The following may be mentioned by way of example, but not by limitation:
[0273]a) Selection markers which confer a resistance to a metabolism inhibitor such as 2-deoxyglucose-6-phosphate (WO 98/45456), antibiotics or biocides, preferably herbicides, such as, for example, kanamycin, G 418, bleomycin or hygromycin, or else phosphinothricin and the like. Especially preferred selection markers are those which confer resistance to herbicides. Examples which may be mentioned are: DNA sequences which encode phosphinothricin acetyl transferases (PAT) and which inactivate glutamin synthase inhibitors (bar and pat genes), 5-enolpyruvylshikimate-3-phosphate synthase genes (EPSP synthase genes), which confer resistance to Glyphosate® (N-(phosphonomethyl)glycine), the gox gene, which encodes Glyphosate®.-degrading enzymes (Glyphosate oxidoreductase), the deh gene (encoding a dehalogenase which inactivates dalapon), sulfonylurea- and imidazolinone-inactivating acetolactate synthases, and bxn genes, which encode bromoxynil-degrading nitrilase enzymes, the aasa gene, which confers resistance to the antibiotic apectinomycin, the streptomycin phosphotransferase (SPT) gene, which allows resistance to streptomycin, the neomycin phosphotransferase (NPTII) gene, which confers resistance to kanamycin or geneticidin, the hygromycin phosphotransferase (HPT) gene, which mediates resistance to hygromycin, the acetolactate synthase gene (ALS), which confers resistance to sulfonylurea herbicides (for example mutated ALS variants with, for example, the S4 and/or Hra mutation).
[0274]b) Reporter genes which encode readily quantifiable proteins and, via their color or enzyme activity, make possible an assessment of the transformation efficacy, the site of expression or the time of expression. Very especially preferred in this context are genes encoding reporter proteins (Schenborn E, Groskreutz D. Mol Biotechnol. 1999; 13(1):29-44) such as the green fluorescent protein (GFP). (Sheen et al. (1995) Plant Journal 8(5):777-784; Haseloff et al. (1997) Proc Natl Acad Sci USA 94(6):2122-2127; Reichel et al. (1996) Proc Natl Acad Sci USA 93(12):5888-5893; Tian et al. (1997) Plant Cell Rep 16:267-271; WO 97/41228; Chui W L et al. (1996) Curr Biol 6:325-330; Leffel S M et al. (1997) Biotechniques. 23(5):912-8), chloramphenicol transferase, a luciferase (Ow et al. (1986) Science 234:856-859; Millar et al. (1992) Plant Mol Biol Rep 10:324-414), the aequorin gene (Prasher et al. (1985) Biochem Biophys Res Commun 126(3):1259-1268), β.-galactosidase, R locus gene (encoding a protein which regulates the production of anthocyanin pigments (red coloring) in plant tissue and thus makes possible the direct analysis of the promoter activity without addition of further auxiliary substances or chromogenic substrates; Dellaporta et al. In: Chromosome Structure and Function: Impact of New Concepts, 18th Stadler Genetics Symposium, 11:263-282, 1988), with β-glucuronidase being very especially preferred (Jefferson et al., EMBO J. 1987, 6, 3901-3907).
[0275]c) Origins of replication, which ensure amplification of the expression cassettes or vectors according to the invention in, for example, E. coli. Examples which may be mentioned are ORI (origin of DNA replication), the pBR322 ori or the P15A ori (Sambrook et al.: Molecular Cloning. A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1989).
[0276]d) Elements which are necessary for Agrobacterium-mediated plant transformation, such as, for example, the right or left border of the T-DNA or the vir region.
[0277]To select cells which have successfully undergone homologous recombination, or else to select transformed cells, it is, as a rule, necessary additionally to introduce a selectable marker, which confers resistance to a biocide (for example herbicide), a metabolism inhibitor such as 2-deoxyglucose-6-phosphate (WO 98/45456) or an antibiotic to the cells which have successfully undergone recombination. The selection marker permits the selection of the transformed cells from untransformed ones (McCormick et al. (1986) Plant Cell Reports 5:81-84).
[0278]The introduction of an expression cassette according to the invention into an organism or cells, tissues, organs, parts or seeds thereof (preferably into plants or plant cells, tissue, organs, parts or seeds) can be effected advantageously using vectors which comprise the expression cassettes. The expression cassette can be introduced into the vector (for example a plasmid) via a suitable restriction cleavage site. The plasmid formed is first introduced into E. coli. Correctly transformed E. coli are selected, grown, and the recombinant plasmid is obtained by the methods familiar to the skilled worker. Restriction analysis and sequencing may serve to verify the cloning step.
[0279]Examples of vectors may be plasmids, cosmids, phages, viruses or else agrobacteria. In an advantageous embodiment, the expression cassette is introduced by means of plasmid vectors. Preferred vectors are those which make possible stable integration of the expression cassette into the host genome.
[0280]The generation of a transformed organism (or of a transformed cell or tissue) requires introducing the DNA, RNA or protein in question into the relevant host cell.
[0281]A multiplicity of methods are available for this procedure, which is termed transformation (or transduction or transfection) (Keown et al. (1990) Methods in Enzymology 185:527-537). For example, the DNA or RNA can be introduced directly by microinjection or by bombardment with DNA-coated microparticles. Also, the cell can be permeabilized chemically, for example using polyethylene glycol, so that DNA can enter the cell by diffusion. The DNA can also be introduced by protoplast fusion with other DNA-containing units such as minicells, cells, lysosomes or liposomes. Another suitable method of introducing DNA is electroporation, where the cells are permeabilized reversibly by an electrical pulse. Suitable methods have been described (for example by Bilang et al. (1991) Gene 100:247-250; Scheid et al. (1991) Mol Gen Genet 228:104-112; Guerche et al. (1987) Plant Science 52:111-116; Neuhause et al. (1987) Theor Appl Genet 75:30-36; Klein et al. (1987) Nature 327:70-73; Howell et al. (1980) Science 208:1265; Horsch et al. (1985) Science 227:1229-1231; DeBlock et al. (1989) Plant Physiology 91:694-701; Methods for Plant Molecular Biology (Weissbach and Weissbach, eds.) Academic Press Inc. (1988); and Methods in Plant Molecular Biology (Schuler and Zielinski, eds.) Academic Press Inc. (1989)).
[0282]In plants, the above-described methods of transforming and regenerating plants from plant tissues or plant cells are exploited for transient or stable transformation. Suitable methods are especially protoplast transformation by polyethylene-glycol-induced DNA uptake, the biolistic method with the gene gun, what is known as the particle bombardment method, electroporation, incubation of dry embryos in DNA-containing solution, and microinjection.
[0283]In addition to these "direct" transformation techniques, transformation can also be effected by bacterial infection by means of Agrobacterium tumefaciens or Agrobacterium rhizogenes. The Agrobacterium-mediated transformation is best suited to dicotyledonous plant cells. The methods are described, for example, by Horsch R B et al. (1985) Science 225: 1229f.
[0284]When agrobacteria are used, the expression cassette must be integrated into specific plasmids, either into a shuttle or intermediate vector, or into a binary vector. If a Ti or Ri plasmid is to be used for the transformation, at least the right border, but in most cases the right and left border, of the Ti or Ri plasmid T-DNA is linked to the expression cassette to be introduced in the form of a flanking region.
[0285]Binary vectors are preferably used. Binary vectors are capable of replication both in E. coli and in Agrobacterium. As a rule, they comprise a selection marker gene and a linker or polylinker flanked by the right and left T-DNA border sequence. They can be transferred directly into Agrobacterium (Holsters et al. (1978) Mol Gen Genet 163:181-187). The selection marker gene permits the selection of transformed agrobacteria and is, for example, the nptII gene, which confers resistance to kanamycin. The Agrobacterium which acts as host organism in this case should already contain a plasmid with the vir region. The latter is required for transferring the T-DNA to the plant cell. An Agrobacterium transformed in this way can be used for transforming plant cells. The use of T-DNA for transforming plant cells has been studied and described intensively (EP 120 516, Hoekema, In: The Binary Plant Vector System, Offsetdrukkerij Kanters B. V., Alblasserdam, Chapter V; An et al. (1985) EMBO J. 4:277-287). Various binary vectors are known, some of which are commercially available such as, for example, pBI101.2 or pBIN19 (Clontech Laboratories, Inc. USA).
[0286]Further promoters which are suitable for expression in plants have been described (Rogers et al. (1987) Meth in Enzymol 153:253-277; Schardl et al. (1987) Gene 61:1-11; Berger et al. (1989) Proc Natl Acad Sci USA 86:8402-8406).
[0287]Direct transformation techniques are suitable for any organism and cell type.
[0288]The plasmid used need not meet any particular requirements in the case of the injection or electroporation of DNA or RNA into plant cells. Simple plasmids such as those of the pUC series can be used. If complete plants are to be regenerated from the transformed cells, it is necessary for an additional selectable marker gene to be located on the plasmid.
[0289]Stably transformed cells, i.e. those which contain the introduced DNA integrated into the DNA of the host cell, can be selected from untransformed cells when a selectable marker is part of the DNA introduced. Examples of genes which can act as markers are all those which are capable of conferring resistance to antibiotics or herbicides (such as kanamycin, G 418, bleomycin, hygromycin or phosphinothricin) (see above). Transformed cells which express such marker genes are capable of surviving in the presence of concentrations of a corresponding antibiotic or herbicide which kill an untransformed wild type. Examples are mentioned above and preferably comprise the bar gene, which confers resistance to the herbicide phosphinothricin (Rathore K S et al. (1993) Plant Mol Biol 21(5):871-884), the nptII gene, which confers resistance to kanamycin, the hpt gene, which confers resistance to hygromycin, or the EPSP gene, which confers resistance to the herbicide Glyphosate. The selection marker permits the selection of transformed cells from untransformed cells (McCormick et al. (1986) Plant Cell Reports 5:81-84). The resulting plants can be bred and hybridized in the customary fashion. Two or more generations should be grown in order to ensure that the genomic integration is stable and hereditary.
[0290]The abovementioned methods are described, for example, in Jenes B et al. (1993) Techniques for Gene Transfer, in: Transgenic Plants, Vol. 1, Engineering and Utilization, edited by S D Kung and R Wu, Academic Press, pp. 128-143 and in Potrykus (1991) Annu Rev Plant Physiol Plant Molec Biol 42:205-225). The construct to be expressed is preferably cloned into a vector which is suitable for the transformation of Agrobacterium tumefaciens, for example pBin19 (Bevan et al. (1984) Nucl Acids Res 12:8711f).
[0291]As soon as a transformed plant cell has been generated, a complete plant can be obtained using methods known to the skilled worker. For example, callus cultures are used as starting material. The development of shoot and root can be induced in this as yet undifferentiated cell biomass in a known fashion. The shoots obtained can be planted out and bred.
[0292]The skilled worker is familiar with such methods of regenerating plant parts and intact plants from plant cells. Methods to do so are described, for example, by Fennell et al. (1992) Plant Cell Rep. 11: 567-570; Stoeger et al (1995) Plant Cell Rep. 14:273-278; Jahne et al. (1994) Theor Appl Genet 89:525-533.
[0293]The method according to the invention can advantageously be combined with further methods which bring about pathogen resistance (for example to insects, fungi, bacteria, nematodes and the like), stress resistance or another improvement of the plant properties. Examples are mentioned, inter alia, by Dunwell J M, Transgenic approaches to crop improvement, J Exp Bot. 2000; 51 Spec No; pages 487-96.
[0294]The invention furthermore relates to the barley RacB protein as shown in SEQ ID NO: 2, and to dominant-negative variant thereof, for example described by SEQ ID NO: 7.
[0295]The invention furthermore relates to nucleic acid sequences encoding the barley RacB protein, preferably the nucleic acid sequence as shown in SEQ ID NO: 1, the nucleic acid sequence complementary thereto, and the sequences derived owing to degeneracy of the genetic code.
[0296]The invention furthermore relates to the polypeptide encoding functional equivalents of the RacB protein from barley as shown in SEQ ID NO: 35, 37 or 39.
[0297]The invention furthermore relates to nucleic acid sequences encoding functional equivalents of the RacB protein from barley, preferably the nucleic acid sequence as shown in SEQ ID NO: 34, 36 or 38, the nucleic acid sequence complementary thereto and the sequences derived by degeneration of the genetic code.
[0298]The invention furthermore relates to transgenic expression cassettes comprising one of the nucleic acid sequences according to the invention. In the transgenic expression cassettes according to the invention, the nucleic acid sequence encoding the barley RacB protein is linked to at least one genetic control element as defined above in such a manner that it is capable of expression (transcription and, if appropriate, translation) in any organism, preferably in plants. Suitable genetic control elements are described above. The transgenic expression cassettes may also comprise further functional elements in accordance with the above definition. The inserted nucleic acid sequence encoding a barley RacB protein can be inserted in the expression cassette in sense or antisense orientation and thus lead to the expression of sense or antisense RNA. Transgenic vectors comprising the transgenic expression cassettes are also in accordance with the invention.
[0299]"Transgenic", for example regarding a nucleic acid sequence, an expression cassette or a vector comprising said nucleic acid sequence or an organism transformed with said nucleic acid sequence, expression cassette or vector, refers to all those constructs originating by recombinant methods in which either
[0300]a) the RacB nucleic acid sequence, or
[0301]b) a genetic control sequence linked operably to the RacB nucleic acid sequence, for example a promoter, or
[0302]c) (a) and (b) are not located in their natural genetic environment or have been modified by recombinant methods, an example of a modification being a substitutions, additions, deletions, inversion or insertions of one or more nucleotide residues. Natural genetic environment refers to the natural chromosomal locus in the organism of origin, or to the presence in a genomic library. In the case of a genomic library, the natural genetic environment of the nucleic acid sequence is preferably retained, at least in part. The environment flanks the nucleic acid sequence at least at one side and has a sequence of at least 50 bp, preferably at least 500 bp, especially preferably at least 1000 bp, very especially preferably at least 5000 bp, in length. A naturally occurring expression cassette--for example the naturally occurring combination of the RacB promoter with the corresponding RacB gene--becomes a transgenic expression cassette when it is modified by non-natural, synthetic "artificial" methods such as, for example, mutagenization. Such methods have been described (U.S. Pat. No. 5,565,350; WO 00/15815; also see above).
[0303]The invention also relates to transgenic organisms transformed with at least one of the nucleic acid sequences according to the invention, expression cassette according to the invention or vector according to the invention, and to cells, cell cultures, tissues, parts--such as, for example, leaves, roots and the like in the case of plant organisms--or propagation material derived from such organisms. The term organism is to be understood in the broad sense and refers to prokaryotic and eukaryotic organisms, preferably bacteria, yeasts, fungi, animal organisms and plant organisms.
[0304]The following are preferred:
[0305]a) fungi such as Aspergillus, Eremothecium, Trichoderma, Ashbya, Neurospora, Fusarium, Beauveria or other fungi described in Indian Chem Eng. Section B. Vol 37, No. 1, 2 (1995) on page 15, Table 6. Especially preferred is the filamentous hemiascomycete Ashbya gossypii or Eremothecium ashbyii,
[0306]b) yeasts such as Candida, Saccharomyces, Hansenula or Pichia, with Saccharomyces cerevisiae or Pichia pastoris (ATCC Accession No. 201178) being especially preferred,
[0307]c) plants in accordance with the above definition of "plants",
[0308]d) vertebrates and invertebrates. Especially preferred vertebrates are nonhuman mammals such as in dog, cat, sheep, goat, chicken, mouse, rat, cattle or horse. Preferred animal cells comprise CHO, COS and HEK293 cells. Preferred invertebrates comprise insect cells such as Drosophila S2 and Spodoptera Sf9 or Sf21 cells,
[0309]e) prokaryotic organisms such as Gram-positive or Gram-negative bacteria such as Acetobacter, Gluconobacter, Corynebacterium, Brevibacterium, Bacillus, Clostridium, Cyanobacter, Escherichia (mainly Escherichia coli), Serratia, Staphylococcus, Aerobacter, Alcaligenes, Penicillium or Klebsiella.
[0310]Host or starting organisms which are preferred as transgenic organisms are mainly plants in accordance with the above definition. Included within the scope of the invention are all genera and species of higher and lower plants of the Plant Kingdom. Furthermore included are the mature plants, seed, shoots and seedlings, and parts, propagation material and cultures derived therefrom, for example cell cultures. Mature plants refers to plants at any developmental stage beyond that of the seedling. The term seedling refers to a young immature plant in an early developmental stage. Plants preferred as host organisms are in particular plants which can be used for the process according to the invention to obtain a pathogen resistance according to the criteria mentioned above. Very particularly preferred are monocotyledonous plants, such as wheat, oats, millet, barley, rye, maize, rice, buckwheat, sorghum, triticale, spelt, linseed, sugar cane, as dicotyledonous crop plants, such as oil seed rape, canola, cress, Arabidopsis, cabbages, soya, alfalfa, pea, beans, peanut, potato, tobacco, tomato, eggplant, bell pepper, sunflower, Tagetes, lettuce, Calendula, melon, pumpkin/squash or zucchini.
[0311]The transgenic organisms can be generated with the above-described methods for the transformation or transfection of organisms.
[0312]The invention furthermore relates to the use of the transgenic organisms according to the invention and of the cells, cell cultures, parts such as, for example, roots, leaves and the like in the case of transgenic plant organisms--derived from them, and to transgenic propagation material such as seeds or fruits, for the production of foodstuffs or feeding stuffs, pharmaceuticals or fine chemicals.
[0313]Furthermore preferred is a method for the recombinant production of pharmaceuticals or fine chemicals in host organisms, wherein a host organism is transformed with one of the above-described expression cassettes and this expression cassette comprises one or more structural genes which encode the desired fine chemical or catalyze the biosynthesis of the desired fine chemical, the transformed host organism is cultured, and the desired fine chemical is isolated from the culture medium. This method can be applied widely to fine chemicals such as enzymes, vitamins, amino acids, sugars, fatty acids, and natural and synthetic flavorings, aroma substances and colorants. Especially preferred is the production of tocopherols and tocotrienols and carotenoids. The transformed host organisms are cultured and the products are isolated from the host organisms or the culture medium by methods known to the skilled worker. The production of pharmaceuticals such as, for example, antibodies or vaccines, is described by Hood E E, Jilka J M. (1999) Curr Opin Biotechnol. 10(4):382-6; Ma J K, Vine N D. (1999) Curr Top Microbiol Immunol. 236:275-92.
[0314]Sequences [0315]1. SEQ ID NO: 1 Nucleic acid sequence encoding the barley (Hordeum vulgare) RacB protein. [0316]2. SEQ ID NO: 2 Amino acid sequence encoding the barley (Hordeum vulgare) RacB protein. [0317]3. SEQ ID NO: 3 Nucleic acid sequence encoding the rice (Oryza sativa) RacB protein. [0318]4. SEQ ID NO: 4 Amino acid sequence encoding the rice (Oryza sativa) RacB protein. [0319]5. SEQ ID NO: 5 Nucleic acid sequence encoding the maize (Zea mays) RacB protein. [0320]6. SEQ ID NO: 6 Amino acid sequence encoding the maize (Zea mays) RacB protein. [0321]7. SEQ ID NO: 7 Amino acid sequence encoding a dominant-negative variant of the RacB protein (Hordeum vulgare). [0322]8. SEQ ID NO: 8 Amino acid sequence encoding a dominant-negative variant of the rice (Oryza sativa) RacB protein. [0323]9. SEQ ID NO: 9 Amino acid sequence encoding a dominant-negative variant of the maize (Zea mays) RacB protein. [0324]10. SEQ ID NO, 10 Oligonucleotide primer ONP-1
TABLE-US-00008 [0324] 5'-GGATCCGATGAGCGCGTCCAGGTT-3'
[0325]11. SEQ ID NO: 11 Oligonucleotide primer ONP-2
TABLE-US-00009 [0325] 5'-TCGACCTTCGCCCTTGTTCTTTGTC-3'
[0326]12. SEQ ID NO: 12 RACE-RacB primer
TABLE-US-00010 [0326] 5'-gtgggcacatagtcggtggggaaggt-3'
[0327]13. SEQ ID NO: 13 GeneRacer® 5' primer:
TABLE-US-00011 [0327] 5'-CGACTGGAGCACGAGGACACTGA-3
[0328]14. SEQ ID NO: 14 GeneRacer® 5' nested primer:
TABLE-US-00012 [0328] 5'-GGACACTGACATGGACTGAAGGAGTA-3
[0329]15. SEQ ID NO: 15 RacB sense primer
TABLE-US-00013 [0329] 5'-gttcatcaagtgcgtcaccgtg-3'
[0330]16. SEQ ID NO: 16 RacB antisense primer
TABLE-US-00014 [0330] 5'-ttagcttcctcagttcttccctg-3'
[0331]17. SEQ ID NO: 17 BAS sense primer
TABLE-US-00015 [0331] 5'-cgcgccgcagccgagtacgac-3'
[0332]18. SEQ ID NO: 18 BAS antisense primer
TABLE-US-00016 [0332] 5'-gtcacaaaaacacatgtaacc-3'
[0333]19. SEQ ID NO: 19 OXLP sense primer
TABLE-US-00017 [0333] 5'-ggccgacatgcattcaccag-3'
[0334]20. SEQ ID NO: 20 OXLP antisense primer
TABLE-US-00018 [0334] 5'-catctgatattgctgggtctg-3'
[0335]21. SEQ ID NO: 21 UBI sense primer
TABLE-US-00019 [0335] 5'-ccaagatgcagatcttcgtga--3'
[0336]22. SEQ ID NO: 22 UBI antisense primer
TABLE-US-00020 [0336] 5'-ttcgcgataggtaaaagagca-3'
[0337]23. SEQ ID NO: 23 M13 fwd primer
TABLE-US-00021 [0337] 5'-GTAAAACGACGGCCAGTG-3'
[0338]24. SEQ ID NO: 24 M13 rev primer
TABLE-US-00022 [0338] 5'-GGAAACAGCTATGACCATG-3'
[0339]25. SEQ ID NO: 25 HvRop6 LEFT PRIMER
TABLE-US-00023 [0339] 5'-GTGGAGGCGCGGCGAGA-3'
[0340]26. SEQ ID NO: 26 HvRop6 RIGHT PRIMER
TABLE-US-00024 [0340] 5'-CCATGCTTCATCTCCATAGTCA-3'
[0341]27. SEQ ID NO: 27 HvRacD LEFT PRIMER
TABLE-US-00025 [0341] 5'-ggatccCGATTCCATCAGGAAAGCAT-3'
[0342]28. SEQ ID NO: 28 HvRacD RIGHT PRIMER
TABLE-US-00026 [0342] 5'-gtcgacGCGAGACACTGCAAAACAAA--3'
[0343]29. SEQ ID NO: 29 HvRop4 LEFT PRIMER
TABLE-US-00027 [0343] 5'-GGATCCttctcgtccatttagccggc-3'
[0344]30. SEQ ID NO: 30 HvRop4 RIGHT PRIMER
TABLE-US-00028 [0344] 5'-GTCGACtgatcacttgaagcatgccag-3'
[0345]31. SEQ ID NO: 31 RacB5' BamHI Primer
TABLE-US-00029 [0345] 5'-GGATCCGATGAGCGCGTCCAGGT-T-3'
[0346]32. SEQ ID NO: 32 RacB3' SalI Primer
TABLE-US-00030 [0346] 5'-GTCGACCTTCGCCCTTGTTCTTTGTC-3'
[0347]33. SEQ ID NO 33 V15 mutagenesis Primer
TABLE-US-00031 [0347] 5'-ACCGTGGGGGACGTCGCCGTCGGCAAGAC-3'
[0348]34. SEQ ID NO: 34 Nucleic acid sequence encoding the RacB homolog HvRop6 from barley (Hordeum vulgare). [0349]35. SEQ ID NO: 35 Amino acid sequence encoding the RacB homolog HvRop6 from barley (1Hordeum vulgare). [0350]36. SEQ ID NO: 36 Nucleic acid sequence encoding the RacB homolog HvRacD from barley (Hordeum vulgare). [0351]37. SEQ ID NO: 37 Amino acid sequence encoding the RacB homolog HvRacD from barley (Hordeum vulgare). [0352]38. SEQ ID NO: 38 Nucleic acid sequence encoding the RacB homolog HvRop4 from barley (Hordeum vulgare). [0353]39. SEQ ID NO: 39 Nucleic acid sequence encoding the RacB homolog HvRop4 from barley (Hordeum vulgare). [0354]40. SEQ ID NO: 40 Nucleic acid sequence encoding the RacB homolog Zea mays ROP6 (GenBank Acc.-No.: AJ278665) [0355]41. SEQ ID NO: 41 Amino acid sequence encoding the RacB homolog Zea mays ROP6 [0356]42. SEQ ID NO: 42 Nucleic acid sequence encoding the RacB homolog Oryza saliva subsp. japonica PACDP (RACD) (GenBank Acc.-No.: AF218381) [0357]43. SEQ ID NO: 43 Amino acid sequence encoding the RacB homolog Oryza saliva subsp. japonica RACDP [0358]44 SEQ ID NO: 44 Nucleic acid sequence encoding the RacB homolog Oryza saliva ROP4 (GenBank Acc.-No.: AF380335) [0359]45. SEQ ID NO: 45 Amino acid sequence encoding the RacB) homolog Oryza sativa ROP4 [0360]46. SEQ ID NO: 46 Nucleic acid sequence encoding the RacB homolog Zea mays RACA (GenBank Acc.-No.: AF126052) [0361]47. SEQ ID NO: 47 Amino acid sequence encoding the RacB homolog Zea mays RACA [0362]48. SEQ ID NO: 48 Nucleic acid sequence encoding an RacB homolog from Hordeum vulgare (GenBank Acc.-No.: BM816965) [0363]49. SEQ ID NO: 49 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At3g51300) [0364]50. SEQ ID NO: 50 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At3g51300) [0365]51. SEQ ID NO: 51 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At2g17800) [0366]52. SEQ ID NO: 52 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At2g17800) [0367]53. SEQ ID NO: 53 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At4g35950) [0368]54. SEQ ID NO: 54 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At4g35950) [0369]55. SEQ ID NO: 55 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At1g75840) [0370]56. SEQ ID NO: 56 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At1g75840) [0371]57. SEQ ID NO: 57 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At4g35020) [0372]58. SEQ ID NO: 58 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At4g35020) [0373]59. SEQ ID NO: 59 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At1g20090) [0374]60. SEQ ID NO: 60 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At1g20090) [0375]61. SEQ ID NO: 61 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At5g45970) [0376]62. SEQ ID NO: 62 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At5g4S970) [0377]63. SEQ ID NO: 63 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At3g48040) [0378]64. SEQ ID NO: 64 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At3g48040) [0379]65. SEQ ID NO: 65 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At5g62880) [0380]66. SEQ ID NO; 66 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At5g62880) [0381]67. SEQ ID NO: 67 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At4g28950) [0382]68. SEQ ID NO: 68 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At4g28950) [0383]69. SEQ ID NO: 79 Nucleic acid sequence encoding an RacB homolog from Arabidopsis thaliana (At7g44690) [0384]70. SEQ ID NO: 70 Amino acid sequence encoding an RacB homolog from Arabidopsis thaliana (At2g44690) [0385]71. SEQ ID NO: 71 Oligonucleotide primer Fra 186
TABLE-US-00032 [0385] 5'-ATGAGCGCGTCCAGGTTCATA-3'
[0386]72. SEQ ID NO: 72 Oligonucleotide primer Fra 187
TABLE-US-00033 [0386] 5'-ATCAAACACGCCCTTCACGTT-3'
[0387]73. SEQ ID NO: 73 Transgenic expression vector pSUN3NIT_AtRacB_s for expression of Arbidopsis thalianan RacB in sense orientation [0388]74. SEQ ID NO: 74 Transgenic expression vector pSUN3NIT_AtRacB_as for expression of Arbidopsis thalianan RacB in antisense orientation [0389]75. SEQ ID NO: 75 Transgenic expression vector pSUN3NIT_HvRacB_s for expression of a barley RacB fragment in sense orientation [0390]76. SEQ ID NO: 76 Transgenic expression vector pSUN3NIT_HvRacB_as for expression of a barley RacB fragment in antisense orientation
FIGURES
[0391]1. FIG. 1: Alignment of the amino acid sequences of barley RacB, rice RacB, maize RacB, and human Rac1 and Rac2 proteins.
[0392]Regions in boxes show the position of the G1 element (GXXXXGKS/T; amino acid 13 to 20), of the 62 effector region (amino acid 29 to 45), of the G3 element (LWDTAGQ; amino acid 58 to 64), of the G4 element (TKXD, amino acid 118 to 121), of the G5 element (EXS) and of the C-terminal isoprenylation motif (CXXX, Hassanain H H et al. (2000) Biochem Biophys Res Commun. 272(3):783-8.). Hyphens indicate sequence gaps. Asterisks denote amino acids which are identical in all homologs. Amino acids which differ between barley on the one hand and maize and rice on the other hand are shown white against black. The position which is advantageously modified to obtain a dominant-negative RacB variant is marked by a black triangle above the sequence.
[0393]2. FIG. 2: Expression of RacB in epidermal tissue
[0394]RT-PCR of RNA from the barley lines Pallas and BCPMla12 (P10) 24 h post-inoculation ("hai" hours after inoculation") with BghA6. To extract the RNA, strips of the abaxial epidermis (E, from inoculated locations of leaves) were removed from the mesophyll and the adaxial epidermis (M). Ubiquitin 1 (Ubi) acted as marker for tissue-unspecific expression, OXLP as positive control for gene expression in the epidermis, and Bas as positive control for gene expression in mesophyll cells. The RT-PCR was carried out over 25 amplification cycles as described hereinbelow. RT-PCR products were denatured in the gel, blotted and detected by means of antisense RNA probes under stringent conditions.
[0395]3. FIG. 3: RacB is expressed constitutively in various resistant barley lines.
[0396]RNA was isolated from the variety Ingrid (Mlo, Ror1, Bgh-susceptible), BCIngrid-mlo5 (mlo5, Ror1, Bgh-resistant) and A 89 (mlo5, ror1, moderately susceptible to BghA6) immediately prior to inoculation (0 O) or 8, 15 or 24 h post-inoculation with Bgh and 24 h thereafter from noninoculated control plants (24 O). Ubiquitin 1 (Ubi) was used as marker for constitutive expression, OXLP as positive control for Bgh-induced gene expression in the epidermal layer. OXLP expression was detected via Northern blot. The RT-PCR for RacB and Ubi was carried out as described over 25 amplification cycles. The PCR products were denatured in the gel, blotted and detected by means of antisense RNA samples under stringent conditions.
[0397]4. FIG. 4: "RNA interference" with RacB-dsRNA reduces the penetration efficacy of barley powdery mildew BghA6 in barley.
[0398]The relative penetration efficacy (RPE) was determined in six individual experiments with inoculation with Bgh from barley cv. Pallas. The RPE was calculated as the difference between the penetration efficacy of cells transformed with RacB-dsRNA and the penetration efficacy of cells transformed with control dsRNA (here: average penetration efficacy 57%). The RPE percentage (%-RPE) is calculated from the RPE minus 1, multiplied by 100.
RPE = [ PE of cells transformed with RacB - dsRNA ] [ PE of cells transformed with control dsRNA ] ##EQU00001## % - RPE = 100 * ( RPE - 1 ) ##EQU00001.2##
[0399]The black columns represent the %-RPE upon evaluation of at least 100 interaction sites for in each case one independent experiment. The white column represents the average %-RPE of the experiments with the RacB-dsRNA ("RACB-dsRNA"). The error bar indicates the standard error.
[0400]"control" represents the parallel experiments with a control dsRNA.
[0401]In cells which had been bombarded with RacB-dsRNA, the %-RPE was markedly reduced in comparison with cells which had been bombarded with a control dsRNA (TR: human thyroid receptor dsRNA).
[0402]5. FIG. 5: Effect of the genetic background on RacB function
[0403]The %-RPE was studied in 5 independent experiments by inoculating barley cv. Pallas, Ingrid or A89, which had previously been transformed with RacB-dsRNA, with BghA6.
[0404]The %-RPE is markedly reduced in Pallas (Mlo Ror1, black bars, experiments 1 and 2) or Ingrid (Mlo Ror1, black bars, experiments 3, 4 and 5). The %-RPE of the susceptible mutant A89 (mlo5 ror1, black bars, experiments 1 to 5), however, was not reduced. White bars indicate the mean, error bars the standard error.
[0405]6. FIG. 6: Overexpression of a constitutively active RacB mutant in barley cv. Pallas
[0406]A constitutively active mutant of barley RACE (exchange G->V in position 15; RacB-V15) was transiently overexpressed in the barley variety Pallas in 5 independent experiments using the expression construct pGY-RacBV15. For comparison, corresponding experiments were carried out using the vector alone, without RacB insert (pGY).
[0407]The expression of a constitutive RacB mutant results in significantly higher susceptibility to pathogen attack by mildew of barley, compared to the controls (FIG. 6-A). In all cases, the relative susceptibility to the fungal pathogen is increased (FIG. 6-B). These results, too, demonstrate the key function of racB in the defense of pathogens.
[0408]7. FIG. 7: Plasmid map for expression vector pGY-1 (Schweizer P et al. (1999) Mol Plant Microbe Interact 12: 647-54; Shinshi H et al. (1990) Plant Mol Biol 14:357-368).
EXAMPLES
General Methods
[0409]The chemical synthesis of oligonucleotides can be effected, for example, in the known fashion using the phosphoamidite method (Voet, Voet, 2nd Edition, Wiley Press New York, pages 896-897). The cloning steps carried out for the purposes of the present invention such as, for example, restriction cleavages, agarose gel electrophoresis, purification of DNA fragments, transfer of nucleic acids to nitrocellulose and nylon membranes, linking DNA fragments, transformation of E. coli cells, growing bacteria, multiplying phages and sequence analysis of recombinant DNA, are carried out as described by Sambrook et al. (1989) Cold Spring Harbor Laboratory Press; ISBN 0-87969-309-6. The sequencing of recombinant DNA molecules is carried out with an MWG-Licor laser fluorescence DNA sequencer following the method of Sanger (Sanger et al. (1977) Proc Natl Acad Sci USA 74:5463-5467).
Example 1
Plants, Pathogens and Inoculation
[0410]The barley variety Ingrid is from James McKey, University of Uppsala, Sweden. The variety Pallas and backcrossed line BCIngrid-mlo5 was donated by Lisa Munk, Department of Plant Pathology, Royal Veterinary and Agricultural University, Copenhagen, Denmark. Its production has been described (K.o slashed.lster P et al. (1986) Crop Sci 26: 903-907). Line A89 was provided by Paul Schulze-Lefert (Max-Plank-Institut fur Zuchtungsforschung, Cologne, Germany).
[0411]Unless otherwise specified, the seed which had been pregerminated on moist filter paper for 12 to 36 hours in the dark was sown along the edge of a square pot (8×8 cm; 5 kernels per pot) in Fruhstorfer soil, type P, covered with soil and watered regularly with tap water. All of the plants were cultured in controlled-environment cabinets or chambers for 5 to 8 days at 16 to 18° C., 50 to 60% relative atmospheric humidity and a 16-hr-light/8-hr-dark rhythm at 3000 or 5000 lux (photon flow density 50 and 60 μmols-1 m-2, respectively) and used in the experiments during the seedling stage. In experiments in which applications to the primary leaves were carried out, the latter were developed fully.
[0412]Before the transient transfection experiments were carried out, the plants were grown in controlled-environment cabinets or chambers at a daytime temperature of 24° C., a nighttime temperature of 20° C., 50 to 60% relative atmospheric humidity and a 16-hr light/8-hr-dark rhythm at 30 000 lux.
[0413]Barley powdery mildew Blumeria graminis (DC) Speer f.sp. hordei Em. Marchal race A6 (Wiberg A (1974) Hereditas 77: 89-148) (BghA6) was used for the inoculation of barley plants. The fungus was provided by the Department of Biometry, JLU Gieβen. Inoculum was growl in controlled-environment cabinets under identical conditions to those described above for the plants by transferring the conidia of infected plant material at a rate of 100 conidia/mm2 to 7-day-old barley plants cv. Golden Promise, which were grown regularly.
[0414]The inoculation with BghA6 was carried out using 7-day-old seedlings by shaking off the conidia of already infected plants in an inoculation tower at a rate of approx. 100 conidia/mm2 (unless otherwise specified).
Example 2
RNA Extraction
[0415]Total RNA was extracted from 8 to 10 primary leaf segments (length 5 cm) by means of "RNA extraction buffer" (AGS, Heidelberg, Germany).
[0416]To this end, the central primary leaf segments 5 cm in length were harvested and homogenized in liquid nitrogen in mortars. The homogenate was stored at -7° C. until the RNA was extracted.
[0417]Total RNA was extracted from the deep-frozen leaf material with the aid of an RNA extraction kit (AGS, Heidelberg). To this end, 200 mg of the deep-frozen leaf material were covered with 1.7 ml RNA extraction buffer (AGS) in a microcentrifuge tube (2 ml) and immediately mixed thoroughly. After addition of 200 μl of chloroform, the mixture was again mixed thoroughly and shaken for 45 minutes on a horizontal shaker at 200 rpm at room temperature. To separate the phases, the tubes were subsequently centrifuged for 15 minutes at 20 000 g and 4° C., and the upper, aqueous phase was transferred into a fresh microcentrifuge tube, while the bottom phase was discarded. The aqueous phase was repurified with 900 μl of chloroform by homogenizing for 10 seconds and recentrifuging (see above) and removing the aqueous phase (3 times). Then, 850 μl of 2-propanol were added and the mixture was homogenized and placed on ice for 30 to 60 minutes in order to precipitate the RNA. Thereafter, the mixture was centrifuged for 20 minutes (see above), the supernatant was carefully decanted off, 2 ml of 70% strength ethanol (-20° C.) were pipetted in, and the mixture was mixed and recentrifuged for 10 minutes.
[0418]Then, the supernatant was again decanted off, and the pellet was carefully freed from residual fluid, using a pipette, and then dried in a stream of clean air in a clean bench. Then, the RNA was dissolved in 50 μl of DEPC water on ice, mixed and centrifuged for 5 minutes (see above). 40 μl of the supernatant, constituting the RNA solution, were transferred into a fresh microcentrifuge tube and stored at -70° C.
[0419]The RNA concentration was determined photometrically. To this end, the RNA solution was diluted 1:99 (v/v) with distilled water, and the absorption was measured at 260 nm (Beckman Photometer DU 7400); (E260 1 at 40 μg RNA/ml). The concentrations of the RNA solutions were subsequently adjusted to 1 μg/μl with DEPC water to match the calculated RNA contents and verified in an agarose gel.
[0420]To verify the RNA concentrations in a horizontal agarose gel (1% agarose in 1×MOPS buffer with 0.2 μg/ml ethidium bromide), 1 μl of RNA solution was treated with 1 μl of 10×MOPS, 1 μl of color marker and 7 μl of DEPC water, separated according to size in 1×MOPS running buffer over 1.5 hours at a voltage of 120 V in the gel, and photographed under UV light. Any differences in concentration of the RNA extracts were adjusted with DEPC water, and the adjustment was rechecked in the gel.
Example 3
Cloning the Barley RacB cDNA Sequence
[0421]The cDNA fragments required for isolating the HvRacB cDNA, cloning it, sequencing it and generating probes were obtained by means of RT-PCR using the "One Step RT-PCR Kit" (Life Technologies, Karlsruhe, Germany or Qiagen, Hilden, Germany). To this end, total RNA from barley seedlings was used as template.
[0422]The RNA was isolated from Pallas 3, 5 and 0.7/days after germination. Moreover, RNA was isolated from Pallas and the backcrossed lines with mlo5, Mlg or Mla12 1, 2 and 5 days after inoculation with BghA6 on day 7 after germination. The following primers are used for the RT-PCR:
TABLE-US-00034 ONP-1 5'-GGATCCGATGAGCGCGTCCAGGTT-3' (SEQ ID NO: 10) and ONP-2 5'-GTCGACCTTCGCCCTTGTTCTTTGTC-3' (SEQ ID NO: 11)
[0423]1000 ng of total RNA, 0.4 mM dNTPs, in each case 0.6 mM OPN-1 and OPN-2 primer, 10 μl of RNase inhibitor and 1 μl of enzyme mix in 1×RT buffer (One Step RT-PCR Kit, Qiagen, Hilden) were employed for each reaction (25 μl batch).
[0424]The following temperature program is used (PTC-100TM model 96V; MJ Research, Inc., Watertown, Mass.):
TABLE-US-00035 1 cycle of 30 min at 50° C. 1 cycle of 150 sec at 94° C. 30 cycles of 45 sec at 94° C., 1 min at 55° C. and 2 min at 72° C. 1 cycle of 7 min at 72° C.
[0425]The PCR product was separated by 2% w/v agarose gel electrophoresis. This gave an RT-PCR product of in total 642 bp which was composed of the RacB sequence (SEQ ID NO: 1) and terminal sequences encoding restriction endonuclease restriction sites. The fragment encodes a 591 bp open reading frame encoding a polypeptide of 197 amino acids. The corresponding cDNA was isolated from an agarose gel and cloned into vector pGEM-T (Promega, Mannheim, Germany) by means of T-overhang ligation. The cDNAs were sequenced starting from the plasmid DNA using the "Thermo Sequenase Fluorescent Labeled Primer Cycle Sequencing Kit" (Amersham, Freiburg, Germany).
[0426]Since a primer has been deduced from the rice RacB sequence as starting primer OPN-1 (GenBank Acc. No.: AF250327), this region (i.e. the 5'-end) of the barley RacB cDNA was reverified by means of RACE technology using the "GeneRacer Kit" (INVITROGENE Life Technologies). To this end, 100 ng of poly-A mRNA, 1 μl of 10×CIP buffer, 10 units of RNAse inhibitor, 10 units of CIP ("calf intestinal phosphatase") and DEPC-treated water were treated for 1 hour at 50° C. in a total volume of 10 μl. To precipitate the RNA, a further 90 μl of DEPC water and 100 μl of phenol:chloroform were added and the mixture was mixed thoroughly for approximately 30 seconds. After centrifugation for 5 minutes at 20 000 g, the top phase was treated with 2 μl of 10 mg/ml mussel glycogen, 10 μl of 3 M sodium acetate (pH 5.2) in a fresh micro reaction vessel. 220 μl of 95% ethanol were added and the mixture was incubated on ice. The RNA was subsequently precipitated by centrifugation for 20 minutes at 20 000 g and 4° C. The supernatant was discarded, 500 μl of 75% ethanol were added, and the mixture was vortexed briefly and recentrifuged for 2 minutes (20 000 g). Again, the supernatant was discarded, and the precipitate was dried in the air for 0.2 minutes at room temperature and subsequently suspended in 6 μl of DEPC water. mRNA CAP structures were removed by adding 1 μl of 10×TAP buffer, 10 units of RNAsin and 1 unit of TAP (tobacco acid pyrophosphatase). The mixture was incubated for 1 h at 37° C. and subsequently cooled on ice. Again, the RNA was precipitated as described above and transferred into a reaction vessel with 0.25 μg of GeneRacer oligonucleotide primer. The oligonucleotide primer was resuspended in the RNA solution, and the mixture was incubated for 5 minutes at 70° C. and then ice-cooled. 1 μl of 10× ligase buffer, 10 mM ATP, 1 unit of RNAsin and 5 units of T4 RNA ligase were added, and the batch was incubated for 1 h at 37° C. Again, the RNA was precipitated as described above and resuspended in 13 μl of DEPC water. 10 pmol of oligo-dT primer were added to the RNA, and the mixture was immediately heated at 70° C. and again cooled on ice. 1 μl of each dNTP solution (25 mM), 2 μl of 10×RT buffer, 5 u (1 μl) of AMV reverse transcriptase and 20 units of RNAsin were added, and the reaction solution was incubated for 1 hour at 42° C. and subsequently for 15 minutes at 85° C. The first-strand cDNA thus prepared was stored at -20° C.
[0427]The following primer was used to amplify the 5'-cDNA ends:
TABLE-US-00036 RACE RacB primer: 5'-gtgggcacatagtcggtggggaaggt-3' (SEQ ID NO: 12) GeneRacer ® 5'-primer: 5'-CGACTGGAGCACGAGGACACTGA-3 (SEQ ID NO: 13) GeneRacer ® 5'-nested primer: 5'-GGACACTGACATGGACTGAAGGAGTA-3 (SEQ ID NO: 14)
[0428]The batch (total volume 25 μl) was composed as follows: [0429]1 μl primer RACE-RacB (5 pmol/μl), [0430]0.5 μl GeneRacer 5'-primer (10 pmol/μl) [0431]2.5 μl 10× buffer Qiagen, [0432]2.5 μl dNTPs (2 mM) [0433]0.5 μl cDNA [0434]0.2 μl QiagenTAG (5 u/μl) [0435]17.8 μl H2O
[0436]The PCR conditions were:
TABLE-US-00037 94° C. denaturation for 5 minutes 5 cycles of 30 seconds at 70° C. (annealing), 1 min at 72° C. (extension), 30 seconds at 94° C. (denaturation) 5 cycles of 30 seconds at 68° C. (annealing), 1 min at 72° C. (extension), 30 seconds at 94° C. (denaturation) 28 cycles of 30 seconds at 66° C. (annealing), 1 min at 72° C. (extension), 30 seconds at 94° C. (denaturation) 72° C. final extension for 10 minutes 4° C. cooling until further use
[0437]The PCR gave a product of approx. 400 bp product. Starting from this product, a nested PCR with the RacB-specific oligonucleotide primer and the "GeneRacer nested 5'-primer" was carried out:
TABLE-US-00038 94° C. denaturation for 5 minutes 30 cycles of 30 sec at 64° C. (annealing), 1 min at 72° C. (extension), 30 sec at 94° C. (denaturation) 72° C. final extension for 10 minutes 4° C. cooling until further use
[0438]The PCR product obtained was isolated via a gel, extracted from the gel, cloned into PGEM-T by means of T-overhang ligation, and sequenced. The sequence in the region of the primer OPN-1 was absolutely identical to the sequence of rice racB, so that no point mutations could be generated by means of primers. Thus, the sequence shown under SEQ ID NO: 1 is identical to the barley RacB sequence.
Example 4
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
[0439]The "One Step RT-PCR Kit" (Qiagen, Hilden, Germany) was used for the semi-quantitative RT-PCR. In doing so, RNA (prepared as above) was first translated into cDNA (reverse transcription) and the sought cDNA was amplified in a subsequent PCR reaction using specific primers. To estimate the initial amount of template RNA, the amplification was interrupted during the exponential phase in order to reflect differences in the target RNA. The PCR products were separated by means of an agarose gel, denatured, blotted onto nylon membranes, and detected with specific non-radiolabeled probes under stringent standard conditions. Hybridization, wash steps and immunodetection were carried out as described under "Northern blot".
[0440]The following components were combined for the individual reactions (25 μl batch) using the "One Step RT-PCR Kit" (Qiagen, Hilden, Germany):
[0441]1000 ng total RNA of a specific sample
[0442]0.4 mM dNTPs
[0443]0.6 μM of each sense and antisense primer
[0444]0.1 μl RNase inhibitor
[0445]1 μl enzyme mix in 1×RT buffer
[0446]cDNA synthesis (reverse transcription) was carried out for 30 minutes at 50° C. The reverse transcriptase was subsequently inactivated for 15 minutes at 95° C., which simultaneously causes activation of DNA polymerase and denaturation of cDNA. A PCR was subsequently carried out with the following program:
TABLE-US-00039 denaturation for 1 minute at 94° C. 25 cycles of 1 minute at 54° C. primer annealing 1 minute at 72° C. primer extension 10 minutes at 72° C. completion of the DNA duplexes then: termination of the reaction at 4° C.
[0447]The PCR products were separated in a 1×TBE agarose gel using ethidium bromide.
[0448]The following oligonucleotide primer pairs were used for the amplifications in the individual batches:
[0449]a) amplification of a 387 bp fragment of the barley RacB cDNA
TABLE-US-00040 RacB sense 5'-gttcatcaagtgcgtcaccgtg-3' (SEQ ID NO: 15) RacB antisense 5'-ttagcttcctcagttcttccctg-3' (SEQ ID NO: 16)
[0450]b) amplification of a 674 bp fragment of barley BAS cDNA (GenBank Acc. No. Z34917)
TABLE-US-00041 BAS sense 5'-cgcgccgcagccgagtacgac-3' (SEQ ID NO: 17) BAS antisense 5'-gtcacaaaaacacatgtaacc-3' (SEQ ID NO: 18)
[0451]c) amplification of a 506 bp OXLP cDNA fragment (GenBank Acc. No. X93171)
TABLE-US-00042 OXLP sense 5'-ggccgacatgcattcaccag-3' (SEQ ID NO: 19) OXLP antisense 5'-catctgatattgctgggtctg-3' (SEQ ID NO: 20)
[0452]d) amplification of a 513 bp Ubi cDNA fragment (GenBank accession M60175)
TABLE-US-00043 UBI sense 5'-ccaagatgcagatcttcgtga-3' (SEQ ID NO: 21) UBI antisense 5'-ttcgcgataggtaaaagagca-3' (SEQ ID NO: 22)
[0453]All of the fragments obtained were additionally ligated into the vector PGEM-T by means of T-overhang ligation and were used as starting plasmids for the generation of probes (for example for Northern blots) or dsRNA. The individual constructs were named pGEMT-RAC1, PGEMT-BAS, pGEMT-OXLP, pGEMT-UBI.
Example 5
Northern Blot Analysis
[0454]To prepare the Northern blotting, the RNA was separated in an agarose gel under denaturing conditions. To this end, part of the RNA solution (corresponding to 5 μg of RNA) was mixed with an identical volume of sample buffer (with ethidium bromide), denatured for 5 minutes at 94° C., placed on ice for 5 minutes, centrifuged briefly and applied to the gel. The 1×MOPS gel (1.5% agarose, ultra pure grade) contained 5 percent by volume of concentrated formaldehyde solution (36.5% [v/v]). The R(NA was separated for 2 hours at 100 V and subsequently blotted.
[0455]Northern blotting was done as an upward capillary RNA transfer. To this end, the gel was first agitated gently for 30 minutes in 25 mM sodium hydrogen/dihydrogen phosphate buffer (pH 6.5) and cut to size. A piece of Whatman paper was prepared in such a way that it rested on a horizontal support and extended on 2 sides into a trough with 25 mM sodium hydrogen/dihydrogen phosphate buffer (pH 6.5). This piece of paper was covered with the gel, uncovered parts of the piece of Whatman paper being covered with a plastic film. The gel was then covered with a positively charged nylon membrane (Boehringer-Mannheim), avoiding air bubbles, whereupon the membrane was recovered to a height of approximately 5 cm with a stack of blotting paper. The blotting paper was additionally weighed down with a sheet of glass and with a 100 g weight. Blotting was carried out overnight at room temperature. The membrane was rinsed briefly in twice-distilled water and irradiated with UV light in a crosslinking apparatus (Biorad) with a light energy of 125 mJ in order to immobilize the RNA. The uniformity of the RNA transfer to the membrane was checked on a UV light bench.
[0456]To detect barley mRNA, 10 μg of total RNA from each sample were resolved in an agarose gel and blotted onto a positively charged nylon membrane by capillary transfer. Detection was effected using the DIG system.
[0457]Probe preparation: Digogygenin- or fluorescein-labeled RNA probes were prepared for hybridization with the mRNAS to be detected. The probes were generated by in-vitro transcription of a PCR product by means of a T7 or SP6 RNA polymerase, using labeled UTPs. The template for the PCR-aided amplification was provided by the above-described plasmid vectors pGEMT-RAC1, pGEMT-BAS, pGEMT-OXLP, PGEMT-UBI.
[0458]Depending on the orientation of the insert, different RNA polymerases were used for generating the antisense strand. T7 RNA polymerase was used for PGEMT-BAS and PGEMT-OXLP, while SP6-RNA polymerase was used for pGEMT-RAC1 and pGEMT-UBI.
[0459]The insert of the individual vector was amplified via PCR using flanking standard primers (M13 fwd and rev). The reaction proceeded with the following end concentrations in a total volume of 50μl of PCR buffer (Silverstar):
TABLE-US-00044 M13-fwd: 5'-GTAAAACGACGGCCAGTG-3' (SEQ ID NO: 23) M13-rev: 5'-GGAAACAGCTATGACCATG-3' (SEQ ID NO: 24)
[0460]10% dimethyl sulfoxide (v/v)
[0461]2 ng/μl of each primer (M13 forward and reversed)
[0462]1.5 mM MgCl2,
[0463]0.2 mM dNTPs,
[0464]4 units Taq polymerase (Silverstar),
[0465]2 ng/μl plasmid DNA.
[0466]The amplification was carried out in a Thermocycler (Perkin-Elmar 2400) with the following temperature program:
TABLE-US-00045 94° C. denaturing for 3 minutes 30 cycles of 30 seconds at 94° C. (denaturing) 30 seconds at 58° C. (annealing), 1.2 minutes at. 72° C. (extension), 72° C. final extension for 5 minutes 4° C. cooling until further use
[0467]The success of the reaction was verified in a 1% agarose gel. The products were subsequently purified using a "High Pure PCR-Product Purification Kit" (Boehringer-Mannheim). This gave approximately 40 μl of column eluate, which was again verified in the gel and stored at -20° C.
[0468]The RNA polymerization, the hybridization and the immunodetection were carried out largely following the kit manufacturer's instructions regarding the nonradioactive RNA detection (DIG System User's Guide, DIG-Luminescence detection Kit, Boehringer-Mannheim, Kogel et al. (1994) Plant Physiol 106:1264-1277). 4 μl of purified PCR product were treated with 2 μl of transcription buffer, 2 μl of NTP labeling mix, 2 μl of NTP mix and 10 μl of DEPC water. Then, 2 μl of the T7 RNA polymerase solution were pipetted in. The reaction was then carried out for 2 hours at 37° C. and then made up to 100 μl with DEPC water. The RNA probe was detected in an ethidium bromide gel and stored at -20° C.
[0469]To prepare the hybridization, the membranes were first agitated gently for 1 hour at 68° C. in 2×SSC (salt, sodium citrate), 0.1% SDS buffer (sodium dodecyl sulfate), the buffer being renewed twice or 3 times. The membranes were subsequently applied to the internal wall of hybridization tubes preheated at 68° C. and incubated for 30 minutes with 10 ml of Dig-Easy hybridization buffer in a preheated hybridization oven. In the meantime, 10 μl of probe solution were denatured for 5 minutes at 94° C. in 80 μl of hybridization buffer, and the mixture was subsequently placed on ice and centrifuged briefly. For the hybridization, the probe was then transferred into 10 ml of hybridization buffer at a temperature of 68° C., and the buffer in the hybridization tube was replaced by this probe buffer. Hybridization was then carried out overnight, likewise at 68° C.
[0470]Prior to the immunodetection of RNA-RNA hybrids, the blots were washed twice under stringent conditions for in each case 20 minutes in 0.1% (w/v) SDS, 0.1×SSC at 68° C.
[0471]For the immunodetection, the blots were first agitated twice for 5 minutes in 2×SSC, 0.1% SDS at RT. 2 stringent wash steps were subsequently carried out for in each case 15 minutes at 68° C. in 0.1×SSC, 0.1% SDS. The solution was then replaced by wash buffer without Tween. The reaction mix was shaken for 1 minute and the solution was exchanged for blocking reagent. After a further 30 minutes' shaking, 10 μl of anti-fluorescein antibody solution were added, and shaking was continued for 60 minutes. This was followed by two 15-minute wash steps in Tween-containing wash buffer. The membrane was subsequently equilibrated for 2 minutes in substrate buffer and, after being left to drain, transferred to a sheet of acetate paper. A mixture of 20 μl CDP-Star® and 2 ml of substrate buffer was then divided uniformly on the "RNA side" of the membrane. The membrane was subsequently covered with a second sheet of acetate paper and the edges were heat-sealed to provide a water-tight seal, avoiding air bubbles. In a dark room, the membrane was then covered for 10 minutes with an X-ray film and the film was subsequently developed. The exposure time was varied as a function of the luminescence reaction.
[0472]Unless otherwise specified, the solutions were part of the kit as delivered (DIG-Luminescence detection Kit, Boehringer-Mannheim). All the others were prepared from the following stock solutions by dilution with autoclaved distilled water. Unless otherwise specified, all the stock solutions were made with DEPC (like DEPC water) and subsequently autoclaved. [0473]DEPC water: distilled water is treated overnight at 37° C. with diethyl pyrocarbonate (DEPC, 0.1%, w/v) and subsequently autoclaved. [0474]10×MOPS buffer: 0.2 M MOPS (morpholine-3-propanesulfonic acic), 0.05 M sodium acetate, 0.01 M EDTA, pH brought to 7.0 with 10 M NaOH. [0475]20×SSC (sodium chloride/sodium citrate, salt/sodium citrate): 3 M NaClo, 0.3 M trisodium citrate×2H2O, pH brought to 7.0 with 4 M HCl. [0476]1% SDS (sodium dodecyl sulfate) sodium dodecyl sulfate (w/v), without DEPC. [0477]RNA sample buffer: 760 μl formamide, 260 μl formaldehyde, 100 μl ethidium bromide (10 mg/ml), 80 μl glycerol, 80 μl bromophenol blue (saturated), 160 μl 10×MOPS, 100 μl water. [0478]10× wash buffer without Tween: 1.0 M maleic acid, 1.5 M NaCl; without DEPC, bring to pH 7.5 with NaOH (solid, approx. 77 g) and 10 M NaOH. [0479]Tween-containing wash buffer: made by adding Tween to wash buffer without Tween (0.3%, v/v). [0480]10× blocking reagent: suspend 50 g of blocking powder (Boehringer-Mannheim) in 500 ml of wash buffer without Tween. [0481]Substrate buffer: bring 100 mM Tris (trishydroxymethylaminomethane)-, 150 mM NaCl to pH 9.5 with 4 M HCl. [0482]10× color marker: 50% glycerol (v/v), 1.0 mM EDTA pH 8.0, 0.25% bromophenol blue (w/v), 0.25% xylene cyanole (w/v).
Example 6
In Vitro Synthesis of the RacB dsRNA
[0483]All of the plasmids (pGEMT-RAC1, pGEMT-BAS, pGEMT-OXLP, pGEMT-UBI) which were employed for in-vitro transcription comprise the T7 and SP6 promoters (pGEM-T, Promega) at the respective ends of the nucleic acid sequence inserted, which makes possible the synthesis of sense or antisense RNA. The plasmids can be linearized with suitable restriction enzymes in order to ensure correct transcription of the nucleic acid sequence inserted and to prevent reading being continued into vectorial sequences.
[0484]To this end, 10 μg of plasmid DNA were cleaved with in each case at the side of the insert which was located distally from the promoter. The cleaved plasmids are extracted in 200μl of water with an identical volume of phenol/chloroform/isoamyl alcohol, transferred into a new Eppendorf reaction vessel (RNAse-free) and centrifuged for 5 minutes at 20 000 g. 180 μl of the plasmid solution were treated with 420 μl of ethanol, and the mixture was placed on ice and subsequently precipitated by centrifugation for 30 minutes at 20 000 g and -4° C. The precipitate was taken up in 10 μl of TE buffer.
[0485]To prepare the RacB dsRNA, the plasmid pGEMT-Rac1 was digested with SpeI, and sense RNA was transcribed using T7 RNA polymerase.
[0486]Furthermore, pGEMT-Rac1 was digested with NcoI, and antisense RNA was transcribed using SP6 RNA polymerase. RNA polymerases were obtained from Roche Molecular Biology, Mannheim, Germany.
[0487]Each transcription reaction contained the following in a volume of 40 μl: [0488]2 μl of linearized plasmid DNA (1 μg) [0489]2 μl of NTPs (25 mM) (1.25 mM of each NTP) [0490]4 μl of 10× reaction buffer (Roche Molecular Biology), [0491]1 μl of RNAsin RNAsin (27 units; Roche Molecular Biology), [0492]2 μl of RNA polymerase (40 units) [0493]29 μl of DEPC water
[0494]Following incubation for 2 hours at 37° C., part of the reactions from the transcription of the sense and antisense strand, respectively, were mixed, denatured for 5 minutes at 95° C. and subsequently hybridized (annealed) by cooling to a final temperature of 37° C. over 30 minutes. Alternatively, it is also possible first to denature the mixture of sense and antisense strand and then to cool it for 30 minutes at -20° C. The protein precipitate which formed during denaturing and hybridization was removed by briefly centrifuging the reaction at 20 800 g, and the supernatant was used directly for coating tungsten particles (see hereinbelow). For analysis, 1 μl of each RNA strand and of the dsRNA were resolved on a nondenaturing agarose gel. Successful hybridization manifested itself by a band shift toward higher molecular weight in comparison with the single strands.
[0495]4 μl of the dsRNA were ethanol-precipitated (by adding 6 μl of water, 1 μl of 3M sodium acetate solution and 25 μl of ethanol, and centrifugation for at least 5 minutes at 20 000 g and 4° C.) and the pellet was resuspended in 500 μl of water. The absorption spectrum between 230 and 300 nm was measured or the absorption at 280 and 260 nm was determined in order to determine the purity and concentration of the dsRNA. As a rule, 80 to 100 μl of dsRNA with an OD260/OD280 ratio of 1.80 to 1.95 were obtained. Digestion with DNase I can optionally be carried out, but has no significant effect on the results which follow.
[0496]The control dsRNA used was the human thyroid receptor dsRNA (starting vector pT7betaSal (Norman C et al. (1988) Cell 55(6):989-1003), provided by Dr. Baniahmad, Department of Genetics, Gieβen, Germany; the sequence of the insert is described under the GenBank Ace. No.: NM--000461). To generate the sense RNA, the plasmid was digested with PvuII, to generate the antisense RNA, it was digested with HindIII, and the RNA was then transcribed with T7 and SP6 RNA polymerase, respectively. The individual process steps for generating the control dsRNA are carried out analogously to those described above for the RacB dsRNA.
Example 7
Transient Transformation, RNAi, and Evaluation of the Development of the Fungal Pathogen
[0497]Barley cv. Pallas leaf segments were transformed with an RacB dsRNA together with a GFP expression vector. The leaves were subsequently inoculated with Bgh, and the result was analyzed after 48 hours by light and fluorescence microscopy. The penetration into GFP-expressing cells was assessed by detecting haustoria in live cells and by assessing the fungal development on precisely these cells. In all six experiments, bombardment of barley cv. Pallas with RacB dsRNA resulted in a reduced number of successfully Bgh-penetrated cells in comparison with cells which had been bombarded with a foreign control dsRNA (human thyroid hormone receptor dsRNA, TR). The resistance-inducing effect of the Racy dsRNA caused an average reduction in Bgh penetration efficacy by 44% (FIG. 4).
[0498]A method was employed for the transient transformation which had already been described for the biolistic introduction of dsRNA into epidermal cells of barley leaves (Schweizer P et al. (1999) Mol Plant Microbe Interact 12:647-54; Schweizer P et al. (2000) Plant J 2000 24: 895-903). Tungsten particles with a diameter of 1.1 μm (particle density 25 mg/ml) were coated with dsRNA (preparation see above) together with plasmid DNA of the vector pGFP (GFP under the control of the CaMV 35S promoter) as transformation marker. For each bombardment, the following amounts of dsRNA and reporter plasmid were used for coating: 1 μg of pGFP and 2 μg of dsRNA. Double-stranded RNA was synthesized in vitro by annealing sense and antisense RNA (see above).
[0499]To prepare microcarriers, 55 mg of tungsten particles (M 17, diameter 1.1 μm; Bio-Rad, Munich) were washed twice with 1 ml of autoclaved distilled water and once with 1 ml of absolute ethanol, dried and taken up in 1 ml of 50% strength glycerol (approx. 50 mg/ml stock solution). The solution was diluted to 25 mg/ml with 50% strength glycerol, mixed thoroughly prior to use, and suspended in an ultrasonic bath. To coat the microcarriers for each bombardment, 1 μg of plasmid, 2 μg of dsRNA (1 μl), 12.5 μl of tungsten particle suspension (25 mg/ml), 12.5 μl of 1 M Ca(NO3)2 solution (pH 10) were combined dropwise with constant mixing, the mixture was left to stand for 10 minutes at RT and then briefly centrifuged, and 20 μl of the supernatant were drawn off. The remainder with the tungsten particles is resuspended (ultrasonic bath) and employed in the experiment.
[0500]Segments (approx. 4 cm in length) of barley primary leaves were used. The tissue was placed on 0.5% Phytagar (GibcoBRL® Life Technologies®, Karlsruhe) supplemented with 20 μg/ml benzimidazole in Petri dishes (diameter 6.5 cm), and the edges were coveted directly prior to particle bombardment with a stencil provided with a rectangular opening of 2.2 cm×2.3 cm. One after the other, the dishes were placed on the bottom of the vacuum chamber (Schweizer P et al. (1999) Mol Plant Microbe Interact 12:647-54) over which a nylon mesh (mesh size 0.2 mm, Millipore, Eschborn) on an apertured plate had been inserted (5 cm above the bottom, 11 cm underneath the macrocarrier, see hereinbelow) to act as diffuser in order to disperse particle aggregates and to slow down the particle stream. For each bombardment, the macrocarrier (plastic syringe filter holder, 13 mm, Gelman Sciences, Swinney, UK), which was attached at the top of the chamber, was loaded with 5.8 μl of DNA-coated tungsten particles (microcarrier, see hereinbelow). The pressure in the chamber was reduced by 0.9 bar using a diaphragm vacuum pump (Vacuubrand, Wertheim), and the surface of the plant tissue was bombarded with the tungsten particles at a helium gas pressure of 9 bar. The chamber was aerated immediately thereafter. To label transformed cells, the leaves were bombarded with the plasmid (pGFP; vector pUC18-based, CaMV 35S promoter/terminator cassette with inserted GFP gene; Schweizer P et al. (1999) Mol Plant Microbe Interact 12:647-54; provided by Dr. P. Schweizer Schweizer P, Institut fur Pflanzengenetik [Department of Plant Genetics] IPK, Gatersleben, Germany). Each time before another plasmid was used for the bombardment, the macrocarrier was cleaned thoroughly with water. Following incubation for four hours after the bombardment with slightly open Petri dishes, RT and daylight, the leaves were inoculated with 100 conidia/mm2 of the barley powdery mildew fungus (race A6) and incubated for a further 36 to 48 hours under identical conditions.
[0501]Leaf segments were bombarded with the coated particles using a particle inflow gun. 312 μg of tungsten particles were applied per bombardment. 4 hours after bombardment, the leaf segments were inoculation inoculated with Blumeria graminis f.sp. hordei mildew (race A6) and, after a further 40 hours, evaluated with regard to infection symptoms. The result (for example the penetration efficacy, defined as percentage of attacked cells with a mature haustorium and a secondary hypha (secondary elongating hyphae) was analyzed by fluorescence and light microscopy. Inoculation with 100 conidia/mm2 results in an attack frequency of approximately 50% of the transformed cells. A minimum of 100 interaction sites were evaluated for each individual experiment. Transformed (GFP-expressing) cells were identified under excitation with blue light. Three different categories of transformed cells were distinguished:
[0502]1. Penetrated cells comprising a readily recognizable haustorium. A cell with more than one haustorium counted as one cell.
[0503]2. Cells which were attacked by a fungal appressorium, but comprise no haustorium. A cell which was attacked repeatedly by Bgh, but contains no haustorium, counted as one cell.
[0504]3. Cells which are not attacked by Bgh.
[0505]Stomatal cells and subsidiary cells were excluded from the evaluation. Surface structures of Bgh were analyzed by light microscopy or fluorescent staining of the fungus with 0.1% Calcofluor (w/v in water) for 30 sec. The fungal development can be evaluated readily by staining with Calcofluor followed by fluorescence microscopy. While the fungus develops a primary germ tube and an appressorial germ tube in cells transformed with RacB dsRNA, it fails to develop a haustorium. The development of haustoria is a precondition for the development of a secondary hyphae.
[0506]The relative penetration efficacies (RPEs) were calculated as the difference between the penetration efficacies in transformed cells (transformation with RacB dsRNA or control dsRNA) and the penetration efficacies of untransformed cells (here: average penetration efficacy 57%). The percentage RPE (%-RPE) is calculated from the RPE minus 1, multiplied by 100.
RPE = [ PE of cells transformed with RacB - dsRNA ] [ PE of cells transformed with control dsRNA ] ##EQU00002## % - RPE = 100 * ( RPE - 1 ) ##EQU00002.2##
[0507]The %-RPE value (deviation from the average penetration efficacy of the control) serves to determine the susceptibility of cells transfected with RacB dsRNA (FIG. 4).
[0508]In the case of the control dsRNA, no difference between the transfection with the control dsRNA and with water was found in five independent experiments with regard to the penetration efficacy of Bgh.
[0509]The deviation of the PE in various genotypes was also studied. To demonstrate the operable linkage with the Mlo gene, an mlo5 genotype (A89, mlo5 ror1, background: Ingrid), which owing to a mutation of the Ror1 gene has only moderate susceptibility to Bgh attack (Freialdenhoven et al. (1996) Plant cell 8:5-14), was employed. In this doubly-mutant genotype, the efficacy of RacB dsRNA was studied in comparison with a wild-type Mlo genotype. However, no prevention of the development of haustoria in A89 was observed in five independent experiments, while in parallel experiments with Pallas and Ingrid the PE was markedly reduced (FIG. 5). Interestingly, the effect of the RacB dsRNA was more pronounced in Pallas than in Ingrid (FIG. 5, Experiments 1 and 2 in comparison with 3, 4 and 5).
[0510]To rule out an effect of the dsRNA on the transformation rate or survival rate of the attacked cells, the number of GFP-expressing cells was compared between control and RacB dsRNA experiments (Table 7). The RacB dsRNA had no effect on the total number, or the number of attacked, GFP-expressing cells.
TABLE-US-00046 TABLE 7 Transformation rates of barley leaves following bombardment with dsRNA Number of GFP - expressing cells per bombardmenta Total Attacked Total Attacked (Control (Control (RacB (RacB Line dsRNA) dsRNA) dsRNA) dsRNA) nc Pallas 34.3 ± 4.6 16.0 ± 2.2 33.9 ± 4.8 15.5 ± 1.4 6 (21) (Mlo Ror1) Ingrid 51.0 ± 8.9 27.6 ± 8.7 49.9 ± 5.6 31.5 ± 7.8 3 (11) (Mlo Ror1) A 89 34.3 ± 5.4 18.1 ± 4.0 34.1 ± 5.5 16.7 ± 3.8 5 (22) (mlo5 ror1) a4 leaves were bombarded per bombardment. The data shown are means and standard error. cNumber of independent experiments (bombardments n in each case for control and RacB dsRNA).
Example 8
Constitutively Active RACB Mutant
[0511]A putatively constitutively active barley RACB mutant (substitution G->V at position 15; RacB-V15) was generated and overexpressed in the barley variety Pallas in order to positively identify RACB as susceptibility factor. First, full-length RACB was synthesized via RT-PCR. The following oligonucleotide primers were employed for this purpose:
TABLE-US-00047 RacB5'BamHI: 5'-GGATCCGATGAGCGCGTCCAGGTT-3' (SEQ ID NO: 31) RacB3'SalI: 5'-GTCGACCTTCGCCCTTGTTCTTTGTC-3' (SEQ ID NO: 32)
[0512]The cDNA was cloned into PGEM-T and subsequently excised via the primer cleavage sites and cloned into pGY-1 (Schweizer P et al. (1999) Mol Plant Microbe Interact 12: 647-54; FIG. 7) via BamHI/SalI cleavage sites. The construct is referred to as pGY1-RacB.
[0513]The nucleic acid sequence encoding the constitutively active RacB mutant RACB-V15 was generated using the "Transformer®Site-Directed Mutagenesis Kit" (Clonetech, Heidelberg), following the manufacturer's instructions. The starting vector employed was pGY1-RacB. The following oligonucleotide was used as mutagenesis primer:
TABLE-US-00048 (SEQ ID NO: 33) V15: 5'-ACCGTGGGGGACGTCGCCGTCGG-CAAGAC-3'
[0514]Then, RACB-V15 was then overexpressed transiently in 5 independent experiments in the barley variety Pallas under the control of the 35S CamV promoter. The experiments were carried out as described by Schultheiss et al. (Schultheiss H et al. (2002) Plant Physiol 128:1447-1454), except that, after the particle bombardment, 24 hours instead of 4 hours elapsed prior to inoculation. The particles were coated as described in Schweizer et al. (Schweizer P et al. (1999) Mol Plant Microbe Interact 12:647-54).
[0515]The expression of a constitutive RacB mutant brings about a significantly increased susceptibility to pathogen attack by powdery mildew of barley in comparison with the controls. Again, these results confirm the key function of RacB in the defense of pathogens. The RACB-V15 effects. (see FIG. 6-A/B) are significant in the t-test when a two-tail paired test is carried out. The relative susceptibility to the fungal pathogen is increased in all cases (FIG. 6-B).
Example 9
Further HvRac Homologs
[0516]All full-length sequences were isolated from RNA using specific primers and cloned into PGEM-T and sequenced (Huckelhoven et al. (2001) Plant Mol Biol; Schultheiss et al. (2002) Plant Physiol 128:1447-1454). In some cases, the sequences are very similar to RacB.
TABLE-US-00049 a) HvRop6: LEFT PRIMER 5'-GTGGAGGCGCGGCGAGA-3' (SEQ ID NO: 25) RIGHT PRIMER 5'-CCATGCTTCATCTCCATAGTCA-3' (SEQ ID NO: 26) b) HvRacD: LEFT PRIMER 5'-ggatccCGATTCCATCAGGAAAGCAT-3' (SEQ ID NO: 27) RIGHT PRIMER 5'-gtcgacGCGAGACACTGCAAAACAAA-3' (SEQ ID NO: 28) c) HvRop4: LEFT PRIMER 5'-GGATCCttctcgtccatttagccggc-3' (SEQ ID NO: 29) RIGHT PRIMER 5'-GTCGACtgatcacttgaagcatgccag-3' (SEQ ID NO: 30)
Example 10
Generation of Sense and Antisense Constructs with the Gene AtRacB for Expression in Arabidopsis thaliana
[0517]A fragment of the Arabidopsis RacB homolog (MIPS-Code: AT4g35950; SEQ ID NO: 53; hereinbelow AtRacB) is isolated via PCR from an Arabidopsis thaliana cDNA library. The primer sequences used are:
TABLE-US-00050 Fra 186: 5'-ATGAGCGCGTCCAGGTTCATA-3' (SEQ ID NO: 71) Fra 187: 5'-ATCAAACACGCCCTTCACGTT-3' (SEQ ID NO: 72)
[0518]The amplification proceeds in a T3 thermocycler from Biometra with the following temperature profile.
[0519]35 cycles of 1 min at 95° C., 0.5 min at 59° C. and 3 min at 72° C. Subsequent extension for 5 min at 72° C.
[0520]The PCR products is cloned into the vector pCR2.1 (in accordance with the pCR Script Cloning Kit, Stratagene, Heidelberg) following the manufacturer's instructions. A fragment is excised from the vector construct via the restriction enzyme EcoRI (Roche, Mannheim). The fragment can be isolated via gel electrophoresis and subsequent purification using anion exchanger columns (QIAex Purification Kit, Qiagen, Hilden). Accordingly, the binary vector pSUN3-Nit is opened up via the enzymes XmaI and EcoRI and subjected to purification by means of gel electrophoresis followed by elution over anion exchanger columns (QIAex Purification Kit, Qiagen, Hilden).
[0521]Since blunt ends have to be generated for the cloning of insert and vector with 5'-overhangs, both the eluted AtRacB fragment and the eluted pSUN3-Nit fragment are treated with 2 μl of dNTP mix I (10 mM each of dATP, dCTP, dCTP, dTTP; Pharmacia, Freiburg) and 1.6 μl of Klenow fragment (USB/Amersham, Braunschweig, 2 U/μl) for filling up the overhang and incubated for 30 min at 37° C. To prevent religation, the vectors are first purified via a QIAquick Spin Column (Qiagen, Hilden), treated with CIAP (Calf Intestinal Alkaline Phosphatase, GibcoBRL, Eggenstein, 1 U/μl) and finally purified via a 0.8% strength agarose gel.
[0522]To prepare the following ligation mix in a total volume of 50 μl, 34 μl of H2O, 5 μl of ligation buffer and 1 μl of T4 ligase (Roche, Mannheim) are additionally added to the 10 μl of cut DNA. This mixture is incubated overnight at 16° C. The ligase is subsequently inactivated for 10 min at 65° C. The ligation is now followed by precipitation with 0.1 volume of sodium acetate (pH 5.2) and 2.5 volumes of ethanol. After centrifugation (30 min, 15 000 g, 4° C.), the pellet is dried in 70% ethanol and resuspended in 10 μl of H2O. 2μl of this pellet are transformed by electroporation (E. coli Pulser, Bio-Rad) into Escherichia coli bacteria, strain DH5α. The DNA-treated bacteria are plated onto LB plates supplemented with the antibiotic ampicillin (50 mg/l). After incubation for 16 h at 37° C., bacteria are scraped from the colonies which have grown and transferred into tubes containing 3 ml of LB-Amp liquid medium each. After incubation for 16 h at 37° C., the cultures, which have grown to great density, are centrifuged. Plasmid DNA is isolated from the bacterial pellets by means of the QIAprep DNA miniprep kit (Qiagen, Hilden) following the manufacturer's instructions and subjected to analytic digests with various enzyme combinations. These control digests allow the isolation of constructs in which the AtRacB gene is cloned in sense or antisense orientation behind the of the A. thaliana nitrilase-1 (nit1) gene, which is constitutively active in plants (GenBank Acc.-No.: Y07648.2, nucleotides 2456-4340, Hillebrand et al. (1996) Gene 170:197-200). These constructs are named pSUN3NIT_AtRacB_s (SEQ ID NO: 73) and pSUN3NIT atRacB_as (SEQ ID NO: 74) and used for the transformation of Arabidopsis plants. The constructs comprise the complete sequence of AtRacB, so that the expression vector pSUN3NIT_HvRacB_s, which comprises the fragment in sense orientation, is capable of expressing a functional AtRacB protein. The vector acts primarily as negative control and leads in most cases to reduced pathogen resistance, but in some cases (see hereinbelow) also to an increase in pathogen resistance, presumably via a cosuppression effect.
Example 11
Generation of Sense and Antisense Constructs with the Gene HvRacB for Expression in Arabidopsis thaliana
[0523]Various nonfunctional fragments of the gene HvRacB are to be prepared for expression in Arabidopsis plants. To this end, the plasmid, which contains the HvRacB gene subcloned into the bacterial vector PGEM-T, is digested with the enzyme combinations BamHI/HindIII (Roche, Mannheim). Overhanging 5'-single strands are filled up by treatment with Klenow polymerase in the presence of a mixture of nucleotides (see above). The resulting HvRacB fragment with these blunt ends is cloned directly into a pSUN3NIT vector, which is opened up with the enzymes BglII and SpeI (Roche, Mannheim) in its multiple cloning site and whose 5'-overhangs are filled up by means of treatment with Klenow polymerase (as described above). For the ligation batches, each of which has a total volume of 50 μl, 34 μl of H2O, 5 μl of ligation buffer and 1 μl of T4 ligase (Roche, Mannheim) are additionally added to the 10 μl of cut DNA. This mixture is incubated overnight at 16° C. The ligase is subsequently inactivated for 10 min at 65° C. The ligation is now followed by precipitation with 0.1 volume of sodium acetate (pH 5.2) and 2.5 volumes of ethanol. After centrifugation (30 min, 15 000 g, 4° C.), the pellet is dried in 70% ethanol and resuspended in 10 μl of H2O. 2 μl of this pellet are transformed by electroporation (E. coli Pulser, Bio-Rad) into Escherichia coli bacteria, strain DH5α. The DNA-treated bacteria are plated onto LB plates supplemented with the antibiotic ampicillin (50 mg/l). After incubation for 16 h at 37° C., bacteria are scraped from the colonies which have grown and transferred into tubes containing 3 ml of LB-Amp liquid medium each. After incubation for 16 h at 37° C., the cultures, which have grown to great density, are centrifuged. Plasmid DNA is isolated from the bacterial pellets by means of the QIAprep DNA miniprep kit (Qiagen, Hilden) following the manufacturer's instructions and subjected to analytic digests with various enzyme combinations. These control digests allow the identification of constructs in which the appropriate gene construct is cloned in sense or antisense orientation behind the promoter of the A. thaliana nitrilase-1 gene, which is constitutively active in plants (see above). These constructs are named pSUN3NIT_HvRacB_s (SEQ ID NO: 75) and pSUN3NIT_HvRacB_as (SEQ ID NO: 76) and used for the transformation of Arabidopsis plants. The constructs comprise a truncated fragment of hvRacB, so that not even the expression vector pSUN3NIT_HvRacB_s, which comprises the fragment in sense orientation, is capable of expressing a functional HvRacB protein. The vector acts primarily as negative control but leads in some cases (see hereinbelow) also to an increase in pathogen resistance, presumably via a cosuppression effect.
Example 12
Transformation of Arabidopsis thaliana, and Analysis of the Fungal Resistance
[0524]Wild-type A. thaliana plants (Columbia) are with the Agrabacterium tumefaciens strain (EHA105) based on a modified method (Steve Clough and Andrew Bent (1998) Plant J 16(6):735-743) of the vacuum infiltration method of Bechtold et al. (Bechtold N et al. (1993) CR Acad Sci Paris, Life Sciences 316:1194-1199).
[0525]The A. tumefaciens cells used are previously transformed with the plasmids pSUN3NIT AtRacB_s (SEQ ID NO: 73), pSUN3NIT_atRacB_as (SEQ ID NO: 74), pSUN3NIT_HvRacB_s (SEQ ID NO: 75) and pSUN3NIT_HvRacB_as (SEQ ID NO: 76).
[0526]Seeds of the Agrobacterium-transformed primary transformants are selected on the basis of their kanamycin resistance. Antibiotic-resistant seedlings are planted in soil and, when grown into fully developed plants, used for biochemical analysis.
[0527]To analyze the resistance of the transgenic Arabidopsis plants to pathogenic fungi, inoculations with the biotrophic fungi Peronospora parasitica and Erysiphe cichoracearum are performed.
[0528]a) Peronospora parasitica
[0529]Plants aged 5 to 8 weeks are sprayed with a conidia spore suspension (approx. 106 spores/ml). The inoculated plants are kept overnight in a refrigerator at approximately 16° C. under dark and damp conditions, being covered with a plastic bag. After one day, the plastic bag is opened slightly and later removed completely. Six days after inoculation, the plants are again covered with the plastic bag overnight, whereby sporulation is induced. On the next day, the leaves are examined for the appearance of conidiophores. Over the next days, the intercellular growth of the fungus leads to the induction of weak chloroses up to severe necroses in the leaves. These symptoms are quantified and tested for significance.
[0530]b) Erysiphe cichoracearum
[0531]The biotrophic mildew fungus is grown on Arabidopsis plants. To infect the 4-week-old transgenic RacB-expressing Arabidopsis plants, conidiophores are removed from the leaf surface with a fine brush and applied to the leaves of the transgenic plants. The plants are incubated for 7 days at 20° C. 7 days after the inoculation, the conidiophores appear on the leaves, and chloroses and necroses can be observed over the following days. These symptoms are quantified and tested for significance.
[0532]c) Results
[0533]The transgenic Arabidopsis plants which express antisense sequences for AtRacB or HvRacB are significantly more resistant to Peronospora parasitica and to Erysiphe cichoracearum than nontransgenic wild-type plants.
[0534]The transgenic Arabidopsis plants which express sense sequences for the complete AtRacB are in are in most cases significantly more susceptible to both Peronospora parasitica and Erysiphe cichoracearum than nontransgenic wildtype plants. In some cases, however, an increased resistance can also be observed (presumably via a cosuppression effect).
[0535]Transgenic Arabidopsis plants which express sense sequences for the an HvRacB fragment are in are in some cases significantly more resistant to both Peronospora parasitica and Erysiphe cichoracearum than nontransgenic wildtype plants.
Sequence CWU
1
761857DNAHordeum vulgareCDS(90)..(680)misc_feature(780)n is a, c, g or t
1atcttaacca gctccctacc tccccttctt cttcctcctc ctcccctgtc tcgcccgcag
60cttcaccggc agcgagggaa agagggagg atg agc gcg tcc agg ttc ata aag
113Met Ser Ala Ser Arg Phe Ile Lys1 5tgc gtc acc gtg ggg
gac ggc gcc gtc ggc aag acc tgc atg ctc atc 161Cys Val Thr Val Gly
Asp Gly Ala Val Gly Lys Thr Cys Met Leu Ile10 15
20tcc tac acc tcc aac acc ttc ccc acc gac tat gtg ccc acg gtg
ttt 209Ser Tyr Thr Ser Asn Thr Phe Pro Thr Asp Tyr Val Pro Thr Val
Phe25 30 35 40gac aac
ttc agt gct aat gtt gtg gtt gat ggc aac act gtc aac ctt 257Asp Asn
Phe Ser Ala Asn Val Val Val Asp Gly Asn Thr Val Asn Leu45
50 55ggg cta tgg gat act gca ggt cag gaa gac tac aac
aga ctg aga ccg 305Gly Leu Trp Asp Thr Ala Gly Gln Glu Asp Tyr Asn
Arg Leu Arg Pro60 65 70ctg agt tat cgt
gga gct gat gtc ttc ctt ctg gcc ttc tcg ctt atc 353Leu Ser Tyr Arg
Gly Ala Asp Val Phe Leu Leu Ala Phe Ser Leu Ile75 80
85agc aag gct agc tat gag aat gtt tca aag aag tgg ata cct
gaa ctg 401Ser Lys Ala Ser Tyr Glu Asn Val Ser Lys Lys Trp Ile Pro
Glu Leu90 95 100aag cat tat gca cca ggt
gtg cct att atc ctc gtg gga aca aag ctt 449Lys His Tyr Ala Pro Gly
Val Pro Ile Ile Leu Val Gly Thr Lys Leu105 110
115 120gat ctt cga gat gac aag cag ttc ttt gtg gac
cat cct ggt gct gtt 497Asp Leu Arg Asp Asp Lys Gln Phe Phe Val Asp
His Pro Gly Ala Val125 130 135cct atc act
act gct cag ggg gag gaa cta aaa aag tta ata ggc gca 545Pro Ile Thr
Thr Ala Gln Gly Glu Glu Leu Lys Lys Leu Ile Gly Ala140
145 150ccc tac tac atc gaa tgc agc tcg aag acc caa cta
aat gtc aag ggt 593Pro Tyr Tyr Ile Glu Cys Ser Ser Lys Thr Gln Leu
Asn Val Lys Gly155 160 165gta ttt gat gcg
gca ata aag gtg gta ctg cag cca cca aag gca aag 641Val Phe Asp Ala
Ala Ile Lys Val Val Leu Gln Pro Pro Lys Ala Lys170 175
180aag aag aaa aag gcg cag agg ggg gct tgc tcc atc ttg
tgatctaatc 690Lys Lys Lys Lys Ala Gln Arg Gly Ala Cys Ser Ile
Leu185 190 195aatcggtaga caaagaacaa
gggcgaagtt gccgccatgc tatattattg ttacgtcttg 750cttcagcgga gctgcactct
catggtcgtn ctnccttccc tcacccccac cccaccctag 810gttacccacc ggcagctgca
acaaggtctc tttgtcgagg catcggg 8572197PRTHordeum vulgare
2Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15Val Gly Lys Thr Cys Met
Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35
40 45Val Asp Gly Asn Thr Val Asn Leu Gly
Leu Trp Asp Thr Ala Gly Gln50 55 60Glu
Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80Phe Leu Leu Ala Phe Ser
Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85 90
95Ser Lys Lys Trp Ile Pro Glu Leu Lys His Tyr Ala Pro Gly Val Pro100
105 110Ile Ile Leu Val Gly Thr Lys Leu
Asp Leu Arg Asp Asp Lys Gln Phe115 120
125Phe Val Asp His Pro Gly Ala Val Pro Ile Thr Thr Ala Gln Gly Glu130
135 140Glu Leu Lys Lys Leu Ile Gly Ala Pro
Tyr Tyr Ile Glu Cys Ser Ser145 150 155
160Lys Thr Gln Leu Asn Val Lys Gly Val Phe Asp Ala Ala Ile
Lys Val165 170 175Val Leu Gln Pro Pro Lys
Ala Lys Lys Lys Lys Lys Ala Gln Arg Gly180 185
190Ala Cys Ser Ile Leu19531113DNAOryza sativaCDS(158)..(748)
3ccttgctttg ctcctccttc aaccttcttc tttcttggag tttcttgaga gagagagaga
60gagagagaga gagagagaga gagagagaga ggggggggag cggtcgcagg aggaggagga
120cggcggcgtc tgctgcgacc gacggggagc ggcgagg atg agc gcg tcc agg ttc
175Met Ser Ala Ser Arg Phe1 5ata aag tgc gtc acc gtc ggg
gac ggc gcc gtc ggc aag acc tgc atg 223Ile Lys Cys Val Thr Val Gly
Asp Gly Ala Val Gly Lys Thr Cys Met10 15
20ctc atc tcc tac acc tcc aac acc ttc ccc act gat tat gtt ccg acg
271Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro Thr Asp Tyr Val Pro Thr25
30 35gtg ttt gac aac ttc agt gcc aac gtc gtg
gtt gat ggt aac acc gtc 319Val Phe Asp Asn Phe Ser Ala Asn Val Val
Val Asp Gly Asn Thr Val40 45 50aac ctc
ggg cta tgg gac act gca ggt cag gag gat tac aac aga ctg 367Asn Leu
Gly Leu Trp Asp Thr Ala Gly Gln Glu Asp Tyr Asn Arg Leu55
60 65 70aga cca ctg agt tat cgt gga
gct gat gtt ttc ctt ctg gcc ttc tcg 415Arg Pro Leu Ser Tyr Arg Gly
Ala Asp Val Phe Leu Leu Ala Phe Ser75 80
85cta atc agc aag gcc agc tat gag aat gtt tca aag aag tgg ata cct
463Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val Ser Lys Lys Trp Ile Pro90
95 100gag ctg aag cat tat gca cct ggt gtt cct
atc atc ctt gtg gga aca 511Glu Leu Lys His Tyr Ala Pro Gly Val Pro
Ile Ile Leu Val Gly Thr105 110 115aag ctt
gat ctt cga gat gac aag cag ttt ttt gtg gac cat cct ggt 559Lys Leu
Asp Leu Arg Asp Asp Lys Gln Phe Phe Val Asp His Pro Gly120
125 130gct gtt cct atc acc act gct cag gga gag gaa cta
aga aag caa ata 607Ala Val Pro Ile Thr Thr Ala Gln Gly Glu Glu Leu
Arg Lys Gln Ile135 140 145
150ggc gcc cca tac tac atc gaa tgc agc tca aag acc caa cta aac gtc
655Gly Ala Pro Tyr Tyr Ile Glu Cys Ser Ser Lys Thr Gln Leu Asn Val155
160 165aag ggc gtt ttc gat gcg gca ata aag
gtg gtg ctg cag cca ccc aag 703Lys Gly Val Phe Asp Ala Ala Ile Lys
Val Val Leu Gln Pro Pro Lys170 175 180gcg
aag aag aag aaa aag gcg caa agg ggg gcg tgc tcc att ttg 748Ala
Lys Lys Lys Lys Lys Ala Gln Arg Gly Ala Cys Ser Ile Leu185
190 195tgatctaatc atcagtagac gacgaagaag aagaacgatg
aagttgccag gctttattat 808tgttgcgtct tgcttcagcg aaacagcatt catggtccgg
ggatcctagt ttactggcag 868ctgcagcaag gcctctttgt cgaggcaatg agcgatccgt
ttgtttcatt ttctcctttc 928tgccttgtga ttatctcgtg tgactgacaa gtcgtggcaa
ttaggtaact ttcctagatg 988gtatttcctg tgtttgagaa aaaaaattct tgttatccct
gtttcataag tagacatgat 1048gtaatcgcac tcagtttatt cttttccttc ttatttcact
tcaatggaaa attatgtttc 1108ccttc
11134197PRTOryza sativa 4Met Ser Ala Ser Arg Phe
Ile Lys Cys Val Thr Val Gly Asp Gly Ala1 5
10 15Val Gly Lys Thr Cys Met Leu Ile Ser Tyr Thr Ser Asn
Thr Phe Pro20 25 30Thr Asp Tyr Val Pro
Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35 40
45Val Asp Gly Asn Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly
Gln50 55 60Glu Asp Tyr Asn Arg Leu Arg
Pro Leu Ser Tyr Arg Gly Ala Asp Val65 70
75 80Phe Leu Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser
Tyr Glu Asn Val85 90 95Ser Lys Lys Trp
Ile Pro Glu Leu Lys His Tyr Ala Pro Gly Val Pro100 105
110Ile Ile Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys
Gln Phe115 120 125Phe Val Asp His Pro Gly
Ala Val Pro Ile Thr Thr Ala Gln Gly Glu130 135
140Glu Leu Arg Lys Gln Ile Gly Ala Pro Tyr Tyr Ile Glu Cys Ser
Ser145 150 155 160Lys Thr
Gln Leu Asn Val Lys Gly Val Phe Asp Ala Ala Ile Lys Val165
170 175Val Leu Gln Pro Pro Lys Ala Lys Lys Lys Lys Lys
Ala Gln Arg Gly180 185 190Ala Cys Ser Ile
Leu19551393DNAZea maysCDS(398)..(988) 5gtcgacccac gcgtccgcgg acgcgtgggc
ggacgcgtgg gtccccaccc accaccgcgc 60cgggccacca ccacccactc taccctcccc
tccccaccac cactagcacc caccgtcccg 120gcgcggagac cgcttccctc cctccgcctc
cgcaaccctc tcccgcctcg cccgcgcctc 180cctccatttg tccgcggctc ccctccctcc
cgatcttaac cacccgccac ccggcttcct 240ctcccccttc ttcctccctc aaaccagacg
ctcgcccccc tttcctccac gcctatcttc 300ttcagacgac cagcaggagg tacgaggaag
accacctagg aggcctctct ctctctctcc 360ccagccaccc ccgtagcgag agggagggcg
gaagagg atg agc gcg tcc agg ttc 415Met Ser Ala Ser Arg Phe1
5ata aag tgc gtc acg gtc ggg gac ggc gcc gtc ggc aag acc tgc atg
463Ile Lys Cys Val Thr Val Gly Asp Gly Ala Val Gly Lys Thr Cys Met10
15 20ctc atc tcc tac acc tcc aac acc ttc ccc
acc gac tat gtt ccg aca 511Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro
Thr Asp Tyr Val Pro Thr25 30 35gtg ttt
gat aac ttc agt gcc aac gtt gtg gtt gat ggt aat act gtc 559Val Phe
Asp Asn Phe Ser Ala Asn Val Val Val Asp Gly Asn Thr Val40
45 50aac ctc ggc ctc tgg gac act gca ggt caa gag gat
tac aac aga ctg 607Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln Glu Asp
Tyr Asn Arg Leu55 60 65
70aga cca ctg agc tat cgt gga gct gat gtt ttt ctt ctg gct ttc tca
655Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val Phe Leu Leu Ala Phe Ser75
80 85ctg atc agt aag gcc agc tat gag aat gtt
tcg aag aag tgg ata cct 703Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val
Ser Lys Lys Trp Ile Pro90 95 100gaa ctg
aag cat tat gca cct ggt gtg cca att att ctc gta ggg aca 751Glu Leu
Lys His Tyr Ala Pro Gly Val Pro Ile Ile Leu Val Gly Thr105
110 115aag ctt gat ctt cga gac gac aag cag ttc ttt gtg
gac cat cct ggt 799Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe Phe Val
Asp His Pro Gly120 125 130gct gtc cct atc
act act gct cag gga gag gag cta aga aag caa ata 847Ala Val Pro Ile
Thr Thr Ala Gln Gly Glu Glu Leu Arg Lys Gln Ile135 140
145 150ggc gct cca tac tac atc gaa tgc agc
tcg aag acc caa cta aac gtg 895Gly Ala Pro Tyr Tyr Ile Glu Cys Ser
Ser Lys Thr Gln Leu Asn Val155 160 165aag
ggc gtc ttc gat gcg gcg ata aag gtt gtg ctg cag ccg cct aag 943Lys
Gly Val Phe Asp Ala Ala Ile Lys Val Val Leu Gln Pro Pro Lys170
175 180gcg aag aag aag aaa aag gtg cag agg ggg gcg
tgc tcc att ttg 988Ala Lys Lys Lys Lys Lys Val Gln Arg Gly Ala
Cys Ser Ile Leu185 190 195tgatctaatc
atcggtagat gaagaaacaa gggcgaaggt gccatggctt tatcatcgtc 1048gcgtcttgct
tcagtggaac agcatgaatg gtccccaccc cctctaggtt tactggcggc 1108tcggctgcag
cgagttctca tctctttgtc gaggcattga gcgatatgtt tgtttcattt 1168tcctccttcc
tgccttgtga ttatctggtg tgtgtgtgtg tgtgactgac gaagtcgcgg 1228cgattaggta
actcgcttag aaggtatttc ccgtgtttga gcaaaagaaa gtatccctgt 1288tatctctgtt
ccataagtta gacatgatgt aatcgtacta agtttatttt tacttatttc 1348acttgaatgg
aaaagtatgc ttcccattta aaaaaaaaaa aaaaa 13936197PRTZea
mays 6Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15Val Gly Lys Thr Cys
Met Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val
Val35 40 45Val Asp Gly Asn Thr Val Asn
Leu Gly Leu Trp Asp Thr Ala Gly Gln50 55
60Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80Phe Leu Leu Ala Phe
Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85 90
95Ser Lys Lys Trp Ile Pro Glu Leu Lys His Tyr Ala Pro Gly Val
Pro100 105 110Ile Ile Leu Val Gly Thr Lys
Leu Asp Leu Arg Asp Asp Lys Gln Phe115 120
125Phe Val Asp His Pro Gly Ala Val Pro Ile Thr Thr Ala Gln Gly Glu130
135 140Glu Leu Arg Lys Gln Ile Gly Ala Pro
Tyr Tyr Ile Glu Cys Ser Ser145 150 155
160Lys Thr Gln Leu Asn Val Lys Gly Val Phe Asp Ala Ala Ile
Lys Val165 170 175Val Leu Gln Pro Pro Lys
Ala Lys Lys Lys Lys Lys Val Gln Arg Gly180 185
190Ala Cys Ser Ile Leu1957197PRTArtificial sequenceDescription of
the artificial sequence dominant-negative mutant of barley RacB
protein 7Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15Val Gly Lys Asn
Cys Met Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn
Val Val35 40 45Val Asp Gly Asn Thr Val
Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50 55
60Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80Phe Leu Leu Ala
Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85 90
95Ser Lys Lys Trp Ile Pro Glu Leu Lys His Tyr Ala Pro Gly
Val Pro100 105 110Ile Ile Leu Val Gly Thr
Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe115 120
125Phe Val Asp His Pro Gly Ala Val Pro Ile Thr Thr Ala Gln Gly
Glu130 135 140Glu Leu Lys Lys Leu Ile Gly
Ala Pro Tyr Tyr Ile Glu Cys Ser Ser145 150
155 160Lys Thr Gln Leu Asn Val Lys Gly Val Phe Asp Ala
Ala Ile Lys Val165 170 175Val Leu Gln Pro
Pro Lys Ala Lys Lys Lys Lys Lys Ala Gln Arg Gly180 185
190Ala Cys Ser Ile Leu1958197PRTArtificial
sequenceDescription of the artificial sequence dominant-negative
mutant of rice RacB protein 8Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr
Val Gly Asp Gly Ala1 5 10
15Val Gly Lys Asn Cys Met Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20
25 30Thr Asp Tyr Val Pro Thr Val Phe Asp Asn
Phe Ser Ala Asn Val Val35 40 45Val Asp
Gly Asn Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50
55 60Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg
Gly Ala Asp Val65 70 75
80Phe Leu Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85
90 95Ser Lys Lys Trp Ile Pro Glu Leu Lys His
Tyr Ala Pro Gly Val Pro100 105 110Ile Ile
Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe115
120 125Phe Val Asp His Pro Gly Ala Val Pro Ile Thr Thr
Ala Gln Gly Glu130 135 140Glu Leu Arg Lys
Gln Ile Gly Ala Pro Tyr Tyr Ile Glu Cys Ser Ser145 150
155 160Lys Thr Gln Leu Asn Val Lys Gly Val
Phe Asp Ala Ala Ile Lys Val165 170 175Val
Leu Gln Pro Pro Lys Ala Lys Lys Lys Lys Lys Ala Gln Arg Gly180
185 190Ala Cys Ser Ile Leu1959197PRTArtificial
sequenceSITE(20)Thr/Asn mutation for dominant-negative phenotype
9Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15Val Gly Lys Asn Cys Met
Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35
40 45Val Asp Gly Asn Thr Val Asn Leu Gly
Leu Trp Asp Thr Ala Gly Gln50 55 60Glu
Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80Phe Leu Leu Ala Phe Ser
Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85 90
95Ser Lys Lys Trp Ile Pro Glu Leu Lys His Tyr Ala Pro Gly Val Pro100
105 110Ile Ile Leu Val Gly Thr Lys Leu
Asp Leu Arg Asp Asp Lys Gln Phe115 120
125Phe Val Asp His Pro Gly Ala Val Pro Ile Thr Thr Ala Gln Gly Glu130
135 140Glu Leu Arg Lys Gln Ile Gly Ala Pro
Tyr Tyr Ile Glu Cys Ser Ser145 150 155
160Lys Thr Gln Leu Asn Val Lys Gly Val Phe Asp Ala Ala Ile
Lys Val165 170 175Val Leu Gln Pro Pro Lys
Ala Lys Lys Lys Lys Lys Val Gln Arg Gly180 185
190Ala Cys Ser Ile Leu1951024DNAArtificial sequenceDescription of
the artificial sequence oligonucleotide primer 10ggatccgatg
agcgcgtcca ggtt
241126DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 11gtcgaccttc gcccttgttc tttgtc
261226DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 12gtgggcacat agtcggtggg
gaaggt 261323DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 13cgactggagc acgaggacac tga
231426DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 14ggacactgac atggactgaa ggagta
261522DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 15gttcatcaag tgcgtcaccg
tg 221623DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 16ttagcttcct cagttcttcc ctg
231721DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 17cgcgccgcag ccgagtacga c
211821DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 18gtcacaaaaa cacatgtaac c
211920DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 19ggccgacatg cattcaccag
202021DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 20catctgatat tgctgggtct g
212121DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 21ccaagatgca gatcttcgtg a
212221DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 22ttcgcgatag gtaaaagagc a
212318DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 23gtaaaacgac ggccagtg
182419DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 24ggaaacagct atgaccatg
192517DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 25gtggaggcgc ggcgaga
172622DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 26ccatgcttca tctccatagt ca
222726DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 27ggatcccgat tccatcagga
aagcat 262826DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 28gtcgacgcga gacactgcaa aacaaa
262926DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 29ggatccttct cgtccattta gccggc
263027DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 30gtcgactgat cacttgaagc
atgccag 273124DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 31ggatccgatg agcgcgtcca ggtt
243226DNAArtificial sequenceDescription of the artificial sequence
oligonucleotide primer 32gtcgaccttc gcccttgttc tttgtc
263329DNAArtificial sequenceDescription of the
artificial sequence oligonucleotide primer 33accgtggggg acgtcgccgt
cggcaagac 2934721DNAHordeum
vulgareCDS(38)..(673)coding for RacB homologue (Rop6) 34gtggaggcgc
ggcgagagcg gcggaggcgg aggagag atg agc gtg acc aag ttc 55Met Ser Val
Thr Lys Phe1 5atc aag tgc gtc acg gtg ggg gac ggc gcc gtc
ggc aag acc tgc atg 103Ile Lys Cys Val Thr Val Gly Asp Gly Ala Val
Gly Lys Thr Cys Met10 15 20ctc atc tgc
tac acc agc aac agg ttc ccc agt gat tac atc ccc acg 151Leu Ile Cys
Tyr Thr Ser Asn Arg Phe Pro Ser Asp Tyr Ile Pro Thr25 30
35gtg ttc gac aac ttc agc gcc aac gtc tcc gtc gac ggc
aac atc gtc 199Val Phe Asp Asn Phe Ser Ala Asn Val Ser Val Asp Gly
Asn Ile Val40 45 50aac ctc ggc cta tgg
gac acc gcc ggg caa gaa gac tac agc cgg ctg 247Asn Leu Gly Leu Trp
Asp Thr Ala Gly Gln Glu Asp Tyr Ser Arg Leu55 60
65 70agg ccg ctg agc tac aga ggc gcc gac gtg
ttc gtg ctc gcc ttc tcc 295Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val
Phe Val Leu Ala Phe Ser75 80 85ctc atc
agc agc gcc agc tac gag aat gtt ctt aag aag tgg atg cca 343Leu Ile
Ser Ser Ala Ser Tyr Glu Asn Val Leu Lys Lys Trp Met Pro90
95 100gag ctc cgc cgg ttc gcg ccg aat gtc ccc att gtt
ctt gtt ggg acc 391Glu Leu Arg Arg Phe Ala Pro Asn Val Pro Ile Val
Leu Val Gly Thr105 110 115aag cta gat ctg
cgt gac cac aga gcc tac ctc gcc gac cac ccc ggt 439Lys Leu Asp Leu
Arg Asp His Arg Ala Tyr Leu Ala Asp His Pro Gly120 125
130gct tca gca atc aca act gca cag ggt gaa gaa ctt agg aag
cag atc 487Ala Ser Ala Ile Thr Thr Ala Gln Gly Glu Glu Leu Arg Lys
Gln Ile135 140 145 150ggc
gcc gcg gct tac atc gag tgc agc tcc aag aca cag cag aac gtc 535Gly
Ala Ala Ala Tyr Ile Glu Cys Ser Ser Lys Thr Gln Gln Asn Val155
160 165aag gct gtg ttt gac acc gcc ata aag gtg gtc
ctc cag ccg ccg agg 583Lys Ala Val Phe Asp Thr Ala Ile Lys Val Val
Leu Gln Pro Pro Arg170 175 180aga agg gag
gtg atg tcc gcc agg aag aaa acc agg cga agc tct gga 631Arg Arg Glu
Val Met Ser Ala Arg Lys Lys Thr Arg Arg Ser Ser Gly185
190 195tgc tcc atc aag cac ttg atc tgc ggg agt acg tgc
gct gct 673Cys Ser Ile Lys His Leu Ile Cys Gly Ser Thr Cys
Ala Ala200 205 210tgaattagca ccatggaggc
ctggactgac tatggagatg aagcatgg 72135212PRTHordeum vulgare
35Met Ser Val Thr Lys Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15Val Gly Lys Thr Cys Met
Leu Ile Cys Tyr Thr Ser Asn Arg Phe Pro20 25
30Ser Asp Tyr Ile Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Ser35
40 45Val Asp Gly Asn Ile Val Asn Leu Gly
Leu Trp Asp Thr Ala Gly Gln50 55 60Glu
Asp Tyr Ser Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80Phe Val Leu Ala Phe Ser
Leu Ile Ser Ser Ala Ser Tyr Glu Asn Val85 90
95Leu Lys Lys Trp Met Pro Glu Leu Arg Arg Phe Ala Pro Asn Val Pro100
105 110Ile Val Leu Val Gly Thr Lys Leu
Asp Leu Arg Asp His Arg Ala Tyr115 120
125Leu Ala Asp His Pro Gly Ala Ser Ala Ile Thr Thr Ala Gln Gly Glu130
135 140Glu Leu Arg Lys Gln Ile Gly Ala Ala
Ala Tyr Ile Glu Cys Ser Ser145 150 155
160Lys Thr Gln Gln Asn Val Lys Ala Val Phe Asp Thr Ala Ile
Lys Val165 170 175Val Leu Gln Pro Pro Arg
Arg Arg Glu Val Met Ser Ala Arg Lys Lys180 185
190Thr Arg Arg Ser Ser Gly Cys Ser Ile Lys His Leu Ile Cys Gly
Ser195 200 205Thr Cys Ala
Ala21036699DNAHordeum vulgareCDS(67)..(657)coding for RacB homologue
(RacD) 36ggatcccgat tccatcagga aagcatatag actagcccag taaatagaaa
taagnaaaga 60tcggcg atg agc gca tct cgg ttc atc aag tgc gtg acg gtg
ggg gac 108Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp1
5 10ggc gcc gtg gga aag aca tgc ctc ctc
atc tca tac aca tcc aac acc 156Gly Ala Val Gly Lys Thr Cys Leu Leu
Ile Ser Tyr Thr Ser Asn Thr15 20 25
30ttc ccc aca gac tat gtc cca aca gtt ttc gac aac ttc agc
gct aac 204Phe Pro Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser
Ala Asn35 40 45gtc gtg gtt gac ggc agc
acc gtc aac ctc gga tta tgg gat act gca 252Val Val Val Asp Gly Ser
Thr Val Asn Leu Gly Leu Trp Asp Thr Ala50 55
60gga caa gaa gac tat aat cga cta cgc cca cta agc tac cgt ggt gcc
300Gly Gln Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala65
70 75gat gtc ttc ctg ctc gcc ttt tct ctc
atc agc aaa gca agc tac gag 348Asp Val Phe Leu Leu Ala Phe Ser Leu
Ile Ser Lys Ala Ser Tyr Glu80 85 90aat
gtc act aag aag tgg att cca gag tta cgg cac tat gct cct ggc 396Asn
Val Thr Lys Lys Trp Ile Pro Glu Leu Arg His Tyr Ala Pro Gly95
100 105 110gtg ccc ata att ctt gtt
gga aca aag ctt gat ctg cgg gat gac aag 444Val Pro Ile Ile Leu Val
Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys115 120
125cag ttt ttt gtg gat cac cct ggg gcg gtt cct att tcc act gct cag
492Gln Phe Phe Val Asp His Pro Gly Ala Val Pro Ile Ser Thr Ala Gln130
135 140ggt gaa gag ctg aag aag gtg att ggc
gcg act gcc tac atc gag tgc 540Gly Glu Glu Leu Lys Lys Val Ile Gly
Ala Thr Ala Tyr Ile Glu Cys145 150 155agc
tca aaa aca cag cag aac atc aag gcg gtg ttt gat gcg gcg atc 588Ser
Ser Lys Thr Gln Gln Asn Ile Lys Ala Val Phe Asp Ala Ala Ile160
165 170aag gtg gtc ctc cag cct ccg aag cag aag cgg
aag aag agg aag tca 636Lys Val Val Leu Gln Pro Pro Lys Gln Lys Arg
Lys Lys Arg Lys Ser175 180 185
190cag aaa gga tgc agc atc ttg taaagctaaa atcccttttg ttttgcagtg
687Gln Lys Gly Cys Ser Ile Leu195tctcgcgtcg ac
69937197PRTHordeum vulgare 37Met Ser Ala
Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1 5
10 15Val Gly Lys Thr Cys Leu Leu Ile Ser Tyr
Thr Ser Asn Thr Phe Pro20 25 30Thr Asp
Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35
40 45Val Asp Gly Ser Thr Val Asn Leu Gly Leu Trp Asp
Thr Ala Gly Gln50 55 60Glu Asp Tyr Asn
Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65 70
75 80Phe Leu Leu Ala Phe Ser Leu Ile Ser
Lys Ala Ser Tyr Glu Asn Val85 90 95Thr
Lys Lys Trp Ile Pro Glu Leu Arg His Tyr Ala Pro Gly Val Pro100
105 110Ile Ile Leu Val Gly Thr Lys Leu Asp Leu Arg
Asp Asp Lys Gln Phe115 120 125Phe Val Asp
His Pro Gly Ala Val Pro Ile Ser Thr Ala Gln Gly Glu130
135 140Glu Leu Lys Lys Val Ile Gly Ala Thr Ala Tyr Ile
Glu Cys Ser Ser145 150 155
160Lys Thr Gln Gln Asn Ile Lys Ala Val Phe Asp Ala Ala Ile Lys Val165
170 175Val Leu Gln Pro Pro Lys Gln Lys Arg
Lys Lys Arg Lys Ser Gln Lys180 185 190Gly
Cys Ser Ile Leu19538677DNAHordeum vulgareCDS(27)..(665)coding for RacB
homologue (Rop4) 38ggatccttct cgtccattta gccggc atg gcg tcc agc gcc tcc
cgg ttc atc 53Met Ala Ser Ser Ala Ser Arg Phe Ile1
5aag tgc gtc acc gtc ggg gac ggc gcc gtc ggc aag acc tgc atg ctc
101Lys Cys Val Thr Val Gly Asp Gly Ala Val Gly Lys Thr Cys Met Leu10
15 20 25atc tgc tac acc agc
aac aag ttc ccc acc gac tac gtg ccc acc gtg 149Ile Cys Tyr Thr Ser
Asn Lys Phe Pro Thr Asp Tyr Val Pro Thr Val30 35
40ttc gac aat ttc agc gcg aac gtg gtg gtg gac ggc acc acc gtg
aac 197Phe Asp Asn Phe Ser Ala Asn Val Val Val Asp Gly Thr Thr Val
Asn45 50 55ctg ggc ctc tgg gac act gca
ggg cag gag gac tac aac aga ttg aga 245Leu Gly Leu Trp Asp Thr Ala
Gly Gln Glu Asp Tyr Asn Arg Leu Arg60 65
70ccg ctg agc tac cgg gga gcc gac gtc ttc gtg ctc tcc ttc tcg ctc
293Pro Leu Ser Tyr Arg Gly Ala Asp Val Phe Val Leu Ser Phe Ser Leu75
80 85gtc agc cga gcc agc tac gag aat gtc atg
aag aag tgg cta ccg gag 341Val Ser Arg Ala Ser Tyr Glu Asn Val Met
Lys Lys Trp Leu Pro Glu90 95 100
105ctt cag cac cat gca ccc ggc gtg cca aca gtg ctg gtt ggt aca
aag 389Leu Gln His His Ala Pro Gly Val Pro Thr Val Leu Val Gly Thr
Lys110 115 120cta gat cta cgt gaa gac aag
caa tac tta ctt gac cac ccc ggc gtg 437Leu Asp Leu Arg Glu Asp Lys
Gln Tyr Leu Leu Asp His Pro Gly Val125 130
135gtg cct gtt act aca gct cag ggg gag gaa ctc cgc aag cac atc ggt
485Val Pro Val Thr Thr Ala Gln Gly Glu Glu Leu Arg Lys His Ile Gly140
145 150gca act tgt tat gtc gaa tgc agc tca
aag aca cag cag aat gtc aaa 533Ala Thr Cys Tyr Val Glu Cys Ser Ser
Lys Thr Gln Gln Asn Val Lys155 160 165gct
gtg ttt gat gct gcc atc aag gta gtg atc aaa cct cca aca aag 581Ala
Val Phe Asp Ala Ala Ile Lys Val Val Ile Lys Pro Pro Thr Lys170
175 180 185cag agg gaa agg agg aag
aag aaa gca cgg caa gga tgt gca tca ttg 629Gln Arg Glu Arg Arg Lys
Lys Lys Ala Arg Gln Gly Cys Ala Ser Leu190 195
200ggt acc ctg tca aga agg aag ctg gca tgc ttc aag tgatcagtcg ac
677Gly Thr Leu Ser Arg Arg Lys Leu Ala Cys Phe Lys205
21039213PRTHordeum vulgare 39Met Ala Ser Ser Ala Ser Arg Phe Ile Lys Cys
Val Thr Val Gly Asp1 5 10
15Gly Ala Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr Ser Asn Lys20
25 30Phe Pro Thr Asp Tyr Val Pro Thr Val Phe
Asp Asn Phe Ser Ala Asn35 40 45Val Val
Val Asp Gly Thr Thr Val Asn Leu Gly Leu Trp Asp Thr Ala50
55 60Gly Gln Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser
Tyr Arg Gly Ala65 70 75
80Asp Val Phe Val Leu Ser Phe Ser Leu Val Ser Arg Ala Ser Tyr Glu85
90 95Asn Val Met Lys Lys Trp Leu Pro Glu Leu
Gln His His Ala Pro Gly100 105 110Val Pro
Thr Val Leu Val Gly Thr Lys Leu Asp Leu Arg Glu Asp Lys115
120 125Gln Tyr Leu Leu Asp His Pro Gly Val Val Pro Val
Thr Thr Ala Gln130 135 140Gly Glu Glu Leu
Arg Lys His Ile Gly Ala Thr Cys Tyr Val Glu Cys145 150
155 160Ser Ser Lys Thr Gln Gln Asn Val Lys
Ala Val Phe Asp Ala Ala Ile165 170 175Lys
Val Val Ile Lys Pro Pro Thr Lys Gln Arg Glu Arg Arg Lys Lys180
185 190Lys Ala Arg Gln Gly Cys Ala Ser Leu Gly Thr
Leu Ser Arg Arg Lys195 200 205Leu Ala Cys
Phe Lys21040945DNAZea maysCDS(37)..(672)coding for RacB homologue (Rop6)
40gagaagaagg gggcctgccg gccggggctg ggagac atg agc gtg acc aag ttc
54Met Ser Val Thr Lys Phe1 5atc aag tgc gtc acg gtg ggc gac
ggc gcg gtg ggc aag acc tgc atg 102Ile Lys Cys Val Thr Val Gly Asp
Gly Ala Val Gly Lys Thr Cys Met10 15
20ctc atc tgc tac acc agc aac aag ttc ccc acg gac tac atc ccc acg
150Leu Ile Cys Tyr Thr Ser Asn Lys Phe Pro Thr Asp Tyr Ile Pro Thr25
30 35gtg ttc gac aac ttc agc gcc aac gtc tcc
gtg gac ggc agc atc gtc 198Val Phe Asp Asn Phe Ser Ala Asn Val Ser
Val Asp Gly Ser Ile Val40 45 50aac ctg
ggc ctc tgg gac acc gcg ggg caa gag gac tac agc agg ctg 246Asn Leu
Gly Leu Trp Asp Thr Ala Gly Gln Glu Asp Tyr Ser Arg Leu55
60 65 70cgg ccg ctg agc tac agg ggc
gcg gac gtg ttc gtg ctg gcc ttc tcc 294Arg Pro Leu Ser Tyr Arg Gly
Ala Asp Val Phe Val Leu Ala Phe Ser75 80
85ctg atc agc agg gcg agc tac gag aac gtt ctt aag aag tgg gtg cca
342Leu Ile Ser Arg Ala Ser Tyr Glu Asn Val Leu Lys Lys Trp Val Pro90
95 100gag ctt cgc aga ttc gcg ccc aac gtc ccg
gtc gtt ctt gtt ggg acc 390Glu Leu Arg Arg Phe Ala Pro Asn Val Pro
Val Val Leu Val Gly Thr105 110 115aag tta
gat ctc cgc gac cac aga gcc tac ctc gcc gac cat cct gga 438Lys Leu
Asp Leu Arg Asp His Arg Ala Tyr Leu Ala Asp His Pro Gly120
125 130gct tca gca gtc acc acg gcg cag ggt gag gaa ctg
agg aag cag atc 486Ala Ser Ala Val Thr Thr Ala Gln Gly Glu Glu Leu
Arg Lys Gln Ile135 140 145
150ggc gct gcg gcc tac atc gag tgc agt tcc aaa acc cag cag aac gtc
534Gly Ala Ala Ala Tyr Ile Glu Cys Ser Ser Lys Thr Gln Gln Asn Val155
160 165aag tct gtc ttc gat acg gcc atc aaa
gtg gtc ctt cag ccc cca cgg 582Lys Ser Val Phe Asp Thr Ala Ile Lys
Val Val Leu Gln Pro Pro Arg170 175 180agg
agg gag gca gtg cct gcc agg aag aag aac agg cgt ggc tcc gga 630Arg
Arg Glu Ala Val Pro Ala Arg Lys Lys Asn Arg Arg Gly Ser Gly185
190 195tgc tct ata atg aac ctt gtg tgt ggc agc aca
tgt gct gct 672Cys Ser Ile Met Asn Leu Val Cys Gly Ser Thr
Cys Ala Ala200 205 210tagggagtct
actagaacac tgaaccggaa gggaggtgaa ggcgtgattc atggtgtgta 732atgtgctgtg
gcaactggca agttagtttg ctatagatga ggatgactgc tgcttttgtt 792ttccttggcc
catctgctgt agttcgtcag gctcttcaag ggctgacttt ttaccagact 852gcagtgttgt
gtaagaagtt tgctagacgc tgtaactgta atgttctccg ctgatgtggt 912ataactaaga
tacgagtaag cttgagcgtg ttc 94541212PRTZea
mays 41Met Ser Val Thr Lys Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15Val Gly Lys Thr Cys
Met Leu Ile Cys Tyr Thr Ser Asn Lys Phe Pro20 25
30Thr Asp Tyr Ile Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val
Ser35 40 45Val Asp Gly Ser Ile Val Asn
Leu Gly Leu Trp Asp Thr Ala Gly Gln50 55
60Glu Asp Tyr Ser Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80Phe Val Leu Ala Phe
Ser Leu Ile Ser Arg Ala Ser Tyr Glu Asn Val85 90
95Leu Lys Lys Trp Val Pro Glu Leu Arg Arg Phe Ala Pro Asn Val
Pro100 105 110Val Val Leu Val Gly Thr Lys
Leu Asp Leu Arg Asp His Arg Ala Tyr115 120
125Leu Ala Asp His Pro Gly Ala Ser Ala Val Thr Thr Ala Gln Gly Glu130
135 140Glu Leu Arg Lys Gln Ile Gly Ala Ala
Ala Tyr Ile Glu Cys Ser Ser145 150 155
160Lys Thr Gln Gln Asn Val Lys Ser Val Phe Asp Thr Ala Ile
Lys Val165 170 175Val Leu Gln Pro Pro Arg
Arg Arg Glu Ala Val Pro Ala Arg Lys Lys180 185
190Asn Arg Arg Gly Ser Gly Cys Ser Ile Met Asn Leu Val Cys Gly
Ser195 200 205Thr Cys Ala
Ala21042850DNAOryza sativaCDS(112)..(702)coding for RacB homologue
(RACDP / RACD) 42agcaagcagc agctgaggtg aggtccgtgg cgttggagtg aggactgagg
aggaagaaga 60gggcgggatc tagggtaccg gatgcgctgg ctgtgctgag tgagagtaga g
atg agc 117Met Ser1gcg tct cgg ttc atc aag tgc gtc acc gtg ggg gac
ggc gcc gtg ggc 165Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp
Gly Ala Val Gly5 10 15aag acc tgc atg
ctc atc tcc tac acc tcc aac acc ttc ccc acg gac 213Lys Thr Cys Met
Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro Thr Asp20 25
30tat gtt cca act gtt ttt gat aac ttc agt gca aat gtt gtg
gtc gat 261Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val
Val Asp35 40 45 50ggg
agc act gtg aac ttg ggg ttg tgg gat aca gca gga caa gag gac 309Gly
Ser Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln Glu Asp55
60 65tac aat agg cta cgc ccg ttg agc tat cgt ggc
gct gat gtt ttc ctg 357Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly
Ala Asp Val Phe Leu70 75 80ctg gcc ttt
tct ctg atc agc aaa gca agc tat gag aat gtt tct aaa 405Leu Ala Phe
Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val Ser Lys85 90
95aag tgg ata cct gaa tta agg cat tat gct cct ggt gtg
cca ata att 453Lys Trp Ile Pro Glu Leu Arg His Tyr Ala Pro Gly Val
Pro Ile Ile100 105 110ctc gtt gga aca aag
ctt gat ctg cgg gat gat aag caa ttt ttc gta 501Leu Val Gly Thr Lys
Leu Asp Leu Arg Asp Asp Lys Gln Phe Phe Val115 120
125 130gat cac cct ggt gct gta cct att tcc act
gct cag ggc gaa gag ctg 549Asp His Pro Gly Ala Val Pro Ile Ser Thr
Ala Gln Gly Glu Glu Leu135 140 145agg aaa
ctc att ggt gca gcg gca tac att gaa tgc agt tca aaa aca 597Arg Lys
Leu Ile Gly Ala Ala Ala Tyr Ile Glu Cys Ser Ser Lys Thr150
155 160cag caa aac atc aag gca gtt ttc gat gct gcg att
aag gtg gtt ctc 645Gln Gln Asn Ile Lys Ala Val Phe Asp Ala Ala Ile
Lys Val Val Leu165 170 175cag cct cca aag
caa aag aag aag aag aaa aag gcg cag aaa gga tgt 693Gln Pro Pro Lys
Gln Lys Lys Lys Lys Lys Lys Ala Gln Lys Gly Cys180 185
190gcc atc ttg taattaaatg gtagacagtg cagtgcagat cgatgtatcc
742Ala Ile Leu195cttcatttgt agcctctggc ttcaatcgtc gcttgtttgt
ataattacgc tagatgccac 802cggcagaaga tataatatag tcctcctgcc tttgtggtgt
tggtctct 85043197PRTOryza sativa 43Met Ser Ala Ser Arg
Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1 5
10 15Val Gly Lys Thr Cys Met Leu Ile Ser Tyr Thr Ser
Asn Thr Phe Pro20 25 30Thr Asp Tyr Val
Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35 40
45Val Asp Gly Ser Thr Val Asn Leu Gly Leu Trp Asp Thr Ala
Gly Gln50 55 60Glu Asp Tyr Asn Arg Leu
Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65 70
75 80Phe Leu Leu Ala Phe Ser Leu Ile Ser Lys Ala
Ser Tyr Glu Asn Val85 90 95Ser Lys Lys
Trp Ile Pro Glu Leu Arg His Tyr Ala Pro Gly Val Pro100
105 110Ile Ile Leu Val Gly Thr Lys Leu Asp Leu Arg Asp
Asp Lys Gln Phe115 120 125Phe Val Asp His
Pro Gly Ala Val Pro Ile Ser Thr Ala Gln Gly Glu130 135
140Glu Leu Arg Lys Leu Ile Gly Ala Ala Ala Tyr Ile Glu Cys
Ser Ser145 150 155 160Lys
Thr Gln Gln Asn Ile Lys Ala Val Phe Asp Ala Ala Ile Lys Val165
170 175Val Leu Gln Pro Pro Lys Gln Lys Lys Lys Lys
Lys Lys Ala Gln Lys180 185 190Gly Cys Ala
Ile Leu195441169DNAOryza sativaCDS(169)..(813)coding for RacB homologue
(Rop 4) 44aacagttcag agaggaagca tgtgacactt ccctctgtcc ctctctctct
ctctagcctc 60caaccatcgc ttcaccaaga agccatcacc tcctcctctc tatcaagttc
tctcccctct 120cttgctgtct ctgcttgctg ctgctgctgc tcgattcggc cggcggcc atg
gcg tcc 177Met Ala Ser1agc gcg tcg cgg ttc atc aag tgc gtc acg gtc
ggg gac ggc gcc gtc 225Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val
Gly Asp Gly Ala Val5 10 15ggc aag acc
tgc atg ctc atc tgc tac acc agc aac aag ttc ccc act 273Gly Lys Thr
Cys Met Leu Ile Cys Tyr Thr Ser Asn Lys Phe Pro Thr20 25
30 35gat tac gta ccc act gtt ttt gac
aat ttc agt gca aac gtg gtg gtc 321Asp Tyr Val Pro Thr Val Phe Asp
Asn Phe Ser Ala Asn Val Val Val40 45
50gac ggc acc acg gtg aat ttg ggt ctc tgg gat act gca ggg cag gaa
369Asp Gly Thr Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln Glu55
60 65gat tac aac aga ttg agg ccg cta agc tac
cgt ggc gcc gat gtc ttt 417Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr
Arg Gly Ala Asp Val Phe70 75 80gtg ctt
gcc ttc tcc cta gtg agc cga gct agc tat gag aat gtc atg 465Val Leu
Ala Phe Ser Leu Val Ser Arg Ala Ser Tyr Glu Asn Val Met85
90 95aag aag tgg tta cca gag ctt cag cat tat gca cca
ggg gtg cca att 513Lys Lys Trp Leu Pro Glu Leu Gln His Tyr Ala Pro
Gly Val Pro Ile100 105 110
115gtg ttg gtt ggg acc aaa ttg gat ctt cgt gaa gat aaa cac tac tta
561Val Leu Val Gly Thr Lys Leu Asp Leu Arg Glu Asp Lys His Tyr Leu120
125 130ctt gac cat cct agc ttg gtg cct gtg
act aca gca cag gga gag gaa 609Leu Asp His Pro Ser Leu Val Pro Val
Thr Thr Ala Gln Gly Glu Glu135 140 145ctc
cgc aag cac att ggc gca acg tgt tac atc gaa tgc agc tca aag 657Leu
Arg Lys His Ile Gly Ala Thr Cys Tyr Ile Glu Cys Ser Ser Lys150
155 160aca cag cag aat gta aaa gct gtg ttt gat gct
gcc atc aag gta gta 705Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala
Ala Ile Lys Val Val165 170 175atc aag cct
cca aca aag cag agg gac agg aag aag aag aaa aca cgg 753Ile Lys Pro
Pro Thr Lys Gln Arg Asp Arg Lys Lys Lys Lys Thr Arg180
185 190 195cgc gga tgt tct ttc ttc tgc
aag ggt gtc atg tcc aga aga agg cta 801Arg Gly Cys Ser Phe Phe Cys
Lys Gly Val Met Ser Arg Arg Arg Leu200 205
210gta tgc ttc aag tgaacaagag gggttctttg atgagcagag cagaggtctg
853Val Cys Phe Lys215tgagacaaaa tgatgtcttg tgtttgataa ttgctttatc
tcaaaagttc cagtttgata 913gttgcatttc caacctatat atatcctgtt tggcaattaa
ctactacctc cgtcccaaaa 973tataacaact tttggctatg aatctgaacg cacagttatc
cagattcata gctaaaaata 1033cttatatttt gggacggagg gagtactagt agtagattac
tatcctgtcc atgtaatgta 1093ttaggaaggt taatagcact ccctacatct cagaatggaa
gttgttttgg ttttaaaaaa 1153aaaaaaaaaa aaaaaa
116945215PRTOryza sativa 45Met Ala Ser Ser Ala Ser
Arg Phe Ile Lys Cys Val Thr Val Gly Asp1 5
10 15Gly Ala Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr
Ser Asn Lys20 25 30Phe Pro Thr Asp Tyr
Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn35 40
45Val Val Val Asp Gly Thr Thr Val Asn Leu Gly Leu Trp Asp Thr
Ala50 55 60Gly Gln Glu Asp Tyr Asn Arg
Leu Arg Pro Leu Ser Tyr Arg Gly Ala65 70
75 80Asp Val Phe Val Leu Ala Phe Ser Leu Val Ser Arg
Ala Ser Tyr Glu85 90 95Asn Val Met Lys
Lys Trp Leu Pro Glu Leu Gln His Tyr Ala Pro Gly100 105
110Val Pro Ile Val Leu Val Gly Thr Lys Leu Asp Leu Arg Glu
Asp Lys115 120 125His Tyr Leu Leu Asp His
Pro Ser Leu Val Pro Val Thr Thr Ala Gln130 135
140Gly Glu Glu Leu Arg Lys His Ile Gly Ala Thr Cys Tyr Ile Glu
Cys145 150 155 160Ser Ser
Lys Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala Ala Ile165
170 175Lys Val Val Ile Lys Pro Pro Thr Lys Gln Arg Asp
Arg Lys Lys Lys180 185 190Lys Thr Arg Arg
Gly Cys Ser Phe Phe Cys Lys Gly Val Met Ser Arg195 200
205Arg Arg Leu Val Cys Phe Lys210
215461127DNAZea maysCDS(190)..(831)coding for RacB homologue (RacA)
46gtcgacccac gcgtccgccc agaagtcacg caccaaacac caccaccaaa gaaggcgaga
60acgtactccg tccctcccct cccctcccct ccccttcccc tcgaggctcc aggaccgtct
120cctcgcctgc tcatccgccg ctgcttccct tctctgggct cggagaaccg gagagaagcg
180cgcgcggcc atg gcg tcc agc gcc tct cgg ttc atc aag tgc gtc acg gtc
231Met Ala Ser Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val1
5 10ggc gac ggt gcc gtg ggc aag aca tgt atg ctc atc tgc
tac acc agc 279Gly Asp Gly Ala Val Gly Lys Thr Cys Met Leu Ile Cys
Tyr Thr Ser15 20 25
30aac aag ttc ccc act gac tac ata cct acg gtg ttc gac aat ttc agt
327Asn Lys Phe Pro Thr Asp Tyr Ile Pro Thr Val Phe Asp Asn Phe Ser35
40 45gca aat gta gtt gtg gat ggc acc act gtg
aat ttg ggc ctt tgg gat 375Ala Asn Val Val Val Asp Gly Thr Thr Val
Asn Leu Gly Leu Trp Asp50 55 60acc gct
ggg cag gaa gat tac aac cgc ctg agg cct cta agc tac cga 423Thr Ala
Gly Gln Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg65
70 75ggt gca gat gtt ttc gtg ctt gca ttc tca ctt gtg
agc cga gct agc 471Gly Ala Asp Val Phe Val Leu Ala Phe Ser Leu Val
Ser Arg Ala Ser80 85 90tat gag aat atc
atg aag aag tgg ata cca gag ctt caa cat tat gca 519Tyr Glu Asn Ile
Met Lys Lys Trp Ile Pro Glu Leu Gln His Tyr Ala95 100
105 110cct ggg gtg ccc gtt gtt ttg gca ggc
aca aaa ttg gat ctt cgt gaa 567Pro Gly Val Pro Val Val Leu Ala Gly
Thr Lys Leu Asp Leu Arg Glu115 120 125gac
aag cac tac ttg atg gac cat cct gga ttg gtg cct gtt acc act 615Asp
Lys His Tyr Leu Met Asp His Pro Gly Leu Val Pro Val Thr Thr130
135 140gca cag ggg gag gaa ctt cgt aga caa att ggt
gct atg tat tac att 663Ala Gln Gly Glu Glu Leu Arg Arg Gln Ile Gly
Ala Met Tyr Tyr Ile145 150 155gaa tgc agc
tca aag aca cag cag aat gtc aaa gct gtg ttc gat gct 711Glu Cys Ser
Ser Lys Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala160
165 170gcc atc aag gta gta atc cag cct cca act aaa ata
aga gaa aag aag 759Ala Ile Lys Val Val Ile Gln Pro Pro Thr Lys Ile
Arg Glu Lys Lys175 180 185
190aag aaa aaa tca cgc aaa gga tgt tct atg atg aac atc ttc ggt gga
807Lys Lys Lys Ser Arg Lys Gly Cys Ser Met Met Asn Ile Phe Gly Gly195
200 205aga aaa atg cta tgc ttc aag tcc
tgaatggttc aagggggtct tacatggact 861Arg Lys Met Leu Cys Phe Lys
Ser210gataccacga gtgtgacccc gagtttgcga agcttgaaat cttgatgtgc tcgttgcgca
921tgtgtatatt tgcacctttg gttattaatg actagaggta ggtaattgaa actagtctgc
981ttaagcgttc tgcactgctg gtgtggttag ctctatgagt taagcagttc gacagaggcc
1041aaaccgacag tgagattttg ttctttcatg gaaatgtgcc aatgtcacag ctttttcgtg
1101aaaaaaaaaa aaaaaaaaaa aaaaaa
112747214PRTZea mays 47Met Ala Ser Ser Ala Ser Arg Phe Ile Lys Cys Val
Thr Val Gly Asp1 5 10
15Gly Ala Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr Ser Asn Lys20
25 30Phe Pro Thr Asp Tyr Ile Pro Thr Val Phe
Asp Asn Phe Ser Ala Asn35 40 45Val Val
Val Asp Gly Thr Thr Val Asn Leu Gly Leu Trp Asp Thr Ala50
55 60Gly Gln Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser
Tyr Arg Gly Ala65 70 75
80Asp Val Phe Val Leu Ala Phe Ser Leu Val Ser Arg Ala Ser Tyr Glu85
90 95Asn Ile Met Lys Lys Trp Ile Pro Glu Leu
Gln His Tyr Ala Pro Gly100 105 110Val Pro
Val Val Leu Ala Gly Thr Lys Leu Asp Leu Arg Glu Asp Lys115
120 125His Tyr Leu Met Asp His Pro Gly Leu Val Pro Val
Thr Thr Ala Gln130 135 140Gly Glu Glu Leu
Arg Arg Gln Ile Gly Ala Met Tyr Tyr Ile Glu Cys145 150
155 160Ser Ser Lys Thr Gln Gln Asn Val Lys
Ala Val Phe Asp Ala Ala Ile165 170 175Lys
Val Val Ile Gln Pro Pro Thr Lys Ile Arg Glu Lys Lys Lys Lys180
185 190Lys Ser Arg Lys Gly Cys Ser Met Met Asn Ile
Phe Gly Gly Arg Lys195 200 205Met Leu Cys
Phe Lys Ser21048901DNAHordeum vulgaremisc_feature(1)..(901)coding for
RacB homologue (RacA) 48cccgggctgc aggaattcgg cacgaggcaa gaagtcacgc
accaaacacc accccccatc 60accgccgctc cgctccccag tcccccaccc ctcctccgcc
cccttcctcg agccgagctc 120cgggggaagg aatcggagag gccggcgcgc ggcgagccat
ggcgtccagc gcctcccggt 180tcatcaagtg cgtcacggtg ggcgacggcg ccgtcggcaa
gacctgcatg ctcatctgct 240acaccagcaa caagttcccc accgactaca tacccacggt
gttcgacaat ttcagcgcga 300acgtggtggc ggacggcacc acggtgaatt tgggcctttg
ggacaccgcc gggcaggagg 360attacaaccg gctgaggcct ctaagctacc gcggcgccga
cgttttcgtg cttgccttct 420cccttgtgag ccgagctagc tatgagaata tcatgaagaa
gtggataccg gagcttcagc 480attacgcgcc cggcgtacct gttgtgctgg taggcacaaa
actggatctt cgtgaagata 540agcactattt gctggaccac cctgggatga tacccgttac
cacagcacag ggggaggaac 600ttcgtaagca agttggtgct ttatattaca tagagtgcag
ctcaaagaca caacagaatg 660tcaaagctgt gtttgatgct gctatcaagg tagtaatcca
gccccccact aaacaaagag 720aaaagaagaa aaagaaacag cgtcggggat gttctatgat
gaacttcagc ggaaggaaat 780gctatgcttc aaatcctgaa tgatgaaaga gaaggttcct
tgcctngaac gattgtcacg 840gttgcgctgc accaatttga caacacctcc aaaccggttg
aatgtgctgg attgcaccgt 900c
90149594DNAArabidopsis thalianaCDS(1)..(591)coding
for RacB homologue 49atg agc gct tcg agg ttc gta aag tgc gtg acg gtt ggt
gat gga gct 48Met Ser Ala Ser Arg Phe Val Lys Cys Val Thr Val Gly
Asp Gly Ala1 5 10 15gtc
gga aaa act tgt ttg ttg att tct tac aca agc aac act ttc cct 96Val
Gly Lys Thr Cys Leu Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20
25 30acg gat tat gtg cct acc gtt ttc gat aat ttc
agt gcc aat gtt gtg 144Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe
Ser Ala Asn Val Val35 40 45gtt aat gga
agc act gtg aat ctt gga ttg tgg gac act gca ggg caa 192Val Asn Gly
Ser Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50 55
60gag gat tac aat aga tta aga cca ctg agt tac cgt gga
gca gat gtt 240Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly
Ala Asp Val65 70 75
80ttc att ttg gcc ttc tct ctt atc agt aaa gcc agt tat gaa aac gtc
288Phe Ile Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85
90 95tcc aaa aag tgg atc ccg gag ttg aaa cat
tac gcg cct ggt gtc ccc 336Ser Lys Lys Trp Ile Pro Glu Leu Lys His
Tyr Ala Pro Gly Val Pro100 105 110atc gtc
ctt gtt gga aca aag ctt gat ctt cga gat gat aaa cag ttc 384Ile Val
Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe115
120 125ttt atc gac cat cct ggt gct gtt ccg att act act
gct cag gga gag 432Phe Ile Asp His Pro Gly Ala Val Pro Ile Thr Thr
Ala Gln Gly Glu130 135 140gag ctg agg aag
caa ata gga gca cct act tac atc gaa tgc agt tcc 480Glu Leu Arg Lys
Gln Ile Gly Ala Pro Thr Tyr Ile Glu Cys Ser Ser145 150
155 160aaa act caa gag aat gtg aag gcg gtg
ttt gac gca gcc atc cga gtg 528Lys Thr Gln Glu Asn Val Lys Ala Val
Phe Asp Ala Ala Ile Arg Val165 170 175gtg
ttg caa ccg cca aag cag aag aag aag aag agc aaa gcg cag aag 576Val
Leu Gln Pro Pro Lys Gln Lys Lys Lys Lys Ser Lys Ala Gln Lys180
185 190gca tgc tcc att cta tga
594Ala Cys Ser Ile Leu195 50197PRTArabidopsis
thaliana 50Met Ser Ala Ser Arg Phe Val Lys Cys Val Thr Val Gly Asp Gly
Ala1 5 10 15Val Gly Lys
Thr Cys Leu Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala
Asn Val Val35 40 45Val Asn Gly Ser Thr
Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50 55
60Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp
Val65 70 75 80Phe Ile
Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85
90 95Ser Lys Lys Trp Ile Pro Glu Leu Lys His Tyr Ala
Pro Gly Val Pro100 105 110Ile Val Leu Val
Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe115 120
125Phe Ile Asp His Pro Gly Ala Val Pro Ile Thr Thr Ala Gln
Gly Glu130 135 140Glu Leu Arg Lys Gln Ile
Gly Ala Pro Thr Tyr Ile Glu Cys Ser Ser145 150
155 160Lys Thr Gln Glu Asn Val Lys Ala Val Phe Asp
Ala Ala Ile Arg Val165 170 175Val Leu Gln
Pro Pro Lys Gln Lys Lys Lys Lys Ser Lys Ala Gln Lys180
185 190Ala Cys Ser Ile Leu19551594DNAArabidopsis
thalianaCDS(1)..(591)coding for RacB homologue 51atg agc gct tcg agg ttc
ata aag tgt gtc acc gtt ggc gac gga gct 48Met Ser Ala Ser Arg Phe
Ile Lys Cys Val Thr Val Gly Asp Gly Ala1 5
10 15gtt ggt aaa acc tgt ttg ctg att tct tac acc agc aac
act ttt cct 96Val Gly Lys Thr Cys Leu Leu Ile Ser Tyr Thr Ser Asn
Thr Phe Pro20 25 30acg gat tat gta ccg
act gtt ttc gat aac ttt agc gca aat gtg gtt 144Thr Asp Tyr Val Pro
Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35 40
45gtt aat gga gcc act gtg aat ctt ggg cta tgg gat acc gca ggg
cag 192Val Asn Gly Ala Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly
Gln50 55 60gag gat tat aac aga tta aga
cct ttg agt tac cgc ggt gct gat gtt 240Glu Asp Tyr Asn Arg Leu Arg
Pro Leu Ser Tyr Arg Gly Ala Asp Val65 70
75 80ttc atc tta gca ttc tct ctt atc agt aag gct agt
tat gag aat gtc 288Phe Ile Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser
Tyr Glu Asn Val85 90 95tcc aag aag tgg
atc cca gag ctg aag cat tat gcc cct ggt gtc cct 336Ser Lys Lys Trp
Ile Pro Glu Leu Lys His Tyr Ala Pro Gly Val Pro100 105
110ata gtt ctt gtt gga acc aaa cta gat ctt cgg gat gac aaa
cag ttc 384Ile Val Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys
Gln Phe115 120 125ttc att gac cac cct ggc
gct gta cca att act act gct cag gga gag 432Phe Ile Asp His Pro Gly
Ala Val Pro Ile Thr Thr Ala Gln Gly Glu130 135
140gaa ctg aag aaa cta att gga gct ccc gca tac atc gag tgc agt tca
480Glu Leu Lys Lys Leu Ile Gly Ala Pro Ala Tyr Ile Glu Cys Ser Ser145
150 155 160aaa aca caa gag
aac gtg aaa gga gta ttt gat gca gcg atc cga gtg 528Lys Thr Gln Glu
Asn Val Lys Gly Val Phe Asp Ala Ala Ile Arg Val165 170
175gtt ctt caa cct cca aag cag aag aaa aag aaa agc aaa gca
caa aaa 576Val Leu Gln Pro Pro Lys Gln Lys Lys Lys Lys Ser Lys Ala
Gln Lys180 185 190gcc tgc tcc att ttg taa
594Ala Cys Ser Ile Leu195
52197PRTArabidopsis thaliana 52Met Ser Ala Ser Arg Phe Ile Lys Cys Val
Thr Val Gly Asp Gly Ala1 5 10
15Val Gly Lys Thr Cys Leu Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20
25 30Thr Asp Tyr Val Pro Thr Val Phe Asp
Asn Phe Ser Ala Asn Val Val35 40 45Val
Asn Gly Ala Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50
55 60Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr
Arg Gly Ala Asp Val65 70 75
80Phe Ile Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85
90 95Ser Lys Lys Trp Ile Pro Glu Leu Lys
His Tyr Ala Pro Gly Val Pro100 105 110Ile
Val Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe115
120 125Phe Ile Asp His Pro Gly Ala Val Pro Ile Thr
Thr Ala Gln Gly Glu130 135 140Glu Leu Lys
Lys Leu Ile Gly Ala Pro Ala Tyr Ile Glu Cys Ser Ser145
150 155 160Lys Thr Gln Glu Asn Val Lys
Gly Val Phe Asp Ala Ala Ile Arg Val165 170
175Val Leu Gln Pro Pro Lys Gln Lys Lys Lys Lys Ser Lys Ala Gln Lys180
185 190Ala Cys Ser Ile
Leu19553594DNAArabidopsis thalianaCDS(1)..(591)coding for RacB homologue
53atg agc gca tca agg ttc ata aag tgc gtc acc gtt ggt gat gga gct
48Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15gtt ggt aaa acc tgt ttg
ctg att tct tat acc agc aac acc ttt ccc 96Val Gly Lys Thr Cys Leu
Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30acg gat tat gtt ccg act gtt ttc gat aac ttt agt gca aat gtg gtt
144Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35
40 45gtt aat gga gcc acg gtg aat ctt gga
ttg tgg gat act gca ggg caa 192Val Asn Gly Ala Thr Val Asn Leu Gly
Leu Trp Asp Thr Ala Gly Gln50 55 60gag
gac tat aac aga tta aga cct ttg agt tac cgt ggt gct gat gtt 240Glu
Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80ttc att ctt gcc ttc tct
ctc att agt aag gct agt tat gag aat gtt 288Phe Ile Leu Ala Phe Ser
Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85 90
95tcc aag aag tgg att cct gag ttg aag cac tat gct cct ggt gtc cca
336Ser Lys Lys Trp Ile Pro Glu Leu Lys His Tyr Ala Pro Gly Val Pro100
105 110att gtc ctt gtt gga acc aaa cta gat
ctt cga gat gac aaa cag ttt 384Ile Val Leu Val Gly Thr Lys Leu Asp
Leu Arg Asp Asp Lys Gln Phe115 120 125ttc
atc gac cat cct ggt gct gtc cct att acc act gtt cag gga gag 432Phe
Ile Asp His Pro Gly Ala Val Pro Ile Thr Thr Val Gln Gly Glu130
135 140gag ctg aag aag cta att gga gcg cca gct tac
atc gag tgc agt tca 480Glu Leu Lys Lys Leu Ile Gly Ala Pro Ala Tyr
Ile Glu Cys Ser Ser145 150 155
160aaa tca caa gag aac gtg aag ggc gtg ttt gat gca gcg atc aga gtg
528Lys Ser Gln Glu Asn Val Lys Gly Val Phe Asp Ala Ala Ile Arg Val165
170 175gtc ctt caa cct cca aag cag aag aaa
aag aag aac aaa gca caa aag 576Val Leu Gln Pro Pro Lys Gln Lys Lys
Lys Lys Asn Lys Ala Gln Lys180 185 190gcc
tgc tcc atc ttg taa 594Ala
Cys Ser Ile Leu19554197PRTArabidopsis thaliana 54Met Ser Ala Ser Arg Phe
Ile Lys Cys Val Thr Val Gly Asp Gly Ala1 5
10 15Val Gly Lys Thr Cys Leu Leu Ile Ser Tyr Thr Ser Asn
Thr Phe Pro20 25 30Thr Asp Tyr Val Pro
Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35 40
45Val Asn Gly Ala Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly
Gln50 55 60Glu Asp Tyr Asn Arg Leu Arg
Pro Leu Ser Tyr Arg Gly Ala Asp Val65 70
75 80Phe Ile Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser
Tyr Glu Asn Val85 90 95Ser Lys Lys Trp
Ile Pro Glu Leu Lys His Tyr Ala Pro Gly Val Pro100 105
110Ile Val Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys
Gln Phe115 120 125Phe Ile Asp His Pro Gly
Ala Val Pro Ile Thr Thr Val Gln Gly Glu130 135
140Glu Leu Lys Lys Leu Ile Gly Ala Pro Ala Tyr Ile Glu Cys Ser
Ser145 150 155 160Lys Ser
Gln Glu Asn Val Lys Gly Val Phe Asp Ala Ala Ile Arg Val165
170 175Val Leu Gln Pro Pro Lys Gln Lys Lys Lys Lys Asn
Lys Ala Gln Lys180 185 190Ala Cys Ser Ile
Leu19555591DNAArabidopsis thalianaCDS(1)..(588)coding for RacB homologue
55atg agt gct tcg agg ttt ata aag tgt gtc acc gtc ggc gat ggt gcc
48Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15gtc gga aaa act tgt atg
ctg att tct tac aca agc aac act ttc cct 96Val Gly Lys Thr Cys Met
Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30acg gac tat gtt cca act gtt ttc gac aac ttc agt gct aat gtg gtt
144Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val35
40 45gta gat ggg aac acg gtg aat ctt gga
ttg tgg gat aca gct ggt caa 192Val Asp Gly Asn Thr Val Asn Leu Gly
Leu Trp Asp Thr Ala Gly Gln50 55 60gaa
gac tat aac agg tta aga ccg ttg agt tac cgt ggt gcc gat gtc 240Glu
Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80ttc att ctt gca ttc tcg
ctt att agc aaa gct agc tac gag aat gta 288Phe Ile Leu Ala Phe Ser
Leu Ile Ser Lys Ala Ser Tyr Glu Asn Val85 90
95gcc aag aag tgg att cct gag ctt agg cat tat gcc cct ggt gtt cct
336Ala Lys Lys Trp Ile Pro Glu Leu Arg His Tyr Ala Pro Gly Val Pro100
105 110ata atc ctc gtt gga acg aaa ctc gat
ctt cga gat gac aag caa ttc 384Ile Ile Leu Val Gly Thr Lys Leu Asp
Leu Arg Asp Asp Lys Gln Phe115 120 125ttc
ata gac cat cct ggt gca gtg cct att act aca aac cag gga gag 432Phe
Ile Asp His Pro Gly Ala Val Pro Ile Thr Thr Asn Gln Gly Glu130
135 140gaa cta aag aaa ctg ata gga tca cca atc tac
att gaa tgt agt tca 480Glu Leu Lys Lys Leu Ile Gly Ser Pro Ile Tyr
Ile Glu Cys Ser Ser145 150 155
160aag act cag cag aat gtg aaa gca gtc ttt gac gca gcc ata aaa gtg
528Lys Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala Ala Ile Lys Val165
170 175gtg ctt cag cca ccg aaa cag aag aag
aag aaa aag aac aag aac cgc 576Val Leu Gln Pro Pro Lys Gln Lys Lys
Lys Lys Lys Asn Lys Asn Arg180 185 190tgc
gtg ttc ttg tga 591Cys
Val Phe Leu19556196PRTArabidopsis thaliana 56Met Ser Ala Ser Arg Phe Ile
Lys Cys Val Thr Val Gly Asp Gly Ala1 5 10
15Val Gly Lys Thr Cys Met Leu Ile Ser Tyr Thr Ser Asn Thr
Phe Pro20 25 30Thr Asp Tyr Val Pro Thr
Val Phe Asp Asn Phe Ser Ala Asn Val Val35 40
45Val Asp Gly Asn Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50
55 60Glu Asp Tyr Asn Arg Leu Arg Pro Leu
Ser Tyr Arg Gly Ala Asp Val65 70 75
80Phe Ile Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu
Asn Val85 90 95Ala Lys Lys Trp Ile Pro
Glu Leu Arg His Tyr Ala Pro Gly Val Pro100 105
110Ile Ile Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln
Phe115 120 125Phe Ile Asp His Pro Gly Ala
Val Pro Ile Thr Thr Asn Gln Gly Glu130 135
140Glu Leu Lys Lys Leu Ile Gly Ser Pro Ile Tyr Ile Glu Cys Ser Ser145
150 155 160Lys Thr Gln Gln
Asn Val Lys Ala Val Phe Asp Ala Ala Ile Lys Val165 170
175Val Leu Gln Pro Pro Lys Gln Lys Lys Lys Lys Lys Asn Lys
Asn Arg180 185 190Cys Val Phe
Leu19557597DNAArabidopsis thalianaCDS(1)..(594)coding for RacB homologue
57atg agt gct tca agg ttt atc aag tgt gtc act gtc ggc gac ggt gct
48Met Ser Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1
5 10 15gtt gga aag act tgt ctt
ctc atc tcc tac act agc aac act ttc ccc 96Val Gly Lys Thr Cys Leu
Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30acg gat tat gtg cca act gtg ttc gat aat ttc agt gcc aat gtg att
144Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Ile35
40 45gtt gat ggc aac act atc aac ttg gga
ttg tgg gat act gca ggg caa 192Val Asp Gly Asn Thr Ile Asn Leu Gly
Leu Trp Asp Thr Ala Gly Gln50 55 60gag
gac tac aat aga cta aga cct ttg agc tat cgc ggt gca gat gtc 240Glu
Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65
70 75 80ttc tta ctt gca ttc tca
ctt gtc agc aaa gct agc tat gaa aat gtt 288Phe Leu Leu Ala Phe Ser
Leu Val Ser Lys Ala Ser Tyr Glu Asn Val85 90
95tct aaa aag tgg gtt cct gaa ctg aga cat tat gct cct ggt gtt ccc
336Ser Lys Lys Trp Val Pro Glu Leu Arg His Tyr Ala Pro Gly Val Pro100
105 110atc atc ctc gtt gga aca aag ctt gat
ctt cga gat gat aag caa ttc 384Ile Ile Leu Val Gly Thr Lys Leu Asp
Leu Arg Asp Asp Lys Gln Phe115 120 125ttt
gcc gag cac cct ggt gct gtg cct atc tct acc gct cag ggt gaa 432Phe
Ala Glu His Pro Gly Ala Val Pro Ile Ser Thr Ala Gln Gly Glu130
135 140gaa cta aag aag ctg att ggg gcg cct gct tat
atc gaa tgc agt gca 480Glu Leu Lys Lys Leu Ile Gly Ala Pro Ala Tyr
Ile Glu Cys Ser Ala145 150 155
160aaa act caa cag aat gtg aaa gca gtg ttt gat gcg gct atc aag gtc
528Lys Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala Ala Ile Lys Val165
170 175gtt ctc cag cca cca aaa aac aag aag
aag aag aag aga aaa tct cag 576Val Leu Gln Pro Pro Lys Asn Lys Lys
Lys Lys Lys Arg Lys Ser Gln180 185 190aaa
ggt tgt tct ata ctc tga 597Lys
Gly Cys Ser Ile Leu19558198PRTArabidopsis thaliana 58Met Ser Ala Ser Arg
Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala1 5
10 15Val Gly Lys Thr Cys Leu Leu Ile Ser Tyr Thr Ser
Asn Thr Phe Pro20 25 30Thr Asp Tyr Val
Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Ile35 40
45Val Asp Gly Asn Thr Ile Asn Leu Gly Leu Trp Asp Thr Ala
Gly Gln50 55 60Glu Asp Tyr Asn Arg Leu
Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val65 70
75 80Phe Leu Leu Ala Phe Ser Leu Val Ser Lys Ala
Ser Tyr Glu Asn Val85 90 95Ser Lys Lys
Trp Val Pro Glu Leu Arg His Tyr Ala Pro Gly Val Pro100
105 110Ile Ile Leu Val Gly Thr Lys Leu Asp Leu Arg Asp
Asp Lys Gln Phe115 120 125Phe Ala Glu His
Pro Gly Ala Val Pro Ile Ser Thr Ala Gln Gly Glu130 135
140Glu Leu Lys Lys Leu Ile Gly Ala Pro Ala Tyr Ile Glu Cys
Ser Ala145 150 155 160Lys
Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala Ala Ile Lys Val165
170 175Val Leu Gln Pro Pro Lys Asn Lys Lys Lys Lys
Lys Arg Lys Ser Gln180 185 190Lys Gly Cys
Ser Ile Leu19559588DNAArabidopsis thalianaCDS(1)..(585)coding for RacB
homologue 59atg gcg tca agg ttt ata aag tgt gtg acc gtc gga gat ggt gcc
gtc 48Met Ala Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala
Val1 5 10 15gga aaa act
tgc atg ctc att tct tac act agc aat act ttt cct act 96Gly Lys Thr
Cys Met Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro Thr20 25
30gat tat gtg cca act gtt ttc gac aac ttc agt gct aat
gtg gtt gtt 144Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn
Val Val Val35 40 45gat ggc aac act gtc
aat ctt gga ttg tgg gat act gct ggt caa gag 192Asp Gly Asn Thr Val
Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln Glu50 55
60gac tac aac agg tta cga cct ttg agt tac cgt ggt gct gat gtt
ttc 240Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val
Phe65 70 75 80att ctt
gct ttc tct ctt att agc aag gct agc tat gag aat ata gcc 288Ile Leu
Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Ile Ala85
90 95aag aag tgg att cct gag ctc agg cat tat gct cct
ggt gtt ccc att 336Lys Lys Trp Ile Pro Glu Leu Arg His Tyr Ala Pro
Gly Val Pro Ile100 105 110atc ctt gtt ggg
aca aaa ctc gat ctt cga gat gac aag caa ttc ttt 384Ile Leu Val Gly
Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe Phe115 120
125ata gat cat cct ggt gct gtg cca att act aca aac cag gga
gag gaa 432Ile Asp His Pro Gly Ala Val Pro Ile Thr Thr Asn Gln Gly
Glu Glu130 135 140ctg aag aaa ctg att gga
tct gct gtc tac att gaa tgt agt tca aag 480Leu Lys Lys Leu Ile Gly
Ser Ala Val Tyr Ile Glu Cys Ser Ser Lys145 150
155 160aca cag cag aac gtg aag gca gtg ttt gat gca
gct ata aaa gtg gtg 528Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala
Ala Ile Lys Val Val165 170 175ctt cag cca
cca aag cag aag aag aag aaa aag aat aag aac cgt tgc 576Leu Gln Pro
Pro Lys Gln Lys Lys Lys Lys Lys Asn Lys Asn Arg Cys180
185 190gcg ttc ttg tga
588Ala Phe Leu19560195PRTArabidopsis thaliana 60Met Ala
Ser Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly Ala Val1 5
10 15Gly Lys Thr Cys Met Leu Ile Ser Tyr
Thr Ser Asn Thr Phe Pro Thr20 25 30Asp
Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala Asn Val Val Val35
40 45Asp Gly Asn Thr Val Asn Leu Gly Leu Trp Asp
Thr Ala Gly Gln Glu50 55 60Asp Tyr Asn
Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp Val Phe65 70
75 80Ile Leu Ala Phe Ser Leu Ile Ser
Lys Ala Ser Tyr Glu Asn Ile Ala85 90
95Lys Lys Trp Ile Pro Glu Leu Arg His Tyr Ala Pro Gly Val Pro Ile100
105 110Ile Leu Val Gly Thr Lys Leu Asp Leu Arg
Asp Asp Lys Gln Phe Phe115 120 125Ile Asp
His Pro Gly Ala Val Pro Ile Thr Thr Asn Gln Gly Glu Glu130
135 140Leu Lys Lys Leu Ile Gly Ser Ala Val Tyr Ile Glu
Cys Ser Ser Lys145 150 155
160Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala Ala Ile Lys Val Val165
170 175Leu Gln Pro Pro Lys Gln Lys Lys Lys
Lys Lys Asn Lys Asn Arg Cys180 185 190Ala
Phe Leu19561606DNAArabidopsis thalianaCDS(1)..(603)coding for RacB
homologue 61atg agc aca gca aga ttc att aag tgt gtg act gtc gga gat gga
gca 48Met Ser Thr Ala Arg Phe Ile Lys Cys Val Thr Val Gly Asp Gly
Ala1 5 10 15gtg gga aag
act tgt atg ctc att tca tat acc agc aat acg ttt cct 96Val Gly Lys
Thr Cys Met Leu Ile Ser Tyr Thr Ser Asn Thr Phe Pro20 25
30acg gat tat gtt cca aca gtt ttc gac aac ttc agc gca
aat gtg gtg 144Thr Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Ser Ala
Asn Val Val35 40 45gtc gac ggg agt acc
gtg aac ctt ggc ctg tgg gat act gcc ggt cag 192Val Asp Gly Ser Thr
Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50 55
60gaa gat tat aat agg ctt agg cct ttg agt tac aga gga gca gat
gtc 240Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala Asp
Val65 70 75 80ttc tta
tta gca ttt tcc ctt ata agc aag gcc agt tac gag aat att 288Phe Leu
Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn Ile85
90 95cac aaa aag tgg ctt ccg gag ctg aaa cat tat gct
cct ggc atc ccc 336His Lys Lys Trp Leu Pro Glu Leu Lys His Tyr Ala
Pro Gly Ile Pro100 105 110att gtg ctc gtc
gga aca aaa tta gat ttg agg gat gac aag cag ttc 384Ile Val Leu Val
Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe115 120
125ttg aag gat cat cca gga gca gct tct ata aca act gct cag
gga gaa 432Leu Lys Asp His Pro Gly Ala Ala Ser Ile Thr Thr Ala Gln
Gly Glu130 135 140gaa tta agg aaa atg att
gga gct gtt agg tac tta gag tgc agc tcc 480Glu Leu Arg Lys Met Ile
Gly Ala Val Arg Tyr Leu Glu Cys Ser Ser145 150
155 160aaa acc caa cag aat gtg aag gca gtg ttt gat
aca gcg ata agg gta 528Lys Thr Gln Gln Asn Val Lys Ala Val Phe Asp
Thr Ala Ile Arg Val165 170 175gct ttg agg
cca cca aag gca aag aaa aag ata aaa cca ttg aag act 576Ala Leu Arg
Pro Pro Lys Ala Lys Lys Lys Ile Lys Pro Leu Lys Thr180
185 190aag aga tca aga ata tgc ttt ttc cta taa
606Lys Arg Ser Arg Ile Cys Phe Phe Leu195
20062201PRTArabidopsis thaliana 62Met Ser Thr Ala Arg Phe Ile Lys Cys
Val Thr Val Gly Asp Gly Ala1 5 10
15Val Gly Lys Thr Cys Met Leu Ile Ser Tyr Thr Ser Asn Thr Phe
Pro20 25 30Thr Asp Tyr Val Pro Thr Val
Phe Asp Asn Phe Ser Ala Asn Val Val35 40
45Val Asp Gly Ser Thr Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50
55 60Glu Asp Tyr Asn Arg Leu Arg Pro Leu Ser
Tyr Arg Gly Ala Asp Val65 70 75
80Phe Leu Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn
Ile85 90 95His Lys Lys Trp Leu Pro Glu
Leu Lys His Tyr Ala Pro Gly Ile Pro100 105
110Ile Val Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gln Phe115
120 125Leu Lys Asp His Pro Gly Ala Ala Ser
Ile Thr Thr Ala Gln Gly Glu130 135 140Glu
Leu Arg Lys Met Ile Gly Ala Val Arg Tyr Leu Glu Cys Ser Ser145
150 155 160Lys Thr Gln Gln Asn Val
Lys Ala Val Phe Asp Thr Ala Ile Arg Val165 170
175Ala Leu Arg Pro Pro Lys Ala Lys Lys Lys Ile Lys Pro Leu Lys
Thr180 185 190Lys Arg Ser Arg Ile Cys Phe
Phe Leu195 20063606DNAArabidopsis
thalianaCDS(1)..(603)coding for RacB homologue 63atg gct tcg agt gct tca
aaa ttc atc aaa tgt gtg act gtt gga gat 48Met Ala Ser Ser Ala Ser
Lys Phe Ile Lys Cys Val Thr Val Gly Asp1 5
10 15ggt gcc gtt gga aaa act tgt atg ctc atc tgc tac act
agc aac aaa 96Gly Ala Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr
Ser Asn Lys20 25 30ttc cct act gac tac
ata cca aca gtt ttt gac aac ttt agt gtt aat 144Phe Pro Thr Asp Tyr
Ile Pro Thr Val Phe Asp Asn Phe Ser Val Asn35 40
45gtt gtg gtt gaa ggc atc act gtg aac tta ggc ctt tgg gac act
gcc 192Val Val Val Glu Gly Ile Thr Val Asn Leu Gly Leu Trp Asp Thr
Ala50 55 60ggg caa gaa gac tat aac aga
cta agg cct tta agt tac aga gga gca 240Gly Gln Glu Asp Tyr Asn Arg
Leu Arg Pro Leu Ser Tyr Arg Gly Ala65 70
75 80gat gtt ttt gtg ttg gct ttc tca ttg atc agc cga
gct agc tat gag 288Asp Val Phe Val Leu Ala Phe Ser Leu Ile Ser Arg
Ala Ser Tyr Glu85 90 95aat gtg ttt aaa
aag tgg atc cct gaa ctc caa cac ttt gca cca gga 336Asn Val Phe Lys
Lys Trp Ile Pro Glu Leu Gln His Phe Ala Pro Gly100 105
110gtc ccc att gtg ctt gtt ggt acc aaa atg gat ctt cgt gaa
gat aga 384Val Pro Ile Val Leu Val Gly Thr Lys Met Asp Leu Arg Glu
Asp Arg115 120 125cat tac ttg tct gat cat
cct gga ctg tcc ccg gta act aca tca cag 432His Tyr Leu Ser Asp His
Pro Gly Leu Ser Pro Val Thr Thr Ser Gln130 135
140gga gag gaa ctc cgc aag cat atc gga gcg act tat tac att gaa tgt
480Gly Glu Glu Leu Arg Lys His Ile Gly Ala Thr Tyr Tyr Ile Glu Cys145
150 155 160agc tca aaa act
caa cag aat gtg aaa gcc gta ttt gat gct gct att 528Ser Ser Lys Thr
Gln Gln Asn Val Lys Ala Val Phe Asp Ala Ala Ile165 170
175aaa gta gta att aaa cca gca gtg aaa caa aag gag aag aag
aag aag 576Lys Val Val Ile Lys Pro Ala Val Lys Gln Lys Glu Lys Lys
Lys Lys180 185 190cag aag cct cgc agc gga
tgt ctc tcg taa 606Gln Lys Pro Arg Ser Gly
Cys Leu Ser195 20064201PRTArabidopsis thaliana 64Met Ala
Ser Ser Ala Ser Lys Phe Ile Lys Cys Val Thr Val Gly Asp1 5
10 15Gly Ala Val Gly Lys Thr Cys Met Leu
Ile Cys Tyr Thr Ser Asn Lys20 25 30Phe
Pro Thr Asp Tyr Ile Pro Thr Val Phe Asp Asn Phe Ser Val Asn35
40 45Val Val Val Glu Gly Ile Thr Val Asn Leu Gly
Leu Trp Asp Thr Ala50 55 60Gly Gln Glu
Asp Tyr Asn Arg Leu Arg Pro Leu Ser Tyr Arg Gly Ala65 70
75 80Asp Val Phe Val Leu Ala Phe Ser
Leu Ile Ser Arg Ala Ser Tyr Glu85 90
95Asn Val Phe Lys Lys Trp Ile Pro Glu Leu Gln His Phe Ala Pro Gly100
105 110Val Pro Ile Val Leu Val Gly Thr Lys Met
Asp Leu Arg Glu Asp Arg115 120 125His Tyr
Leu Ser Asp His Pro Gly Leu Ser Pro Val Thr Thr Ser Gln130
135 140Gly Glu Glu Leu Arg Lys His Ile Gly Ala Thr Tyr
Tyr Ile Glu Cys145 150 155
160Ser Ser Lys Thr Gln Gln Asn Val Lys Ala Val Phe Asp Ala Ala Ile165
170 175Lys Val Val Ile Lys Pro Ala Val Lys
Gln Lys Glu Lys Lys Lys Lys180 185 190Gln
Lys Pro Arg Ser Gly Cys Leu Ser195 20065648DNAArabidopsis
thalianaCDS(1)..(645)coding for RacB homologue 65atg gct tca agt gct tca
aag ttc atc aag tgt gtg act gtt ggt gat 48Met Ala Ser Ser Ala Ser
Lys Phe Ile Lys Cys Val Thr Val Gly Asp1 5
10 15ggt gct gtt ggt aaa acc tgt atg ctc atc tgc tac acc
agc aat aaa 96Gly Ala Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr
Ser Asn Lys20 25 30ttc ccc act gac tac
ata cca aca gtt ttt gac aac ttt agt gca aat 144Phe Pro Thr Asp Tyr
Ile Pro Thr Val Phe Asp Asn Phe Ser Ala Asn35 40
45gtt gtt gtt gaa ggc acc act gtc aat ttg ggg ctt tgg gac act
gct 192Val Val Val Glu Gly Thr Thr Val Asn Leu Gly Leu Trp Asp Thr
Ala50 55 60ggg caa gaa gac tat aac aga
tta agg cct tta agt tac agg gga gca 240Gly Gln Glu Asp Tyr Asn Arg
Leu Arg Pro Leu Ser Tyr Arg Gly Ala65 70
75 80gat gtt ttc gtc ttg tct ttc tca tta gtc agc cga
gct agc tac gag 288Asp Val Phe Val Leu Ser Phe Ser Leu Val Ser Arg
Ala Ser Tyr Glu85 90 95aat gtt ttt aaa
aag tgg atc cct gaa ctc caa cac ttt gct cca gga 336Asn Val Phe Lys
Lys Trp Ile Pro Glu Leu Gln His Phe Ala Pro Gly100 105
110gtt ccc ctt gtc ctt gtt ggt acc aaa tta gat ctt cgt gaa
gat aag 384Val Pro Leu Val Leu Val Gly Thr Lys Leu Asp Leu Arg Glu
Asp Lys115 120 125cat tat ttg gct gat cat
cct gga cta tcc cct gta act act gca cag 432His Tyr Leu Ala Asp His
Pro Gly Leu Ser Pro Val Thr Thr Ala Gln130 135
140gga gag gag ttg cgt aag cta att ggt gcg acg tat tac att gag tgt
480Gly Glu Glu Leu Arg Lys Leu Ile Gly Ala Thr Tyr Tyr Ile Glu Cys145
150 155 160agt tca aaa act
caa cag aat gtg aaa gca gtt ttt gat tct gcg ata 528Ser Ser Lys Thr
Gln Gln Asn Val Lys Ala Val Phe Asp Ser Ala Ile165 170
175aag gaa gtg atc aaa cct ctg gtt aaa caa aag gag aag act
aag aag 576Lys Glu Val Ile Lys Pro Leu Val Lys Gln Lys Glu Lys Thr
Lys Lys180 185 190aag aag aag caa aag tcg
aat cac ggc tgt tta tca aat gtt ctg tgt 624Lys Lys Lys Gln Lys Ser
Asn His Gly Cys Leu Ser Asn Val Leu Cys195 200
205ggg agg ata gtg act cgg cat tga
648Gly Arg Ile Val Thr Arg His210
21566215PRTArabidopsis thaliana 66Met Ala Ser Ser Ala Ser Lys Phe Ile Lys
Cys Val Thr Val Gly Asp1 5 10
15Gly Ala Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr Ser Asn Lys20
25 30Phe Pro Thr Asp Tyr Ile Pro Thr Val
Phe Asp Asn Phe Ser Ala Asn35 40 45Val
Val Val Glu Gly Thr Thr Val Asn Leu Gly Leu Trp Asp Thr Ala50
55 60Gly Gln Glu Asp Tyr Asn Arg Leu Arg Pro Leu
Ser Tyr Arg Gly Ala65 70 75
80Asp Val Phe Val Leu Ser Phe Ser Leu Val Ser Arg Ala Ser Tyr Glu85
90 95Asn Val Phe Lys Lys Trp Ile Pro Glu
Leu Gln His Phe Ala Pro Gly100 105 110Val
Pro Leu Val Leu Val Gly Thr Lys Leu Asp Leu Arg Glu Asp Lys115
120 125His Tyr Leu Ala Asp His Pro Gly Leu Ser Pro
Val Thr Thr Ala Gln130 135 140Gly Glu Glu
Leu Arg Lys Leu Ile Gly Ala Thr Tyr Tyr Ile Glu Cys145
150 155 160Ser Ser Lys Thr Gln Gln Asn
Val Lys Ala Val Phe Asp Ser Ala Ile165 170
175Lys Glu Val Ile Lys Pro Leu Val Lys Gln Lys Glu Lys Thr Lys Lys180
185 190Lys Lys Lys Gln Lys Ser Asn His Gly
Cys Leu Ser Asn Val Leu Cys195 200 205Gly
Arg Ile Val Thr Arg His210 21567630DNAArabidopsis
thalianaCDS(1)..(627)coding for RacB homologue 67atg agt gct tcg aag ttc
ata aaa tgt gtt act gtt gga gat ggg gct 48Met Ser Ala Ser Lys Phe
Ile Lys Cys Val Thr Val Gly Asp Gly Ala1 5
10 15gtt ggg aag aca tgt atg ctt atc tgt tac act agc aac
aag ttt cct 96Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr Ser Asn
Lys Phe Pro20 25 30act gat tat ata ccg
act gtg ttc gac aat ttc agt gcc aat gta gct 144Thr Asp Tyr Ile Pro
Thr Val Phe Asp Asn Phe Ser Ala Asn Val Ala35 40
45gtg gat gga caa atc gtt aat tta ggg cta tgg gac act gcc ggt
caa 192Val Asp Gly Gln Ile Val Asn Leu Gly Leu Trp Asp Thr Ala Gly
Gln50 55 60gaa gat tac agt agg tta aga
cca ttg agt tat aga gga gct gat atc 240Glu Asp Tyr Ser Arg Leu Arg
Pro Leu Ser Tyr Arg Gly Ala Asp Ile65 70
75 80ttc gtc tta gcc ttt tcg ctt att agc aag gcg agt
tac gaa aat gta 288Phe Val Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser
Tyr Glu Asn Val85 90 95ctc aag aag tgg
atg cct gaa ctt cgt cgg ttt gcg cca aat gtt ccc 336Leu Lys Lys Trp
Met Pro Glu Leu Arg Arg Phe Ala Pro Asn Val Pro100 105
110ata gtt ctt gtt ggt aca aag cta gat ctc cgg gat gac aag
gga tac 384Ile Val Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys
Gly Tyr115 120 125ctc gcg gat cac acc aat
gtc att acc tct act cag gga gag gaa ttg 432Leu Ala Asp His Thr Asn
Val Ile Thr Ser Thr Gln Gly Glu Glu Leu130 135
140agg aag caa att ggt gca gct gct tat att gag tgt agt tcc aag act
480Arg Lys Gln Ile Gly Ala Ala Ala Tyr Ile Glu Cys Ser Ser Lys Thr145
150 155 160caa caa aat gtg
aaa gca gtg ttt gat aca gcg atc aag gtg gtt ctt 528Gln Gln Asn Val
Lys Ala Val Phe Asp Thr Ala Ile Lys Val Val Leu165 170
175cag cct cca agg agg aaa gag gtc ccg agg agg agg aag aat
cat aga 576Gln Pro Pro Arg Arg Lys Glu Val Pro Arg Arg Arg Lys Asn
His Arg180 185 190aga tcc ggt tgc tcc att
gcg agt att gtc tgt gga ggt tgc acc gct 624Arg Ser Gly Cys Ser Ile
Ala Ser Ile Val Cys Gly Gly Cys Thr Ala195 200
205gct taa
630Ala68209PRTArabidopsis thaliana 68Met Ser Ala Ser Lys Phe Ile Lys
Cys Val Thr Val Gly Asp Gly Ala1 5 10
15Val Gly Lys Thr Cys Met Leu Ile Cys Tyr Thr Ser Asn Lys Phe
Pro20 25 30Thr Asp Tyr Ile Pro Thr Val
Phe Asp Asn Phe Ser Ala Asn Val Ala35 40
45Val Asp Gly Gln Ile Val Asn Leu Gly Leu Trp Asp Thr Ala Gly Gln50
55 60Glu Asp Tyr Ser Arg Leu Arg Pro Leu Ser
Tyr Arg Gly Ala Asp Ile65 70 75
80Phe Val Leu Ala Phe Ser Leu Ile Ser Lys Ala Ser Tyr Glu Asn
Val85 90 95Leu Lys Lys Trp Met Pro Glu
Leu Arg Arg Phe Ala Pro Asn Val Pro100 105
110Ile Val Leu Val Gly Thr Lys Leu Asp Leu Arg Asp Asp Lys Gly Tyr115
120 125Leu Ala Asp His Thr Asn Val Ile Thr
Ser Thr Gln Gly Glu Glu Leu130 135 140Arg
Lys Gln Ile Gly Ala Ala Ala Tyr Ile Glu Cys Ser Ser Lys Thr145
150 155 160Gln Gln Asn Val Lys Ala
Val Phe Asp Thr Ala Ile Lys Val Val Leu165 170
175Gln Pro Pro Arg Arg Lys Glu Val Pro Arg Arg Arg Lys Asn His
Arg180 185 190Arg Ser Gly Cys Ser Ile Ala
Ser Ile Val Cys Gly Gly Cys Thr Ala195 200
205Ala69600DNAArabidopsis thalianaCDS(1)..(597)coding for RacB homologue
69atg gct gca aca tca aca tca tca gca aca gct aca acg ttt ata aag
48Met Ala Ala Thr Ser Thr Ser Ser Ala Thr Ala Thr Thr Phe Ile Lys1
5 10 15tgt gtc act gtt ggc gat
gga gct ctt ttg gtg act gtt gag atc ttg 96Cys Val Thr Val Gly Asp
Gly Ala Leu Leu Val Thr Val Glu Ile Leu20 25
30tta tta cag gat tat gtt cca aca gtg ttc gac aat ttc aat gct aat
144Leu Leu Gln Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Asn Ala Asn35
40 45gtt tta gtc gat ggt aaa act gtc aat
ctg ggt ctc tgg gat act gct 192Val Leu Val Asp Gly Lys Thr Val Asn
Leu Gly Leu Trp Asp Thr Ala50 55 60ggt
caa gaa gac tac aat agg gtt aga cca ttg agt tac aga gga gca 240Gly
Gln Glu Asp Tyr Asn Arg Val Arg Pro Leu Ser Tyr Arg Gly Ala65
70 75 80gat gtt ttc att ctt gcc
ttc tca ctt att agc agg cct agc ttt gag 288Asp Val Phe Ile Leu Ala
Phe Ser Leu Ile Ser Arg Pro Ser Phe Glu85 90
95aac att gct aaa aag tgg gta ccc gag ctg aga cat tat gcc ccg act
336Asn Ile Ala Lys Lys Trp Val Pro Glu Leu Arg His Tyr Ala Pro Thr100
105 110gtg cct att gtt ctt gtg gga acc aaa
tca gat cta aga gac aac atg 384Val Pro Ile Val Leu Val Gly Thr Lys
Ser Asp Leu Arg Asp Asn Met115 120 125cag
ttc cca aag aat tat cca ggt gct tgc aca atc ttc cca gaa cag 432Gln
Phe Pro Lys Asn Tyr Pro Gly Ala Cys Thr Ile Phe Pro Glu Gln130
135 140ggt caa gaa cta aga aag gaa ata gga gca tta
gca tac ata gag tgc 480Gly Gln Glu Leu Arg Lys Glu Ile Gly Ala Leu
Ala Tyr Ile Glu Cys145 150 155
160agc tca aaa gca caa atg aac gta aaa gcc gtg ttt gat gaa gcg atc
528Ser Ser Lys Ala Gln Met Asn Val Lys Ala Val Phe Asp Glu Ala Ile165
170 175aaa gta gtt tta cat cct cct tca aag
act aag aag cga aag aga aag 576Lys Val Val Leu His Pro Pro Ser Lys
Thr Lys Lys Arg Lys Arg Lys180 185 190atc
ggt tta tgc cat gtt ctt tga 600Ile
Gly Leu Cys His Val Leu19570199PRTArabidopsis thaliana 70Met Ala Ala Thr
Ser Thr Ser Ser Ala Thr Ala Thr Thr Phe Ile Lys1 5
10 15Cys Val Thr Val Gly Asp Gly Ala Leu Leu Val
Thr Val Glu Ile Leu20 25 30Leu Leu Gln
Asp Tyr Val Pro Thr Val Phe Asp Asn Phe Asn Ala Asn35 40
45Val Leu Val Asp Gly Lys Thr Val Asn Leu Gly Leu Trp
Asp Thr Ala50 55 60Gly Gln Glu Asp Tyr
Asn Arg Val Arg Pro Leu Ser Tyr Arg Gly Ala65 70
75 80Asp Val Phe Ile Leu Ala Phe Ser Leu Ile
Ser Arg Pro Ser Phe Glu85 90 95Asn Ile
Ala Lys Lys Trp Val Pro Glu Leu Arg His Tyr Ala Pro Thr100
105 110Val Pro Ile Val Leu Val Gly Thr Lys Ser Asp Leu
Arg Asp Asn Met115 120 125Gln Phe Pro Lys
Asn Tyr Pro Gly Ala Cys Thr Ile Phe Pro Glu Gln130 135
140Gly Gln Glu Leu Arg Lys Glu Ile Gly Ala Leu Ala Tyr Ile
Glu Cys145 150 155 160Ser
Ser Lys Ala Gln Met Asn Val Lys Ala Val Phe Asp Glu Ala Ile165
170 175Lys Val Val Leu His Pro Pro Ser Lys Thr Lys
Lys Arg Lys Arg Lys180 185 190Ile Gly Leu
Cys His Val Leu1957121DNAArtificial sequenceDescription of the artificial
sequence oligonucleotide primer 71atgagcgcgt ccaggttcat a
217221DNAArtificial
sequenceDescription of the artificial sequence oligonucleotide
primer 72atcaaacacg cccttcacgt t
217310828DNAArtificial sequenceDescription of the artificial
sequence transgenic expression vector pSUN3NIT_AtRacB_s for
expression of A-thalianan RacB sense RNA 73ttccatggac atacaaatgg
acgaacggat aaaccttttc acgccctttt aaatatccga 60ttattctaat aaacgctctt
ttctcttagg tttacccgcc aatatatcct gtcaaacact 120gatagtttaa actgaaggcg
ggaaacgaca atcagatcta gtaggaaaca gctatgacca 180tgattacgcc aagcttgcat
gcctgcaggt cgactctaga ggatccccca tcaagatctt 240ggtgatgtag caagagctaa
gttgtacttc gatycggttg gacattactc gagaccagat 300gttttacact tgaccgtaaa
tgagcacccg aagaaaccgg taacattcat ttcgaaggta 360gagaaagcgg aagatgactc
aaacaagtaa tcggttgtga ttcgtcagtt catgtcactc 420ctatgaagga gtcaagttca
aaatgttatg ttgagtttca aacttttatg ctaaactttt 480tttcttaatt ttcgttaata
atggaagaga accaattctc ttgtatctaa agattatcca 540tctatcatcc aatttgagtg
ttcaattctg gatgttgtgt taccctacat tctacaacca 600tgtagccaat tattatgaat
ctggctttga tttcagttgt gttcttttct ttttttcttt 660gcatatttgc atttagaatg
tttaataatt aagttactgt atttccacat acattagttc 720caagaatata catatattaa
tttatttttc ttaaaaatgt tttggaatga ctaatattga 780caacgaaaat agaagctatg
ctaaaccatt acgtatatgt gacttcacat gttgttgttt 840tacattccct atatatatgg
atggctgtca caatcagaaa cgtgatcgaa aaaagacaaa 900cagtgtttgc ataaaaagac
tatttcgttt cattgacaat ttgtgtttat ttgtaaagaa 960aagtggcaaa gtggaatttg
agttcctgca agtaagaaag atgaaataaa agacttgagt 1020gtgtgttttt ttcttttatc
tgaaagctgc aatgaaatat tcctaccaag cccgtttgat 1080tattaattgg ggtttggttt
tcttgatgcg aactaattgg ttatataaga aactatacaa 1140tccatgttaa ttcaaaaatt
ttgatttctc ttgtaggaat atgatttact atatgagact 1200ttcttttcgc caataatagt
aaatccaaag atatttgacc ggaccaaaac acattgatct 1260attttttagt ttatttaatc
cagtttctct gagataattc attaaggaaa acttagtatt 1320aacccatcct aagattaaat
aggagccaaa ctcacatttc aaatattaaa taacataaaa 1380tggatttaaa aaatctatac
gtcaaatttt atttatgaca tttcttattt aaatttatat 1440ttaatgaaat acagctaaga
caaaccaaaa aaaaaatact ttctaagtgg tccaaaacat 1500caattccgtt caatattatt
aggtagaatc gtacgaccaa aaaaggtagg ttaatacgaa 1560attacaaaca tatctatata
catagtatat attattacct attatgagga atcaaaatgc 1620atcaaatatg gatttaagga
atccataaaa gaataaattc tacggaaaaa aaaaaaagaa 1680taaattcttt taagttttta
atttgttttt tatttggtag ttctccattt tgttttattt 1740cgtttggatt tattgtgtcc
aaatactttg taaaccaccg ttgtaattct taaacggggt 1800tttcacttct tttttatatt
cagacataaa gcatcggctg gtttaatcaa tcaatagatt 1860ttatttttct tctcaattat
tagtaggttt gatgtgaact ttacaaaaaa aacaaaaaca 1920aatcaatgca gagaaaagaa
accacgtggg ctagtcccac cttgtttcat ttccaccaca 1980ggttcgatct tcgttaccgt
ctccaatagg aaaataaacg tgaccacaaa aaaaaaacaa 2040aaaaaaagtc tatatattgc
ttctctcaag tctctgagtg tcatgaacca aagtaaaaaa 2100caaagactcg agtggatccc
cggaattcgc ccttatgagc gcatcaaggt tcataaagtg 2160cgtcaccgtt ggtgatggag
ctgttggtaa aacctgtttg ctgatttctt ataccagcaa 2220cacctttccc acggntattc
atcaatcatt ctccctcctt tttttgatat ctgattcatt 2280tgattctgat tgtgccatat
atgaattggg atccatacat actaaaaatg ttgtatacat 2340ttccattgga acaggattat
gttccgactg ttttcgataa ctttagtgca aatgtggttg 2400tcaatggggc cacggtgaat
cttggattgt gggatactgc aggtaaatga atgatgatcc 2460aattcataat ccttggtgag
agagctttcc gtgatgaatc agaggtcgaa attatgtttt 2520tggtttatgc agggcaagag
gactataaca gattaagacc tttgagttac cgtggtgctg 2580atgttttcat tcttgccttc
tctctcatta gtaaggctag ttatgagaat gtttccaaga 2640aggtcagttt cgtccgaact
ggtcgactat ttaacaattg agagttccaa attttgatgc 2700ttcttttctt ttacagtgga
ttcctgagtt gaagcactat gctcctggtg tcccaattgt 2760ccttgttgga accaaactag
gttacttcct cctctcacat ttgtccttgt ttatgcattt 2820atttatatat atgtctgatt
cccatgctta cactgccatt ttccttttca ctttattaag 2880ctgctctggt ataatatata
tcatgacatt agtggacata aaacttcacc ttctcttgat 2940tgtggttaaa acttgtacat
gttcaagatg tattcgttag gtgaaactga gggtagtttt 3000cagagaatat cattggtcaa
caggcttctt cttgtatctt gcacttcttg tgataaagca 3060accgtatcct ataacacacg
cctttaagca tcctccaatg aaatagctac tgtatagcaa 3120gtgtatacct ttataaaaga
cacttgcaag atcttcagtc aactcatgat cctggccttt 3180ttgattgtct aaccttggtt
gttgtcagat cttcgagatg acaaacagtt tttcatcgac 3240catcctggtg ctgtccctat
taccactgtt caggtaagaa tacagttatt tcctcagtgc 3300aattttatca gctttaccac
cgttaagcat tttccctctc tgcatggaag ggagaggagc 3360tgaagaagct aattggagcg
ccagcttaca tcgagtgcag ttcaaaatca caagaggtaa 3420acgaataaaa gacatctcat
gaatcatctt ttcggtgtta gattcttctt tttttgatga 3480aaacaatgtg actataactg
cagaacgtga agggcgtgtt tgataagggc gaattaattc 3540actggccgtc gttttacaac
gactcagagc ttgacaggag gcccgatcta gtaacataga 3600tgacaccgcg cgcgataatt
tatcctagtt tgcgcgctat attttgtttt ctatcgcgta 3660ttaaatgtat aattgcggga
ctctaatcat aaaaacccat ctcataaata acgtcatgca 3720ttacatgtta attattacat
gcttaacgta attcaacaga aattatatga taatcatcgc 3780aagaccggca acaggattca
atcttaagaa actttattgc caaatgtttg aacgatcggg 3840gatcatccgg gtctgtggcg
ggaactccac gaaaatatcc gaacgcagca agatctagag 3900cttgggtccc gctcagaaga
actcgtcaag aaggcgatag aaggcgatgc gctgcgaatc 3960gggagcggcg ataccgtaaa
gcacgaggaa gcggtcagcc cattcgccgc caagctcttc 4020agcaatatca cgggtagcca
acgctatgtc ctgatagcgg tccgccacac ccagccggcc 4080acagtcgatg aatccagaaa
agcggccatt ttccaccatg atattcggca agcaggcatc 4140gccatgggtc acgacgagat
cctcgccgtc gggcatgcgc gccttgagcc tggcgaacag 4200ttcggctggc gcgagcccct
gatgctcttc gtccagatca tcctgatcga caagaccggc 4260ttccatccga gtacgtgctc
gctcgatgcg atgtttcgct tggtggtcga atgggcaggt 4320agccggatca agcgtatgca
gccgccgcat tgcatcagcc atgatggata ctttctcggc 4380aggagcaagg tgagatgaca
ggagatcctg ccccggcact tcgcccaata gcagccagtc 4440ccttcccgct tcagtgacaa
cgtcgagcac agctgcgcaa ggaacgcccg tcgtggccag 4500ccacgatagc cgcgctgcct
cgtcctgcag ttcattcagg gcaccggaca ggtcggtctt 4560gacaaaaaga accgggcgcc
cctgcgctga cagccggaac acggcggcat cagagcagcc 4620gattgtctgt tgtgcccagt
catagccgaa tagcctctcc acccaagcgg ccggagaacc 4680tgcgtgcaat ccatcttgtt
caatcatgcg aaacgatcca gatccggtgc agattatttg 4740gattgagagt gaatatgaga
ctctaattgg ataccgaggg gaatttatgg aacgtcagtg 4800gagcattttt gacaagaaat
atttgctagc tgatagtgac cttaggcgac ttttgaacgc 4860gcaataatgg tttctgacgt
atgtgcttag ctcattaaac tccagaaacc cgcggctgag 4920tggctccttc aacgttgcgg
ttctgtcagt tccaaacgta aaacggcttg tcccgcgtca 4980tcggcggggg tcataacgtg
actcccttaa ttctccgctc atgatcagat tgtcgtttcc 5040cgccttcagt ttaaactatc
agtgtttgac aggatcctgc ttggtaataa ttgtcattag 5100attgttttta tgcatagatg
cactcgaaat cagccaattt tagacaagta tcaaacggat 5160gttaattcag tacattaaag
acgtccgcaa tgtgttatta agttgtctaa gcgtcaattt 5220gtttacacca caatatatcc
tgccaccagc cagccaacag ctccccgacc ggcagctcgg 5280cacaaaatca ccacgcgtta
ccaccacgcc ggccggccgc atggtgttga ccgtgttcgc 5340cggcattgcc gagttcgagc
gttccctaat catcgaccgc acccggagcg ggcgcgaggc 5400cgccaaggcc cgaggcgtga
agtttggccc ccgccctacc ctcaccccgg cacagatcgc 5460gcacgcccgc gagctgatcg
accaggaagg ccgcaccgtg aaagaggcgg ctgcactgct 5520tggcgtgcat cgctcgaccc
tgtaccgcgc acttgagcgc agcgaggaag tgacgcccac 5580cgaggccagg cggcgcggtg
ccttccgtga ggacgcattg accgaggccg acgccctggc 5640ggccgccgag aatgaacgcc
aagaggaaca agcatgaaac cgcaccagga cggccaggac 5700gaaccgtttt tcattaccga
agagatcgag gcggagatga tcgcggccgg gtacgtgttc 5760gagccgcccg cgcacgtctc
aaccgtgcgg ctgcatgaaa tcctggccgg tttgtctgat 5820gccaagctgg cggcctggcc
ggccagcttg gccgctgaag aaaccgagcg ccgccgtcta 5880aaaaggtgat gtgtatttga
gtaaaacagc ttgcgtcatg cggtcgctgc gtatatgatg 5940cgatgagtaa ataaacaaat
acgcaagggg aacgcatgaa ggttatcgct gtacttaacc 6000agaaaggcgg gtcaggcaag
acgaccatcg caacccatct agcccgcgcc ctgcaactcg 6060ccggggccga tgttctgtta
gtcgattccg atccccaggg cagtgcccgc gattgggcgg 6120ccgtgcggga agatcaaccg
ctaaccgttg tcggcatcga ccgcccgacg attgaccgcg 6180acgtgaaggc catcggccgg
cgcgacttcg tagtgatcga cggagcgccc caggcggcgg 6240acttggctgt gtccgcgatc
aaggcagccg acttcgtgct gattccggtg cagccaagcc 6300cttacgacat atgggccacc
gccgacctgg tggagctggt taagcagcgc attgaggtca 6360cggatggaag gctacaagcg
gcctttgtcg tgtcgcgggc gatcaaaggc acgcgcatcg 6420gcggtgaggt tgccgaggcg
ctggccgggt acgagctgcc cattcttgag tcccgtatca 6480cgcagcgcgt gagctaccca
ggcactgccg ccgccggcac aaccgttctt gaatcagaac 6540ccgagggcga cgctgcccgc
gaggtccagg cgctggccgc tgaaattaaa tcaaaactca 6600tttgagttaa tgaggtaaag
agaaaatgag caaaagcaca aacacgctaa gtgccggccg 6660tccgagcgca cgcagcagca
aggctgcaac gttggccagc ctggcagaca cgccagccat 6720gaagcgggtc aactttcagt
tgccggcgga ggatcacacc aagctgaaga tgtacgcggt 6780acgccaaggc aagaccatta
ccgagctgct atctgaatac atcgcgcagc taccagagta 6840aatgagcaaa tgaataaatg
agtagatgaa ttttagcggc taaaggaggc ggcatggaaa 6900atcaagaaca accaggcacc
gacgccgtgg aatgccccat gtgtggagga acgggcggtt 6960ggccaggcgt aagcggctgg
gttgtctgcc ggccctgcaa tggcactgga acccccaagc 7020ccgaggaatc ggcgtgagcg
gtcgcaaacc atccggcccg gtacaaatcg gcgcggcgct 7080gggtgatgac ctggtggaga
agttgaaggc cgcgcaggcc gcccagcggc aacgcatcga 7140ggcagaagca cgccccggtg
aatcgtggca agcggccgct gatcgaatcc gcaaagaatc 7200ccggcaaccg ccggcagccg
gtgcgccgtc gattaggaag ccgcccaagg gcgacgagca 7260accagatttt ttcgttccga
tgctctatga cgtgggcacc cgcgatagtc gcagcatcat 7320ggacgtggcc gttttccgtc
tgtcgaagcg tgaccgacga gctggcgagg tgatccgcta 7380cgagcttcca gacgggcacg
tagaggtttc cgcagggccg gccggcatgg ccagtgtgtg 7440ggattacgac ctggtactga
tggcggtttc ccatctaacc gaatccatga accgataccg 7500ggaagggaag ggagacaagc
ccggccgcgt gttccgtcca cacgttgcgg acgtactcaa 7560gttctgccgg cgagccgatg
gcggaaagca gaaagacgac ctggtagaaa cctgcattcg 7620gttaaacacc acgcacgttg
ccatgcagcg tacgaagaag gccaagaacg gccgcctggt 7680gacggtatcc gagggtgaag
ccttgattag ccgctacaag atcgtaaaga gcgaaaccgg 7740gcggccggag tacatcgaga
tcgagctagc tgattggatg taccgcgaga tcacagaagg 7800caagaacccg gacgtgctga
cggttcaccc cgattacttt ttgatcgatc ccggcatcgg 7860ccgttttctc taccgcctgg
cacgccgcgc cgcaggcaag gcagaagcca gatggttgtt 7920caagacgatc tacgaacgca
gtggcagcgc cggagagttc aagaagttct gtttcaccgt 7980gcgcaagctg atcgggtcaa
atgacctgcc ggagtacgat ttgaaggagg aggcggggca 8040ggctggcccg atcctagtca
tgcgctaccg caacctgatc gagggcgaag catccgccgg 8100ttcctaatgt acggagcaga
tgctagggca aattgcccta gcaggggaaa aaggtcgaaa 8160aggtctcttt cctgtggata
gcacgtacat tgggaaccca aagccgtaca ttgggaaccg 8220gaacccgtac attgggaacc
caaagccgta cattgggaac cggtcacaca tgtaagtgac 8280tgatataaaa gagaaaaaag
gcgatttttc cgcctaaaac tctttaaaac ttattaaaac 8340tcttaaaacc cgcctggcct
gtgcataact gtctggccag cgcacagccg aagagctgca 8400aaaagcgcct acccttcggt
cgctgcgctc cctacgcccc gccgcttcgc gtcggcctat 8460cgcggccgct ggccgctcaa
aaatggctgg cctacggcca ggcaatctac cagggcgcgg 8520acaagccgcg ccgtcgccac
tcgaccgccg gcgcccacat caaggcaccc tgcctcgcgc 8580gtttcggtga tgacggtgaa
aacctctgac acatgcagct cccggagacg gtcacagctt 8640gtctgtaagc ggatgccggg
agcagacaag cccgtcaggg cgcgtcagcg ggtgttggcg 8700ggtgtcgggg cgcagccatg
acccagtcac gtagcgatag cggagtgtat actggcttaa 8760ctatgcggca tcagagcaga
ttgtactgag agtgcaccat atgcggtgtg aaataccgca 8820cagatgcgta aggagaaaat
accgcatcag gcgctcttcc gcttcctcgc tcactgactc 8880gctgcgctcg gtcgttcggc
tgcggcgagc ggtatcagct cactcaaagg cggtaatacg 8940gttatccaca gaatcagggg
ataacgcagg aaagaacatg tgagcaaaag gccagcaaaa 9000ggccaggaac cgtaaaaagg
ccgcgttgct ggcgtttttc cataggctcc gcccccctga 9060cgagcatcac aaaaatcgac
gctcaagtca gaggtggcga aacccgacag gactataaag 9120ataccaggcg tttccccctg
gaagctccct cgtgcgctct cctgttccga ccctgccgct 9180taccggatac ctgtccgcct
ttctcccttc gggaagcgtg gcgctttctc atagctcacg 9240ctgtaggtat ctcagttcgg
tgtaggtcgt tcgctccaag ctgggctgtg tgcacgaacc 9300ccccgttcag cccgaccgct
gcgccttatc cggtaactat cgtcttgagt ccaacccggt 9360aagacacgac ttatcgccac
tggcagcagc cactggtaac aggattagca gagcgaggta 9420tgtaggcggt gctacagagt
tcttgaagtg gtggcctaac tacggctaca ctagaaggac 9480agtatttggt atctgcgctc
tgctgaagcc agttaccttc ggaaaaagag ttggtagctc 9540ttgatccggc aaacaaacca
ccgctggtag cggtggtttt tttgtttgca agcagcagat 9600tacgcgcaga aaaaaaggat
ctcaagaaga tcctttgatc ttttctacgg ggtctgacgc 9660tcagtggaac gaaaactcac
gttaagggat tttggtcatg catgatatat ctcccaattt 9720gtgtagggct tattatgcac
gcttaaaaat aataaaagca gacttgacct gatagtttgg 9780ctgtgagcaa ttatgtgctt
agtgcatcta acgcttgagt taagccgcgc cgcgaagcgg 9840cgtcggcttg aacgaatttc
tagctagaca ttatttgccg actaccttgg tgatctcgcc 9900tttcacgtag tggacaaatt
cttccaactg atctgcgcgc gaggccaagc gatcttcttc 9960ttgtccaaga taagcctgtc
tagcttcaag tatgacgggc tgatactggg ccggcaggcg 10020ctccattgcc cagtcggcag
cgacatcctt cggcgcgatt ttgccggtta ctgcgctgta 10080ccaaatgcgg gacaacgtaa
gcactacatt tcgctcatcg ccagcccagt cgggcggcga 10140gttccatagc gttaaggttt
catttagcgc ctcaaataga tcctgttcag gaaccggatc 10200aaagagttcc tccgccgctg
gacctaccaa ggcaacgcta tgttctcttg cttttgtcag 10260caagatagcc agatcaatgt
cgatcgtggc tggctcgaag atacctgcaa gaatgtcatt 10320gcgctgccat tctccaaatt
gcagttcgcg cttagctgga taacgccacg gaatgatgtc 10380gtcgtgcaca acaatggtga
cttctacagc gcggagaatc tcgctctctc caggggaagc 10440cgaagtttcc aaaaggtcgt
tgatcaaagc tcgccgcgtt gtttcatcaa gccttacggt 10500caccgtaacc agcaaatcaa
tatcactgtg tggcttcagg ccgccatcca ctgcggagcc 10560gtacaaatgt acggccagca
acgtcggttc gagatggcgc tcgatgacgc caactacctc 10620tgatagttga gtcgatactt
cggcgatcac cgcttccccc atgatgttta actttgtttt 10680agggcgactg ccctgctgcg
taacatcgtt gctgctccat aacatcaaac atcgacccac 10740ggcgtaacgc gcttgctgct
tggatgcccg aggcatagac tgtaccccaa aaaaacagtc 10800ataacaagcc atgaaaaccg
ccactgcg 108287410828DNAArtificial
sequenceDescription of the artificial sequence transgenic
expression vector pSUN3NIT_AtRacB_as for expression of A-thalianan
RacB antisense RNA 74ttccatggac atacaaatgg acgaacggat aaaccttttc
acgccctttt aaatatccga 60ttattctaat aaacgctctt ttctcttagg tttacccgcc
aatatatcct gtcaaacact 120gatagtttaa actgaaggcg ggaaacgaca atcagatcta
gtaggaaaca gctatgacca 180tgattacgcc aagcttgcat gcctgcaggt cgactctaga
ggatccccca tcaagatctt 240ggtgatgtag caagagctaa gttgtacttc gatycggttg
gacattactc gagaccagat 300gttttacact tgaccgtaaa tgagcacccg aagaaaccgg
taacattcat ttcgaaggta 360gagaaagcgg aagatgactc aaacaagtaa tcggttgtga
ttcgtcagtt catgtcactc 420ctatgaagga gtcaagttca aaatgttatg ttgagtttca
aacttttatg ctaaactttt 480tttcttaatt ttcgttaata atggaagaga accaattctc
ttgtatctaa agattatcca 540tctatcatcc aatttgagtg ttcaattctg gatgttgtgt
taccctacat tctacaacca 600tgtagccaat tattatgaat ctggctttga tttcagttgt
gttcttttct ttttttcttt 660gcatatttgc atttagaatg tttaataatt aagttactgt
atttccacat acattagttc 720caagaatata catatattaa tttatttttc ttaaaaatgt
tttggaatga ctaatattga 780caacgaaaat agaagctatg ctaaaccatt acgtatatgt
gacttcacat gttgttgttt 840tacattccct atatatatgg atggctgtca caatcagaaa
cgtgatcgaa aaaagacaaa 900cagtgtttgc ataaaaagac tatttcgttt cattgacaat
ttgtgtttat ttgtaaagaa 960aagtggcaaa gtggaatttg agttcctgca agtaagaaag
atgaaataaa agacttgagt 1020gtgtgttttt ttcttttatc tgaaagctgc aatgaaatat
tcctaccaag cccgtttgat 1080tattaattgg ggtttggttt tcttgatgcg aactaattgg
ttatataaga aactatacaa 1140tccatgttaa ttcaaaaatt ttgatttctc ttgtaggaat
atgatttact atatgagact 1200ttcttttcgc caataatagt aaatccaaag atatttgacc
ggaccaaaac acattgatct 1260attttttagt ttatttaatc cagtttctct gagataattc
attaaggaaa acttagtatt 1320aacccatcct aagattaaat aggagccaaa ctcacatttc
aaatattaaa taacataaaa 1380tggatttaaa aaatctatac gtcaaatttt atttatgaca
tttcttattt aaatttatat 1440ttaatgaaat acagctaaga caaaccaaaa aaaaaatact
ttctaagtgg tccaaaacat 1500caattccgtt caatattatt aggtagaatc gtacgaccaa
aaaaggtagg ttaatacgaa 1560attacaaaca tatctatata catagtatat attattacct
attatgagga atcaaaatgc 1620atcaaatatg gatttaagga atccataaaa gaataaattc
tacggaaaaa aaaaaaagaa 1680taaattcttt taagttttta atttgttttt tatttggtag
ttctccattt tgttttattt 1740cgtttggatt tattgtgtcc aaatactttg taaaccaccg
ttgtaattct taaacggggt 1800tttcacttct tttttatatt cagacataaa gcatcggctg
gtttaatcaa tcaatagatt 1860ttatttttct tctcaattat tagtaggttt gatgtgaact
ttacaaaaaa aacaaaaaca 1920aatcaatgca gagaaaagaa accacgtggg ctagtcccac
cttgtttcat ttccaccaca 1980ggttcgatct tcgttaccgt ctccaatagg aaaataaacg
tgaccacaaa aaaaaaacaa 2040aaaaaaagtc tatatattgc ttctctcaag tctctgagtg
tcatgaacca aagtaaaaaa 2100caaagactcg agtggatccc cggaattcgc ccttatcaaa
cacgcccttc acgttctgca 2160gttatagtca cattgttttc atcaaaaaaa gaagaatcta
acaccgaaaa gatgattcat 2220gagatgtctt ttattcgttt acctcttgtg attttgaact
gcactcgatg taagctggcg 2280ctccaattag cttcttcagc tcctctccct tccatgcaga
gagggaaaat gcttaacggt 2340ggtaaagctg ataaaattgc actgaggaaa taactgtatt
cttacctgaa cagtggtaat 2400agggacagca ccaggatggt cgatgaaaaa ctgtttgtca
tctcgaagat ctgacaacaa 2460ccaaggttag acaatcaaaa aggccaggat catgagttga
ctgaagatct tgcaagtgtc 2520ttttataaag gtatacactt gctatacagt agctatttca
ttggaggatg cttaaaggcg 2580tgtgttatag gatacggttg ctttatcaca agaagtgcaa
gatacaagaa gaagcctgtt 2640gaccaatgat attctctgaa aactaccctc agtttcacct
aacgaataca tcttgaacat 2700gtacaagttt taaccacaat caagagaagg tgaagtttta
tgtccactaa tgtcatgata 2760tatattatac cagagcagct taataaagtg aaaaggaaaa
tggcagtgta agcatgggaa 2820tcagacatat atataaataa atgcataaac aaggacaaat
gtgagaggag gaagtaacct 2880agtttggttc caacaaggac aattgggaca ccaggagcat
agtgcttcaa ctcaggaatc 2940cactgtaaaa gaaaagaagc atcaaaattt ggaactctca
attgttaaat agtcgaccag 3000ttcggacgaa actgaccttc ttggaaacat tctcataact
agccttacta atgagagaga 3060aggcaagaat gaaaacatca gcaccacggt aactcaaagg
tcttaatctg ttatagtcct 3120cttgccctgc ataaaccaaa aacataattt cgacctctga
ttcatcacgg aaagctctct 3180caccaaggat tatgaattgg atcatcattc atttacctgc
agtatcccac aatccaagat 3240tcaccgtggc cccattgaca accacatttg cactaaagtt
atcgaaaaca gtcggaacat 3300aatcctgttc caatggaaat gtatacaaca tttttagtat
gtatggatcc caattcatat 3360atggcacaat cagaatcaaa tgaatcagat atcaaaaaaa
ggagggagaa tgattgatga 3420atanccgtgg gaaaggtgtt gctggtataa gaaatcagca
aacaggtttt accaacagct 3480ccatcaccaa cggtgacgca ctttatgaac cttgatgcgc
tcataagggc gaattaattc 3540actggccgtc gttttacaac gactcagagc ttgacaggag
gcccgatcta gtaacataga 3600tgacaccgcg cgcgataatt tatcctagtt tgcgcgctat
attttgtttt ctatcgcgta 3660ttaaatgtat aattgcggga ctctaatcat aaaaacccat
ctcataaata acgtcatgca 3720ttacatgtta attattacat gcttaacgta attcaacaga
aattatatga taatcatcgc 3780aagaccggca acaggattca atcttaagaa actttattgc
caaatgtttg aacgatcggg 3840gatcatccgg gtctgtggcg ggaactccac gaaaatatcc
gaacgcagca agatctagag 3900cttgggtccc gctcagaaga actcgtcaag aaggcgatag
aaggcgatgc gctgcgaatc 3960gggagcggcg ataccgtaaa gcacgaggaa gcggtcagcc
cattcgccgc caagctcttc 4020agcaatatca cgggtagcca acgctatgtc ctgatagcgg
tccgccacac ccagccggcc 4080acagtcgatg aatccagaaa agcggccatt ttccaccatg
atattcggca agcaggcatc 4140gccatgggtc acgacgagat cctcgccgtc gggcatgcgc
gccttgagcc tggcgaacag 4200ttcggctggc gcgagcccct gatgctcttc gtccagatca
tcctgatcga caagaccggc 4260ttccatccga gtacgtgctc gctcgatgcg atgtttcgct
tggtggtcga atgggcaggt 4320agccggatca agcgtatgca gccgccgcat tgcatcagcc
atgatggata ctttctcggc 4380aggagcaagg tgagatgaca ggagatcctg ccccggcact
tcgcccaata gcagccagtc 4440ccttcccgct tcagtgacaa cgtcgagcac agctgcgcaa
ggaacgcccg tcgtggccag 4500ccacgatagc cgcgctgcct cgtcctgcag ttcattcagg
gcaccggaca ggtcggtctt 4560gacaaaaaga accgggcgcc cctgcgctga cagccggaac
acggcggcat cagagcagcc 4620gattgtctgt tgtgcccagt catagccgaa tagcctctcc
acccaagcgg ccggagaacc 4680tgcgtgcaat ccatcttgtt caatcatgcg aaacgatcca
gatccggtgc agattatttg 4740gattgagagt gaatatgaga ctctaattgg ataccgaggg
gaatttatgg aacgtcagtg 4800gagcattttt gacaagaaat atttgctagc tgatagtgac
cttaggcgac ttttgaacgc 4860gcaataatgg tttctgacgt atgtgcttag ctcattaaac
tccagaaacc cgcggctgag 4920tggctccttc aacgttgcgg ttctgtcagt tccaaacgta
aaacggcttg tcccgcgtca 4980tcggcggggg tcataacgtg actcccttaa ttctccgctc
atgatcagat tgtcgtttcc 5040cgccttcagt ttaaactatc agtgtttgac aggatcctgc
ttggtaataa ttgtcattag 5100attgttttta tgcatagatg cactcgaaat cagccaattt
tagacaagta tcaaacggat 5160gttaattcag tacattaaag acgtccgcaa tgtgttatta
agttgtctaa gcgtcaattt 5220gtttacacca caatatatcc tgccaccagc cagccaacag
ctccccgacc ggcagctcgg 5280cacaaaatca ccacgcgtta ccaccacgcc ggccggccgc
atggtgttga ccgtgttcgc 5340cggcattgcc gagttcgagc gttccctaat catcgaccgc
acccggagcg ggcgcgaggc 5400cgccaaggcc cgaggcgtga agtttggccc ccgccctacc
ctcaccccgg cacagatcgc 5460gcacgcccgc gagctgatcg accaggaagg ccgcaccgtg
aaagaggcgg ctgcactgct 5520tggcgtgcat cgctcgaccc tgtaccgcgc acttgagcgc
agcgaggaag tgacgcccac 5580cgaggccagg cggcgcggtg ccttccgtga ggacgcattg
accgaggccg acgccctggc 5640ggccgccgag aatgaacgcc aagaggaaca agcatgaaac
cgcaccagga cggccaggac 5700gaaccgtttt tcattaccga agagatcgag gcggagatga
tcgcggccgg gtacgtgttc 5760gagccgcccg cgcacgtctc aaccgtgcgg ctgcatgaaa
tcctggccgg tttgtctgat 5820gccaagctgg cggcctggcc ggccagcttg gccgctgaag
aaaccgagcg ccgccgtcta 5880aaaaggtgat gtgtatttga gtaaaacagc ttgcgtcatg
cggtcgctgc gtatatgatg 5940cgatgagtaa ataaacaaat acgcaagggg aacgcatgaa
ggttatcgct gtacttaacc 6000agaaaggcgg gtcaggcaag acgaccatcg caacccatct
agcccgcgcc ctgcaactcg 6060ccggggccga tgttctgtta gtcgattccg atccccaggg
cagtgcccgc gattgggcgg 6120ccgtgcggga agatcaaccg ctaaccgttg tcggcatcga
ccgcccgacg attgaccgcg 6180acgtgaaggc catcggccgg cgcgacttcg tagtgatcga
cggagcgccc caggcggcgg 6240acttggctgt gtccgcgatc aaggcagccg acttcgtgct
gattccggtg cagccaagcc 6300cttacgacat atgggccacc gccgacctgg tggagctggt
taagcagcgc attgaggtca 6360cggatggaag gctacaagcg gcctttgtcg tgtcgcgggc
gatcaaaggc acgcgcatcg 6420gcggtgaggt tgccgaggcg ctggccgggt acgagctgcc
cattcttgag tcccgtatca 6480cgcagcgcgt gagctaccca ggcactgccg ccgccggcac
aaccgttctt gaatcagaac 6540ccgagggcga cgctgcccgc gaggtccagg cgctggccgc
tgaaattaaa tcaaaactca 6600tttgagttaa tgaggtaaag agaaaatgag caaaagcaca
aacacgctaa gtgccggccg 6660tccgagcgca cgcagcagca aggctgcaac gttggccagc
ctggcagaca cgccagccat 6720gaagcgggtc aactttcagt tgccggcgga ggatcacacc
aagctgaaga tgtacgcggt 6780acgccaaggc aagaccatta ccgagctgct atctgaatac
atcgcgcagc taccagagta 6840aatgagcaaa tgaataaatg agtagatgaa ttttagcggc
taaaggaggc ggcatggaaa 6900atcaagaaca accaggcacc gacgccgtgg aatgccccat
gtgtggagga acgggcggtt 6960ggccaggcgt aagcggctgg gttgtctgcc ggccctgcaa
tggcactgga acccccaagc 7020ccgaggaatc ggcgtgagcg gtcgcaaacc atccggcccg
gtacaaatcg gcgcggcgct 7080gggtgatgac ctggtggaga agttgaaggc cgcgcaggcc
gcccagcggc aacgcatcga 7140ggcagaagca cgccccggtg aatcgtggca agcggccgct
gatcgaatcc gcaaagaatc 7200ccggcaaccg ccggcagccg gtgcgccgtc gattaggaag
ccgcccaagg gcgacgagca 7260accagatttt ttcgttccga tgctctatga cgtgggcacc
cgcgatagtc gcagcatcat 7320ggacgtggcc gttttccgtc tgtcgaagcg tgaccgacga
gctggcgagg tgatccgcta 7380cgagcttcca gacgggcacg tagaggtttc cgcagggccg
gccggcatgg ccagtgtgtg 7440ggattacgac ctggtactga tggcggtttc ccatctaacc
gaatccatga accgataccg 7500ggaagggaag ggagacaagc ccggccgcgt gttccgtcca
cacgttgcgg acgtactcaa 7560gttctgccgg cgagccgatg gcggaaagca gaaagacgac
ctggtagaaa cctgcattcg 7620gttaaacacc acgcacgttg ccatgcagcg tacgaagaag
gccaagaacg gccgcctggt 7680gacggtatcc gagggtgaag ccttgattag ccgctacaag
atcgtaaaga gcgaaaccgg 7740gcggccggag tacatcgaga tcgagctagc tgattggatg
taccgcgaga tcacagaagg 7800caagaacccg gacgtgctga cggttcaccc cgattacttt
ttgatcgatc ccggcatcgg 7860ccgttttctc taccgcctgg cacgccgcgc cgcaggcaag
gcagaagcca gatggttgtt 7920caagacgatc tacgaacgca gtggcagcgc cggagagttc
aagaagttct gtttcaccgt 7980gcgcaagctg atcgggtcaa atgacctgcc ggagtacgat
ttgaaggagg aggcggggca 8040ggctggcccg atcctagtca tgcgctaccg caacctgatc
gagggcgaag catccgccgg 8100ttcctaatgt acggagcaga tgctagggca aattgcccta
gcaggggaaa aaggtcgaaa 8160aggtctcttt cctgtggata gcacgtacat tgggaaccca
aagccgtaca ttgggaaccg 8220gaacccgtac attgggaacc caaagccgta cattgggaac
cggtcacaca tgtaagtgac 8280tgatataaaa gagaaaaaag gcgatttttc cgcctaaaac
tctttaaaac ttattaaaac 8340tcttaaaacc cgcctggcct gtgcataact gtctggccag
cgcacagccg aagagctgca 8400aaaagcgcct acccttcggt cgctgcgctc cctacgcccc
gccgcttcgc gtcggcctat 8460cgcggccgct ggccgctcaa aaatggctgg cctacggcca
ggcaatctac cagggcgcgg 8520acaagccgcg ccgtcgccac tcgaccgccg gcgcccacat
caaggcaccc tgcctcgcgc 8580gtttcggtga tgacggtgaa aacctctgac acatgcagct
cccggagacg gtcacagctt 8640gtctgtaagc ggatgccggg agcagacaag cccgtcaggg
cgcgtcagcg ggtgttggcg 8700ggtgtcgggg cgcagccatg acccagtcac gtagcgatag
cggagtgtat actggcttaa 8760ctatgcggca tcagagcaga ttgtactgag agtgcaccat
atgcggtgtg aaataccgca 8820cagatgcgta aggagaaaat accgcatcag gcgctcttcc
gcttcctcgc tcactgactc 8880gctgcgctcg gtcgttcggc tgcggcgagc ggtatcagct
cactcaaagg cggtaatacg 8940gttatccaca gaatcagggg ataacgcagg aaagaacatg
tgagcaaaag gccagcaaaa 9000ggccaggaac cgtaaaaagg ccgcgttgct ggcgtttttc
cataggctcc gcccccctga 9060cgagcatcac aaaaatcgac gctcaagtca gaggtggcga
aacccgacag gactataaag 9120ataccaggcg tttccccctg gaagctccct cgtgcgctct
cctgttccga ccctgccgct 9180taccggatac ctgtccgcct ttctcccttc gggaagcgtg
gcgctttctc atagctcacg 9240ctgtaggtat ctcagttcgg tgtaggtcgt tcgctccaag
ctgggctgtg tgcacgaacc 9300ccccgttcag cccgaccgct gcgccttatc cggtaactat
cgtcttgagt ccaacccggt 9360aagacacgac ttatcgccac tggcagcagc cactggtaac
aggattagca gagcgaggta 9420tgtaggcggt gctacagagt tcttgaagtg gtggcctaac
tacggctaca ctagaaggac 9480agtatttggt atctgcgctc tgctgaagcc agttaccttc
ggaaaaagag ttggtagctc 9540ttgatccggc aaacaaacca ccgctggtag cggtggtttt
tttgtttgca agcagcagat 9600tacgcgcaga aaaaaaggat ctcaagaaga tcctttgatc
ttttctacgg ggtctgacgc 9660tcagtggaac gaaaactcac gttaagggat tttggtcatg
catgatatat ctcccaattt 9720gtgtagggct tattatgcac gcttaaaaat aataaaagca
gacttgacct gatagtttgg 9780ctgtgagcaa ttatgtgctt agtgcatcta acgcttgagt
taagccgcgc cgcgaagcgg 9840cgtcggcttg aacgaatttc tagctagaca ttatttgccg
actaccttgg tgatctcgcc 9900tttcacgtag tggacaaatt cttccaactg atctgcgcgc
gaggccaagc gatcttcttc 9960ttgtccaaga taagcctgtc tagcttcaag tatgacgggc
tgatactggg ccggcaggcg 10020ctccattgcc cagtcggcag cgacatcctt cggcgcgatt
ttgccggtta ctgcgctgta 10080ccaaatgcgg gacaacgtaa gcactacatt tcgctcatcg
ccagcccagt cgggcggcga 10140gttccatagc gttaaggttt catttagcgc ctcaaataga
tcctgttcag gaaccggatc 10200aaagagttcc tccgccgctg gacctaccaa ggcaacgcta
tgttctcttg cttttgtcag 10260caagatagcc agatcaatgt cgatcgtggc tggctcgaag
atacctgcaa gaatgtcatt 10320gcgctgccat tctccaaatt gcagttcgcg cttagctgga
taacgccacg gaatgatgtc 10380gtcgtgcaca acaatggtga cttctacagc gcggagaatc
tcgctctctc caggggaagc 10440cgaagtttcc aaaaggtcgt tgatcaaagc tcgccgcgtt
gtttcatcaa gccttacggt 10500caccgtaacc agcaaatcaa tatcactgtg tggcttcagg
ccgccatcca ctgcggagcc 10560gtacaaatgt acggccagca acgtcggttc gagatggcgc
tcgatgacgc caactacctc 10620tgatagttga gtcgatactt cggcgatcac cgcttccccc
atgatgttta actttgtttt 10680agggcgactg ccctgctgcg taacatcgtt gctgctccat
aacatcaaac atcgacccac 10740ggcgtaacgc gcttgctgct tggatgcccg aggcatagac
tgtaccccaa aaaaacagtc 10800ataacaagcc atgaaaaccg ccactgcg
108287510036DNAArtificial sequenceDescription of the
artificial sequence transgenic expression vector pSUN3NIT_HvRacB_s
for expression of barley RacB sense RNA fragment 75ttccatggac
atacaaatgg acgaacggat aaaccttttc acgccctttt aaatatccga 60ttattctaat
aaacgctctt ttctcttagg tttacccgcc aatatatcct gtcaaacact 120gatagtttaa
actgaaggcg ggaaacgaca atcagatcta gtaggaaaca gctatgacca 180tgattacgcc
aagcttgcat gcctgcaggt cgactctaga ggatccccca tcaagatctt 240ggtgatgtag
caagagctaa gttgtacttc gatycggttg gacattactc gagaccagat 300gttttacact
tgaccgtaaa tgagcacccg aagaaaccgg taacattcat ttcgaaggta 360gagaaagcgg
aagatgactc aaacaagtaa tcggttgtga ttcgtcagtt catgtcactc 420ctatgaagga
gtcaagttca aaatgttatg ttgagtttca aacttttatg ctaaactttt 480tttcttaatt
ttcgttaata atggaagaga accaattctc ttgtatctaa agattatcca 540tctatcatcc
aatttgagtg ttcaattctg gatgttgtgt taccctacat tctacaacca 600tgtagccaat
tattatgaat ctggctttga tttcagttgt gttcttttct ttttttcttt 660gcatatttgc
atttagaatg tttaataatt aagttactgt atttccacat acattagttc 720caagaatata
catatattaa tttatttttc ttaaaaatgt tttggaatga ctaatattga 780caacgaaaat
agaagctatg ctaaaccatt acgtatatgt gacttcacat gttgttgttt 840tacattccct
atatatatgg atggctgtca caatcagaaa cgtgatcgaa aaaagacaaa 900cagtgtttgc
ataaaaagac tatttcgttt cattgacaat ttgtgtttat ttgtaaagaa 960aagtggcaaa
gtggaatttg agttcctgca agtaagaaag atgaaataaa agacttgagt 1020gtgtgttttt
ttcttttatc tgaaagctgc aatgaaatat tcctaccaag cccgtttgat 1080tattaattgg
ggtttggttt tcttgatgcg aactaattgg ttatataaga aactatacaa 1140tccatgttaa
ttcaaaaatt ttgatttctc ttgtaggaat atgatttact atatgagact 1200ttcttttcgc
caataatagt aaatccaaag atatttgacc ggaccaaaac acattgatct 1260attttttagt
ttatttaatc cagtttctct gagataattc attaaggaaa acttagtatt 1320aacccatcct
aagattaaat aggagccaaa ctcacatttc aaatattaaa taacataaaa 1380tggatttaaa
aaatctatac gtcaaatttt atttatgaca tttcttattt aaatttatat 1440ttaatgaaat
acagctaaga caaaccaaaa aaaaaatact ttctaagtgg tccaaaacat 1500caattccgtt
caatattatt aggtagaatc gtacgaccaa aaaaggtagg ttaatacgaa 1560attacaaaca
tatctatata catagtatat attattacct attatgagga atcaaaatgc 1620atcaaatatg
gatttaagga atccataaaa gaataaattc tacggaaaaa aaaaaaagaa 1680taaattcttt
taagttttta atttgttttt tatttggtag ttctccattt tgttttattt 1740cgtttggatt
tattgtgtcc aaatactttg taaaccaccg ttgtaattct taaacggggt 1800tttcacttct
tttttatatt cagacataaa gcatcggctg gtttaatcaa tcaatagatt 1860ttatttttct
tctcaattat tagtaggttt gatgtgaact ttacaaaaaa aacaaaaaca 1920aatcaatgca
gagaaaagaa accacgtggg ctagtcccac cttgtttcat ttccaccaca 1980ggttcgatct
tcgttaccgt ctccaatagg aaaataaacg tgaccacaaa aaaaaaacaa 2040aaaaaaagtc
tatatattgc ttctctcaag tctctgagtg tcatgaacca aagtaaaaaa 2100caaagactcg
agtggatccc cgggccgcca tggccgcggg atggatccga tgagcgcgtc 2160caggttcata
aagtgcgtca cggtcgggga cggcgccgtc ggcaagacct gcatgctcat 2220ctcctacacc
tccaacacct tccccaccga ctatgttccg acagtgtttg ataacttcag 2280tgccaacgtt
gtggttgatg gtaatactgt caacctcggc ctctgggaca ctgcaggtca 2340agaggattac
aacagactga gaccactgag ctatcgtgga gctgatgttt ttcttctggc 2400tttctcactg
atcagtaagg ccagctatga gaatgtttcg aagaagtgga tacctgaact 2460gaagcattat
gcacctggtg tgccaattat tctcgtaggg acaaagcttg atcttcgaga 2520cgacaagcag
ttctttgtgg accatcctgg tgctgtccct atcactactg ctcagggaga 2580ggagctaaga
aagcaaatag gcgctccata ctacatcgaa tgcagctcga agacccaact 2640aaacgtgaag
ggcgtcttcg atgcggcgat aaaggttgtg ctgcagccgc ctaaggcgaa 2700gaagaagaaa
aaggtgcaga ggggggcgtg ctccattttg tgaaattcac tggccgtcgt 2760tttacaacga
ctcagagctt gacaggaggc ccgatctagt aacatagatg acaccgcgcg 2820cgataattta
tcctagtttg cgcgctatat tttgttttct atcgcgtatt aaatgtataa 2880ttgcgggact
ctaatcataa aaacccatct cataaataac gtcatgcatt acatgttaat 2940tattacatgc
ttaacgtaat tcaacagaaa ttatatgata atcatcgcaa gaccggcaac 3000aggattcaat
cttaagaaac tttattgcca aatgtttgaa cgatcgggga tcatccgggt 3060ctgtggcggg
aactccacga aaatatccga acgcagcaag atctagagct tgggtcccgc 3120tcagaagaac
tcgtcaagaa ggcgatagaa ggcgatgcgc tgcgaatcgg gagcggcgat 3180accgtaaagc
acgaggaagc ggtcagccca ttcgccgcca agctcttcag caatatcacg 3240ggtagccaac
gctatgtcct gatagcggtc cgccacaccc agccggccac agtcgatgaa 3300tccagaaaag
cggccatttt ccaccatgat attcggcaag caggcatcgc catgggtcac 3360gacgagatcc
tcgccgtcgg gcatgcgcgc cttgagcctg gcgaacagtt cggctggcgc 3420gagcccctga
tgctcttcgt ccagatcatc ctgatcgaca agaccggctt ccatccgagt 3480acgtgctcgc
tcgatgcgat gtttcgcttg gtggtcgaat gggcaggtag ccggatcaag 3540cgtatgcagc
cgccgcattg catcagccat gatggatact ttctcggcag gagcaaggtg 3600agatgacagg
agatcctgcc ccggcacttc gcccaatagc agccagtccc ttcccgcttc 3660agtgacaacg
tcgagcacag ctgcgcaagg aacgcccgtc gtggccagcc acgatagccg 3720cgctgcctcg
tcctgcagtt cattcagggc accggacagg tcggtcttga caaaaagaac 3780cgggcgcccc
tgcgctgaca gccggaacac ggcggcatca gagcagccga ttgtctgttg 3840tgcccagtca
tagccgaata gcctctccac ccaagcggcc ggagaacctg cgtgcaatcc 3900atcttgttca
atcatgcgaa acgatccaga tccggtgcag attatttgga ttgagagtga 3960atatgagact
ctaattggat accgagggga atttatggaa cgtcagtgga gcatttttga 4020caagaaatat
ttgctagctg atagtgacct taggcgactt ttgaacgcgc aataatggtt 4080tctgacgtat
gtgcttagct cattaaactc cagaaacccg cggctgagtg gctccttcaa 4140cgttgcggtt
ctgtcagttc caaacgtaaa acggcttgtc ccgcgtcatc ggcgggggtc 4200ataacgtgac
tcccttaatt ctccgctcat gatcagattg tcgtttcccg ccttcagttt 4260aaactatcag
tgtttgacag gatcctgctt ggtaataatt gtcattagat tgtttttatg 4320catagatgca
ctcgaaatca gccaatttta gacaagtatc aaacggatgt taattcagta 4380cattaaagac
gtccgcaatg tgttattaag ttgtctaagc gtcaatttgt ttacaccaca 4440atatatcctg
ccaccagcca gccaacagct ccccgaccgg cagctcggca caaaatcacc 4500acgcgttacc
accacgccgg ccggccgcat ggtgttgacc gtgttcgccg gcattgccga 4560gttcgagcgt
tccctaatca tcgaccgcac ccggagcggg cgcgaggccg ccaaggcccg 4620aggcgtgaag
tttggccccc gccctaccct caccccggca cagatcgcgc acgcccgcga 4680gctgatcgac
caggaaggcc gcaccgtgaa agaggcggct gcactgcttg gcgtgcatcg 4740ctcgaccctg
taccgcgcac ttgagcgcag cgaggaagtg acgcccaccg aggccaggcg 4800gcgcggtgcc
ttccgtgagg acgcattgac cgaggccgac gccctggcgg ccgccgagaa 4860tgaacgccaa
gaggaacaag catgaaaccg caccaggacg gccaggacga accgtttttc 4920attaccgaag
agatcgaggc ggagatgatc gcggccgggt acgtgttcga gccgcccgcg 4980cacgtctcaa
ccgtgcggct gcatgaaatc ctggccggtt tgtctgatgc caagctggcg 5040gcctggccgg
ccagcttggc cgctgaagaa accgagcgcc gccgtctaaa aaggtgatgt 5100gtatttgagt
aaaacagctt gcgtcatgcg gtcgctgcgt atatgatgcg atgagtaaat 5160aaacaaatac
gcaaggggaa cgcatgaagg ttatcgctgt acttaaccag aaaggcgggt 5220caggcaagac
gaccatcgca acccatctag cccgcgccct gcaactcgcc ggggccgatg 5280ttctgttagt
cgattccgat ccccagggca gtgcccgcga ttgggcggcc gtgcgggaag 5340atcaaccgct
aaccgttgtc ggcatcgacc gcccgacgat tgaccgcgac gtgaaggcca 5400tcggccggcg
cgacttcgta gtgatcgacg gagcgcccca ggcggcggac ttggctgtgt 5460ccgcgatcaa
ggcagccgac ttcgtgctga ttccggtgca gccaagccct tacgacatat 5520gggccaccgc
cgacctggtg gagctggtta agcagcgcat tgaggtcacg gatggaaggc 5580tacaagcggc
ctttgtcgtg tcgcgggcga tcaaaggcac gcgcatcggc ggtgaggttg 5640ccgaggcgct
ggccgggtac gagctgccca ttcttgagtc ccgtatcacg cagcgcgtga 5700gctacccagg
cactgccgcc gccggcacaa ccgttcttga atcagaaccc gagggcgacg 5760ctgcccgcga
ggtccaggcg ctggccgctg aaattaaatc aaaactcatt tgagttaatg 5820aggtaaagag
aaaatgagca aaagcacaaa cacgctaagt gccggccgtc cgagcgcacg 5880cagcagcaag
gctgcaacgt tggccagcct ggcagacacg ccagccatga agcgggtcaa 5940ctttcagttg
ccggcggagg atcacaccaa gctgaagatg tacgcggtac gccaaggcaa 6000gaccattacc
gagctgctat ctgaatacat cgcgcagcta ccagagtaaa tgagcaaatg 6060aataaatgag
tagatgaatt ttagcggcta aaggaggcgg catggaaaat caagaacaac 6120caggcaccga
cgccgtggaa tgccccatgt gtggaggaac gggcggttgg ccaggcgtaa 6180gcggctgggt
tgtctgccgg ccctgcaatg gcactggaac ccccaagccc gaggaatcgg 6240cgtgagcggt
cgcaaaccat ccggcccggt acaaatcggc gcggcgctgg gtgatgacct 6300ggtggagaag
ttgaaggccg cgcaggccgc ccagcggcaa cgcatcgagg cagaagcacg 6360ccccggtgaa
tcgtggcaag cggccgctga tcgaatccgc aaagaatccc ggcaaccgcc 6420ggcagccggt
gcgccgtcga ttaggaagcc gcccaagggc gacgagcaac cagatttttt 6480cgttccgatg
ctctatgacg tgggcacccg cgatagtcgc agcatcatgg acgtggccgt 6540tttccgtctg
tcgaagcgtg accgacgagc tggcgaggtg atccgctacg agcttccaga 6600cgggcacgta
gaggtttccg cagggccggc cggcatggcc agtgtgtggg attacgacct 6660ggtactgatg
gcggtttccc atctaaccga atccatgaac cgataccggg aagggaaggg 6720agacaagccc
ggccgcgtgt tccgtccaca cgttgcggac gtactcaagt tctgccggcg 6780agccgatggc
ggaaagcaga aagacgacct ggtagaaacc tgcattcggt taaacaccac 6840gcacgttgcc
atgcagcgta cgaagaaggc caagaacggc cgcctggtga cggtatccga 6900gggtgaagcc
ttgattagcc gctacaagat cgtaaagagc gaaaccgggc ggccggagta 6960catcgagatc
gagctagctg attggatgta ccgcgagatc acagaaggca agaacccgga 7020cgtgctgacg
gttcaccccg attacttttt gatcgatccc ggcatcggcc gttttctcta 7080ccgcctggca
cgccgcgccg caggcaaggc agaagccaga tggttgttca agacgatcta 7140cgaacgcagt
ggcagcgccg gagagttcaa gaagttctgt ttcaccgtgc gcaagctgat 7200cgggtcaaat
gacctgccgg agtacgattt gaaggaggag gcggggcagg ctggcccgat 7260cctagtcatg
cgctaccgca acctgatcga gggcgaagca tccgccggtt cctaatgtac 7320ggagcagatg
ctagggcaaa ttgccctagc aggggaaaaa ggtcgaaaag gtctctttcc 7380tgtggatagc
acgtacattg ggaacccaaa gccgtacatt gggaaccgga acccgtacat 7440tgggaaccca
aagccgtaca ttgggaaccg gtcacacatg taagtgactg atataaaaga 7500gaaaaaaggc
gatttttccg cctaaaactc tttaaaactt attaaaactc ttaaaacccg 7560cctggcctgt
gcataactgt ctggccagcg cacagccgaa gagctgcaaa aagcgcctac 7620ccttcggtcg
ctgcgctccc tacgccccgc cgcttcgcgt cggcctatcg cggccgctgg 7680ccgctcaaaa
atggctggcc tacggccagg caatctacca gggcgcggac aagccgcgcc 7740gtcgccactc
gaccgccggc gcccacatca aggcaccctg cctcgcgcgt ttcggtgatg 7800acggtgaaaa
cctctgacac atgcagctcc cggagacggt cacagcttgt ctgtaagcgg 7860atgccgggag
cagacaagcc cgtcagggcg cgtcagcggg tgttggcggg tgtcggggcg 7920cagccatgac
ccagtcacgt agcgatagcg gagtgtatac tggcttaact atgcggcatc 7980agagcagatt
gtactgagag tgcaccatat gcggtgtgaa ataccgcaca gatgcgtaag 8040gagaaaatac
cgcatcaggc gctcttccgc ttcctcgctc actgactcgc tgcgctcggt 8100cgttcggctg
cggcgagcgg tatcagctca ctcaaaggcg gtaatacggt tatccacaga 8160atcaggggat
aacgcaggaa agaacatgtg agcaaaaggc cagcaaaagg ccaggaaccg 8220taaaaaggcc
gcgttgctgg cgtttttcca taggctccgc ccccctgacg agcatcacaa 8280aaatcgacgc
tcaagtcaga ggtggcgaaa cccgacagga ctataaagat accaggcgtt 8340tccccctgga
agctccctcg tgcgctctcc tgttccgacc ctgccgctta ccggatacct 8400gtccgccttt
ctcccttcgg gaagcgtggc gctttctcat agctcacgct gtaggtatct 8460cagttcggtg
taggtcgttc gctccaagct gggctgtgtg cacgaacccc ccgttcagcc 8520cgaccgctgc
gccttatccg gtaactatcg tcttgagtcc aacccggtaa gacacgactt 8580atcgccactg
gcagcagcca ctggtaacag gattagcaga gcgaggtatg taggcggtgc 8640tacagagttc
ttgaagtggt ggcctaacta cggctacact agaaggacag tatttggtat 8700ctgcgctctg
ctgaagccag ttaccttcgg aaaaagagtt ggtagctctt gatccggcaa 8760acaaaccacc
gctggtagcg gtggtttttt tgtttgcaag cagcagatta cgcgcagaaa 8820aaaaggatct
caagaagatc ctttgatctt ttctacgggg tctgacgctc agtggaacga 8880aaactcacgt
taagggattt tggtcatgca tgatatatct cccaatttgt gtagggctta 8940ttatgcacgc
ttaaaaataa taaaagcaga cttgacctga tagtttggct gtgagcaatt 9000atgtgcttag
tgcatctaac gcttgagtta agccgcgccg cgaagcggcg tcggcttgaa 9060cgaatttcta
gctagacatt atttgccgac taccttggtg atctcgcctt tcacgtagtg 9120gacaaattct
tccaactgat ctgcgcgcga ggccaagcga tcttcttctt gtccaagata 9180agcctgtcta
gcttcaagta tgacgggctg atactgggcc ggcaggcgct ccattgccca 9240gtcggcagcg
acatccttcg gcgcgatttt gccggttact gcgctgtacc aaatgcggga 9300caacgtaagc
actacatttc gctcatcgcc agcccagtcg ggcggcgagt tccatagcgt 9360taaggtttca
tttagcgcct caaatagatc ctgttcagga accggatcaa agagttcctc 9420cgccgctgga
cctaccaagg caacgctatg ttctcttgct tttgtcagca agatagccag 9480atcaatgtcg
atcgtggctg gctcgaagat acctgcaaga atgtcattgc gctgccattc 9540tccaaattgc
agttcgcgct tagctggata acgccacgga atgatgtcgt cgtgcacaac 9600aatggtgact
tctacagcgc ggagaatctc gctctctcca ggggaagccg aagtttccaa 9660aaggtcgttg
atcaaagctc gccgcgttgt ttcatcaagc cttacggtca ccgtaaccag 9720caaatcaata
tcactgtgtg gcttcaggcc gccatccact gcggagccgt acaaatgtac 9780ggccagcaac
gtcggttcga gatggcgctc gatgacgcca actacctctg atagttgagt 9840cgatacttcg
gcgatcaccg cttcccccat gatgtttaac tttgttttag ggcgactgcc 9900ctgctgcgta
acatcgttgc tgctccataa catcaaacat cgacccacgg cgtaacgcgc 9960ttgctgcttg
gatgcccgag gcatagactg taccccaaaa aaacagtcat aacaagccat 10020gaaaaccgcc
actgcg
100367610036DNAArtificial sequenceDescription of the artificial sequence
transgenic expression vector pSUN3NIT_HvRacB_as for
expression of barley RacB anti sense RNA fragment 76ttccatggac
atacaaatgg acgaacggat aaaccttttc acgccctttt aaatatccga 60ttattctaat
aaacgctctt ttctcttagg tttacccgcc aatatatcct gtcaaacact 120gatagtttaa
actgaaggcg ggaaacgaca atcagatcta gtaggaaaca gctatgacca 180tgattacgcc
aagcttgcat gcctgcaggt cgactctaga ggatccccca tcaagatctt 240ggtgatgtag
caagagctaa gttgtacttc gatycggttg gacattactc gagaccagat 300gttttacact
tgaccgtaaa tgagcacccg aagaaaccgg taacattcat ttcgaaggta 360gagaaagcgg
aagatgactc aaacaagtaa tcggttgtga ttcgtcagtt catgtcactc 420ctatgaagga
gtcaagttca aaatgttatg ttgagtttca aacttttatg ctaaactttt 480tttcttaatt
ttcgttaata atggaagaga accaattctc ttgtatctaa agattatcca 540tctatcatcc
aatttgagtg ttcaattctg gatgttgtgt taccctacat tctacaacca 600tgtagccaat
tattatgaat ctggctttga tttcagttgt gttcttttct ttttttcttt 660gcatatttgc
atttagaatg tttaataatt aagttactgt atttccacat acattagttc 720caagaatata
catatattaa tttatttttc ttaaaaatgt tttggaatga ctaatattga 780caacgaaaat
agaagctatg ctaaaccatt acgtatatgt gacttcacat gttgttgttt 840tacattccct
atatatatgg atggctgtca caatcagaaa cgtgatcgaa aaaagacaaa 900cagtgtttgc
ataaaaagac tatttcgttt cattgacaat ttgtgtttat ttgtaaagaa 960aagtggcaaa
gtggaatttg agttcctgca agtaagaaag atgaaataaa agacttgagt 1020gtgtgttttt
ttcttttatc tgaaagctgc aatgaaatat tcctaccaag cccgtttgat 1080tattaattgg
ggtttggttt tcttgatgcg aactaattgg ttatataaga aactatacaa 1140tccatgttaa
ttcaaaaatt ttgatttctc ttgtaggaat atgatttact atatgagact 1200ttcttttcgc
caataatagt aaatccaaag atatttgacc ggaccaaaac acattgatct 1260attttttagt
ttatttaatc cagtttctct gagataattc attaaggaaa acttagtatt 1320aacccatcct
aagattaaat aggagccaaa ctcacatttc aaatattaaa taacataaaa 1380tggatttaaa
aaatctatac gtcaaatttt atttatgaca tttcttattt aaatttatat 1440ttaatgaaat
acagctaaga caaaccaaaa aaaaaatact ttctaagtgg tccaaaacat 1500caattccgtt
caatattatt aggtagaatc gtacgaccaa aaaaggtagg ttaatacgaa 1560attacaaaca
tatctatata catagtatat attattacct attatgagga atcaaaatgc 1620atcaaatatg
gatttaagga atccataaaa gaataaattc tacggaaaaa aaaaaaagaa 1680taaattcttt
taagttttta atttgttttt tatttggtag ttctccattt tgttttattt 1740cgtttggatt
tattgtgtcc aaatactttg taaaccaccg ttgtaattct taaacggggt 1800tttcacttct
tttttatatt cagacataaa gcatcggctg gtttaatcaa tcaatagatt 1860ttatttttct
tctcaattat tagtaggttt gatgtgaact ttacaaaaaa aacaaaaaca 1920aatcaatgca
gagaaaagaa accacgtggg ctagtcccac cttgtttcat ttccaccaca 1980ggttcgatct
tcgttaccgt ctccaatagg aaaataaacg tgaccacaaa aaaaaaacaa 2040aaaaaaagtc
tatatattgc ttctctcaag tctctgagtg tcatgaacca aagtaaaaaa 2100caaagactcg
agtggatccc cggtcacaaa atggagcacg cccccctctg cacctttttc 2160ttcttcttcg
ccttaggcgg ctgcagcaca acctttatcg ccgcatcgaa gacgcccttc 2220acgtttagtt
gggtcttcga gctgcattcg atgtagtatg gagcgcctat ttgctttctt 2280agctcctctc
cctgagcagt agtgataggg acagcaccag gatggtccac aaagaactgc 2340ttgtcgtctc
gaagatcaag ctttgtccct acgagaataa ttggcacacc aggtgcataa 2400tgcttcagtt
caggtatcca cttcttcgaa acattctcat agctggcctt actgatcagt 2460gagaaagcca
gaagaaaaac atcagctcca cgatagctca gtggtctcag tctgttgtaa 2520tcctcttgac
ctgcagtgtc ccagaggccg aggttgacag tattaccatc aaccacaacg 2580ttggcactga
agttatcaaa cactgtcgga acatagtcgg tggggaaggt gttggaggtg 2640taggagatga
gcatgcaggt cttgccgacg gcgccgtccc cgaccgtgac gcactttatg 2700aacctggacg
cgctcatcgg atccatcccg cggccatggc ggcaattcac tggccgtcgt 2760tttacaacga
ctcagagctt gacaggaggc ccgatctagt aacatagatg acaccgcgcg 2820cgataattta
tcctagtttg cgcgctatat tttgttttct atcgcgtatt aaatgtataa 2880ttgcgggact
ctaatcataa aaacccatct cataaataac gtcatgcatt acatgttaat 2940tattacatgc
ttaacgtaat tcaacagaaa ttatatgata atcatcgcaa gaccggcaac 3000aggattcaat
cttaagaaac tttattgcca aatgtttgaa cgatcgggga tcatccgggt 3060ctgtggcggg
aactccacga aaatatccga acgcagcaag atctagagct tgggtcccgc 3120tcagaagaac
tcgtcaagaa ggcgatagaa ggcgatgcgc tgcgaatcgg gagcggcgat 3180accgtaaagc
acgaggaagc ggtcagccca ttcgccgcca agctcttcag caatatcacg 3240ggtagccaac
gctatgtcct gatagcggtc cgccacaccc agccggccac agtcgatgaa 3300tccagaaaag
cggccatttt ccaccatgat attcggcaag caggcatcgc catgggtcac 3360gacgagatcc
tcgccgtcgg gcatgcgcgc cttgagcctg gcgaacagtt cggctggcgc 3420gagcccctga
tgctcttcgt ccagatcatc ctgatcgaca agaccggctt ccatccgagt 3480acgtgctcgc
tcgatgcgat gtttcgcttg gtggtcgaat gggcaggtag ccggatcaag 3540cgtatgcagc
cgccgcattg catcagccat gatggatact ttctcggcag gagcaaggtg 3600agatgacagg
agatcctgcc ccggcacttc gcccaatagc agccagtccc ttcccgcttc 3660agtgacaacg
tcgagcacag ctgcgcaagg aacgcccgtc gtggccagcc acgatagccg 3720cgctgcctcg
tcctgcagtt cattcagggc accggacagg tcggtcttga caaaaagaac 3780cgggcgcccc
tgcgctgaca gccggaacac ggcggcatca gagcagccga ttgtctgttg 3840tgcccagtca
tagccgaata gcctctccac ccaagcggcc ggagaacctg cgtgcaatcc 3900atcttgttca
atcatgcgaa acgatccaga tccggtgcag attatttgga ttgagagtga 3960atatgagact
ctaattggat accgagggga atttatggaa cgtcagtgga gcatttttga 4020caagaaatat
ttgctagctg atagtgacct taggcgactt ttgaacgcgc aataatggtt 4080tctgacgtat
gtgcttagct cattaaactc cagaaacccg cggctgagtg gctccttcaa 4140cgttgcggtt
ctgtcagttc caaacgtaaa acggcttgtc ccgcgtcatc ggcgggggtc 4200ataacgtgac
tcccttaatt ctccgctcat gatcagattg tcgtttcccg ccttcagttt 4260aaactatcag
tgtttgacag gatcctgctt ggtaataatt gtcattagat tgtttttatg 4320catagatgca
ctcgaaatca gccaatttta gacaagtatc aaacggatgt taattcagta 4380cattaaagac
gtccgcaatg tgttattaag ttgtctaagc gtcaatttgt ttacaccaca 4440atatatcctg
ccaccagcca gccaacagct ccccgaccgg cagctcggca caaaatcacc 4500acgcgttacc
accacgccgg ccggccgcat ggtgttgacc gtgttcgccg gcattgccga 4560gttcgagcgt
tccctaatca tcgaccgcac ccggagcggg cgcgaggccg ccaaggcccg 4620aggcgtgaag
tttggccccc gccctaccct caccccggca cagatcgcgc acgcccgcga 4680gctgatcgac
caggaaggcc gcaccgtgaa agaggcggct gcactgcttg gcgtgcatcg 4740ctcgaccctg
taccgcgcac ttgagcgcag cgaggaagtg acgcccaccg aggccaggcg 4800gcgcggtgcc
ttccgtgagg acgcattgac cgaggccgac gccctggcgg ccgccgagaa 4860tgaacgccaa
gaggaacaag catgaaaccg caccaggacg gccaggacga accgtttttc 4920attaccgaag
agatcgaggc ggagatgatc gcggccgggt acgtgttcga gccgcccgcg 4980cacgtctcaa
ccgtgcggct gcatgaaatc ctggccggtt tgtctgatgc caagctggcg 5040gcctggccgg
ccagcttggc cgctgaagaa accgagcgcc gccgtctaaa aaggtgatgt 5100gtatttgagt
aaaacagctt gcgtcatgcg gtcgctgcgt atatgatgcg atgagtaaat 5160aaacaaatac
gcaaggggaa cgcatgaagg ttatcgctgt acttaaccag aaaggcgggt 5220caggcaagac
gaccatcgca acccatctag cccgcgccct gcaactcgcc ggggccgatg 5280ttctgttagt
cgattccgat ccccagggca gtgcccgcga ttgggcggcc gtgcgggaag 5340atcaaccgct
aaccgttgtc ggcatcgacc gcccgacgat tgaccgcgac gtgaaggcca 5400tcggccggcg
cgacttcgta gtgatcgacg gagcgcccca ggcggcggac ttggctgtgt 5460ccgcgatcaa
ggcagccgac ttcgtgctga ttccggtgca gccaagccct tacgacatat 5520gggccaccgc
cgacctggtg gagctggtta agcagcgcat tgaggtcacg gatggaaggc 5580tacaagcggc
ctttgtcgtg tcgcgggcga tcaaaggcac gcgcatcggc ggtgaggttg 5640ccgaggcgct
ggccgggtac gagctgccca ttcttgagtc ccgtatcacg cagcgcgtga 5700gctacccagg
cactgccgcc gccggcacaa ccgttcttga atcagaaccc gagggcgacg 5760ctgcccgcga
ggtccaggcg ctggccgctg aaattaaatc aaaactcatt tgagttaatg 5820aggtaaagag
aaaatgagca aaagcacaaa cacgctaagt gccggccgtc cgagcgcacg 5880cagcagcaag
gctgcaacgt tggccagcct ggcagacacg ccagccatga agcgggtcaa 5940ctttcagttg
ccggcggagg atcacaccaa gctgaagatg tacgcggtac gccaaggcaa 6000gaccattacc
gagctgctat ctgaatacat cgcgcagcta ccagagtaaa tgagcaaatg 6060aataaatgag
tagatgaatt ttagcggcta aaggaggcgg catggaaaat caagaacaac 6120caggcaccga
cgccgtggaa tgccccatgt gtggaggaac gggcggttgg ccaggcgtaa 6180gcggctgggt
tgtctgccgg ccctgcaatg gcactggaac ccccaagccc gaggaatcgg 6240cgtgagcggt
cgcaaaccat ccggcccggt acaaatcggc gcggcgctgg gtgatgacct 6300ggtggagaag
ttgaaggccg cgcaggccgc ccagcggcaa cgcatcgagg cagaagcacg 6360ccccggtgaa
tcgtggcaag cggccgctga tcgaatccgc aaagaatccc ggcaaccgcc 6420ggcagccggt
gcgccgtcga ttaggaagcc gcccaagggc gacgagcaac cagatttttt 6480cgttccgatg
ctctatgacg tgggcacccg cgatagtcgc agcatcatgg acgtggccgt 6540tttccgtctg
tcgaagcgtg accgacgagc tggcgaggtg atccgctacg agcttccaga 6600cgggcacgta
gaggtttccg cagggccggc cggcatggcc agtgtgtggg attacgacct 6660ggtactgatg
gcggtttccc atctaaccga atccatgaac cgataccggg aagggaaggg 6720agacaagccc
ggccgcgtgt tccgtccaca cgttgcggac gtactcaagt tctgccggcg 6780agccgatggc
ggaaagcaga aagacgacct ggtagaaacc tgcattcggt taaacaccac 6840gcacgttgcc
atgcagcgta cgaagaaggc caagaacggc cgcctggtga cggtatccga 6900gggtgaagcc
ttgattagcc gctacaagat cgtaaagagc gaaaccgggc ggccggagta 6960catcgagatc
gagctagctg attggatgta ccgcgagatc acagaaggca agaacccgga 7020cgtgctgacg
gttcaccccg attacttttt gatcgatccc ggcatcggcc gttttctcta 7080ccgcctggca
cgccgcgccg caggcaaggc agaagccaga tggttgttca agacgatcta 7140cgaacgcagt
ggcagcgccg gagagttcaa gaagttctgt ttcaccgtgc gcaagctgat 7200cgggtcaaat
gacctgccgg agtacgattt gaaggaggag gcggggcagg ctggcccgat 7260cctagtcatg
cgctaccgca acctgatcga gggcgaagca tccgccggtt cctaatgtac 7320ggagcagatg
ctagggcaaa ttgccctagc aggggaaaaa ggtcgaaaag gtctctttcc 7380tgtggatagc
acgtacattg ggaacccaaa gccgtacatt gggaaccgga acccgtacat 7440tgggaaccca
aagccgtaca ttgggaaccg gtcacacatg taagtgactg atataaaaga 7500gaaaaaaggc
gatttttccg cctaaaactc tttaaaactt attaaaactc ttaaaacccg 7560cctggcctgt
gcataactgt ctggccagcg cacagccgaa gagctgcaaa aagcgcctac 7620ccttcggtcg
ctgcgctccc tacgccccgc cgcttcgcgt cggcctatcg cggccgctgg 7680ccgctcaaaa
atggctggcc tacggccagg caatctacca gggcgcggac aagccgcgcc 7740gtcgccactc
gaccgccggc gcccacatca aggcaccctg cctcgcgcgt ttcggtgatg 7800acggtgaaaa
cctctgacac atgcagctcc cggagacggt cacagcttgt ctgtaagcgg 7860atgccgggag
cagacaagcc cgtcagggcg cgtcagcggg tgttggcggg tgtcggggcg 7920cagccatgac
ccagtcacgt agcgatagcg gagtgtatac tggcttaact atgcggcatc 7980agagcagatt
gtactgagag tgcaccatat gcggtgtgaa ataccgcaca gatgcgtaag 8040gagaaaatac
cgcatcaggc gctcttccgc ttcctcgctc actgactcgc tgcgctcggt 8100cgttcggctg
cggcgagcgg tatcagctca ctcaaaggcg gtaatacggt tatccacaga 8160atcaggggat
aacgcaggaa agaacatgtg agcaaaaggc cagcaaaagg ccaggaaccg 8220taaaaaggcc
gcgttgctgg cgtttttcca taggctccgc ccccctgacg agcatcacaa 8280aaatcgacgc
tcaagtcaga ggtggcgaaa cccgacagga ctataaagat accaggcgtt 8340tccccctgga
agctccctcg tgcgctctcc tgttccgacc ctgccgctta ccggatacct 8400gtccgccttt
ctcccttcgg gaagcgtggc gctttctcat agctcacgct gtaggtatct 8460cagttcggtg
taggtcgttc gctccaagct gggctgtgtg cacgaacccc ccgttcagcc 8520cgaccgctgc
gccttatccg gtaactatcg tcttgagtcc aacccggtaa gacacgactt 8580atcgccactg
gcagcagcca ctggtaacag gattagcaga gcgaggtatg taggcggtgc 8640tacagagttc
ttgaagtggt ggcctaacta cggctacact agaaggacag tatttggtat 8700ctgcgctctg
ctgaagccag ttaccttcgg aaaaagagtt ggtagctctt gatccggcaa 8760acaaaccacc
gctggtagcg gtggtttttt tgtttgcaag cagcagatta cgcgcagaaa 8820aaaaggatct
caagaagatc ctttgatctt ttctacgggg tctgacgctc agtggaacga 8880aaactcacgt
taagggattt tggtcatgca tgatatatct cccaatttgt gtagggctta 8940ttatgcacgc
ttaaaaataa taaaagcaga cttgacctga tagtttggct gtgagcaatt 9000atgtgcttag
tgcatctaac gcttgagtta agccgcgccg cgaagcggcg tcggcttgaa 9060cgaatttcta
gctagacatt atttgccgac taccttggtg atctcgcctt tcacgtagtg 9120gacaaattct
tccaactgat ctgcgcgcga ggccaagcga tcttcttctt gtccaagata 9180agcctgtcta
gcttcaagta tgacgggctg atactgggcc ggcaggcgct ccattgccca 9240gtcggcagcg
acatccttcg gcgcgatttt gccggttact gcgctgtacc aaatgcggga 9300caacgtaagc
actacatttc gctcatcgcc agcccagtcg ggcggcgagt tccatagcgt 9360taaggtttca
tttagcgcct caaatagatc ctgttcagga accggatcaa agagttcctc 9420cgccgctgga
cctaccaagg caacgctatg ttctcttgct tttgtcagca agatagccag 9480atcaatgtcg
atcgtggctg gctcgaagat acctgcaaga atgtcattgc gctgccattc 9540tccaaattgc
agttcgcgct tagctggata acgccacgga atgatgtcgt cgtgcacaac 9600aatggtgact
tctacagcgc ggagaatctc gctctctcca ggggaagccg aagtttccaa 9660aaggtcgttg
atcaaagctc gccgcgttgt ttcatcaagc cttacggtca ccgtaaccag 9720caaatcaata
tcactgtgtg gcttcaggcc gccatccact gcggagccgt acaaatgtac 9780ggccagcaac
gtcggttcga gatggcgctc gatgacgcca actacctctg atagttgagt 9840cgatacttcg
gcgatcaccg cttcccccat gatgtttaac tttgttttag ggcgactgcc 9900ctgctgcgta
acatcgttgc tgctccataa catcaaacat cgacccacgg cgtaacgcgc 9960ttgctgcttg
gatgcccgag gcatagactg taccccaaaa aaacagtcat aacaagccat 10020gaaaaccgcc
actgcg 10036
User Contributions:
Comment about this patent or add new information about this topic:
| People who visited this patent also read: | |
| Patent application number | Title |
|---|---|
| 20210257974 | DISTRIBUTED AMPLIFIERS WITH CONTROLLABLE LINEARIZATION |
| 20210257973 | RADIO-FREQUENCY POWER-AMPLIFYING ELEMENT |
| 20210257972 | Power Amplifier System and Transfer Learning-based Autotuning Optimization Method Thereof |
| 20210257971 | SUPPLY MODULATOR AND WIRELESS COMMUNICATION APPARATUS INCLUDING THE SAME |
| 20210257970 | SYSTEM AND METHOD OF IMPROVING BLOCKING IMMUNITY OF RADIO FREQUENCY TRANSCEIVER FRONT END |