Patent application title: TRANSCRIPT OPTIMIZED EXPRESSION ENHANCEMENT FOR HIGH-LEVEL PRODUCTION OF PROTEINS AND PROTEIN DOMAINS
Inventors:
Thomas B. Acton (New Brunswick, NJ, US)
Stephen Anderson (New Brunswick, NJ, US)
Yuanpeng Janet Huang (New Brunswick, NJ, US)
Gaetano Montelione (New Brunswick, NJ, US)
IPC8 Class: AC12N1570FI
USPC Class:
435 691
Class name: Chemistry: molecular biology and microbiology micro-organism, tissue cell culture or enzyme using process to synthesize a desired chemical compound or composition recombinant dna technique included in method of making a protein or polypeptide
Publication date: 2014-09-18
Patent application number: 20140273091
Abstract:
The present invention relates to a system for high-level production of
recombinant proteins and protein domains.Claims:
1. A method of preparing an expression vector, wherein the expression
vector comprises, in order of position: a first nucleic acid sequence
encoding a 5' untranslated region of an expressed mRNA that comprises a
ribosome binding site (RBS); a second nucleic acid sequence encoding a
polypeptide tag; and a cloning site, wherein the cloning site enables a
target protein coding sequence to be inserted into the vector in-frame
with the second nucleic acid sequence to encode a fusion protein
comprising the polypeptide tag and the target protein; and wherein the
method comprises specifically modifying the nucleic acid sequence
encoding (i) the 5' untranslated region and (ii) the adjacent polypeptide
tag to minimize RNA secondary structure both within and/or between these
two regions of the mRNA.
2. The method of claim 1, further comprising specifically modifying the second nucleic acid sequence to reduce the presence of rare codons.
3-4. (canceled)
5. The method of claim 1, wherein the expression vector further comprises a target protein coding sequence inserted into the vector in-frame with the nucleic acid tag sequence to encode a fusion protein comprising the polypeptide tag and the target protein.
6. The method of claim 5, wherein the target protein coding sequence is not modified to minimize RNA secondary structure and/or is not modified to reduce the presence of rare codons.
7. (canceled)
8. The method of claim 1, wherein the second nucleic acid sequence encodes at least one affinity purification tag.
9-12. (canceled)
13. The method of claim 1, wherein the second nucleic acid sequence encodes at least one solubility enhancement tag.
14-18. (canceled)
19. The method of claim 5, wherein the target protein coding sequence encodes a transcription factor, a transcription factor domain, an epigenetic regulatory factor, or an epigenetic regulatory factor domain.
20. (canceled)
21. The method of claim 5, wherein the target protein coding sequence encodes a protein antigen for producing an affinity capture reagent.
22-23. (canceled)
24. The method of claim 5, wherein the expression of the target protein is 1.5 fold greater than the expression of a target protein generated from an expression vector that was not modified as described in claim 1.
25. An expression vector prepared using the method of claim 1.
26. An expression vector comprising, in order of position: a first nucleic acid sequence encoding a 5' untranslated region of an expressed mRNA that comprises a ribosome binding site (RBS); a second nucleic acid sequence encoding a polypeptide tag; and a cloning site, wherein the cloning site enables a target protein coding sequence to be inserted into the vector in-frame with the second nucleic acid sequence to encode a fusion protein comprising the polypeptide tag and the target protein; and wherein the nucleic acid sequence encoding (i) the 5' untranslated region and (ii) the adjacent polypeptide tag has been specifically modified to minimize RNA secondary structure both within and/or between these two regions of the mRNA.
27. The expression vector of claim 26, wherein the second nucleic acid sequence has been specifically modified to reduce the presence of rare codons.
28. The expression vector of any one of claims 26-27, wherein nucleotides within about the last 100 nucleotides of the first nucleic acid sequence have been modified.
29. The expression vector of any one of claims 26-28, wherein nucleotides within about the first 90 nucleotides of the second nucleic acid sequence have been modified.
30. The expression vector of claim 26, further comprising a target protein coding sequence inserted into the vector in-frame with the nucleic acid tag sequence to encode a fusion protein comprising the polypeptide tag and the target protein.
31. The expression vector of claim 30, wherein the target protein coding sequence has not been modified to minimize RNA secondary structure and/or has not been modified to eliminate rare codons.
32. (canceled)
33. The expression vector of claim 26, wherein the second nucleic acid sequence encodes at least one affinity purification tag.
34-37. (canceled)
38. The expression vector of claim 26, wherein the second nucleic acid sequence encodes at least one solubility enhancement tag.
39-43. (canceled)
44. The expression vector of claim 30, wherein the target protein coding sequence encodes a transcription factor, a transcription factor domain, an epigenetic regulatory factor, or an epigenetic regulatory factor domain.
45-48. (canceled)
49. The expression vector of claim 30, wherein the target protein is expressed at a 1.5-fold higher level than a target protein generated from an expression vector that was not modified as described in claim 26.
50. A host cell comprising the expression vector of claim 30.
51. A method for expressing a target protein in a host cell, comprising culturing the host cell of claim 50 for a period of time under conditions permitting expression of the target protein.
52-54. (canceled)
Description:
CROSS-REFERENCE TO RELATED APPLICATION(S)
[0001] This application claims priority from U.S. Provisional Application No. 61/558,277, filed Nov. 10, 2011, which application is herein incorporated by reference.
BACKGROUND
[0003] The production of recombinant proteins and protein domains as reagents is extremely valuable to biomedical researchers and the entire biotechnology industry. Escherichia coli expression systems are the most cost effective and widely utilized expression systems for this task. However, production of certain proteins can be challenging in this bacterial system. Often proteins or protein domains fail to express at sufficient levels to allow for the purification of the protein reagents. This is especially true of the protein coding sequences derived from higher eukaryotes (such as humans). For example, using a standard pET E. coli expression system (Acton et al., 2011), nearly one-third of human protein targets produced in a large scale screen of protein expression had no detectable expression levels.
[0004] Thus, there is a need for agents and methods for high-level production of recombinant proteins and protein domains that do not require RNA optimization for each individual target gene.
SUMMARY OF CERTAIN EMBODIMENTS OF THE INVENTION
[0005] This invention relates to a system for high-level production of recombinant proteins and protein domains that does not require RNA optimization for each individual target gene.
[0006] Certain embodiments of the invention provide a method of preparing an expression vector, wherein the expression vector comprises, in order of position: a first nucleic acid sequence encoding a 5' untranslated region of an expressed mRNA that comprises a ribosome binding site (RBS); a second nucleic acid sequence encoding a polypeptide tag; and a cloning site, wherein the cloning site enables a target protein coding sequence to be inserted into the vector in-frame with the second nucleic acid sequence to encode a fusion protein comprising the polypeptide tag and the target protein; and wherein the method comprises specifically modifying the nucleic acid sequence encoding (i) the 5' untranslated region and (ii) the adjacent polypeptide tag to minimize RNA secondary structure both within and/or between these two regions of the mRNA.
[0007] Certain embodiments of the invention provide an expression vector designed using the methods described herein.
[0008] Certain embodiments of the invention provide an expression vector comprising, in order of position: a first nucleic acid sequence encoding a 5' untranslated region of an expressed mRNA that comprises a ribosome binding site (RBS); a second nucleic acid sequence encoding a polypeptide tag; and a cloning site, wherein the cloning site enables a target protein coding sequence to be inserted into the vector in-frame with the second nucleic acid sequence to encode a fusion protein comprising the polypeptide tag and the target protein; and wherein the nucleic acid sequence encoding (i) the 5' untranslated region and (ii) the adjacent polypeptide tag has been specifically modified to minimize RNA secondary structure both within and/or between these two regions of the mRNA.
[0009] Certain embodiments of the invention provide a host cell comprising an expression vector as described herein.
[0010] The details of one or more embodiments of the invention are set forth in the description below. Other features, objects, and advantages of the invention will be apparent from the description and from the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] FIG. 1 is a set of diagrams showing sequences of Avi-tag and Nano-tag based Transcript-Optimized Expression Enhancement Technology (TOEET) expression vectors. The pNESG_Avi6HT Avi-tag sequence (top) (DNA, RNA and protein sequence), the His-tag sequences and the TEV Protease Recognition Site sequences are shown as indicated. Similarly, for pNESG_Nano6HT (bottom) the Nano-tag sequences, the His-tag sequences and TEV Protease Recognition Site sequences are shown as indicated. The T7 RNA transcript produced by each vector is shown under each vector with untranslated sequences indicated with brackets. The Multiple Cloning Site (MCS) is also shown after the tag sequences, including the positions and identity of restriction sites available for cloning.
[0012] FIG. 2 is a diagram showing the predicted mRNA secondary structure resulting from T7-RNA Polymerase based transcription off of the pNESG_Avi6HT T7 promoter. Numbering of the transcript from nucleotides 1-156 is indicated; negative numbers (in italics) show the estimated strength, in kcal/mole, of the predicted base-paired regions. The arrow indicates a predicted open structure (lack of base pairing) at the RBS/translation initiation region. RNA secondary structure predictions were done using GeneBee-NET (http://www.genebee.msu.su/services/rna2_reduced.html).
[0013] FIG. 3 is a set of photographs showing representative SDS-PAGE analysis of expression and solubility for two human protein domains cloned into each of the three vectors pET15_NESG, pNESG_Nano6HT and pNESG_Avi6HT. Left Panel shows the expression and solubility of HR7724C (HUGO ID: ZNF281) residues 291-374. Right Panel shows the expression and solubility of HR8241 (HUGO ID: NR4A21) residues 261-342. Total cell lysate (Tot) and the soluble portion (Sol) of the cell lysate are run in adjacent lanes for each of the two protein domains and the three expression vectors. An asterisk (*) indicates an overexpressed band of the correct size. Note the lack of protein expression in the case of pET15_NESG constructs.
[0014] FIG. 4. Wild-Type and TOEET-Optimized Pyrococcus furiosus (PfR) Maltose Binding Protein (MBP). The sequences at the top corresponds to the first 30 residues of the wild-type PfR-MBP DNA sequence lacking the native secretion signal. The protein open reading frame (DNA sequence) is shown above the corresponding protein sequence. Directly below is the T7 RNA polymerase mediated RNA transcript resulting from the cloning of the PfR-MBP into the pET15_NESG backbone. The Ribosome Binding Site (RBS) is underlined and highlighted in bold, the translation initiation codon is shown in bold-italics. The lower set of sequences correspond to TOEET-optimized PfR-MBP. Bold nucleotides with arrows indicate positions where silent mutations were introduced for codon optimization, predicted decrease in RNA secondary structure in the regions of the RBS and translation initiation codon, or both. The RNA transcript for the TOEET optimized sequence is also shown following the parameters outlined above. The silent mutations were introduced using primers incorporating the nucleotide changes and 5 successive rounds of PCR, negating the need for expensive total gene synthesis.
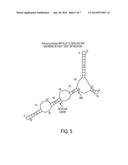
[0015] FIG. 5. The predicted mRNA secondary structure resulting from T7-RNA Polymerase based transcription off of the pET15_NESG vector backbone with Pyrococcus furiosus (PfR) Maltose Binding Protein (MBP) without TOEET optimization. The arrows indicate significant secondary structure (base pairing) at both the Ribosome Binding Site (RBS) and the translation initiation site (Initiation Codon). RNA secondary structure predictions were performed using GeneBee-NET (http://www.genebee.msu.su/services/rna2_reduced.html).
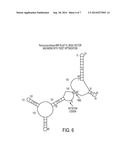
[0016] FIG. 6. The predicted mRNA secondary structure resulting from T7-RNA Polymerase based transcription off of the pET15_NESG vector backbone with Pyrococcus furiosus (PfR) Maltose Binding Protein (MBP) after TOEET optimization. The arrows indicates the Ribosome Binding Site (RBS) and the translation initiation site (Initiation Codon) and the prediction of significantly greater open structure (lack of base pairing) after TOEET optimization. RNA secondary structure predictions were done using GeneBee-NET (http://www.genebee.msu.su/services/rna2_reduced.html).
[0017] FIG. 7. Histogram plots comparing Expression scores (E ranging from 0 to 5) using the TOEET technology (E_TOEET) compared to expression scores for the same target protein using a pET vector lacking TOEET technology (E_pET). The data shown in FIG. 7a is for 98 protein target genes cloned into the pNESG_Avi6HT TOEET vector compared with the exact same genes cloned into the pET15_NESG vector (lacking TOEET). The data shown in FIG. 7b is for 94 protein target genes cloned into the pNESG_Nano6HT TOEET vector compared with the exact same genes cloned into pET15_NESG vector (lacking TOEET). In these histogram plots, a value E_TOEET-E_pET=0 indicates that the expression levels for both vectors were identical; values E_TOEET-E_pET>0 indicate that the TOEET technology provided higher level expression, values E_TOEET-E_pET<0 indicate that the TOEET technology provided lower level expression.
DETAILED DESCRIPTION
[0018] mRNA stem-loop structures often inhibit translation initiation and therefore reduce recombinant protein expression (Nomura et al., 1984). High level expression of proteins is affected by a lack of mRNA secondary structure near the translation start site (Kudla et al., 2009; Rocha et al., 1999). In addition, rare codons present within the first ten residues of a protein have deleterious effects on protein expression levels (Gonzalez de Valdivia and Isaksson, 2004). E. coli, like all organisms, prefers to use a subset of the possible codons. The codons that an organism utilizes only infrequently are termed "rare codons" of that organism.
[0019] Heterologous genes from other organisms, which generally have a different codon bias, often contain E. coli rare codons. Decreasing or minimizing mRNA secondary structure near the Ribosome Binding Site (RBS) and translation initiation site, and separately that a lack of rare codons near the start of translation, are important for high level E. coli protein expression (Gonzalez de Valdivia and Isaksson, 2004; Kudla et al., 2009). However, the DNA coding sequence of a target gene destined for heterologous expression in E. coli has evolved under different conditions and may intrinsically contain deleterious rare codons and mRNA secondary structure when cloned into an expression vector. Deleterious rare codons and mRNA secondary structure features are particularly problematic when expressing domains or specific segments of target proteins; e.g., gene segments coding for fragments other than the native N-terminal region of the protein have not evolved to provide for efficient translation initiation. Total gene synthesis, or the chemical synthesis of a protein coding region, may address these problems to some extent, since the DNA sequence can be optimized to reduce these issues (Quan et al., 2011). However, the costs of total gene synthesis are prohibitive for large sets of protein targets, and generally is not suitable for large-scale screening or projects involving expression of many different proteins.
[0020] This invention is based, at least in part, on an unexpected discovery of a new methodology for achieving high-level production of recombinant proteins and protein domains. RNA sequence optimization is a well-known approach for improving protein expression. A feature of the system described herein is that RNA sequence optimization is required only in DNA comprising the vector backbone, including the DNA coding for the 5'-UTR and a common N-terminal polypeptide tag. Each target gene, coding for various target proteins, that is cloned into this vector backbone, need not be optimized individually. Hence, the optimized vector backbone can be used to enhance expression of many different target proteins without the need for target-protein-specific gene sequence optimization. Unlike certain previous methods, gene-by-gene RNA transcript sequence optimization is not required in certain embodiments of the methods described herein. The methodology includes, among others, jointly designing and optimizing sequences encoding 5' untranslated and 5' translated regions of the mRNA transcript produced by an expression vector so as to minimize RNA secondary structure and/or optimize codon usage in the mRNA transcript.
[0021] In one aspect, this invention addresses, among others, the problems associated with mRNA secondary structure and codon bias. Accordingly, the invention provides systems for high-level production of recombinant proteins and protein domains based on the Transcript-Optimized Expression Enhancement Technology (TOEET). As disclosed herein, TOEET is used to design expression vectors that produce mRNA transcripts with minimal RNA secondary structure and optimum codon usage in the nucleotide region around the Ribosomal Binding Site (RBS) and the translation initiation site, as well as minimal RNA secondary structure and optimal codon usage in a region of the transcript coding for an N-terminal polypeptide tag that is encoded directly downstream of the translation initiation site. Optimization can extend up to approximately 100 or more nucleotides on each of the 5' and 3' sides of the RBS. This generally will involve producing a protein with an N-terminal polypeptide tag, which is called an Expression Enhancement Tag (EET). This EET may be designed with other features that support protein production, such as solubility enhancing properties or affinity purification sequence motifs. Solubility enhancing tags known from the literature include the maltose-binding protein, the B1 domain of protein G, and domain of myxococcus protein S, to name a few representative examples. Expression vectors designed with TOEET allow most genes of interest to be produced with enhanced expression.
[0022] An advantage of the TOEET strategy over target gene optimization by total gene synthesis is that unless the 5' end of the synthetic gene is optimized in the context of the untranslated vector sequences, detrimental mRNA secondary structure may form near or around the RBS/translation initiation site. More specifically, even if the 5' translated region of the target gene is optimized by gene synthesis or by specific mutations, enhanced expression may not be realized unless the 5'-translated and 5'-untranslated regions of the transcript are jointly optimized, as described herein. Furthermore, by using a sufficiently long N-terminal EET tag, translated from an optimized RNA sequence that is encoded by the vector itself, there is no need to optimize the sequence of the target gene, avoiding the need for gene-specific synthesis or modification. This feature allows the TOEET technology to be used for target protein expression enhancement in high throughput applications, including expression screening studies and projects involving expression of many different proteins, where gene-specific synthesis or modification would be costly or impractical. The roughly 30 amino-acid residue (or larger) EETs effectively shift any deleterious RNA features of the target gene transcript significantly downstream of the RBS/translation initiation site, so that any potential RNA secondary structure formation with the 5' end of the transcript is avoided, and any RNA secondary structure within the RNA coded for by the target gene itself will likely have little or no effect on expression. This TOEET strategy, which is independent of the target gene sequence, could be used more generally to enhance the expression levels of proteins produced with almost any expression vector or system.
[0023] Accordingly, certain embodiments of the invention provide a method of preparing an expression vector, wherein the expression vector comprises, in order of position: a first nucleic acid sequence encoding a 5' untranslated region (UTR) of an expressed mRNA that comprises a ribosome binding site (RBS); a second nucleic acid sequence encoding a polypeptide tag (i.e., at the N-terminal end of the expressed target protein); and a cloning site, wherein the cloning site enables a target protein coding sequence to be inserted into the vector in-frame with the second nucleic acid sequence to encode a fusion protein comprising the polypeptide tag and the target protein; and wherein the method comprises specifically modifying the nucleic acid sequence encoding (i) the 5' untranslated region and (ii) the adjacent polypeptide tag to minimize RNA secondary structure both within and/or between these two regions of the mRNA.
[0024] As used herein, a vector refers to a nucleic acid molecule capable of transporting another nucleic acid to which it has been linked. The vector can be capable of autonomous replication or integrate into a host DNA. Examples of the vector include a plasmid, cosmid, or viral vector. The vector of this invention includes a nucleic acid in a form suitable for expression of the nucleic acid in a host cell. Preferably the vector includes one or more regulatory sequences operatively linked to the nucleic acid sequence to be expressed. A "regulatory sequence" includes promoters, enhancers, repressor binding sites, and other expression control elements (e.g., polyadenylation signals). Regulatory sequences include those that direct constitutive expression of a nucleotide sequence, as well as tissue-specific regulatory and/or inducible sequences. For example, in certain embodiments of the invention, an expression vector described herein comprises a 5' upstream sequence encoding an operable promoter and associated regulatory sequences. The design of the expression vector can depend on such factors as the choice of the host cell to be transformed, the level of expression of protein desired, and the like.
[0025] As used herein, the 5'UTR of the encoded messenger RNA is transcribed from a promoter and includes a ribosome binding site several nucleotides preceding the start codon.
[0026] As used herein, a "cloning site" enables a sequence, such as, e.g., a target protein coding sequence, to be inserted into an expression vector. For example, the cloning site may be a multiple cloning site (MCS), also known as a polylinker, which is a short nucleic acid sequence that contains many restriction sites. For example, FIG. 1 shows a multiple cloning site, comprising a series of restriction enzyme recognition sites. In certain embodiments, the sequence is inserted in-frame, enabling expression of the inserted sequence. In certain embodiments, after the sequence, such as, e.g., the target protein coding sequence, has been inserted into the cloning site of the vector, a portion of the cloning site remains as flanking sequence on one or both sides of the inserted sequence. In other embodiments, the cloning site no longer remains after the insertion of the sequence into the cloning site of the vector.
[0027] As described herein, the nucleic acid sequence encoding (i) the 5' untranslated region and (ii) the adjacent polypeptide tag may be specifically modified to minimize RNA secondary structure both within and/or between these two regions of the mRNA. In certain embodiments, one feature of the method described herein is that RNA optimization is required only in DNA comprising the vector backbone, including the DNA coding for the 5'-UTR and a common N-terminal polypeptide tag, and each gene coding for various target proteins, that is cloned into this vector backbone, need not be optimized individually. Accordingly, nucleic acids within the specific sequence encoding the 5' untranslated region and the adjacent polypeptide tag are replaced with different nucleic acids to minimize RNA secondary structure of the expressed mRNA as described herein. In particular, in certain embodiments, the RNA secondary structure is minimized in the region surrounding the RBS and/or translation initiation site of the expressed mRNA. For example, nucleic acids are replaced to reduce base pairing with the RBS and/or translation initiation site of the expressed mRNA. In certain embodiments, the nucleic acid sequence directly surrounding the RBS site and/or the translation initiation site (e.g., the consensus sequences and sequences between these two sites) is minimally modified or not modified. For example, after modification the RBS site and the translation initiation site remain functionally active. In certain embodiments, nucleotides within the nucleic acid sequence encoding the polypeptide tag are modified in a manner that results in silent mutations.
[0028] Prediction of RNA secondary structure can be readily determined by one skilled in the art using techniques and tools known in the art. For example, a skilled artisan may use RNA structure prediction software, including CentroidFold (Hamada et al., 2009), CentroidHomfold (Hamada et al., 2009), CONTRAfold (Do et al., 2006), CyloFold (Bindewald et al.), KineFold (Xayaphoummine et al., 2005; Xayaphoummine et al., 2003), Mfold (Zuker and Stiegler, 1981), GeneBee-NET (Brodskii et al., 1995), (Pknots (Rivas and Eddy, 1999), PknotsRG (Reeder et al., 2007), RNAl23 (www.rna123.com), RNAfold (Gruber et al., 2008), RNAshapes (Voss et al., 2006), RNAstructure (Mathews et al., 2004), Sfold (Ding et al., 2004), UNAFold (Markham and Zuker, 2008), Crumple (Schroeder et al., 2011), and Sliding Windows & Assembly (Schroeder et al., 2011) among others.
[0029] As described herein, a target protein may refer to any of the following non-limiting embodiments: a full-length naturally occurring protein, a polypeptide sequence corresponding to a fragment or domain of a naturally occurring protein sequence, a mutant or modified form of a full-length protein or protein fragment, or a polypeptide sequence coding for a non-natural protein, such as proteins that have been engineered or designed by artificial methods.
[0030] Certain embodiments of the invention provide a method of preparing an expression vector, wherein the expression vector comprises, in order of position, a 5' upstream sequence encoding an operable promoter and associated regulatory signals, a sequence encoding the 5' untranslated region of the messenger RNA transcribed from the promoter including a ribosome binding site several nucleotides preceding the translation start codon, a sequence beginning with the start codon encoding a polypeptide tag, and a cloning site that enables "target protein" coding sequences to be inserted into the vector in-frame with the polypeptide tag thus allowing their expression as fusions to the polypeptide tag, wherein the method comprises specifically modifying the entire sequence encoding the 5' untranslated region of the messenger RNA through and including the sequence encoding the polypeptide tag sequence in order to minimize RNA secondary structure upstream of the target insertion site.
[0031] In certain embodiments, the method further comprises specifically modifying the second nucleic acid sequence to reduce the presence of rare codons (i.e. mRNA codons for which the corresponding tRNAs are in low abundance in the host cell). For example, rare codons are replaced with high frequency codons to increase expression of any target protein expressed by the vector. Codons that are considered rare are dependent on the selected host cell that is used for expression of the vector and are known to and/or can be readily determined by one skilled in the art. For example, rare codons may be identified using computer software programs known in the art, for example, the Rare Codon Calculator (RaCC) for E. coli (http://nihserver.mbi.ucla.edu/RACC/), http://www.jcat.de/, or http://genomes.urv.es/OPTIMIZER/.
[0032] In certain embodiments, the modified region of the nucleic acid sequence spans from the first 5' nucleotide in the expressed mRNA to the last nucleotide of the polypeptide tag.
[0033] In certain embodiments, nucleotides within about the last 20 nucleotides of the first nucleic acid sequence are modified (i.e., from the nucleotide that directly precedes the encoded start codon to 20 nucleotides upstream). In certain embodiments, nucleotides within about the last, e.g., 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 225, 250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500, 525, 550, 575, 600, 625, 650, 675, 700, 725, 750, 775, 800, 825, 850, 875, 900, 925, 950, 975 or 1,000 nucleotides of the first nucleic acid sequence are modified.
[0034] In certain embodiments, nucleotides within about the first 20 nucleotides of the second nucleic acid sequence are modified (i.e., from the first nucleotide within the encoded start codon to 20 nucleotides downstream). In certain embodiments, nucleotides within about the first, e.g., 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 225, 250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500, 525, 550, 575, 600, 625, 650, 675, 700, 725, 750, 775, 800, 825, 850, 875, 900, 925, 950, 975 or 1,000 nucleotides of the second nucleic acid sequence are modified.
[0035] In certain embodiments, the expression vector further comprises a target protein coding sequence inserted into the vector in-frame with the nucleic acid tag sequence to encode a fusion protein comprising the polypeptide tag and the target protein.
[0036] In certain embodiments, the target protein coding sequence is not modified to minimize RNA secondary structure.
[0037] In certain embodiments, the target protein coding sequence is not modified to reduce the presence of rare codons.
[0038] In certain embodiments, the target protein coding sequence is modified to minimize RNA secondary structure.
[0039] In certain embodiments, the target protein coding sequence is modified to reduce the presence of rare codons.
[0040] As used herein, the second nucleic acid sequence encodes at least one polypeptide tag. In certain embodiments, the second nucleic acid sequence encodes more than one polypeptide tag. As used herein, when the second nucleic acid sequence encodes more than one polypeptide tag, the respective sequences that encode each polypeptide tag are joined in-frame to result in a fusion protein that comprises each polypeptide tag. In certain embodiments, the second nucleic acid sequence encodes, e.g., two, three, four, five, etc. polypeptide tags.
[0041] As used herein, the second nucleic acid sequence may encode any polypeptide tag appropriate to the particular chosen application or selected target protein (e.g., an affinity purification tag and/or a solubility enhancement tag). Polypeptide tags are known to those skilled in the art. For example, the encoded polypeptide tag may be an Avi-tag, Calmodulin-tag, FLAG-tag, HA-tag, His-tag, Myc-tag, S-tag, SBP-tag, Softag 1, Softag 3, V5 tag, Xpress tag, Isopeptag, Spy tag, BCCP, Glutathione-S-transferase-tag, Green fluorescent protein-tag, Maltose binding protein-tag, Nus-tag, Strep-tag, Thioredoxin-tag, TC tag, Ty tag, Nano-tag, Halo-tag, protein G B1 domain tag, a myxococcus protein S tag or Protein A tag.
[0042] Accordingly, in certain embodiments, the at least one encoded polypeptide tag is selected from an Avi-tag, Calmodulin-tag, FLAG-tag, HA-tag, His-tag, Myc-tag, S-tag, SBP-tag, Softag 1, Softag 3, V5 tag, Xpress tag, Isopeptag, Spy tag, BCCP, Glutathione-S-transferase-tag, Green fluorescent protein-tag, Maltose binding protein-tag, Nus-tag, Strep-tag, Thioredoxin-tag, TC tag, Ty tag, Nano-tag, Halo-tag, protein G B1 domain tag, a myxococcus protein S tag or Protein A tag.
[0043] In certain embodiments, the second nucleic acid sequence encodes at least one affinity purification tag.
[0044] In certain embodiments, the second nucleic acid sequence encodes more than one affinity purification tag.
[0045] In certain embodiments, the second nucleic acid sequence encodes two affinity purification tags.
[0046] In certain embodiments, the encoded affinity purification tag(s) is/are selected from a Streptavidin binding moiety, a maltose binding protein moiety, and a HIS tag.
[0047] In certain embodiments, the Streptavidin binding moiety is a Nano-tag or a biotinylated Avi-tag.
[0048] In certain embodiments, the second nucleic acid sequence encodes no affinity purification tags.
[0049] In certain embodiments, the second nucleic acid sequence encodes at least one solubility enhancement tag.
[0050] In certain embodiments, the second nucleic acid sequence encodes more than one solubility enhancement tag.
[0051] In certain embodiments, the second nucleic acid sequence encodes two solubility enhancement tags.
[0052] In certain embodiments, the encoded solubility enhancement tag(s) is/are selected from a maltose binding protein tag, a protein G B1 domain tag, and a myxococcus protein S tag.
[0053] In certain embodiments, the second nucleic acid sequence encodes no solubility enhancement tags.
[0054] In certain embodiments, the second nucleic acid sequence further encodes at least one protease recognition site. In certain embodiments, the second nucleic acid sequence encodes more than one protease recognition site.
[0055] As used herein, when the second nucleic acid sequence further encodes a protease recognition site(s), the sequence that encodes this/these site(s) is/are inserted in-frame with the sequence(s) that encode the at least one polypeptide tag to result in a fusion protein that comprises the polypeptide tag(s) and the protease recognition site(s). In certain embodiments, the encoded protease recognition site(s) is/are downstream of the encoded polypeptide tag(s). In certain embodiments, the encoded protease recognition site is/are between a series of encoded polypeptide tag(s).
[0056] In certain embodiments, the protease recognition site(s) is/are a Tobacco Etch Virus (TEV), Thrombin, Factor Xa and/or a human rhinovirus (HRV) 3C (e.g., PreScission Protease, GE Healthcare Life Sciences, Pittsburgh, Pa.) protease recognition site.
[0057] As described herein, the PreScission Protease is a genetically engineered protein consisting of human rhinovirus 3C protease. It is often produced as a fusion protein with a hexaHis or GST affinity purification tag. It specifically cleaves between the Gln and Gly residues of the recognition sequence of LeuGluValLeuPheGln/GlyPro.
[0058] In certain embodiments, the second nucleic acid sequence is at least about 21 nucleotides in length. In certain embodiments, the second nucleic acid sequence is at least about, e.g., 24, 27, 30, 33, 36, 39, 42, 45, 48, 51, 54, 57, 60, 63, 66, 69, 72, 75, 78, 81, 84, 87, 90, 93, 96, 99, 102, 105, 108, 111, 114, 117, 120, 123, 126, 129, 132, 135, 138, 141, 144, 147, 150, 201, 252, 303, 354, 405, 456, 507, 558, 609, 660, 711, 762, 813, 864, 915, 966, or 1,017 nucleotides in length.
[0059] In certain embodiments, the target protein coding sequence encodes a transcription factor, a transcription factor domain, an epigenetic regulatory factor, or an epigenetic regulatory factor domain.
[0060] In certain embodiments, the target protein coding sequence encodes a polypeptide sequence described in Table 2. As described herein, the target protein coding sequence may also encode a polypeptide sequence that has substantial identity to or is a functional equivalent of a polypeptide sequence described in Table 2.
[0061] In certain embodiments, the target protein coding sequence encodes a protein antigen for producing an affinity capture reagent.
[0062] In certain embodiments, the affinity capture reagent is an antibody, an antibody fragment, or an aptamer.
[0063] In certain embodiments, the target protein coding sequence encodes a protein antigen for producing an antibody or Fab by phage display.
[0064] In certain embodiments, the expression of the target protein is about 1.5 fold greater than the expression of a target protein generated from an expression vector that was not modified as described herein. In certain embodiments, the expression of the target protein is, e.g., about 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, 10, 10.5, 11, 11.5, 12, 12.5, 13, 13.5, 14, 14.5, 15, 15.5, 16, 16.5, 17, 17.5, 18, 18.5, 19, 19.5, or 20, etc., fold greater than the expression of a target protein generated from an expression vector that was not modified as described herein.
[0065] As described herein, in certain embodiments, expression of a target protein from a vector that is not TOEET modified as described herein is undetectable, whereas expression of the same target protein from a vector that has been modified as described herein is detectable.
[0066] Certain embodiments of the invention provide an expression vector prepared using a method as described herein.
[0067] Certain embodiments of the invention provide a target protein expression vector (e.g. a target protein expression vector) comprising, in order of position: a first nucleic acid sequence encoding a 5' untranslated region of an expressed mRNA that comprises a ribosome binding site (RBS); a second nucleic acid sequence encoding a polypeptide tag; and a cloning site, wherein the cloning site enables a target protein coding sequence to be inserted into the vector in-frame with the second nucleic acid sequence to encode a fusion protein comprising the polypeptide tag and the target protein; and wherein the nucleic acid sequence encoding (i) the 5' untranslated region and (ii) the adjacent polypeptide tag has been specifically modified to minimize RNA secondary structure both within and/or between these two regions of the mRNA.
[0068] In certain embodiments, the second nucleic acid sequence has been specifically modified to reduce the presence of rare codons.
[0069] In certain embodiments, the modified region of the nucleic acid sequence spans from the first 5' nucleotide in the expressed mRNA to the last nucleotide of the polypeptide tag.
[0070] In certain embodiments, nucleotides within about the last 20 nucleotides of the first nucleic acid sequence have been modified (i.e., from the nucleotide that directly precedes the encoded start codon to 20 nucleotides upstream). In certain embodiments, nucleotides within about the last, e.g., 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 225, 250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500, 525, 550, 575, 600, 625, 650, 675, 700, 725, 750, 775, 800, 825, 850, 875, 900, 925, 950, 975 or 1,000 nucleotides of the first nucleic acid sequence have been modified.
[0071] In certain embodiments, nucleotides within about the first 20 nucleotides of the second nucleic acid sequence have been modified (i.e., from the first nucleotide within the encoded start codon to 20 nucleotides downstream). In certain embodiments, nucleotides within about the first, e.g., 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 225, 250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500, 525, 550, 575, 600, 625, 650, 675, 700, 725, 750, 775, 800, 825, 850, 875, 900, 925, 950, 975 or 1,000 nucleotides of the second nucleic acid sequence have been modified.
[0072] In certain embodiments, an expression vector as described herein, further comprises a target protein coding sequence inserted into the vector in-frame with the nucleic acid tag sequence to encode a fusion protein comprising the polypeptide tag and the target protein.
[0073] In certain embodiments, the target protein coding sequence has not been modified to minimize RNA secondary structure.
[0074] In certain embodiments, the target protein coding sequence has not been modified to eliminate rare codons.
[0075] In certain embodiments, the target protein coding sequence has been modified to minimize RNA secondary structure.
[0076] In certain embodiments, the target protein coding sequence has been modified to eliminate rare codons.
[0077] In certain embodiments, the second nucleic acid sequence encodes at least one affinity purification tag.
[0078] In certain embodiments, the second nucleic acid sequence encodes more than one polypeptide tag. As used herein, when the second nucleic acid sequence encodes more than one polypeptide tag, the respective sequences that encode each polypeptide tag are joined in-frame to result in a fusion protein that comprises each polypeptide tag. In certain embodiments, the second nucleic acid sequence encodes, e.g., two, three, four, five, etc. polypeptide tags.
[0079] As used herein, the second nucleic acid sequence may encode any polypeptide tag appropriate to the particular chosen application or selected target protein (e.g., an affinity purification tag or a solubility enhancement tag). Polypeptide tags are known to those skilled in the art. For example, the encoded polypeptide tag may be an Avi-tag, Calmodulin-tag, FLAG-tag, HA-tag, His-tag, Myc-tag, S-tag, SBP-tag, Softag 1, Softag 3, V5 tag, Xpress tag, Isopeptag, Spy tag, BCCP, Glutathione-S-transferase-tag, Green fluorescent protein-tag, Maltose binding protein-tag, Nus-tag, Strep-tag, Thioredoxin-tag, TC tag, Ty tag, Nano-tag, Halo-tag, protein G B1 domain tag, a myxococcus protein S tag or Protein A tag.
[0080] Accordingly, in certain embodiments, the at least one encoded polypeptide tag is selected from an Avi-tag, Calmodulin-tag, FLAG-tag, HA-tag, His-tag, Myc-tag, S-tag, SBP-tag, Softag 1, Softag 3, V5 tag, Xpress tag, Isopeptag, Spy tag, BCCP, Glutathione-S-transferase-tag, Green fluorescent protein-tag, Maltose binding protein-tag, Nus-tag, Strep-tag, Thioredoxin-tag, TC tag, Ty tag, Nano-tag, Halo-tag, protein. G B1 domain tag, a myxococcus protein S tag or Protein A tag.
[0081] In certain embodiments, the second nucleic acid sequence encodes more than one affinity purification tag.
[0082] In certain embodiments, the second nucleic acid sequence encodes two affinity purification tags.
[0083] In certain embodiments, the encoded affinity purification tag(s) is/are selected from a Streptavidin binding moiety, a maltose binding protein moiety, and a HIS tag.
[0084] In certain embodiments the Streptavidin binding moiety is a Nano-tag or a biotinylated Avi-tag.
[0085] In certain embodiments, the second nucleic acid sequence encodes no affinity purification tags.
[0086] In certain embodiments, the second nucleic acid sequence encodes at least one solubility enhancement tag.
[0087] In certain embodiments, the second nucleic acid sequence encodes more than one solubility enhancement tag.
[0088] In certain embodiments, the second nucleic acid sequence encodes two solubility enhancement tags.
[0089] In certain embodiments, the encoded solubility enhancement tag(s) is/are selected from a maltose binding protein tag, a protein G B1 domain tag, and a myxococcus protein S tag.
[0090] In certain embodiments, the second nucleic acid sequence encodes at least one protease recognition site.
[0091] As used herein, when the second nucleic acid sequence further encodes a protease recognition site(s), the sequence that encodes this/these site(s) is/are inserted in-frame with the sequence(s) that encode the at least one polypeptide tag to result in a fusion protein that comprises the polypeptide tag(s) and the protease recognition site(s). In certain embodiments, the encoded protease recognition site(s) is/are downstream of the encoded polypeptide tag(s). In certain embodiments, the encoded protease recognition site is/are between a series of encoded polypeptide tag(s).
[0092] In certain embodiments, the protease recognition site(s) is/are a Tobacco Etch Virus (TEV), Thrombin, Factor Xa and/or a HRV 3C protease recognition site.
[0093] In certain embodiments, the second nucleic acid sequence is at least about 21 nucleotides in length. In certain embodiments, the second nucleic acid sequence is at least about, e.g., 24, 27, 30, 33, 36, 39, 42, 45, 48, 51, 54, 57, 60, 63, 66, 69, 72, 75, 78, 81, 84, 87, 90, 93, 96, 99, 102, 105, 108, 111, 114, 117, 120, 123, 126, 129, 132, 135, 138, 141, 144, 147, 150, 201, 252, 303, 354, 405, 456, 507, 558, 609, 660, 711, 762, 813, 864, 915, 966, or 1,017 nucleotides in length.
[0094] In certain embodiments, the target protein coding sequence encodes a transcription factor, a transcription factor domain, an epigenetic regulatory factor, or an epigenetic regulatory factor domain.
[0095] In certain embodiments, the target protein coding sequence encodes a polypeptide sequence described in Table 2. As described herein, the target protein coding sequence may also encode a polypeptide sequence that has substantial identity to or is a functional equivalent of a polypeptide sequence described in Table 2.
[0096] In certain embodiments, the target protein coding sequence encodes a protein antigen for producing an affinity capture reagent.
[0097] In certain embodiments, the affinity capture reagent is an antibody, an antibody fragment, or an aptamer.
[0098] In certain embodiments, the target protein coding sequence encodes a protein antigen for producing an antibody or Fab by phage display.
[0099] In certain embodiments, the target protein is expressed at about a 1.5 fold higher level than a target protein generated from an expression vector that was not modified as described herein. In certain embodiments, the target protein is expressed at about, e.g., a 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, 10, 10.5, 11, 11.5, 12, 12.5, 13, 13.5, 14, 14.5, 15, 15.5, 16, 16.5, 17, 17.5, 18, 18.5, 19, 19.5, or 20, etc., higher level than a target protein generated from an expression vector that was not modified as described herein.
[0100] As described herein, in certain embodiments, expression of a target protein from a vector not modified as described herein is undetectable, whereas expression of the same target protein from a vector that has been modified as described herein is detectable.
[0101] Certain embodiments of the invention provide a host cell comprising the expression vector as described herein. Host cells are used for the expression of vectors and are known in the art. For example, a host cell may be a bacterial cell, such as E. coli.
[0102] Certain embodiments of the invention provide a method for expressing a target protein in a host cell, comprising culturing the host cell as described herein for a period of time under conditions permitting expression of the target protein.
[0103] In certain embodiments, the target protein is a protein antigen for producing an affinity capture reagent.
[0104] In certain embodiments, the affinity capture reagent is an antibody, an antibody fragment, or an aptamer.
[0105] In certain embodiments, the target protein is a protein antigen for producing an antibody or Fab by phage display.
[0106] In one aspect, the invention features a method of designing an expression vector for expressing a recombinant protein in a host cell, e.g., bacterial cell (such as E. coli. cell). The method includes steps of: obtaining a first sequence encoding the recombinant protein; obtaining an expression vector containing an insertion site for the first sequence, wherein once inserted at the insertion site, the first sequence is joined in frame with a 5' sequence from the expression vector to form a first fusion sequence that encodes a RNA sequence, the RNA sequence having a Ribosomal Binding Site (RBS) and a translation initiation site; modifying the RNA sequence by (i) designing the RNA sequence so as to minimize RNA secondary structure in a region around the RBS site or translation initiation site, or (ii) optimizing codon usage in the RNA sequence based on codon usage of the host cell, to obtain a second fusion sequence; and cloning the second fusion sequence into the expression vector in such a way to replace the first fusion sequence.
[0107] In one embodiment, the designing step or optimizing step is carried out using Transcript-Optimized Expression Enhancement Technology (TOEET) as shown and described herein. In another, the designing step or optimizing step is carried out by introducing a third sequence encoding a N-terminal polypeptide expression-enhancement tag (EET) directly downstream of the initiation site.
[0108] The expression-enhancement tag can be an affinity purification tag, such as one having the sequence of an Avi tag, a Nano-tag, or a 6×His tag.
[0109] In a second aspect, the invention provides an expression vector that is designed using the method described above. In the expression vector, the second fusion sequence can have a sequence selected from the sequences shown in FIG. 1. In one example, the expression vector is selected from the group consisting of pNESG_Avi6HT and pNESG_Nano6HT. The invention also provides a host cell having the expression vector.
[0110] In a third aspect, the invention features a method for increasing the expression and solubility of a recombinant protein in a host cell. The method includes obtaining the just described host cell; culturing the host cell in a culture for period of time; and recovering the recombinant protein from the host cell or the culture. To that end, the recombinant protein can be a protein antigen for producing an affinity capture reagent (such as an antibody, an antibody fragment, or an aptamer) or a protein antigen for producing antibody or Fab by phage display.
[0111] In a fourth aspect, the invention provides an immunogenic composition having the recombinant protein produced by the method described above. The composition can be administered to a subject in need thereof for generating an immune response in the subject.
[0112] In a fifth aspect, the invention provides a method of generating an antibody (either polyclonal or monoclonal) by, among others, administrating to a subject the immunogenic composition described above.
[0113] The invention also provides an isolated polypeptide, a nucleic acid encoding it, a high throughput method for identifying a soluble protein or protein domain, and a high throughput method for isolating a soluble protein or protein domain substantially as shown and described herein.
[0114] The term "nucleic acid" refers to deoxyribonucleotides (DNA, e.g., a cDNA or genomic DNA), ribonucleotides (RNA, e.g., an mRNA), or a DNA or RNA analog and polymers thereof, in either single- or double-stranded form, but preferably is double-stranded DNA, made of monomers (nucleotides) containing a sugar, phosphate and a base that is either a purine or pyrimidine. A DNA or RNA analog can be synthesized from nucleotide analogs. Unless specifically limited, the term encompasses nucleic acids containing known analogs of natural nucleotides that have similar binding properties as the reference nucleic acid and are metabolized in a manner similar to naturally occurring nucleotides. Unless otherwise indicated, a particular nucleic acid sequence also encompasses conservatively modified variants thereof (e.g., degenerate codon substitutions) and complementary sequences, as well as the sequence explicitly indicated. Specifically, degenerate codon substitutions may be achieved by generating sequences in which the third position of one or more selected (or all) codons is substituted with mixed-base and/or deoxyinosine residues.
[0115] The term "nucleotide sequence" refers to a polymer of DNA or RNA which can be single-stranded or double-stranded, optionally containing synthetic, non-natural or altered nucleotide bases capable of incorporation into DNA or RNA polymers. The terms "nucleic acid," "nucleic acid molecule," or "polynucleotide" are used interchangeably.
[0116] Certain embodiments of the invention encompass isolated or substantially purified nucleic acid compositions. An "isolated nucleic acid" is a nucleic acid the structure of which is not identical to that of any naturally occurring nucleic acid or to that of any fragment of a naturally occurring genomic nucleic acid. The term therefore covers, for example, (a) a DNA which has the sequence of part of a naturally occurring genomic DNA molecule but is not flanked by both of the coding sequences that flank that part of the molecule in the genome of the organism in which it naturally occurs; (b) a nucleic acid incorporated into a vector or into the genomic DNA of a prokaryote or eukaryote in a manner such that the resulting molecule is not identical to any naturally occurring vector or genomic DNA; (c) a separate molecule such as a cDNA, a genomic fragment, a fragment produced by polymerase chain reaction (PCR), or a restriction fragment; and (d) a recombinant nucleotide sequence that is part of a hybrid gene, i.e., a gene encoding a fusion protein. Specifically excluded from this definition are nucleic acids present in mixtures of different (i) DNA molecules, (ii) transfected cells, or (iii) cell clones, e.g., as these occur in a DNA library such as a cDNA or genomic DNA library. The nucleic acid described above can be used to express a fusion protein of this invention. For this purpose, one can operatively link the nucleic acid to suitable regulatory sequences to generate an expression vector. The following terms are used to describe the sequence relationships between two or more nucleotide sequences: (a) "reference sequence," (b) "comparison window," (c) "sequence identity," (d) "percentage of sequence identity," and (e) "substantial identity."
[0117] (a) As used herein, "reference sequence" is a defined sequence used as a basis for sequence comparison. A reference sequence may be a subset or the entirety of a specified sequence; for example, as a segment of a full-length cDNA or gene sequence, or the complete cDNA or gene sequence.
[0118] (b) As used herein, "comparison window" makes reference to a contiguous and specified segment of a polynucleotide sequence, wherein the polynucleotide sequence in the comparison window may comprise additions or deletions (i.e., gaps) compared to the reference sequence (which does not comprise additions or deletions) for optimal alignment of the two sequences. Generally, the comparison window is at least 20 contiguous nucleotides in length, and optionally can be 30, 40, 50, 100, or longer. Those of skill in the art understand that to avoid a high similarity to a reference sequence due to inclusion of gaps in the polynucleotide sequence a gap penalty is typically introduced and is subtracted from the number of matches.
[0119] Methods of alignment of sequences for comparison are well-known in the art. Thus, the determination of percent identity between any two sequences can be accomplished using a mathematical algorithm. Non-limiting examples of such mathematical algorithms are the algorithm of Myers and Miller (Myers and Miller, CABIOS, 4, 11 (1988)); the local homology algorithm of Smith et al. (Smith et al., Adv. Appl. Math., 2, 482 (1981)); the homology alignment algorithm of Needleman and Wunsch (Needleman and Wunsch, JMB, 48, 443 (1970)); the search-for-similarity-method of Pearson and Lipman (Pearson and Lipman, Proc. Natl. Acad. Sci. USA, 85, 2444 (1988)); the algorithm of Karlin and Altschul (Karlin and Altschul, Proc. Natl. Acad. Sci. USA, 87, 2264 (1990)), modified as in Karlin and Altschul (Karlin and Altschul, Proc. Natl. Acad. Sci. USA 90, 5873 (1993)).
[0120] Computer implementations of these mathematical algorithms can be utilized for comparison of sequences to determine sequence identity. Such implementations include, but are not limited to: CLUSTAL in the PC/Gene program (available from Intelligenetics, Mountain View, Calif.); the ALIGN program (Version 2.0) and GAP, BESTFIT, BLAST, FASTA, and TFASTA in the Wisconsin Genetics Software Package, Version 8 (available from Genetics Computer Group (GCG), 575 Science Drive, Madison, Wis., USA). Alignments using these programs can be performed using the default parameters. The CLUSTAL program is well described by Higgins et al. (Higgins et al., CABIOS, 5, 151 (1989)); Corpet et al. (Corpet et al., Nucl. Acids Res., 16, 10881 (1988)); Huang et al. (Huang et al., CABIOS, 8, 155 (1992)); and Pearson et al. (Pearson et al., Meth. Mol. Biol., 24, 307 (1994)). The ALIGN program is based on the algorithm of Myers and Miller, supra. The BLAST programs of Altschul et al. (Altschul et al., JMB, 215, 403 (1990)) are based on the algorithm of Karlin and Altschul supra.
[0121] Software for performing BLAST analyses is publicly available through the National Center for Biotechnology Information. This algorithm involves first identifying high scoring sequence pairs (HSPs) by identifying short words of length W in the query sequence, which either match or satisfy some positive-valued threshold score T when aligned with a word of the same length in a database sequence. T is referred to as the neighborhood word score threshold. These initial neighborhood word hits act as seeds for initiating searches to find longer HSPs containing them. The word hits are then extended in both directions along each sequence for as far as the cumulative alignment score can be increased. Cumulative scores are calculated using, for nucleotide sequences, the parameters M (reward score for a pair of matching residues; always >0) and N (penalty score for mismatching residues; always <0). For amino acid sequences, a scoring matrix is used to calculate the cumulative score. Extension of the word hits in each direction are halted when the cumulative alignment score falls off by the quantity X from its maximum achieved value, the cumulative score goes to zero or below due to the accumulation of one or more negative-scoring residue alignments, or the end of either sequence is reached.
[0122] In addition to calculating percent sequence identity, the BLAST algorithm also performs a statistical analysis of the similarity between two sequences. One measure of similarity provided by the BLAST algorithm is the smallest sum probability (P(N)), which provides an indication of the probability by which a match between two nucleotide or amino acid sequences would occur by chance. For example, a test nucleic acid sequence is considered similar to a reference sequence if the smallest sum probability in a comparison of the test nucleic acid sequence to the reference nucleic acid sequence is less than about 0.1, less than about 0.01, or even less than about 0.001.
[0123] To obtain gapped alignments for comparison purposes, Gapped BLAST (in BLAST 2.0) can be utilized. Alternatively, PSI-BLAST (in BLAST 2.0) can be used to perform an iterated search that detects distant relationships between molecules. When utilizing BLAST, Gapped BLAST, PSI-BLAST, the default parameters of the respective programs (e.g., BLASTN for nucleotide sequences, BLASTX for proteins) can be used. The BLASTN program (for nucleotide sequences) uses as defaults a wordlength (W) of 11, an expectation (E) of 10, a cutoff of 100, M=5, N=-4, and a comparison of both strands. For amino acid sequences, the BLASTP program uses as defaults a wordlength (W) of 3, an expectation (E) of 10, and the BLOSUM62 scoring matrix. Alignment may also be performed manually by inspection.
[0124] For purposes of the present invention, comparison of nucleotide sequences for determination of percent sequence identity to the promoter sequences disclosed herein may be made using the BlastN program (version 1.4.7 or later) with its default parameters or any equivalent program. By "equivalent program" is intended any sequence comparison program that, for any two sequences in question, generates an alignment having identical nucleotide or amino acid residue matches and an identical percent sequence identity when compared to the corresponding alignment generated by the program.
[0125] (c) As used herein, "sequence identity" or "identity" in the context of two nucleic acid or polypeptide sequences makes reference to a specified percentage of residues in the two sequences that are the same when aligned for maximum correspondence over a specified comparison window, as measured by sequence comparison algorithms or by visual inspection. When percentage of sequence identity is used in reference to proteins it is recognized that residue positions which are not identical often differ by conservative amino acid substitutions, where amino acid residues are substituted for other amino acid residues with similar chemical properties (e.g., charge or hydrophobicity) and therefore do not change the functional properties of the molecule. When sequences differ in conservative substitutions, the percent sequence identity may be adjusted upwards to correct for the conservative nature of the substitution. Sequences that differ by such conservative substitutions are said to have "sequence similarity" or "similarity." Means for making this adjustment are well known to those of skill in the art. Typically this involves scoring a conservative substitution as a partial rather than a full mismatch, thereby increasing the percentage sequence identity. Thus, for example, where an identical amino acid is given a score of 1 and a non-conservative substitution is given a score of zero, a conservative substitution is given a score between zero and 1. The scoring of conservative substitutions is calculated, e.g., as implemented in the program PC/GENE (Intelligenetics, Mountain View, Calif.).
[0126] (d) As used herein, "percentage of sequence identity" means the value determined by comparing two optimally aligned sequences over a comparison window, wherein the portion of the polynucleotide sequence in the comparison window may comprise additions or deletions (i.e., gaps) as compared to the reference sequence (which does not comprise additions or deletions) for optimal alignment of the two sequences. The percentage is calculated by determining the number of positions at which the identical nucleic acid base or amino acid residue occurs in both sequences to yield the number of matched positions, dividing the number of matched positions by the total number of positions in the window of comparison, and multiplying the result by 100 to yield the percentage of sequence identity.
[0127] (e)(i) The term "substantial identity" of polynucleotide sequences means that a polynucleotide comprises a sequence that has at least 70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, or 94%, or even at least 95%, 96%, 97%, 98%, or 99% sequence identity, compared to a reference sequence using one of the alignment programs described using standard parameters. One of skill in the art will recognize that these values can be appropriately adjusted to determine corresponding identity of proteins encoded by two nucleotide sequences by taking into account codon degeneracy, amino acid similarity, reading frame positioning, and the like. Substantial identity of amino acid sequences for these purposes normally means sequence identity of at least 70%, 80%, 90%, or even at least 95%.
[0128] Another indication that nucleotide sequences are substantially identical is if two molecules hybridize to each other under stringent conditions. Generally, stringent conditions are selected to be about 5° C. lower than the thermal melting point (Tm) for the specific sequence at a defined ionic strength and pH. However, stringent conditions encompass temperatures in the range of about 1° C. to about 20° C., depending upon the desired degree of stringency as otherwise qualified herein. Nucleic acids that do not hybridize to each other under stringent conditions are still substantially identical if the polypeptides they encode are substantially identical. This may occur, e.g., when a copy of a nucleic acid is created using the maximum codon degeneracy permitted by the genetic code. One indication that two nucleic acid sequences are substantially identical is when the polypeptide encoded by the first nucleic acid is immunologically cross reactive with the polypeptide encoded by the second nucleic acid.
[0129] (e)(ii) The term "substantial identity" in the context of a peptide indicates that a peptide comprises a sequence with at least 70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, or 94%, or even 95%, 96%, 97%, 98% or 99%, sequence identity to the reference sequence over a specified comparison window. In certain embodiments, optimal alignment is conducted using the homology alignment algorithm of Needleman and Wunsch (Needleman and Wunsch, JMB, 48, 443 (1970)). An indication that two peptide sequences are substantially identical is that one peptide is immunologically reactive with antibodies raised against the second peptide. Thus, a peptide is substantially identical to a second peptide, for example, where the two peptides differ only by a conservative substitution. Thus, certain embodiments of the invention provide nucleic acid molecules that are substantially identical to the nucleic acid molecules described herein.
[0130] For sequence comparison, typically one sequence acts as a reference sequence to which test sequences are compared. When using a sequence comparison algorithm, test and reference sequences are input into a computer, subsequence coordinates are designated if necessary, and sequence algorithm program parameters are designated. The sequence comparison algorithm then calculates the percent sequence identity for the test sequence(s) relative to the reference sequence, based on the designated program parameters.
[0131] As noted above, another indication that two nucleic acid sequences are substantially identical is that the two molecules hybridize to each other under stringent conditions. The phrase "hybridizing specifically to" refers to the binding, duplexing, or hybridizing of a molecule only to a particular nucleotide sequence under stringent conditions when that sequence is present in a complex mixture (e.g., total cellular) DNA or RNA. "Bind(s) substantially" refers to complementary hybridization between a probe nucleic acid and a target nucleic acid and embraces minor mismatches that can be accommodated by reducing the stringency of the hybridization media to achieve the desired detection of the target nucleic acid sequence.
[0132] "Stringent hybridization conditions" and "stringent hybridization wash conditions" in the context of nucleic acid hybridization experiments such as Southern and Northern hybridizations are sequence dependent, and are different under different environmental parameters. Longer sequences hybridize specifically at higher temperatures. The thermal melting point (Tm) is the temperature (under defined ionic strength and pH) at which 50% of the target sequence hybridizes to a perfectly matched probe. Specificity is typically the function of post-hybridization washes, the critical factors being the ionic strength and temperature of the final wash solution. For DNA-DNA hybrids, the Tm can be approximated from the equation of Meinkoth and Wahl (1984); Tm 81.5° C.+16.6 (log M)+0.41 (% GC)-0.61 (% form)-500/L; where M is the molarity of monovalent cations, % GC is the percentage of guanosine and cytosine nucleotides in the DNA, % form is the percentage of formamide in the hybridization solution, and L is the length of the hybrid in base pairs. Tm is reduced by about 1° C. for each 1% of mismatching; thus, Tm, hybridization, and/or wash conditions can be adjusted to hybridize to sequences of the desired identity. For example, if sequences with >90% identity are sought, the Tm can be decreased 10° C. Generally, stringent conditions are selected to be about 5° C. lower than the Tm for the specific sequence and its complement at a defined ionic strength and pH. However, severely stringent conditions can utilize a hybridization and/or wash at 1, 2, 3, or 4° C. lower than the Tm; moderately stringent conditions can utilize a hybridization and/or wash at 6, 7, 8, 9, or 10° C. lower than the Tm; low stringency conditions can utilize a hybridization and/or wash at 11, 12, 13, 14, 15, or 20° C. lower than the Tm. Using the equation, hybridization and wash compositions, and desired temperature, those of ordinary skill will understand that variations in the stringency of hybridization and/or wash solutions are inherently described. If the desired degree of mismatching results in a temperature of less than 45° C. (aqueous solution) or 32° C. (formamide solution), the SSC concentration is increased so that a higher temperature can be used. Generally, highly stringent hybridization and wash conditions are selected to be about 5° C. lower than the Tm for the specific sequence at a defined ionic strength and pH.
[0133] An example of highly stringent wash conditions is 0.15 M NaCl at 72° C. for about 15 minutes. An example of stringent wash conditions is a 0.2×SSC wash at 65° C. for 15 minutes. Often, a high stringency wash is preceded by a low stringency wash to remove background probe signal. An example medium stringency wash for a duplex of, e.g., more than 100 nucleotides, is 1×SSC at 45° C. for 15 minutes. For short nucleotide sequences (e.g., about 10 to 50 nucleotides), stringent conditions typically involve salt concentrations of less than about 1.5 M, less than about 0.01 to 1.0 M, Na ion concentration (or other salts) at pH 7.0 to 8.3, and the temperature is typically at least about 30° C. and at least about 60° C. for long probes (e.g., >50 nucleotides). Stringent conditions may also be achieved with the addition of destabilizing agents such as formamide. In general, a signal to noise ratio of 2× (or higher) than that observed for an unrelated probe in the particular hybridization assay indicates detection of a specific hybridization. Nucleic acids that do not hybridize to each other under stringent conditions are still substantially identical if the proteins that they encode are substantially identical. This occurs, e.g., when a copy of a nucleic acid is created using the maximum codon degeneracy permitted by the genetic code.
[0134] Very stringent conditions are selected to be equal to the Tm for a particular probe. An example of stringent conditions for hybridization of complementary nucleic acids that have more than 100 complementary residues on a filter in a Southern or Northern blot is 50% formamide, e.g., hybridization in 50% formamide, 1 M NaCl, 1% SDS at 37° C., and a wash in 0.1×SSC at 60 to 65° C. Exemplary low stringency conditions include hybridization with a buffer solution of 30 to 35% formamide, 1 M NaCl, 1% SDS (sodium dodecyl sulphate) at 37° C., and a wash in 1× to 2×SSC (20×SSC=3.0 M NaCl/0.3 M trisodium citrate) at 50 to 55° C. Exemplary moderate stringency conditions include hybridization in 40 to 45% formamide, 1.0 M NaCl, 1% SDS at 37° C., and a wash in 0.5× to 1×SSC at 55 to 60° C.
[0135] In addition to the chemical optimization of stringency conditions, analytical models and algorithms can be applied to hybridization data-sets (e.g. microarray data) to improve stringency.
[0136] An expression vector as described herein can be introduced into host cells to produce a fusion protein of this invention. Also within the scope of this invention is a host cell that contains the above-described nucleic acid. Examples include E. coli cells, insect cells (e.g., using baculovirus expression vectors), yeast cells, plant cells, or mammalian cells. See e.g., Goeddel, (1990) Gene Expression Technology: Methods in Enzymology 185, Academic Press, San Diego, Calif. To produce a fusion protein of this invention, one can culture a host cell in a medium under conditions permitting expression of the protein encoded by a nucleic acid of this invention, and isolate the protein from the cultured cell or the medium of the cell. The presence of the fusion protein in an occlusion body allows one to prepare the protein from the host cell by simply separating the occlusion body from the host cell. Alternatively, the nucleic acid of this invention can be transcribed and translated in vitro, for example, using T7 promoter regulatory sequences and T7 polymerase.
[0137] The terms "peptide," "polypeptide," and "protein" are used herein interchangeably to describe the arrangement of amino acid residues in a polymer. A peptide, polypeptide, or protein can be composed of the standard 20 naturally occurring amino acid, in addition to rare amino acids and synthetic amino acid analogs. They can be any chain of amino acids, regardless of length or post-translational modification (for example, glycosylation or phosphorylation). The peptide, polypeptide, or protein "of this invention" includes recombinantly or synthetically produced fusion versions having the particular domains or portions that are soluble. The term also encompasses polypeptides that have an added amino-terminal methionine (useful for expression in prokaryotic cells).
[0138] A "recombinant" peptide, polypeptide, or protein refers to a peptide, polypeptide, or protein produced by recombinant DNA techniques; i.e., produced from cells transformed by an exogenous DNA construct encoding the desired peptide. A "synthetic" peptide, polypeptide, or protein refers to a peptide, polypeptide, or protein prepared by chemical synthesis. The term "recombinant" when used with reference, e.g., to a cell, or nucleic acid, protein, or vector, indicates that the cell, nucleic acid, protein or vector, has been modified by the introduction of a heterologous nucleic acid or protein or the alteration of a native nucleic acid or protein, or that the cell is derived from a cell so modified.
[0139] Within the scope of this invention are fusion proteins containing one or more of the afore-mentioned sequences and a heterologous sequence. A heterologous polypeptide, nucleic acid, or gene is one that originates from a foreign species, or, if from the same species, is substantially modified from its original form. Two fused domains or sequences are heterologous to each other if they are not adjacent to each other in a naturally occurring protein or nucleic acid.
[0140] An "isolated" peptide, polypeptide, or protein refers to a peptide, polypeptide, or protein that has been separated from other proteins, lipids, and nucleic acids with which it is naturally associated. The polypeptide/protein can constitute at least 10% (i.e., any percentage between 10% and 100%, e.g., 20%, 30%, 40%, 50%, 60%, 70%, 80%, 85%, 90%, 95%, and 99%) by dry weight of the purified preparation. Purity can be measured by any appropriate standard method, for example, by column chromatography, polyacrylamide gel electrophoresis, or HPLC analysis. An isolated polypeptide/protein described in the invention can be purified from a natural source, produced by recombinant DNA techniques, or by chemical methods.
[0141] A functional equivalent of a peptide, polypeptide, or protein of this invention refers to a polypeptide derivative of the peptide, polypeptide, or protein, e.g., a protein having one or more point mutations, insertions, deletions, truncations, a fusion protein, or a combination thereof. It retains substantially the activity of the corresponding unmodified peptide/polypeptide/protein (e.g., the activity of transcription factor). The isolated polypeptide can contain a sequence of a protein as listed in Table 1 or 2 or a functional fragment thereof. In general, the functional equivalent is at least 75% (e.g., any number between 75% and 100%, inclusive, e.g., 70%, 80%, 85%, 90%, 95%, and 99%) identical to the corresponding unmodified peptide/polypeptide/protein.
[0142] The amino acid composition of the above-mentioned peptide/polypeptide/protein may vary without disrupting their biological activity, e.g., a transcription factor activity, i.e., ability to bind to a DNA element and/or trigger or inhibit the respective cellular response. For example, it can contain one or more conservative amino acid substitutions. A "conservative amino acid substitution" is one in which the amino acid residue is replaced with an amino acid residue having a similar side chain. Families of amino acid residues having similar side chains have been defined in the art. These families include amino acids with basic side chains (e.g., lysine, arginine, histidine), acidic side chains (e.g., aspartic acid, glutamic acid), uncharged polar side chains (e.g., glycine, asparagine, glutamine, serine, threonine, tyrosine, cysteine), nonpolar side chains (e.g., alanine, valine, leucine, isoleucine, proline, phenylalanine, methionine, tryptophan), β-branched side chains (e.g., threonine, valine, isoleucine) and aromatic side chains (e.g., tyrosine, phenylalanine, tryptophan, histidine). Thus, a predicted nonessential amino acid residue in a polypeptide is preferably replaced with another amino acid residue from the same side chain family. Alternatively, mutations can be introduced randomly along all or part of the sequences, such as by saturation mutagenesis, and the resultant mutants can be screened for the respective biological activities.
[0143] A polypeptide described in this invention can be obtained as a recombinant polypeptide. To prepare a recombinant polypeptide, a nucleic acid encoding it can be linked to another nucleic acid encoding a fusion partner, e.g., the tags disclosed herein, glutathione-s-transferase (GST), 6×-His epitope tag (or Hexa-His), 8×-His (or Octa-His) epitope tag, or M13 Gene 3 protein. The resultant fusion nucleic acid expresses in suitable host cells a fusion protein that can be isolated by methods known in the art. The isolated fusion protein can be further treated, e.g., by enzymatic digestion (e.g., TEV protease digestion), to remove the fusion partner and obtain the recombinant polypeptide of this invention.
[0144] The peptide/polypeptide/protein of this invention covers chemically modified versions. Examples of chemically modified peptide/protein include those subjected to conformational change, addition or deletion of a sugar chain, and those to which a compound such as polyethylene glycol has been bound. Once purified and tested by standard methods or according to the methods described in the examples below, the peptide/polypeptide/protein can be included in a composition, e.g., a pharmaceutical composition or an immunogenic composition.
[0145] The term "immunogenic" refers to a capability of producing an immune response in a host animal against an antigen or antigens. This immune response forms the basis of the protective immunity elicited by a vaccine against a specific infectious organism. "Immune response" refers to a response elicited in an animal, which may refer to cellular immunity (CMI); humoral immunity or both. "Antigenic agent," "antigen," or "immunogen" means a substance that induces a specific immune response in a host animal. The antigen can be a protein described above, a vector encoding it, a cell having the vector or protein, or any combination thereof.
[0146] The term "animal" includes all vertebrate animals including humans. It also includes an individual animal in all stages of development, including embryonic and fetal stages. In particular, the term "vertebrate animal" includes, but not limited to, humans, canines (e.g., dogs), felines (e.g., cats); equines (e.g., horses), bovines (e.g., cattle), porcine (e.g., pigs), as well as in avians. The term "avian" refers to any species or subspecies of the taxonomic class ava, such as, but not limited to, chickens (breeders, broilers and layers), turkeys, ducks, a goose, a quail, pheasants, parrots, finches, hawks, crows and ratites including ostrich, emu and cassowary.
[0147] The immunogenic composition can be used to generate antibodies against the peptide/polypeptide/protein of this invention. As used herein, "antibody" is used in the broadest sense and specifically covers monoclonal antibodies (including full length monoclonal antibodies), polyclonal antibodies, multispecific antibodies (e.g., bispecific antibodies), and antibody fragments so long as they exhibit the desired biological activity.
[0148] As used herein, "antibody fragments", may comprise a portion of an intact antibody, generally including the antigen binding or variable region of the intact antibody, the Fab region of the antibody, or the Fc region of an antibody which retains FcR binding capability. Examples of antibody fragments include linear antibodies; single-chain antibody molecules; and multispecific antibodies formed from antibody fragments. The antibody fragments preferably retain at least part of the hinge and optionally the CH1 region of an IgG heavy chain. More preferably, the antibody fragments retain the entire constant region of an IgG heavy chain, and include an IgG light chain.
[0149] As used herein, Affinity Capture Reagents are cognate molecules capable or recognizing and binding to a protein antigen, including protein antigens produced by TOEET-optimized expression vectors. Affinity Capture reagents include (but are not limited to) monoclonal and polyclonal antibodies, Fab or Fab fragments generated by phage and related antigen display methods, RNA aptamers, and various protein binding scaffolds which can be used to generate antigen-recognizing molecules.
[0150] As used herein, the term "Fc fragment" or "Fc region" is used to define a C-terminal region of an immunoglobulin heavy chain. The "Fc region" may be a native sequence Fc region or a variant Fc region. Although the boundaries of the Fc region of an immunoglobulin heavy chain might vary, the human IgG heavy chain Fc region is usually defined to stretch from an amino acid residue at position Cys226, or from Pro230, to the carboxyl-terminus thereof.
[0151] A "native sequence Fc region" comprises an amino acid sequence identical to the amino acid sequence of an Fc region found in nature. A "variant Fc region" as appreciated by one of ordinary skill in the art comprises an amino acid sequence which differs from that of a native sequence Fc region by virtue of at least one "amino acid modification." Preferably, the variant Fc region has at least one amino acid substitution compared to a native sequence Fc region or to the Fc region of a parent polypeptide, e.g., from about one to about ten amino acid substitutions, and preferably from about one to about five amino acid substitutions in a native sequence Fc region or in the Fc region of the parent polypeptide. The variant Fc region herein will preferably possess at least about 80% homology with a native sequence Fc region and/or with an Fc region of a parent polypeptide, and more preferably at least about 90% homology therewith, more preferably at least about 95% homology therewith, even more preferably, at least about 99% homology therewith.
[0152] Within the scope of this invention is a composition that contains a suitable carrier and one or more of the agents described above. The composition can be a pharmaceutical composition that contains a pharmaceutically acceptable carrier. The term "pharmaceutical composition" refers to the combination of an active agent with a carrier, inert or active, making the composition especially suitable for diagnostic or therapeutic use in vivo or ex vivo. A "pharmaceutically acceptable carrier," after administered to or upon a subject, does not cause undesirable physiological effects. The carrier in the pharmaceutical composition must be "acceptable" also in the sense that it is compatible with the active ingredient and can be capable of stabilizing it. One or more solubilizing agents can be utilized as pharmaceutical carriers for delivery of an active compound. Examples of a pharmaceutically acceptable carrier include, but are not limited to, biocompatible vehicles, adjuvants, additives, and diluents to achieve a composition usable as a dosage form. Examples of other carriers include colloidal silicon oxide, magnesium stearate, cellulose, and sodium lauryl sulfate.
[0153] As used herein, a "subject" refers to a human and a non-human animal. Examples of a non-human animal include all vertebrates, e.g., mammals, such as non-human mammals, non-human primates (particularly higher primates), dog, rodent (e.g., mouse or rat), guinea pig, cat, and rabbit, and non-mammals, such as birds, amphibians, reptiles, etc. In one embodiment, the subject is a human. In another embodiment, the subject is an experimental, non-human animal or animal suitable as a disease model.
[0154] The composition of this invention can include an adjuvant agent or adjuvant. As used herein, the term "adjuvant agent" or "adjuvant" means a substance added to an immunogenic composition or a vaccine to increase the immunogenic composition or the vaccine's immunogenicity. Examples of an adjuvant include a cholera toxin, Escherichia coli heat-labile enterotoxin, liposome, unmethylated DNA (CpG) or any other innate immune-stimulating complex. Various adjuvants that can be used to further increase the immunological response depend on the host species and include Freund's adjuvant (complete and incomplete), mineral gels such as aluminum hydroxide, surface-active substances such as lysolecithin, pluronic polyols, polyanions, peptides, oil emulsions, keyhole limpet hemocyanin, and dinitrophenol. Useful human adjuvants include BCG (bacille Calmette-Guerin) and Corynebacterium parvum.
[0155] Pharmaceutical compositions comprising an adjuvant and an antigen may be manufactured by means of conventional mixing, dissolving, granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping or lyophilizing processes. Pharmaceutical compositions may be formulated in conventional manner using one or more physiologically acceptable carriers, diluents, excipients or auxiliaries which facilitate processing of the antigens of the invention into preparations which can be used pharmaceutically. Proper formulation is dependent upon the route of administration chosen.
[0156] A pharmaceutical composition of this invention can be administered parenterally, orally, nasally, rectally, topically, or buccally. The term "parenteral" as used herein refers to subcutaneous, intracutaneous, intravenous, intramuscular, intraarticular, intraarterial, intrasynovial, infrasternal, intrathecal, intralesional, or intracranial injection, as well as any suitable infusion technique. For injection, immunogenic or vaccine preparations may be formulated in aqueous solutions, preferably in physiologically compatible buffers such as Hanks's solution, Ringer's solution, phosphate buffered saline, or any other physiological saline buffer. The solution may contain formulatory agents such as suspending, stabilizing and/or dispersing agents. Alternatively, the peptides, polypeptides, or proteins may be in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
[0157] Determination of an effective amount of the immunogenic or vaccine formulation for administration is well within the capabilities of those skilled in the art, especially in light of the detailed disclosure provided herein. An effective dose can be estimated initially from in vitro assays. For example, a dose can be formulated in animal models to achieve an induction of an immune response using techniques that are well known in the art. One having ordinary skill in the art could readily optimize administration to all animal species based on results described herein. Dosage amount and interval may be adjusted individually. For example, when used as a vaccine, the vaccine formulations of the invention may be administered in about 1 to 3 doses for a 1-36 week period. Preferably, 1 or 2 doses are administered, at intervals of about 3 weeks to about 4 months, and booster vaccinations may be given periodically thereafter. Alternative protocols may be appropriate for individual animals. A suitable dose is an amount of the vaccine formulation that, when administered as described above, is capable of raising an immune response in an immunized animal sufficient to protect the animal from an infection for at least 4 to 12 months. In general, the amount of the antigen present in a dose ranges from about 1 pg to about 100 mg per kg of host, typically from about 10 pg to about 1 mg, and preferably from about 100 pg to about 1 pg. Suitable dose range will vary with the route of injection and the size of the patient, but will typically range from about 0.1 ml to about 5 ml.
[0158] This invention also provides methods for making antibodies against the above-described proteins. The antibodies can be either polyclonal or monoclonal.
[0159] Polyclonal antibodies against a protein of the invention can be obtained as follows. After verifying that a desired serum antibody level has been reached, blood is withdrawn from the mammal sensitized with the antigen. Serum is isolated from this blood using well-known methods. The serum containing the polyclonal antibody may be used as the polyclonal antibody, or according to needs, the polyclonal antibody-containing fraction may be further isolated from the serum. For instance, a fraction of antibodies that specifically recognize the protein of the invention may be prepared by using an affinity column to which the protein is coupled. Then, the fraction may be further purified by using a Protein A or Protein G column in order to prepare immunoglobulin G or immunoglobulin M.
[0160] To obtain monoclonal antibodies, after verifying that the desired serum antibody level has been reached in the mammal sensitized with the above-described antigen, immunocytes are taken from the mammal and used for cell fusion. For this purpose, splenocytes can be preferable immunocytes. As parent cells fused with the above immunocytes, mammalian myeloma cells are preferably used. More preferably, myeloma cells that have acquired the feature, which can be used to distinguish fusion cells by agents, are used as the parent cell.
[0161] The cell fusion between the above immunocytes and myeloma cells can be conducted according to known methods, for example, the method of Milstein et al. (Methods Enzymol., 73:3-46, 1981). The hybridoma obtained from cell fusion is selected by culturing the cells in a standard selective culture medium, for example, HAT culture medium (hypoxanthine, aminopterin, thymidine-containing culture medium). The culture in this HAT medium is continued for a period sufficient enough for cells (non-fusion cells) other than the objective hybridoma to perish, usually from a few days to a few weeks. Next, the usual limiting dilution method is carried out, and the hybridoma producing the objective antibody is screened and cloned.
[0162] Other than the above method for obtaining hybridomas, by immunizing an animal other than humans with the antigen, a hybridoma producing the objective human antibodies having the activity to bind to proteins can be obtained by the method of sensitizing human lymphocytes, for example, human lymphocytes infected with the EB virus, with proteins, protein-expressing cells, or lysates thereof in vitro, fusing the sensitized lymphocytes with myeloma cells derived from human having a permanent cell division ability.
[0163] The obtained monoclonal antibodies can be purified by, for example, ammonium sulfate precipitation, protein A or protein G column, DEAE ion exchange chromatography, an affinity column to which the protein of the present invention is coupled, and so on. The antibody may be useful for the purification or detection of a protein of the invention. It may also be a candidate for an agonist or antagonist of the protein. Furthermore, it is possible to use it for the antibody treatment of diseases in which the protein is implicated. For in vivo administration (in such antibody treatment), human antibodies or humanized antibodies may be favorably used because of their reduced antigenicity.
[0164] For example, a human antibody against a protein can be obtained using hybridomas made by fusing myeloma cells with antibody-producing cells obtained by immunizing a transgenic animal comprising a repertoire of human antibody genes with an antigen such as a protein, protein-expressing cells, or a cell lysate thereof. Other than producing antibodies by using hybridoma, antibody--producing immunocytes, such as sensitized lymphocytes that are immortalized by oncogenes, may also be used.
[0165] Such monoclonal antibodies can also be obtained as recombinant antibodies produced by using the genetic engineering technique. Recombinant antibodies are produced by cloning the encoding DNA from immunocytes, such as hybridoma or antibody-producing sensitized lymphocytes, incorporating this into a suitable vector, and introducing this vector into a host to produce the antibody. The present invention encompasses such recombinant antibodies as well.
[0166] Moreover, the antibody of the present invention may be an antibody fragment or a modified-antibody, so long as it binds to a protein of the invention. For example, Fab, F (ab')2, Fv, or single chain Fv in which the H chain Fv and the L chain Fv are suitably linked by a linker (scFv, Huston et al., Proc. Natl. Acad. Sci. USA, 85:5879-5883, 1988) can be given as antibody fragments. Specifically, antibody fragments are produced by treating antibodies with enzymes, for example, papain, pepsin, and such, or by constructing a gene encoding an antibody fragment, introducing this into an expression vector, and expressing this vector in suitable host cells (for example, Co et al., J. Immunol., 152:2968-2976, 1994; Better et al., Methods Enzymol., 178:476-496, 1989; Pluckthun et al., Methods Enzymol., 178:497-515, 1989; Lamoyi, Methods Enzymol., 121:652-663, 1986; Rousseaux et al., Methods Enzymol., 121:663-669, 1986; Bird et al., Trends Biotechnol., 9:132-137, 1991).
[0167] As modified antibodies, antibodies bound to various molecules such as polyethylene glycol (PEG) can be used. The antibody of the present invention encompasses such modified antibodies as well. To obtain such a modified antibody, chemical modifications are done to the obtained antibody. These methods are already established in the field.
[0168] The antibody of the invention may be obtained as a chimeric antibody, comprising non-human antibody-derived variable region and human antibody-derived constant region, or as a humanized antibody comprising non-human antibody-derived complementarity determining region (CDR), human antibody-derived framework region (FR), and human antibody-derived constant region by using conventional methods.
[0169] Antibodies thus obtained can be purified to uniformity. The separation and purification methods used in the present invention for separating and purifying the antibody may be any method usually used for proteins. For instance, column chromatography, such as affmity chromatography, filter, ultrafiltration, salt precipitation, dialysis, SDS-polyacrylamide gel electrophoresis, isoelectric point electrophoresis, and so on, may be appropriately selected and combined to isolate and purify the antibodies (Antibodies: a laboratory manual. Ed Harlow and David Lane, Cold Spring Harbor Laboratory, 1988), but is not limited thereto. Antibody concentration of the above mentioned antibody can be assayed by measuring the absorbance, or by the enzyme-linked immunosorbent assay (ELISA), etc. Protein A or Protein G column can be used for the affmity chromatography. Protein A column may be, for example, Hyper D, POROS, Sepharose F.F., and so on.
[0170] Other chromatography may also be used, such as ion exchange chromatography, hydrophobic chromatography, gel filtration, reverse phase chromatography, and adsorption chromatography (Strategies for Protein Purification and Characterization: A laboratory Course Manual. Ed. by Marshak D.R. et al., Cold Spring Harbor Laboratory Press, 1996). These may be performed on liquid chromatography such as HPLC or FPLC.
[0171] Examples of methods that assay the antigen-binding activity of the antibodies of the invention include, for example, measurement of absorbance, enzyme-linked immunosorbent assay (ELISA), enzyme immunoassay (EIA), radio immunoassay (RIA), or fluorescent antibody method. For example, when using ELISA, a protein of the invention is added to a plate coated with the antibodies of the invention, and next, the objective antibody sample, for example, culture supernatants of antibody-producing cells, or purified antibodies are added. Then, secondary antibody recognizing the antibody, which is labeled by alkaline phosphatase and such enzymes, is added, the plate is incubated and washed, and the absorbance is measured to evaluate the antigen-binding activity after adding an enzyme substrate such as p-nitrophenyl phosphate. As the protein, a protein fragment, for example, a fragment comprising a C-terminus, or a fragment comprising an N-terminus may be used. To evaluate the activity of the antibody of the invention, BIAcore may be used.
[0172] The following non-limiting examples set forth herein below illustrate certain aspects of the invention.
Example 1
[0173] This example describes two specific EET tags designed utilizing TOEET. These EETs were engineered and subcloned into the pET15_NESG expression vector (Acton et al., 2011). They contain dual tandem protein purification tags and a protease cleavage site to facilitate purification of the resulting proteins. These include the 6×-His tag (Crowe et al., 1994), and one of two Streptavidin binding moieties, either the Avi-tag (Scholle et al., 2004) or the Nano-tag (Lamla and Erdmann, 2004). The Nano-tag binds directly to streptavidin (Lamla and Erdmann, 2004); the Avi-tag is a substrate for the enzyme BirA which can be used to catalyze the covalent attachment of biotin to the Avi Tag (Scholle et al., 2004). These tandem tags allow for two separate affinity purification steps, (i) Ni-based immobilized metal affinity chromatography (IMAC) and (ii) high-affinity Streptavidin-based chromatography. This dual purification strategy allows preparation of highly purified proteins using high-throughput affinity purification methods. The Tobacco Etch Virus (TEV) protease recognition site (Kapust et al., 2002) engineered into these EETs allows removal of the affinity tags, if required, after expression and purification of the protein target.
[0174] Briefly, in designing the DNA sequences coding for these EETs, the coding sequence of one of the two Streptavidin binding moieties i.e., Avi-tag (SEQ ID NO:1--MSGLNDIFEAQKIEWHE) or Nano-tag (SEQ ID NO:2--MDVEAWLDERVPLVET) (Lamla and Erdmann, 2004; Scholle et al., 2004), a 6×-His tag (Crowe et al., 1994), and a TEV protease recognition site (Kapust et al., 2002) were fused in frame and optimized to have a high Codon Adaptation Index (Sharp and Li, 1987) (FIG. 1). The DNA sequence coding for the EET was optimized with TOEET, together with the 5'-untranslated region of the pET15-NESG expression vector, to generate the expression vectors pNESG_Avi6HT and pNESG_Nano6HT, shown in FIG. 1. These features functioned together to enhance translation initiation and protein expression levels.
[0175] Using these expression vectors (FIG. 1), protein expression resulted in T7 RNA Polymerase mediated transcription producing an mRNA transcript consisting of (i) vector sequence (pET15_NESG-5'-untranslated region), (ii) nucleotides coding for the EET, and (iii) nucleotides coding for the target protein sequence. Both the untranslated region of the vector upstream of the EET-coding region, and the RNA coding for the EET itself were optimized to avoid secondary structure formation within and between these regions of the mRNA transcript. In this particular implementation, the length of the optimized nucleotide sequence coding for the EET was about 90 nucleotides. Together with the 70 upstream 5'-untranslated nucleotides of the transcript driven by the T7 promoter of the vector, the 5'-region of the transcript was optimized as a unit of about 160 nucleotides. Longer optimized nucleotide sequences, and potentially somewhat shorter optimized nucleotide sequences may also be effective in creating TOEET-based expression-enhanced vectors.
[0176] The optimized regions of the pNESG_Avi6HT and pNESG_Nano6HT based TOEET vectors are shown in FIG. 1. The figure shows the DNA sequences, RNA sequences, and the translated protein tag (SEQ ID NO:3--MSGLNDIFEAQKIEWHEHHHHHHENLYFQSH and SEQ ID NO:4--MDVEAWLDERVPLVETHHHHHHENLYFQSH, respectively) sequences of the expression vectors, along with the DNA sequence coding for the multiple cloning site (MCS), a series of restriction endonuclease sites used for cloning into the expression plasmids. FIG. 2 shows, as an example, the predicted RNA secondary structure in transcripts generated from the pNESG_Avi6HT vector, highlighting the lack of predicted RNA secondary structure near the RBS/translation initiation site.
[0177] A third vector comprising the Pyrococcus furiosus (PfR) Maltose Binding Protein (MBP) was also constructed and optimized using TOEET. The MBP from Pyrococcus furiosus is much more thermally stable than that of E. coli, and is expected to provide a more robust solubilization enhancement tag and affinity purification tag. Proteins that are expressed but not soluble in cell extracts can be solubilized and used successfully as antigens using various methods of solublization, including urea and guanidine denaturtants (Agaton et al, 2003). The PfR MBP provides improved purification of target proteins under such partially denaturing conditions or other harsh conditions. The sequences shown at the top of FIG. 4 correspond to the first 30 residues of the wild-type PfR-MBP DNA sequence lacking the native secretion signal. The protein open reading frame (DNA sequence) is shown above the corresponding protein sequence and directly below is the T7 RNA polymerase mediated RNA transcript resulting from the cloning of the PfR-MBP into the pET15_NESG backbone. The lower set of sequences shown in FIG. 4 correspond to TOEET optimized PfR-MBP. Silent mutations were introduced for codon optimization or to decrease the predicted RNA secondary structure in the regions of the RBS and translation initiation codon, or both. The silent mutations were introduced using primers incorporating the nucleotide changes and 5 successive rounds of PCR, negating the need for expensive total gene synthesis.
[0178] The predicted mRNA secondary structure resulting from T7-RNA Polymerase based transcription off of the pET15_NESG vector backbone with Pyrococcus furiosus (PfR) Maltose Binding Protein (MBP) without TOEET optimization is shown in FIG. 5. Significant secondary structure (base pairing) at both the Ribosome Binding Site (RBS) and the translation initiation site (Initiation Codon) is predicted. The predicted mRNA secondary structure resulting from T7-RNA Polymerase based transcription off of the pET15_NESG vector backbone with Pyrococcus furiosus (PfR) Maltose Binding Protein (MBP) after TOEET optimization is shown in FIG. 6. As illustrated by FIG. 6, significantly greater open structure (lack of base pairing) after TOEET optimization is predicted.
Example 2
[0179] The results obtained from expression studies with the above-described new vectors demonstrated that the TOEET strategy is both extremely successful and robust. In this example, similar expression and solubility studies were carried out using a high throughput methodology for the identification and isolation of soluble proteins and protein domains.
[0180] As mentioned above, the isolation of soluble, well-folded proteins and protein domains is of great use and importance to the biotechnology industry and biological researchers as a whole. However, the production of such protein reagents remains extremely challenging, especially in the cost effective, commonly used bacterial expression systems. These Escherichia coli expression systems are often successful in the production of simple bacterial proteins but are far less amenable to the production of eukaryotic, mulitdomain proteins or protein complexes, often resulting in no or low levels of expression and/or solubility (greatly complicating or thwarting their production as a protein reagent). There are a variety of reasons that contribute to the lower success rate of these proteins in bacterial expression systems including the fact that eukaryotic proteins are frequently multidomain in nature, this often results in misfolding when expressed using simple prokaryotic expression systems (Netzer and Hartl, 1997). Another major reason for the higher attrition rate relates to the increased levels of disordered regions in human and other eukaryotic proteins in comparison to simpler organisms (Lui et al., 2002). These disordered regions likely cause aggregation and misfolding in E. coli expression systems leading to proteins or domains with low expression and/or solubility, again, greatly interfering with their production.
[0181] To circumvent these issues, the NESG Construct Optimization Software and High ThroughPut (HTP) Molecular Cloning and Expression Screening Platform and Automated Purification Pipeline methods were developed for assaying multiple alternative constructs to identify soluble proteins or domains (Methods in Enzymology, Vol. 493, Burlington: Academic Press, 2011, pp. 21-60.). Briefly, the NESG Construct Optimization Software used reports from the from the DisMeta Server (http://www-nmr.cabm.rutgers.edu/bioinformatics/disorder), a metaserver that generated a consensus analysis of eight sequence-based disorder predictors to identify protein regions that are likely to be disordered. In addition, secondary structure, transmembrane and signal peptides among others were also predicted. This data along with multiple sequence alignments of homologous proteins were used to predict possible structural domain boundaries. Based on this information, the NESG Construct Optimization software generated nested sets of alternative constructs, for full-length proteins, multidomain constructs, and single domain constructs. Primers for cloning were then designed using the software Primer Primer (Everett, J.K.; Acton, T.B.; Montelione, G.T.J. Struct. Funct. Genomics 2004, 5: 13-21. Primer Prim'r: A web based server for automated primer design.). Thus for a single targeted region, multiple open reading frames were generally designed varying the N and/or C-terminal sequences. These alternative constructs often possessed significantly better expression, solubility and biophysical behavior than their full-length parent sequences, increasing the possibility of successfully producing a protein reagent.
[0182] Although the NESG Construct Optimization Software identified protein subsequences that were more likely to produce soluble well-behaved samples, several variants of each were assayed to identify constructs amenable to protein sample production. Therefore the high-throughput NESG Molecular Cloning and Expression Screening Platform was developed utilizing 96-well parallel cloning/E. coli expression and Qiagen BioRobotS000-based liquid handling. Briefly, protein target sequences (constructs) were PCR amplified from Reverse Transcriptase (RT) generated cDNA pools or genomic DNA, gel purified and extracted in 96-well format (robotic liquid handling) and subcloned into pET_NESG, a series of T7 based (Novagen) bacterial expression vectors generated at Rutgers, using InFusion (Clonetech) Ligation Independent Cloning (LIC). The RT generated cDNA pools were derived from normal and disease tissue (tumor cells and cell lines) allowing for the isolation of wild-type and polymorphic proteins. Correct clones (containing the desired protein open reading frame) were identified using plate based-PCR assays. An automated DNA Miniprep Protocol isolated the nascent expression vectors and a 96-well transformation protocol was used to introduce the plasmids into the BI21(DE3) pMgK E. coli expression strain. Following overnight growth, a single representative colony from each well (96) was transferred to LB in a 96-well S-Block and incubated for 6 hours. Automated liquid handling was then utilized to produce a 500 microliter overnight subculture of each of the 96 constructs in a single 96-well S-block. An aliquot of each well was then subcultured into the corresponding well of one of four 24-well blocks containing 2 ml of fresh media and incubated at 37° C. until mid-log phase growth. Protein expression is induced with IPTG (Isopropyl13-D-1-thiogalactopyranoside) and incubated overnight at 17° C. The cells were harvested using automated liquid handling and sonicated in 96-well format. The expression and solubility of each construct was visualized by SDS-PAGE analysis and constructs suitable for protein production were identified.
[0183] The soluble expression constructs were then fermented in large volume using parallel fermentation system, consisting of 2.5-L baffled Ultra Yield® Fembach flasks, low-cost platform shakers, controlled temperature rooms and specialized MJ9 media (Jansson et al. 1996). This generally produced 10-100 mg of protein per liter of culture. The resulting proteins were then purified using high-throughput AKTAxpress-based parallel protein purification system. This consisted of a two-step automated Ni-affinity purification (pET_NESG imparts a 6×-His tag) followed by gel filtration chromatography. The purified proteins were then analyzed for quality including molecular weight validation by MALDI-TOF mass spectrometry, homogeneity analysis by SDS-PAGE, aggregation screening by analytical gel filtration with static light scattering, and finally concentration determination was performed.
[0184] Together the NESG Construct Optimization Software, Molecular Cloning and Expression Screening Platform and Automated Purification Pipeline allow for identification and isolation of large numbers of soluble well-behaved protein reagents in a time efficient and cost effective manner. Without this technology, many of the proteins would prove elusive in regard to production as a protein reagent.
[0185] In this process, target protein expression constructs were designed using proprietary bioinformatics methods, cloning was done using robotic methods and protocols, and Expression (E, ranging from 0 to 5) and Solubility (S, ranging from 0 to 5) screening were performed in a high throughput fashion and assessed using SDS-PAGE analysis. The read out (ES score=E score×S score, ranging from 0 to 25) provided a measure of the usability of a particular target construct and expression vector system combination for large-scale protein sample production. In general, constructs providing ES scores≧9 in this high throughout expression and solubility assay provided milligram-per-liter (or tens-of-milligram per liter) quantities of protein samples in medium scale (0.5-3 L) shake flask fermentations.
[0186] As a demonstration of the TOEET technology, a set of approximately 96 human transcription factor genes and epigenetic regulatory factor genes were cloned into the pET15_NESG vector (Acton et al., 2011) lacking a TOEET sequence, and into both the pNESG_Avi6HT and pNESG_Nano6HT vectors. These expression vectors were constructed, and the expression and solubility of target proteins assessed, using the technology outlined above. The results of this study are summarized in Table 1.
[0187] It was found that, using the pET15_NESG vector, only 20 of 99 constructs provided expression and solubility levels that can support scale-up protein sample production (ES score≧9; highlighted in grey shade in Table 1). In contrast, using the pNESG_Nano6HT or pNESG_Avi6HT on this same set of target genes provided a significant increase in the number of highly-expressed and soluble targets suitable for scale-up production. As shown in Table 1, 42 of 98 tested, and 34 of 94 tested protein targets exhibited an ES score≧9 (highlighted in grey shade in Table 1) in the pNESG_Avi6HT and pNESG_Nano6HT vectors, respectively. Several SDS-PAGE gels illustrating these expression and solubility enhancements are shown in FIG. 3. Not only were more of these 99 human protein target genes expressed using TOEET, but both expression levels and solubility were generally increased. For example, while about half of the 99 protein targets had expression value E=0 (i.e. no detectable expression) in the pET15_NESG vector (lacking TOEET), 95 of the 99 protein targets had expression values E≧2 in either the pNESG_Nano6HT and pNESG_Avi6HT vectors (Table 1); many have E values E=5 (the maximum level typically observed) in the expression vectors using TOEET.
[0188] Construct designs for a larger set of more than 2,000 human transcription factor proteins and domains are listed in Table 2. A large number of the proteins listed in Table 2 have been cloned into vectors optimized by TOEET, such as the pNESG_Nano6HT and pNESG_Avi6HT vectors, and exhibit high levels expression and solubility. Analysis of these data indicates that both the pNESG_Nano6HT vector and pNESG_Avi6HT vectors produced greater expression and solubility levels than a standard pET15_NESG vector that has not been optimized using the TOETT technology described in this disclosure.
[0189] Overall, TOEET allows for the production of a significantly greater number of human proteins and protein domains. The higher ES values obtained using TOETT also allow for simpler production and purification of the target proteins, since high ES scores mean that the cell extract has a larger amount of the target protein relative to background proteins.
[0190] The pNESG_Avi6HT also allows for the production of protein samples that can be readily biotinylated in the EET tag sequence. The pNESG_Nano6HT tag also provides a means for simple production of a streptavidin-binding protein (Scholle et al., 2004). Such biotinylated or Nano-tagged protein samples can be used for a variety of processes, including phage display antibody production, as well as for screening and discovering protein-protein and protein--nucleic acid interactions.
Example 3
[0191] In certain applications, proteins that are expressed but not soluble in cell extracts can be solubilized and used successfully as antigens using various methods of solubilization, including urea and guanidine denaturants (Agaton et al. 2003). Accordingly, the ability to express a protein target, even it is not soluble in the high throughput Expression-Solubility screen described above [NESG High ThroughPut (HTP) Molecular Cloning and Expression Screening Platform methods] is critical, since if the protein cannot be expressed at all it is not possible to generate a suitable antigen. Accordingly, a particularly important value of the TOEET technology is enhancement of protein expression (E), regardless of the resulting solubility. To illustrate this point, histogram plots are presented in FIGS. 7a and 7b comparing Expression scores (E ranging from 0 to 5) using the TOEET technology (E_TOEET) compared to expression scores for the same target protein using a pET vector lacking TOEET technology (E_pET). The data shown in FIG. 7a is for 98 protein target genes cloned into the pNESG_Avi6HT TOEET vector compared with the exact same genes cloned into the pET15_NESG vector (lacking TOEET). The data shown in FIG. 7b is for 94 protein target genes cloned pNESG_Nano6HT TOEET vectors compared with the exact same genes cloned into pET15_NESG vector (lacking TOEET). In these histogram plots, a value E_TOEET-E_pET=0 indicates that the expression levels for both vectors were identical; values E_TOEET-E_pET>0 indicate that the TOEET technology provided higher level expression, values E_TOEET-E_pET<0 indicate that the TOEET technology provided lower level expression. For both target sets, the vast majority of genes exhibit much higher expression in the pNESG_Avi6HT TOEET and pNESG_Nano6HT TOEET vectors compared with the pET15_NESG vector (lacking TOEET). In many cases, E_TOEET-E_pET is 4 or 5, indicating that the expression in the non-TOEET vector was 0 or 1, which is too low to be useful for antigen production. Thus the TOEET vectors often provide high level expression of proteins which cannot be expressed at all, or those with are otherwise expressed as such marginal levels as to be useless for antigen production.
Example 4
[0192] A representative method for practicing certain embodiments of the invention is described below.
[0193] The first step in the method is to identify the residues of the chosen tag/protein and the corresponding DNA sequences to be modified, for example, the 1st 30 residues of the tag/protein. Low usage codons are identified and are changed to optimal codons either manually or using servers, for example, such as http://www.jcat.de/ or http://genomes.urv.es/OPTIMIZER/, among others (Step 2). The transcription start site of vector and the resulting 5' untranslated region is then identified (Step 3). The 5' UTR RNA sequence is fused in silico with the optimized RNA sequence encoding the tag/protein (e.g., the first 30 residues of the tag/protein) (Step 4). Various RNA secondary structure prediction methods may then be used to analyze the fused sequence, such as, for example: http://www.genebee.msu.su/services/rna2_reduced.html, http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi (Maximum Free Energy with partition function) or http://www.ncrna.org/centroidfold/ (Centroid Estimators-Statistical Decision Theory) (Step 5). The RBS and Initiation codon (IC) are then identified in the secondary structure prediction and the RNA positions in the first, e.g., 30 residues of the tag/protein that pair to the RBS/IC regions are determined (Step 6). Subsequently, alternative high frequency codons for the given residues base pairing with the RBS/IC are substituted and secondary structure is recalculated (Step 7). Steps 5 through 7 may be repeated until the secondary structure in RBS/IC is minimized and there is general agreement with the between the prediction servers (e.g., multiple predication servers may be used, such as the three servers listed above). This information is then used to design and produce the TOEET-optimized expression vector. Target proteins may then be cloned and expressed into the resulting expression system using the NESG Construct Optimization Software and High ThroughPut (HTP) Molecular Cloning and Expression Screening Platform and Automated Purification Pipeline methods, as outlined above.
TABLE-US-00001 TABLE 1 Expression Results ##STR00001## ##STR00002## E = Expression; E = 0-5 (no to high expression) S = Solubility; S = 0-5 (no to high solubility) ES = E * S = (0-25) ES ≧ 9 usability (highlighted with grey fill) ES ≧ 9 (typically results in ≧5 milligrams of protein per one liter of E. coli Fermentation)
TABLE-US-00002 TABLE 2 Human transcription factor protein and domain constructs designed using the NESG Construct Optimization Software for production using TOEET technologies. Each line in the table describes a unique protein construct for RT-PCR cloning, defined by the NESG Vector ID, the HUGO protein identifier, the Uniprot protein identifier, the first 15 amino acid residues in the targeted construct, the last 15 amino acid residues in the target construct, and the length of the targeted gene. The actual length of the targeted gene obtained by RT-PCR may be shorter or longer than indicated in the table due RNA spicing variations. Construct Vector HUGO Uniprot First 15aa Last 15aa Length HR7152A-140-202-TEV ADAR P55265 PVHYNGPSKAGYVDF YSHGLPRCSPYKKLT 63 HR7675A-754-849-NHT ADNP Q9H2P0 LDPKGHEDDSYEARK KHEMDFDAEWLFENH 96 HR7633A-1032-1131-NHT ADNP2 Q6IQ32 KDEALQILALDPKKY ELKNVKHRLNFEYEP 100 HR4425-1-595-15 AHR P35869 MNSSSANITYASRKR ILTYVQDSLSKSPFI 595 HR4425B-277-391-14 AHR P35869 MILEIRTKNFIFRTK DYIIVTQRPLTDEEG 116 HR4425B-277-406-14 AHR P35869 MILEIRTKNFIFRTK TEHLRKRNTKLPFMF 131 HR4425B-282-386-14 AHR P35869 MTKNFIFRTKHKLDF KNGRPDYIIVTQRPL 106 HR4425B-282-403-14 AHR P35869 MTKNFIFRTKHKLDF EEGTEHLRKRNTKLP 123 HR4425C-102-179-15 AHR P35869 MRAANFREGLNLQEG EDRAEFQRQLHWALN 79 HR4425C-108-178-14 AHR P35869 MEGLNLQEGEFLLQA TEDRAEFQRQLHWAL 72 HR4425C-97-184-15 AHR P35869 MGQDNCRAANFREGL FQRQLHWALNPSQCT 89 HR4425D-318-386-14 AHR P35869 MRGSGYQFIHAADML KNGRPDYIIVTQRPL 70 HR6398A-1-104-15 AIRE O43918 MATDAALRRLLRLHR YGRLQPILDSFPKDV 104 HR6398A-1-91-15 AIRE O43918 MATDAALRRLLRLHR FWRVLFKDYNLERYG 91 HR6398A-1-96-15 AIRE O43918 MATDAALRRLLRLHR FKDYNLERYGRLQPI 96 HR4766B-14-107-14 AKAP8 O43823 MGPANTQGAYGTGVA IAKINQRLDMMSKEG 95 HR4766B-19-107-14 AKAP8 O43823 MQGAYGTGVASWQGY IAKINQRLDMMSKEG 90 HR8040A-384-551-Av6HT AKAP8L Q9ULX6 VERIQFVCSLCKYRT KKLERYLKGENPFTD 168 HR6457-14 ALX1 Q15699 MEFLSEKFALKSPPS RMKAKEHTANISWAM 326 HR7916A-159-235-Av6HT ALX3 O95076 TFSTFQLEELEKVFQ RNPFTAAYDISVLPR 77 HR4490C-209-280-NHT ALX4 Q9H161 SNKGKKRRNRTTFTS RAKWRKRERFGQMQQ 72 HR6941A-510-703-NHT ANAPC2 Q9UJX6 GSKDLFINEYRSLLA VALLRRRMSVWLQQG 194 HR6941A-511-695-Av6HT ANAPC2 Q9UJX6 SKDLEINEYRSLLAD LSKAVKMPVALLRRR 185 HR6941B-732-822-Av6HT ANAPC2 Q9UJX6 SDDESDSGMASQADQ LVYSAGVYRLPKNCS 91 HR6941B-765-822-Av6HT ANAPC2 Q9UJX6 LESLSLDRIYNMLRM LVYSAGVYRLPKNCS 58 HR6941C-498-713-Av6HT ANAPC2 Q9UJX6 SSDIISLLVSIYGSK WLQQGVLREEPPGTF 216 HR6941C-510-713-Av6HT ANAPC2 Q9UJX6 GSKDLFINEYRSLLA WLQQGVLREEPPGTF 204 HR8423A-486-593-Av6HT ANKZF1 Q9H8Y5 AKAPGQPELWNALLA STRNEFRRFMEKNPD 108 HR5083-14 APEX2 Q9UBZ4 MLRVVSWNINGIRRP DPSSRCNFFLWSRPS 518 HR5083A-1-319-14 APEX2 Q9UBZ4 MLRVVSWNINGIRRP CPVGAVLSVSSVPAK 319 HR5083A-1-323-14 APEX2 Q9UBZ4 MLRVVSWNINGIRRP AVLSVSSVPAKQCPP 323 HR5083A-1-352-14 APEX2 Q9UBZ4 MLRVVSWNINGIRRP KILRFLVPLEQSPVL 352 HR5083A-1-357-14 APEX2 Q9UBZ4 MLRVVSWNINGIRRP LVPIEQSPVLEQSTL 357 HR8294A-15-116-TEV APTX Q7Z2E3 RVCWLVRQDSRHQRI HMVNELYPYIVEFEE 100 HR7650B-267-331-TEV ARHGAP35 Q9NRY4 SQQIATAKDKYEWLV AKKLFLQHIHRLKHE 65 HR7542A-507-616-NHT ARID2 Q68CP9 QHVAPPPGIVEIDSE RAIPLPIQMYYQQQP 110 HR4394C-14 ARID3A Q99856 MPDHGDWTYEEQFKQ ELQAAIDSNRREGRR 135 HR4394C-15 ARID3A Q99856 MPDHGDWTYEEQFKQ ELQAAIDSNRREGRR 135 HR4394C-218-351-Av6HT ARID3A Q99856 MPDHGDWTYEEQFKQ ELQAAIDSNRREGRR 135 HR4394C-218-351-TEV ARID3A Q99856 PDHGDWTYEEQFKQL ELQAAIDSNRREGRR 134 HR8410A-318-424-TEV ARID5B Q14865 RADEQAFLVALYKYM KGEEDKPLPPIKPRK 107 HR7845A-354-470-TEV ARNT P27540 SNVCQPTEFISRHNI YIICTNTNVKNSSQE 116 HR7821A-334-439-TEV ARNT2 Q9HBZ2 PTEFLSRHNSDGIIT SDEIEYIICTNTNVK 106 HR7274A-178-295-NHT ARNTL2 Q8WYA1 QDNELRHLILKTAEG SFFCRIKSCKISVKE 118 HR6915A-334-389-TEV ARX Q96Q53 TFTSYQLEELERAFQ WFQNRRAKWRKREKA 56 HR4461B-112-194-14 ASCL1 P50553 MLPQQQPAAVARRNE VSAAFQAGVLSPTIS 84 HR4461B-112-210-14 ASCL1 P50553 MLPQQQPAAVARRNE NYSNDLNSMAGSPVS 100 HR4461B-132-189-14 ASCL1 P50553 MKLVNLGFATLREHV DEHDAVSAAFQAGVL 59 HR4461B-132-206-14 ASCL1 P50553 MKLVNLGFATLREHV TISPNYSNDLNSMAG 76 HR4461B-146-206-14 ASCL1 P50553 MPNGAANKKMSKVET TISPNYSNDLNSMAG 62 HR4510B-64-138-14 ASCL2 Q99929 MKLVNLGFQALRQHV AVRPSAPRGPPGTTP 76 HR7137A-2665-2824-TEV ASH1L Q9NR48 YLMRDSRRTPDGHPV PKKLTPKKDFSPHYV 160 HR3149-106-270-14 ATF1 P18846 SGQYIAIAPNGALQL IEELKTLKDLYSNKS 165 HR3149-14 ATF1 P18846 MEDSHKSTTSETAPQ EELKTLKDLYSNKSV 271 HR3149-15 ATF1 P18846 MEDSHKSTTSETAPQ EELKTLKDLYSNKSV 271 HR3149-21 ATF1 P18846 MEDSHKSTTSETAPQ EELKTLKDLYSNKSV 271 HR3149-87-270-14 ATF1 P18846 GVSAAVTSMSVPTPI IEELKTLKDLYSNKS 184 HR3149-96-270-14 ATF1 P18846 SVPTPIYQTSSGQYI IEELKTLKDLYSNKS 175 HR4498B-354-414-TEV ATF2 P15386 KRRKFLERNRAAASR LLRNEVAQLKQLLLA 61 HR4572-21-181-14 ATF3 P18847 MPCLSPPGSLVFEDF RNLFIQQIKEGTLQS 162 HR4572B-103-170-14 ATF3 P18847 MCRNKKKEKTECLQK RAQNGRTPEDERNLF 69 HR4572B-103-181-14 ATF3 P18847 MCRNKKKEKTECLQK RNLFIQQIKEGTLQS 80 HR4572B-77-181-14 ATF3 P18847 MTKAEVAPEEDERKK RNLFIQQIKEGTLQS 106 HR6914A-280-341-Av6HT ATF4 P18848 MKKLKKMEQNKTAAT LAKEIQYLKDLIEEV 63 HR6914A-280-341-TEV ATF4 P18848 KKLKKMEQNKTAATR LAKEIQYLKDLIEEV 62 HR4531-39-469-14 ATF7 P17544 MPARTDSVIIADQTP SAAEAVATSVLTQMA 432 HR8374A-151-218-Av6HT ATOH1 Q92858 KQVNGVQKQRRLAAN AQIYINALSELLQTP 68 HR7270A-225-288-NHT ATOH8 Q96SQ7 KALQQTRRLLANARE IACNYILSLARLADL 64 HR7350A-7-128-TEV BACH1 O14867 SVFAYESSVHSTNVI SVHNIEESCFQFLKF 122 HR8413A-12-132-Av6HT BACH2 Q9BYV9 YVYESTVHCTNILLG MHNLEDSCFSFLQTQ 121 HR7112A-169-265-NHT BARHL1 Q9BZE3 DSPPVRLKKPRKART SALQRMFPSPYFYPQ 97 HR7390A-223-314-NHT BARHL2 Q9NY43 ESPPVRAKKPRKART EAGNYSALQRMFPSP 92 HR7183A-133-199-TEV BARX1 Q9HBU1 GEPGTKAKKGRRSRT QVKTWYQNRRMKWKK 67 HR7561-1-174-Av6HT BARX2 Q9UMQ3 HCHAELRLSSPGQLK TPDRLDLAQSLGLTQ 173 HR7561-1-187-Av6HT BARX2 Q9UMQ3 HCHAELRLSSPGQLK TQLQVKTWYQNRRMK 186 HR7561-1-196-Av6HT BARX2 Q9UMQ3 HCHAELRLSSPGQLK QNRRMKWKKMVLKGG 195 HR7561A-118-196-Av6HT BARX2 Q9UMQ3 SSESETEQPTPRQKK QNRRMKWKKMVLKGG 79 HR6459-34-125-14 BATF Q16520 EKNRIAAQKSRQRQT HAFHQPHVSSPRFQP 92 HR6459-34-125-15 BATF Q16520 EKNRIAAQKSRQRQT HAFHQPHVSSPRFQP 92 HR6459A-19-125-14 BATF Q16520 GKQDSSDDVRRVQRR HAFHQPHVSSPRFQP 107 HR6459A-34-118-14 BATF Q16520 EKNRIAAQKSRQRQT PEVVYSAHAFHQPHV 85 HR7115A-1107-1202-NHT BAZ1A Q9NRL2 RSYKTVLDRWRESLL GDWFCPECRPKQRSR 96 HR7115B-420-468-Av6HT BAZ1A Q9NRL2 LPPEIFGDALMVLEF LEVLEEALVGNDSEG 49 HR7115B-420-486-Av6HT BAZ1A Q9NRL2 LPPEIFGDALMVLEF ELLFFFLTAIFQAIA 67 HR7115C-1408-1534-Av6HT BAZ1A Q9NRL2 CRKRQSPEPSPVTLG TRLQAFFHIQAQKLG 127 HR7115D-1420-1534-Av6HT BAZ1A Q9NRL2 TLGRRSSGRQGGVHE TRLQAFFHIQAQKLG 115 HR7115D-1432-1534-Av6HT BAZ1A Q9NRL2 VHELSAFEQLVVELV TRLQAFFHIQAQKLG 103 HR7115E-1-122-Av6HT BAZ1A Q9NRL2 PLLHRKPFVRQKPPA IFAYVKDRYFVEETV 121 HR7115E-1-129-Av6HT BAZ1A Q9NRL2 PLLHRKPFVRQKPPA RYFVEETVEVIRNNG 128 HR7115E-1-142-Av6HT BAZ1A Q9NRL2 PLLHRKPFVRQKPPA NGARLQCRILEVLPP 141 HR7115E-22-122-Av6HT BAZ1A Q9NRL2 EEVFYCKVTNEIFRH IFAYVKDRYFVEETV 101 HR7115E-22-129-Av6HT BAZ1A Q9NRL2 EEVFYCKVTNEIFRH RYFVEETVEVIRNNG 108 HR7115E-22-142-Av6HT BAZ1A Q9NRL2 EEVFYCKVTNEIFRH NGARLQCRILEVLPP 121 HR7190A-1634-1742-NHT BAZ2A Q9UIF9 SYEITPRIRVWRQTL VEGEFTQKPGFPKRG 109 HR8090A-2062-2166-TEV BAZ2B Q9UIF8 DSKDLALCSMILTEM NMRKYFEKKWTDTFK 105 HR7285A-80-154-TEV BBX Q8WY36 ARRPMNAFLLFCKRH FMKANPGYKWCPTTN 75 HR4436B-523-602-14 BCL6 P41182 MCDCRFSEEASLKRH NLKTHTRIHSGEKPY 81 HR4436B-523-606-14 BCL6 P41182 MCDCRFSEEASLKRH HTRIHSGEKPYKCET 85 HR4436B-528-601-14 BCL6 P41182 MSEEASLKRHTLQTH ANLKTHTRIHSGEKP 75 HR4436B-540-602-14 BCL6 P41182 MTHSDKPYKCDRCQA NLKTHTRIHSGEKPY 64 HR4436B-542-598-14 BCL6 P41182 MSDKPYKCDRCQASF NRPANLKTHTRIHSG 58 HR4436C-5-129-TEV BCL6 P41182 ADSCIQFTRHASDVL EHVVDTCRKFIKASE 125 HR7156A-284-387-Av6HT BDP1 A6H8Y1 ERGSTTTYSSFRKNY KVLAEEEKRKQKSVK 104 HR8401A-71-161-Av6HT BHLHA15 Q7RTS1 DSSIQRRLESNERER PKLYQHYQQQQQVAG 91 HR7639A-64-125-Av6HT BHLHA9 Q7RTU4 KARRMAANVRERKRI IHRIAALSLVLRASP 62 HR8288A-236-314-Av6HT BHLHE22 Q8NFJ8 KSKEQKALRLNINAR LEEMRRLVAYLNQGQ 79 HR7576A-47-183-NHT BHLHE4 O14503 EDSKETYKLPHRLIE SQLVTHLHRVVSELL 137 HR7576B-142-174-Av6HT BHLHE40 O14503 FCSGFQTCAREVLQY HENTRDLKSSQLVTH 33 HR7576B-142-181-Av6HT BHLHE40 O14503 FCSGFQTCAREVLQY KSSQLVTHLHRVVSE 40 HR7518A-44-116-NHT BHLHE41 Q9C0J9 TYKLPHRLIEKKRRD LTEQQHQKIIALQNG 73 HR3082 1-125 pET15TEV_NESG (in BLOC1S1 P78537 MLSRLLKEHQAKQNE ALEYVYKGQLQSAPS 125 progress) HR3082-1-119-14 BLOC1S1 P78537 MLSRLLKEHQAKQNE MRTIATALEYVYKGQ 119 HR3082-14 BLOC1S1 P78537 MLSRLLKEHQAKQNE ALEYVYKGQLQSAPS 125 HR3082-MBP3 BLOC1S1 P78537 MLSRLLKEHQAKQNE LEYVYKGQLQSAPS* 126
HR3082A-32-125-14 BLOC1S1 P78537 TCLTEALVDHLNVGV ALEYVYKGQLQSAPS 94 HR3082B-43-125-15 BLOC1S1 P78537 MNVGVAQAYMNQRKL ALEYVYKGQLQSAPS 84 HR7816A-294-396-NHT BMP2 P12643 SSCKRHPLYVDFSDV LKNYQDMVVEGCGCR 103 HR7816A-294-396-TEV BMP2 P12643 SSCKRHPLYVDFSDV LKNYQDMVVEGCGCR 103 HR7409A-9-128-TEV BOLA1 Q9Y3E2 GLVSMAGRVCLCQGS WRENSQIDTSPPCLG 120 HR8185-1-86-TEV BOLA2B Q9H3K6 ASAKSLDRWKARLLE EYLREKLQRDLEAEH 85 HR7562-8-107-Av6HT BOLA3 Q53533 AAAPLLRGIRGLPLH KEMHGLRIFTSVPKR 100 HR7886A-6-308-TEV BPNT1 O95861 TVLMRLVASAYSIAQ YASRVPESIKNALVP 303 HR7955A-134-243-TEV BRD9 Q9H8M2 KDKIVANEYKSVTEF EPEGNACSLTDSTAE 110 HR6995B-633-746-TEV BRPF1 P55201 FLILLRKTLEQLQEK GAVLRQARRQAEKMG 114 HR8142A-104-176-Av6HT BSX Q3C1V8 PGKHGRRRKARTVFS RMKHKKQLRKSQDEP 73 HR1875-14 C12orf28 Q96LU7 MAFCALTIVALYILS IFFTDYFFYFYRRCA 275 HR7476A-824-884-NHT C14orf43 Q6PIG2 TYHYTGSDQWKMAER FYYTYKKQVKIGRNG 61 HR7019A-867-954-TEV CAMTA1 Q9Y6Y1 SGRVFMVTDYSPEWS NNQIISNSVVFEYKA 88 HR7019B-108-183-TEV CAMTA1 Q9Y6Y1 ILYNRKKVKYRKDGY LQNPDIVLVHYLNVP 76 HR7019B-69-183-TEV CAMTA1 Q9Y6Y1 KERHRWNTNEEIAAY LQNPDIVLVHYLNVP 115 HR7019B-73-183-TEV CAMTA1 Q9Y6Y1 RWNTNEEIAAYLITF LQNPDIVLVHYLNVP 111 HR7019C-1029-1162-Av6HT CAMTA1 Q9Y6Y1 ALGSCFESRVVVVCE LGIARSRGHVKLAEC 134 HR7019C-1029-1168-Av6HT CAMTA1 Q9Y6Y1 ALGSCFESRVVVVCE RGHVKLAECLEHLQR 140 HR7019C-1058-1162-Av6HT CAMTA1 Q9Y6Y1 IHSKTFRGMTLLHLA LGIARSRGHVKLAEC 105 HR7019C-1058-1168-Av6HT CAMTA1 Q9Y6Y1 IHSKTFRGMTLLHLA RGHVKLAECLEHLQR 111 HR7019D-1486-1624-Av6HT CAMTA1 Q9Y6Y1 KPNLPSAADWSEFLS CGKRRQARRTAVIVQ 139 HR7019D-1486-1660-Av6HT CAMTA1 Q9Y6Y1 KPNLPSAADWSEFLS FLRRCRHSPLVDHRL 175 HR7019D-1501-1673-Av6HT CAMTA1 Q9Y6Y1 ASTSEKVENEFAQLT RLYKRSERIEKGQGT 173 HR7019D-1513-1624-Av6HT CAMTA1 Q9Y6Y1 QLTLSDHEQRELYEA CGKRRQARRTAVIVQ 112 HR7019D-1513-1660-Av6HT CAMTA1 Q9Y6Y1 QLTLSDHEQRELYEA FLRRCRHSPLVDHRL 148 HR7295A-60-130-Av6HT CARHSP1 Q9Y2V2 GPVYKGVCKCFCRSK PKNEKLQAVEVVITH 71 HR8150A-1916-1982-Av6HT CASP8AP2 Q9UKL3 KNVIKKKGEIIILWT RFQQLMKLFEKSKCR 67 HR7269A-2-135-Av6HT CBFB Q13951 MPRVVPDQRSKFENE GMGCLEFDEERAQQE 135 HR7269A-2-135-TEV CBFB Q13951 PRVVPDQRSKFENEE GMGCLEFDEERAQQE 134 HR7615A-104-190-NHT CBLL1 Q75N03 TPVHFCDKCGLPIKI YLSQRDLQAHINHRH 87 HR6520A-9-62-Av6HT CBX2 Q14781 MEQVFAAECILSKRL NILDPRLLLAFQKKE 55 HR6520A-9-62-TEV CBX2 Q14781 EQVFAAECILSKRLR NILDPRLLLAFQKKE 54 HR8494A-624-717-Av6HT CCDC79 Q8NA31 IVEAEDRYKSELRKS QQGRKAVDLAHKYHK 94 HR8086A-57-112-TEV CDC5L Q99459 SIKKIEWSREEEEKL EHYEFLLDKAAQRDN 56 HR7252A-160-214-Av6HT CDX1 P47902 VYTDHQRLELEKEFH IWFQNRRAKERKVNK 55 HR7064A-185-251-Av6HT CDX2 Q99626 TKDKYRVVYTDHQRL RRAKERKINKKKLQQ 67 HR7957A-172-246-Av6HT CDX4 O14627 TKEKYRVVYTDHQRL IKKKISQFENSGGSV 75 HR7823A-281-340-TEV CEBPA P49715 NSNEYRVRRERNNIA KRVEQLSRELDTLRG 60 HR4764B-273-336-TEV CEBPB P17676 EYKIRRERNNIAVRK RELSTLRNLFKQLPE 64 HR7557A-190-272-15 CEBPE Q15744 MAGPLHKGKKAVNKD DTLRNLFRQIPEAAN 84 HR7557A-195-268-15 CEBPE Q15744 MKGKKAVNKDSLEYR TQELDTLRNLFRQIP 75 HR7557A-195-281-15 CEBPE Q15744 MKGKKAVNKDSLEYR IPEAANLIKGVGGCS 88 HR7557A-203-281-15 CEBPE Q15744 MDSLEYRLRRERNNI IPEAANLIKGVGGCS 80 HR6439-59-150-Av6HT CEBPG P53567 DRNSDEYRQRRERNN ISTENTTADGDNAGQ 92 HR8022A-431-525-Av6HT CENPT Q96BT3 EPAEPLLVRHPPRPR KPEDLELLMRRQGLV 95 HR7210A-268-373-Av6HT CHD1 O14646 MEEEFETIERFMDCR TKRWLKNASPEDVEY 107 HR7210A-268-373-TEV CHD1 O14646 EEEFETIERFMDCRI TKRWLKNASPEDVEY 106 HR7330A-260-394-NHT CHD2 O14647 SETIEKVLDSRLGKK VERVIAVKTSKSTLG 135 HR7397A-371-431-TEV CHD6 Q8TD26 NPDYVEVDRILEVAH DVDPAKVKEFESLQV 61 HR7397B-679-941-Av6HT CHD6 Q8TD26 LRRLKDDVEKNLAPK LDKAVLQDINRKGGT 262 HR7397B-679-968-Av6HT CHD6 Q8TD26 LRRLKDDVEKNLAPK DLLRKGAYGALMDEE 289 HR7397B-679-974-Av6HT CHD6 Q8TD26 LRRLKDDVEKNLAPK AYGALMDEEDEGSKF 295 HR7397B-679-997-Av6HT CHD6 Q8TD26 LRRLKDDVEKNLAPK LQRRTHTITIQSEGK 318 HR8217A-2631-2715-TEV CHD7 Q9P2D1 RNPNKLDINTLTGEE DRLLTGPVVRGEGAS 85 HR8217B-2561-2614-TEV CHD7 Q9P2D1 GQLDPDTRIPVINLE TYTVDMPSYVPKNAD 54 HR7629A-1-98-NHT CHRAC1 Q9NRG0 ADVVVGKDKGGEQRL SETFQFLADILPKKI 97 HR7688A-98-186-NHT CLOCK O15516 QDWKPTFLSNEEFTQ THLLESDSLTPEYLK 89 HR7654A-1987-2362-TEV CNOT1 A5YKK6 QLPYHRIFIMLLLEL EIEKLFQSVAQCCMG 376 HR7654B-1987-2369-TEV CNOT1 A5YKK6 QLPYHRIFIMLLLEL SVAQCCMGQKQAQQV 383 HR7654B-1987-2376-TEV CNOT1 A5YKK6 QLPYHRIFIMLLLEL GQKQAQQVMEGTGAS 390 HR2981-28-443-15 COPS2 P61201 MPNVDLENQYYNSKA NQLNSLNQAVVSKLA 417 HR2981-301-418-14 COPS2 P61201 PYKNDPEILAMTNLV QVNQLLELDHQKRGG 118 HR2981-45-443-15 COPS2 P61201 MDDPKAALSSFQKVL NQLNSLNQAVVSKLA 400 HR2981A-306-415-14 COPS2 P61201 PEILAMTNLVSAYQN RIDQVNQLLELDHQK 110 HR2981B-339-418-14 COPS2 P61201 DDPFIREHIEELLRN QVNQLLELDHQKRGG 80 HR2981C-45-163-15 COPS2 P61201 MDDPKAALSSFQKVL FKTNTKLGKLYLERE 120 HR2981C-45-184-15 COPS2 P61201 MDDPKAALSSFQKVL KILRQLHQSCQTDDG 141 HR2981C-45-210-15 COPS2 P61201 MDDPKAALSSFQKVL EIYALEIQMYTAQKN 167 HR3016-1-411-14 COPS3 Q9UNS2 MASALEQFVNSVRQL ITVNPQFVQKSMGSQ 411 HR3016-14 COPS3 Q9UNS2 MASALEQFVNSVRQL GSQEDDSGNKPSSYS 423 HR3016A-49-114-15 COPS3 Q9UNS2 LDVQEHSLGVLAVLF FAGLCHQLTNALVER 66 HR3016B-88-154-15 COPS3 Q9UNS2 CNGEHIRYATDTFAG SIHADLCQLCLLAKC 67 HR3016C-270-368-15 COPS3 Q9UNS2 NNPSELRNLVNKHSE KDGMVSFHDNPEKYN 99 HR3016C-270-396-15 COPS3 Q9UNS2 NNPSELRNLVNKHSE KCIELDERLKAMDQE 127 HR3016C-270-411-15 COPS3 Q9UNS2 NNPSELRNLVNKHSE ITVNPQFVQKSMGSQ 142 HR3016D-352-413-15 COPS3 Q9UNS2 NQKDGMVSFHDNPEK VNPQFVQKSMGSQED 62 HR3016D-358-409-15 COPS3 Q9UNS2 VSFHDNPEKYNNPAM QEITVNPQFVQKSMG 52 HR3105-1-292-15 COPS4 Q9BT78 MAAAVRQDLAQLMNS LQEFAAMLMPHQKAT 292 HR3105-1-297-15 COPS4 Q9BT78 MAAAVRQDLAQLMNS AMLMPHQKATTADGS 297 HR3105-15 COPS4 Q9BT78 MAAAVRQDLAQLMNS APEWTAQAMEAQMAQ 406 HR6309A-15 CPSF4 O95639 MSGEKTVVCKHWLRG NKECPFLHIDPESKI 62 HR7458A-62-130-NHT CPSF4L A6NMK7 GEKMVVCKHWLRGLC KPAFKSQDCPWYDQG 69 HR3140-21 CREB1 P16220 MTMESGAENQQSGDA EELKALKDLYCHKSD 341 HR6927-139-461-Av6HT CREB3L3 Q68CJ9 PVIQVPEASVTIDLE TGSGRAGLEAAGDEL 323 HR7960-1-298-Av6HT CREB3L4 Q8TEY5 DLGIPDLLDAWLEPP IAQTSNKAAQTSTCV 297 HR6873A-34-89-Av6HT CRX O43186 SAPRKQRRERTTFTR KINLPESRVQVWFKN 56 HR6873A-45-103-Av6HT CRX O43186 TFTRSQLEELEALFA NRRAKCRQQRQQQKQ 59 HR8272A-84-160-NHT CSDA P16989 KKVLATKVLGTVKWF VEGEKGAEAANVTGP 77 HR7792B-673-744-TEV CSDE1 O75534 LRRATVECVKDQFGF CSACNVWRVCEGPKA 72 HR7173A-399-462-TEV CTCF P49711 RTHSGEKPYECYICH RKSDLGVHLRKQHSY 64 HR7173B-515-592-Av6HT CTCF P49711 MRTHTGEKPYACSHC AGPDGVEGENGGETK 79 HR7173B-515-592-TEV CTCF P49711 RTHTGEKPYACSHCD AGPDGVEGENGGETK 78 HR7558A-411-776-TEV CUL1 Q13616 AQSSSKSPELLARYC LERVDGEKDTYSYLA 365 HR7558B-15-410-Av6HT CUL1 Q13616 IGLDQIWDDLRAGIQ DKACGRFINNNAVTK 396 HR3327B-643-745-14 CUL2 Q13617 NFSSKRTKFKITTSM IERSQASADEYSYVA 103 HR3327B-655-745-14 CUL2 Q13617 TSMQKDTPQEMEQTR IERSQASADEYSYVA 91 HR3327C-1-408-15 CUL2 Q13617 MSLKPRVVDFDETWN YCDNLLKKSAKGMTE 408 HR3327D-4-745-TEV CUL2 Q13617 KPRVVDFDETWNKLL IERSQASADEYSYVA 742 HR3437D-677-768-TEV CUL3 Q13618 VAAKQGESDPERKET LARTPEDRKVYTYVA 92 HR3342C-672-759-TEV CUL4A Q13619 IQMKETVEEQVSTTE MERDKDNPNQYHYVA 88 HR7263A-808-895-TEV CUL4B Q13620 IQMKETVEEQASTTE MERDKENPNQYNYIA 88 HR3340C-7-395-Av6HT CUL5 Q93034 LKNKGSLQFEDKWDF TIFKLELPLKQKGVG 389 HR8510A-825-917-TEV CUX2 O14529 PRGDEAPVPPEDEAA RQVKEKLAKNGICQR 93 HR7807A-15-94-Av6HT CXXC1 Q9P0U4 EDSKSENGENAPIYC LEIRYRHKKSRERDG 80 HR7690A-184-282-TEV DACH1 Q9UI36 TPQNNECKMVDLRGA LISRKDFETLYNDCT 99 HR6867A-61-162-TEV DACH2 Q96NX9 GNTNTNECRMVDMHG TRKDFETLFTDCTNA 102 HR7176A-249-325-Av6HT DBP Q10586 VPEEQKDEKYWSRRY YRAVLSRYQAQHGAL 77 HR7176A-254-325-Av6HT DBP Q10586 KDEKYWSRRYKNNEA YRAVLSRYQAQHGAL 72 HR7911A-178-244-Av6HT DBX2 Q6ZNG2 DSNSKARRGILRRAV VKIWFQNRRMKWRNS 67 HR7702A-201-279-NHT DEAF1 O75398 SELPVRCRNISGTLY CLIQDGILNPHAASG 79 HR7922A-11-108-TEV DEPDC1A Q5TB30 YRATKLWNEVTTSFR SENVDDNNQLFRFPA 98 HR7073A-153-209-TEV DLX2 Q07687 RKPRTIYSSFQLAAL QVKIWFQNRRSKFKK 57 HR8208A-130-186-TEV DLX3 O60479 RKPRTIYSSYQLAAL QVKIWFQNRRSKFKK 57 HR7595A-138-194-TEV DLX5 P56178 RKPRTIYSSFQLAAL QVKIWFQNKRSKIKK 57 HR8524A-46-106-TEV DLX6 P56179 GKKIRKPRTIYSSLQ QVKIWFQNKRSKFKK 61 HR4696-44-404-15 DMAP1 Q9NPF5 MTLTFKRPEGMHREV MLRHRHEALARAGVL 362 HR4696-49-403-15 DMAP1 Q9NPF5 MRPEGMHREVYALLY QMLRHRHEALARAGV 356 HR4696B-208-404-15 DMAP1 Q9NPF5 MVPGTDLKIPVFDAG MLRHRHEALARAGVL 198 HR4696B-213-404-15 DMAP1 Q9NPF5 MLKIPVFDAGHERRR MLRHRHEALARAGVL 193 HR4696B-236-403-15 DMAP1 Q9NPF5 MRTPEQVAEEEYLLQ QMLRHRHEALARAGV 169 HR7582A-79-146-Av6HT DMBX1 Q8NFW5 TAQQLEALEKTFQKT SLQKEQLQKQKEAEG 68 HR7142-1-340-TEV DMC1 Q14565 KEDQVVAEEPGFQDE ATFAITAGGIGDAKE 339 HR8371A-114-182-Av6HT DMRT2 Q9Y5R5 PRKLSRTPKCARCRN LRRQQATEDKKGLSG 69 HR7805A-20-88-NHT DMRT3 Q9NQL9 RAPLQRTPKCARCRN LRRQQANESLESLIP 69
HR6947A-318-361-NHT DMRTA1 Q5VZB9 SLPTVSSRPRDPLDI GILRFCKGDVVQAIE 44 HR7753A-1-53-NHT DMRTB1 Q96MA1 ADKMVRTPKCSRCRN KCYLISERQKIMAAQ 52 HR7387A-44-84-Av6HT DMRTC2 Q8IXT2 RCRNHGVTAHLKGHK KCVLILERRRVMAAQ 41 HR8011A-205-276-15 DMTF1 Q9Y222 MSTEPGDIVTQGVSW RIAELDVADENDINW 78 HR8011A-205-293-15 DMTF1 Q9Y222 MSTEPGDIVTQGVSW LAEGWSSVRSPQWLR 90 HR8011B-255-339-15 DMTF1 Q9Y22 MDEINLILRIAELDV QKNNPTLLENKSGSG 86 HR8011B-255-356-15 DMTF1 Q9Y222 MDEINLILRIAELDV NSNTNSSVQHVQIRV 103 HR8011B-268-339-15 DMTF1 Q9Y222 MVADENDINWDLLAE QKNNPTLLENKSGSG 73 HR8011B-268-356-15 DMTF1 Q9Y222 MVADENDINWDLLAE NSNTNSSVQHVQIRV 90 HR6887A-327-385-TEV DNAIC1 Q96KC8 APEWTEEDLSQLTRS AKQLKDSVTCSPGMV 59 HR7581A-1-76-NHT DNAJC21 Q5F1R6 KCHYEALGVRRDASE RAWYDNHREALLKGG 75 HR8109A-314-391-Av6HT DPF2 Q92785 AAVKTYRWQCIECKC LLKEKASIYQNQNSS 77 HR8202A-15-83-Av6HT DPRX A6NFQ7 HSHRKRTMFTKKQLE AKLKKAKCKHIHQKQ 69 HR7601-1-176-Av6HT DR1 Q01658 MASSSGNDDDLTIPR NQAGSSQDEEDDDDI 176 HR7601-1-176-TEV DR1 Q01658 ASSSGNDDDLTIPRA NQAGSSQDEEDDDDI 175 HR6975A-1-77-TEV DRAP1 Q14919 PSKKKKYNARFPPAR KTMTTSHLKQCIELE 76 HR7517A-25-174-NHT DUSP12 Q9UNI6 GQMLEVQPGLYFGGA WQLKLYQAMGYEVDT 150 HR7523A-86-164-NHT DUXA A6NLW8 SQGQDQPGVEFQSRE QNRRSRLLLQRKREP 79 HR4713B-251-345-TEV DVL1 O14640 TVTLNMERHHFLGIS ISLTVAKCWDPTPRS 95 HR5191A-15 DVL1L1 P54792 MTVTLNMERHHFLGI ISLTVAKAWDPTPRS 96 HR4606C-408-551-14 DVL2 O14641 MLPDGCEGRGLSVHT APLPGATPWPLLPTF 145 HR4606C-412-526-14 DVL2 O14641 MCEGRGLSVHTDMAS CESYLVNLSLNDNDG 116 HR4606C-417-519-14 DVL2 O14641 MLSVHTDMASVTKAM FGDLSGGCESYLVNL 104 HR4606C-417-551-14 DVL2 O14641 MLSVHTDMASVTKAM APLPGATPWPLLPTF 136 HR4606D-260-358-TEV DVL2 O14641 TMSLNIITVTLNMEK PGPIVLTVAKCWDPS 99 HR5528A-14 DVL2 O14641 MTITSGSSLPDGCEG SEQCYYVFGDLSGGC 113 HR5528A-15 DVL2 O14641 MTITSGSSLPDGCEG SEQCYYVFGDLSGGC 113 HR7051A-248-338-TEV DVL3 Q92997 ITVTLNMEKYNFLGI HKPGPITLTVAKGWD 91 HR7051B-397-504-15 DVL3 Q92997 MDTERLDDFHLSIHS CYYIFGDLCGNMANL 109 HR7051B-397-511-15 DVL3 Q92997 MDTERLDDFHLSIHS LCGNMANLSLHDHDG 116 HR7051B-397-530-15 DVL3 Q92997 MDTERLDDFHLSIHS SDQDTLAPLPHPGAA 135 HR7051B-403-504-15 DVL3 Q92997 MDFHLSIHSDMAAIV CYYIFGDCGGNMANL 103 HR7051B-403-530-15 DVL3 Q92997 MDFHLSIHSDMAAIV SDQDTLAPLPHPGAA 129 HR7051C-1-79-TEV DVL3 Q92997 GETKIIYHLDGQETP AKLPCFNGRVVSWLV 78 HR4672B-14 E2F1 Q01094 PGEKSRYETSLNLTT QGPIDVFLCPEETVG 183 HR4672C-116-196-14 E2F1 Q01094 MGKGVKSPGEKSRYE KKSKNHIQWLGSHTT 82 HR4672C-121-192-14 E2F1 Q01094 MSPGEKSRYETSLNL QLIAKKSKNHIQWLG 73 HR4672C-122-196-14 E2F1 Q01094 MPGEKSRYETSLNLT KKSKNHIQWLGSHTT 76 HR4672C-127-192-14 E2F1 Q01094 MRYETSLNLTTKRFL QLIAKKSKNHIQWLG 67 HR4672C-147-192-14 E2F1 Q01094 MADGVVDLNWAAEVL QLIAKKSKNHIQWLG 47 HR4672D-192-301-TEV E2F1 Q01094 GSHTTVGVGGRLEGL KSKQGPIDVFLCPEE 110 HR6383-65-437-14 E2F2 Q14209 ATPHGPEGQVVRCLP SDLFDSYDLGDLLIN 373 HR6383-70-437-14 E2F2 Q14209 PEGQVVRCLPAGRLP SDLFDSYDLGDLLIN 368 HR6383A-195-308-15 E2F2 Q14209 RGMFEDPTRPGKQQQ TQGPIEVYLCPEEVQ 114 HR6383A-198-296-15 E2F2 Q14209 FEDPTRPGKQQQLGQ RTEDNLQIYLKSTQG 99 HR6383B-114-200-15 E2F2 Q14209 MGLPSPKTPKSPGEK AKNNIQWVGRGMFED 88 HR6383B-114-204-15 E2F2 Q14209 MGLPSPKTPKSPGEK IQWVGRGMFEDPTRP 92 HR6383B-119-195-15 E2F2 Q14209 MKTPKSPGEKTRYDT LIRKKAKNNIQWVGR 78 HR6383B-119-195-Av6HT E2F2 Q14209 KTPKSPGEKTRYDTS LIRKKAKNNIQWVGR 77 HR6383B-119-115-TEV E2F2 Q14209 KTPKSPGEKTRYDTS LIRKKAKNNIQWVGR 77 HR6383C-126-200-15 E2F2 Q14209 MEKTRYDTSLGLLTK AKNNIQWVGRGMFED 76 HR6383C-131-195-15 E2F2 Q14209 MDTSLGLLTKKFIYL LIRKKAKNNIQWVGR 66 HR6383C-131-202-15 E2F2 Q14209 MDTSLGLLTKKFIYL NNIQWVGRGMFEDPT 73 HR4418C-14 E2F3 O00716 KTRYDTSLGLLTKKF QGPIEVYLCPEETET 182 HR4418D-14 E2F3 O00716 NNVQWMGCSLSEDGG LCPEETETHSPMKTN 128 HR4470C-84-203-14 E2F4 Q16254 MVGPGCNTREIADKL PIEVLLVNKEAWSSP 121 HR4470C-89-200-14 E2F4 Q16254 MNTREIADKLIELKA VSGPIEVLLVNKEAW 113 HR4470D-11-86-TEV E2F4 Q16254 PPGTPSRHEKSLGLL EKKSKNSIQWKGVGP 76 HR4678B-113-232-14 E2F5 Q15329 MQWKGVGAGCNTKEV KSHSGPIHVLLINKE 121 HR4678B-119-232-14 E2F5 Q15329 MAGCNTKEVIDRLRY KSHSGPIHVLLINKE 115 HR4622-1-237-15 E2F6 O75461 MSQQRPARKLPSLLL HIRSTNGPIDVYLCE 237 HR4622-1-242-15 E2F6 O75461 MSQQRPARKLPSLLL NGPIDVYLCEVEQGQ 242 HR4622-19-242-15 E2F6 O75461 MEETVRRRCRDPINV NGPIDVYLCEVEQGQ 225 HR4622-19-281-15 E2F6 O75461 MEETVRRRCRDPINV EENPQQSEELLEVSN 264 HR4622-24-237-15 E2F6 O75461 MRRCRDPINVEGLLP HIRSTNGPIDVYLCE 215 HR4622-24-281-15 E2F6 O75461 MRRCRDPINVEGLLP EENPQQSEELLEVSN 259 HR4622-24-281-Av6HT E2F6 O75461 RRCRDPINVEGLLPS EENPQQSEELLEVSN 258 HR4622-24-281-TEV E2F6 O75461 RRCRDPINVEGLLPS EENPQQSEELLEVSN 258 HR4622B-128-247-15 E2F6 O75461 GSDLSNFGAVPQQKK VYLCEVEQGQTSNKR 120 HR46228-133-243-15 E2F6 O75461 NFGAVPQQKKLQEEL GPIDVYLCEVEQGQT 111 HR4622C-127-242-15 E2F6 O75461 IGSDLSNFGAVPQQK NGPIDVYLCEVEQGQ 116 HR4622C-132-237-15 E2F6 O75461 SNFGAVPQQKKLQEE HIRSTNGPIDVYLCE 106 HR4622D-54-137-15 E2F6 O75461 RKALKVKRPRFDVSL HIRWIGSDLSNFGAV 84 HR4622D-54-180-15 E2F6 O75461 RKALKVKRPSFDVSL QQLFELTDDKENERL 127 HR4622D-54-242-15 E2F6 O75461 RKALKVKRPRFDVSL NGPIDVYLCEVEQGQ 189 HR4622D-58-132-15 E2F6 O75461 KVKRPRFDVSLVYLT KKSKNHIRWIGSDLS 75 HR4622D-58-175-15 E2F6 O75461 KVKRPRFDVSLVYLT IKDCAQQLFELTDDK 118 HR4622D-58-237-15 E2F6 O75461 KVKRPRFDVSLVYLT HIRSTNGPIDVYLCE 180 HR8499A-141-251-Av6HT E2F7 Q96AV8 SRKQKSLGLLCQKFL YLQQKELDLIDYKFG 111 HR7611A-112-223-NHT E2F8 A0AVK6 SRKEKSLGLLCHKFL IKKKEYEQEFDFIKS 112 HR8342-1-508-Av6HT E4F1 Q66K89 EGAMAVRVTAAHTAE GDCGKLYKTIAHVRG 507 HR8342-1-600-Av6HT E4F1 Q66K89 EGAMAVRVTAAHTAE EHGTLNRHLRTKGGC 599 HR8342A-522-586-15 E4F1 Q66K89 MPKCGKRYKTKNAQQ EKPFKCYKCGRGFAE 66 HR8342A-523-600-15 E4F1 Q66K89 MKCGKRYKTKNAQQV EHGTLNRHLRTKGGC 79 HR8342A-527-581-15 E4F1 Q66K89 MRYKTKNAQQVHFRT RHHTGEKPFKCYKCG 56 HR8342A-527-600-15 E4F1 Q66K89 MRYKTKNAQQVHFRT EHGTLNRHLRTKGGC 75 HR8342B-51-219-15 E4F1 Q66K89 MEEDEDDVHRCGRCQ SILKAHMVTHSSRKD 170 HR8342B-51-231-15 E4F1 Q66K89 MEEDEDDVHRCGRCQ RKDHECKLCGASFRT 182 HR8342B-51-249-15 E4F1 Q66K89 MEEDEDDVHRCGRCQ LIRHHRRHTDERPYK 200 HR8342B-56-214-15 E4F1 Q66K89 MDVHRCGRCQAEFTA TFKTGSILKAHMVTH 160 HR8342B-56-226-15 E4F1 Q66K89 MDVHRCGRCQAEFTA VTHSSRKDHECKLCG 172 HR8342B-56-244-15 E4F1 Q66K89 MDVHRCGRCQAEFTA RTKGSLIRHHRRHTD 190 HR3014A-10-250-TEV EBF1 Q9UH73 RSGSSMKEEPLGSGM NNSKHGRRARRLDPS 241 HR7745A-10-250-TEV EBF3 Q9H4W6 RGGTTMKEEPLGSGM NNSKHGRRARRLDPS 241 HR6883A-10-251-TEV EBF4 Q9BQW3 NLKEEPLLPAGLGSV HGRRARRLDPSEAAT 242 HR7307A-71-148-Av6HT EDF1 O60869 MDRVTLEVGKVIQQG GKDIGKPIEKGPRAK 79 HR7307A-71-148-TEV EDF1 O60869 DRVTLEVGKVIQQGR GKDIGKPIEKGPRAK 78 HR7944A-1347-1411-TEV EEA1 Q15075 RKWAEDNEVQNCMAC KPVRVCDACFNDLQG 65 HR4555D-366-418-Av6HT EGR1 P18146 MKPFQCRICMRNFSR KFARSDERKRHTKIH 54 HR4555D-366-418-TEV EGR1 P18146 KPFQCRICMRNFSRS KFARSDERKRHTKIH 53 HR8206A-368-420-TEV EGR2 P11161 KPFQCRICMRNFSRS KFARSDERKRHTKIH 53 HR8198A-273-328-TEV EGR3 Q06889 RPHACPAEGCDRRFS FSRSDHLTTHIRTHT 56 HR8048A-204-299-TEV EHF Q9NZC4 PRGTHLWEFIRDILL VYKFGKNARGWRENE 96 HR7395A-770-879-NHT EIF3C Q99613 PEADKVRTMLVRKIQ SLVENNERVFDHKQG 110 HR2095-14 EIF3K Q9UBQ5 MAMFEQMRANVGKLL KIDFDSVSSIMASSQ 218 HR564-14 EIF3K Q9UBQ5 MAMFEQMRANVGKLL KIDFDSVSSIMASSQ 218 HR6332A-198-348-15 ELF1 P32519 KKNKDGKGNTIYLWE SPGVKGGATTVLKPG 151 HR6332A-198-353-15 ELF1 P32519 KKNKDGKGNTIYLWE GGATTVLKPGNSKAA 156 HR6332A-203-348-15 ELF1 P32519 GKGNTIYLWEFLLAL SPGVKGGATTVLKPG 146 HR6332B-152-304-15 ELF1 P32519 ETQQVQEKYADSPGA KEMPKDLIYINDEDP 153 HR6332B-157-299-15 ELF1 P32519 QEKYADSPGASSPEQ LVYQFKEMPKDLIYI 143 HR6332B-157-304-15 ELF1 P32519 QEKYADSPGASSPEQ KEMPKDLIYINDEDP 148 HR6332B-198-304-15 ELF1 P32519 KKNKDGKGNTIYLWE KEMPKDLIYINDEDP 107 HR6332C-203-353-15 ELF1 P32519 GKGNTIYLWEFLLAL GGATTVLKPGNSKAA 151 HR7067A-150-308-15 ELF2 Q15723 MLWEFLLDLLQDKNT GVARVVNITSPGHDA 160 HR7067A-157-303-15 ELF2 Q15723 MLLQDKNTCPRYIKW SRAEKGVARVVNITS 148 HR7067A-200-308-15 ELF2 Q15723 MNYETMGRALRYYYQ GVARVVNITSPGHDA 110 HR7867A-45-132-TEV ELF3 P78545 SNPQMSLEGTEKASW GDQLHAQLRDLTSSS 88 HR7867B-269-371-TEV ELF3 P78545 APRGTHLWEFIRDIL NSSGWKEEEVLQSRN 103 HR8186A-1-104-TEV ELF4 Q99607 AITLQPSDLIFEFAS HTMSTAEVLLNMESP 103 HR8186A-1-87-TEV ELF4 Q99607 AITLQPSDLIFEFAS QILEGSFLLTDDNEA 86 HR8186A-1-94-TEV ELF4 Q99607 AITLQPSDLIFEFAS LLTDDNEATSHTMST 93 HR7396A-166-265-TEV ELF5 Q9UKW6 SRTSLQSSHLWEFVR YKFGKNAHGWQEDKL 100 HR7616A-1-93-TEV ELF3 P41970 ESAITLWQFLLQLLL KFVYKFVSFPEILKM 92 HR4449C-1-93-TEV ELK4 P28324 DSAITLWQFLLQLLQ KFVYKFVSYPEILNM 92 HR8153A-249-313-Av6HT EN2 P19622 TAFTAEQLQRLKAEF IKKATGNKNTLAVHL 66
HR7174A-264-457-Av6HT EOMES O95936 GFRAHVYLCNRPLWL LKIDHNPFAKGFRDN 194 HR4540F-1221-1288-14 EP300 Q09472 MQPQTTINKEQFSKR GCLKKSARTRKENKF 69 HR4540F-1226-1281-14 EP300 Q09472 MINKEQFSKRKNDTL PAGFVCDGCLKKSAR 57 HR4540F-1236-1281-14 EP300 Q09472 MNDTLDPELFVECTE PAGFVCDGCLKKSAR 47 HR4540G-323-423-TEV EP300 Q09472 GSGAHTADPEKRKLI HDCPVCLPLKNAGDK 100 HR4540H-1045-1161-Av6HT EP300 Q09472 KKKIFKPEELRQALM EVFEQEIDPVMQSLG 117 HR4540I-1726-1817-Av6HT EP300 Q09472 SPGDSRRLSIQRCIQ VPFCLNIKQKLRQQQ 92 HR4540J-1135-1205-15 EP300 Q09472 MTSRVYKYCSKLSEV YYSYQNRYHFCEKCF 72 HR4540J-1135-1220-15 EP300 Q09472 MTSRVYKYCSKLSEV NEIQGESVSLGDDPS 87 HR4540J-1165-1205-15 EP300 Q09472 MGRKLEFSPQTLCCY YYSYQNRYHFCEKCF 42 HR4540J-1165-1220-15 EP300 Q09472 MGRKLEFSPQTLCCY NEIQGESVSLGDDPS 57 HR7040A-1285-1379-Av6HT EP400 Q96L91 HVLKCRLSNRQKALY RDFWKEADLSMFDLI 95 HR8188A-239-350-TEV EPAS1 Q99814 LDSKTFLSRHSMDMK CIMCVNYVLSEIEKN 112 HR6944A-8-123-Av6HT ERF P50548 GFAFPDWAYKPESSP NKLVLVNYPFIDVGL 116 HR6944A-8-160-NHT ERF P50548 GFAFPDWAYKPESSP PSTPSEVLSPTEDPR 153 HR6944B-24-123-Av6HT ERF P50548 SRQIQLWHFILELLR NKLVLVNYPFIDVGL 109 HR6944B-24-160-Av6HT ERF P50548 SRQIQLWHFILELLR PSTPSEVLSPTEDPR 137 HR4801B-180-254-TEV ESR1 P03372 KETRYCAVCNDYASG CRLRKGYEVGMMKGG 75 HR4685B-144-218-TEV ESR2 Q92731 RDAHFCAVCSDYASG CRLRKCYEVGMVKCG 75 HR7097A-77-146-15 ESRRA P11474 MRLCLVCGDVASGYH QACRFTKCLRVGMLK 71 HR7097A-77-146-Av6HT ESRRA P11474 RLCLVCGDVASGYHY QACRFTKCLRVGMLK 70 HR7097A-77-146-TEV ESRRA P11474 RLCLVCGDVASGYHY QACRFTKCLRVGMLK 70 HR7097A-77-168-15 ESRRA P11474 MRLCLVCGDVASGYH VRGGRQKYKRRPEVD 93 HR7097A-77-168-Av6HT ESRRA P11474 RLCLVCGDVASGYHY VRGGRQKYKRRPEVD 92 HR7097A-77-168-TEV ESRRA P11474 RLCLVCGDVASGYHY VRGGRQKYKRRPEVD 92 HR7097B-179-423-15 ESRRA P11474 MGPLAVAGGPRKTAA PMHKLFLEMLEAMMD 246 HR7097B-179-423-Av6HT ESRRA P11474 GPLAVAGGPRKTAAP PMHKLFLEMLEAMMD 245 HR7097B-179-423-TEV ESRRA P11474 GPLAVAGGPRKTAAP PMHKLFLEMLEAMMD 245 HR7097C-193-423-15 ESRRA P11474 MPVNALVSHLLVVEP PMHKLFLEMLEAMMD 232 HR7097C-193-423-Av6HT ESRRA P11474 PVNALVSHLLVVEPE PMHKLFLEMLEAMMD 231 HR7097C-193-423-TEV ESRRA P11474 PVNALVSHLLVVEPE PMHKLFLEMLEAMMD 231 HR8438A-101-433-15 ESRRB O95718 MRLCLVCGDIASGYH VPMHKLFLEMLEAKA 334 HR8438A-78-435-15 ESRRB O95718 MDCASGIMEDSAIKC MHKLFLEMLEAKAWA 359 HR8438B-169-433-15 ESRRB O95718 MLKEGVRLDRVRGGR VPMHKLFLEMLEAKA 266 HR8438B-182-433-15 ESRRB O95718 MRQKYKRRLDSESSP VPMHKLFLEMLEAKA 253 HR8438B-203-433-15 ESRRB O95718 MPPAKKPLTKIVSYL VPMHKLFLEMLEAKA 232 HR7566D-122-219-Av6HT ESRRG P62508 MSMPKRLCLVCGDIA GGRQKYKRRIDAENS 99 HR7566D-122-219-TEV ESRRG P62508 SMPKRLCLVCGDIAS GGRQKYKRRIDAENS 98 HR6900A-130-214-NHT ESX1 Q8N693 AEGPQPPERKRRRRT VLMLRNTATADLAHP 85 HR8013A-320-415-Av5HT ETS1 P14921 VIPAAALAGYTGSGP IIHKTAGKRYVYRFV 96 HR8013A-320-415-TEV ETS1 P14921 VIPAAALAGYTGSGP IIHKTAGKRYVYRFV 96 HR5529-1-329-15 ETS2 P15036 MNDFGIKNMDQVAPV EDDCSQSLCLNKPTM 329 HR5529A-14 ETS2 P15036 MHDSANCELPLLTPC EHLEQMIKENQEKTE 116 HR5529A-15 ETS2 P15036 MHDSANCELPLLTPC EHLEQMIKENQEKTE 116 HR8505A-240-333-Av6HT ETV2 O00321 IQLWQFLLELLHDGA FGGRVPSLAYPDCAG 94 HR7364A-15-174-NHT ETV3 P41162 GGYQFPDWAYKTESS PTNDVQPGRFSASSL 160 HR6967A-1-136-NHT ETV3L Q6ZN32 HCSCLAEGIPANPGN SKLIVVNYPLWEVRA 135 HR5533A-14 ETV4 P43268 MREGPPYQRRGALQL QRPALKAEFDRPVSE 122 HR5533A-15 ETV4 P43268 MREGPPYQRRGALQL QRPALKAEFDRPVSE 122 HR8084A-311-445-Av6HT ETV4 P43268 CVVPEKFEGDIKQEG AFPDNQRPALKAEFD 135 HR7423A-333-470-NHT ETV5 P41161 LYFDDTCVVPERLEG SMAFPDNQRPFLKAE 138 HR6884A-338-443-TEV ETV6 P41212 CRLLWDYVYQLLSDS GRTDRLEHLESQELD 106 HR6884B-47-129-TEV ETV6 P41212 SIRLPAHLRLQPIYW ELLQHILKQRKPRIL 83 HR7437A-8-133-NHT ETV7 Q9Y603 ISPISPVAAMPPLGT ALVCGPFFGGIFRLK 126 HR7509A-183-242-TEV EVX1 P49640 RRYRTAFTREQIARL KVWFQNRRMKDKRQR 59 HR7284A-188-247-TEV EVX2 Q03828 VRRYRTAFTREQIAR KVWFQNRRMKDKRQR 60 HR7802A-349-453-TEV EWSR1 Q01844 PPVDPDEDSDNSAIY LKVSLARKKPPMNSM 105 HR7511A-1-99-TEV EXOC2 Q96KP1 SRSRQPPLVTGISPN TSTVSFKLLKPEKIG 98 HR6516A-463-729-NHT EZH1 Q92800 KTCKQVFQFAVKESL RAIQAGEELFFDYRY 267 HR6323-214-746-14 EZH2 Q15910 PPRKFPSDKIFEAIS DALKYVGIEREMEIP 533 HR7273-1-589-TEV FARSB Q9NSD9 PTVSVKRDLLFQALG TMPCSSLEINVGPFL 588 HR8271C-2054-2125-NHT FBN1 P35555 QDLRMSYCYAKFEGG CPYGSGIIVGPDDSA 72 HR6868A-99-166-NHT FERD3L Q96RJ6 TYAQRQAANIRERKR FMTELLESCEKKESG 68 HR6882A-43-139-TEV FEV Q99581 GSGQIQLWQFLLELL RFDFQGLAQACQPPP 97 HR6968A-258-310-NHT FEZF1 A0PJY2 KVFTCEVCGKVFNAH GFRQASTLCRHKIIH 53 HR7661A-275-327-NHT FEZF2 Q8TBJ5 KNFTCEVGGKVFNAH GFRQASTLCRHKIIH 53 HR3605C-806-930-14 FGD1 P98174 MRRRSILEKQASVAA LGRAGRGDTFCPGPT 126 HR3605C-811-925-14 FGD1 P98174 MLEKQASVAAENSVI RWMAVLGRAGRGDTF 116 HR8434A-55-150-Av6HT FIGLA Q6QHK4 SSTENLQLVLERRRV SYSNNSSESHTSSAR 96 HR8078A-77-129-Av6HT FIZ1 Q96SL8 RPYRCSACPKGFRDS RFSSRSSLGRHLKRQ 53 HR4739B-114-198-Av6HT FLI1 Q01543 MPPNMTTNERRVIVP TEVLLSHLSYLRESS 86 HR4739B-114-198-TEV FLI1 Q01543 PPNMTTNERRVIVPA TEVLLSHLSYLRESS 85 HR6395-41-355-15 FOS P01100 MGSPVNAQDFCTDLA FVFTYPEADSFPSCA 316 HR6395-41-361-15 FOS P01100 MGSPVNAQDFCTDLA EADSFPSCAAAHRKG 322 HR6395-46-350-15 FOS P01100 MAQDFCTDLAVSSAN AYTSSFVFTYPEADS 306 HR6395-46-361-15 FOS P01100 MAQDFCTDLAVSSAN EADSFPSCAAAHRKG 317 HR3160-41-293-15 FOSL2 P15408 MPGSGSAFIPTINAI NLVFTYPSVLEQESP 254 HR3160-44-288-15 FOSL2 P15408 MGSAFIPTINAITTS TPGTSNLVFTYPSVL 246 HR7662A-167-264-NHT FOXA1 P55317 PHAKPPYSYISLITM SGNMFENGCYLRRQK 98 HR7840A-114-211-NHT FOXA3 P55318 AHAKPPYSYISLITM SGNMFENGCYLRRQK 98 HR7656A-11-100-NHT FOXB1 Q99853 DQKPPYSYISLTAMA FWALHPSCGDMFENG 90 HR7565A-15-100-NHT FOXB2 Q5VYV0 PYSYISLTAMAIQHS FWALHPDCGDMFENG 86 HR8399A-76-168-TEV FOXC1 Q12948 VKPPYSYIALITMAI LDPDSYNMFENGSFL 92 HR6945A-70-162-TEV FOXC2 Q99958 LVKPPYSYIALITMA LDPDSYNMFENGSFL 93 HR8366A-126-222-NHT FOXD2 O60548 VKPPYSYIALITMAI ADMFDNGSFLRRRKR 97 HR8366A-126-222-TEV FOXD2 O60548 VKPPYSYIALITMAI ADMFDNGSFLRRRKR 97 HR7150A-140-236-Av6HT FOXD3 Q9UJU5 VKPPYSYIALITMAI EDMFDNGSFLRRRKR 97 HR7150A-140-236-TEV FOXD3 Q9UJU5 VKPPYSYIALITMAI EDMFDNGSFLRRRKR 97 HR7841A-104-204-TEV FOXD4 Q12950 KPPSSYIALITMAIL NGSFLRRRKRFQRHQ 101 HR6889A-107-207-TEV FOXD4L1 Q9NU39 KPPSSYIALITMAIL NGSFLRRRKRFKRHQ 101 HR8496-108-208-Av6HT FOXD4L3 Q6VB84 KPPYSYIALITMAIL NGSFLRRRKRFKRHQ 101 HR7982-108-208-Av6HT FOXD4L4 Q6VB85 KPPYSYIALITMAIL NGSFLRRRKRFKRHQ 101 HR7029A-108-208-TEV FOXD4L5 Q5VV16 KPPYSYIALITMAIL NGSFLRRRKRFKRHQ 101 HR7874-108-208-Av6HT FOXD4L6 Q3SYB3 KPPYSYIALITMAIL NGSFLRRRKRFKRHQ 101 HR5544A-14 FOXE1 O00358 MAGAGVPGEATGRGA FLRRRKRFKRSDLST 131 HR5544A-15 FOXE1 O00358 MAGAGVPGEATGRGA FLRRRKRFKRSDLST 131 HR6991A-51-146-NHT FOXE1 O00358 RGKPPYSYIALIAMA NAEDMFESGSFLRRR 96 HR7179A-69-165-NHT FOXE3 Q13461 RGKPPYSYIALIAMA AADMFDNGSFLRRRK 97 HR8233A-48-138-15 FOXF1 Q12946 MKPPYSYIALIVMAI IDPASEFMFEEGSFR 92 HR8233A-48-138-Av6HT FOXF1 Q12946 KPPYSYIALIVMAIQ IDPASEFMFEEGSFR 91 HR7975A-101-190-Av6HT FOXF2 Q12947 PPYSYIALIVMAIQS IDPASEFMFEEGSFR 90 HR4505B-182-298-14 FOXG1 P55316 PPFSYNALIMMAIRQ LAFKRGARLTSTGLT 117 HR4505B-183-294-14 FOXG1 P55316 PFSYNALIMMAIRQS SRAKLAFKRGARLTS 112 HR4505C-183-276-Av6HT FOXG1 P55316 PFSYNALIMMAIRQS DDVFIGGTTGKLRRR 94 HR5526A-14 FOXH1 O75593 MYLRHDKPPYTYLAM RLQNTALCRRWQNGG 109 HR5526A-15 FOXH1 O75593 MYLRHDKPPYTYLAM RLQNTALCRRWQNGG 109 HR8014A-138-239-Av6HT FOXI3 A8MTJ6 EDLMKMVRPPYSYSA CEKMFDNGNFRRKRK 102 HR6903A-121-211-NHT FOXJ1 Q92949 KPPYSYATLICMAMQ IDPQYAERLLSGAFK 91 HR8000A-64-153-Av6HT FOXJ2 Q9P0K8 DGKPRYSYATLITYA YWTIDTCPDISRKRR 90 HR7453A-82-173-NHT FOXJ3 Q9UPW0 SYASLITFAINSSPK KEDVLPTRPKKRARS 92 HR7148A-303-403-TEV FOXK1 P85037 ESKPPFSYAQLIVQA LVEQAFRKRRQRGVS 101 HR8426A-256-353-Av6HT FOXK2 Q01167 MDSKPPYSYAQLIVQ ESKLIEQAFRKRRPR 99 HR8426A-256-353-TEV FOXK2 Q01167 DSKPPYSYAQLIVQA ESKLIEQAFRKRRPR 98 HR8426B-34-133-Av6HT FOXK2 Q01167 GWAVARLEGREFEYL NGVFVDGVFQRRGAP 100 HR8426B-34-153-Av6HT FOXK2 Q01167 GWAVARLEGREFEYL RVCTFRFPSTNIKIT 120 HR8426B-58-139-TEV FOXK2 Q01167 RNSSQGSVDVSMGHS GVFQRRGAPPLQLPR 82 HR8426B-58-143-TEV FOXK2 Q01167 RNSSQGSVDVSMGHS RRGAPPLQLPRVCTF 86 HR8426B-63-133-TEV FOXK2 Q01167 GSVDVSMGHSSFISR NGVFVDGVFQRRGAP 71 HR8426B-63-139-TEV FOXK2 Q01167 GSVDVSMGHSSFISR GVFQRRGAPPLQLPR 77 HR8426B-63-153-Av6HT FOXK2 Q01167 GSVDVSMGHSSFISR RVCTFRFPSTNIKIT 91 HR8426B-70-133-Av6HT FOXK2 Q01167 GHSSFISRRHLEIFT NGVFVDGVFQRRGAP 64 HR8426B-70-153-Av6HT FOXK2 Q01167 GHSSFISRRHLEIFT RVCTFRFPSTNIKIT 84 HR7608A-10-139-Av6HT FOXL1 Q12952 PALAASPMLYLYGPE LDPRCLDMFENGNYR 130 HR7608A-43-139-15 FOXL1 Q12952 MRAETPQKPPYSYIA LDPRCLDMFENGNYR 98 HR7608A-48-111-15 FOXL1 Q12952 MQKPPYSYIALIAMA IRHNLSLNDCFVKVP 65
HR7608A-48-139-15 FOXL1 Q12952 MQKPPYSYIALIAMA LDPRCLDMFENGNYR 93 HR7608B-10-134-Av6HT FOXL1 Q12952 PALAASPMLYLYGPE GSYWTLDPRCLDMFE 125 HR7608B-50-164-Av6HT FOXL1 Q12952 PPYSYIALIAMAIQD GAPEAKRPRAETHQR 115 HR7161A-56-143-NHT FOXL2 P58012 PYSYVALIAMAIRES TLDPACEDMFEKGNY 88 HR6909A-222-360-Av6HT FOXM1 Q08050 MPSRPSASWQNSVSE NPELRRNMTIKTELP 140 HR6909A-222-360-TEV FOXM1 Q08050 PSRPSASWQNSVSER NPELRRNMTIKTELP 139 HR7300A-268-368-NHT FOXN1 Q15353 LFPKPIYSYSILIFM DKMQEELQKWKRKDP 101 HR7988A-110-208-Av6HT FOXN2 P32314 TSKPPYSFSLLIYMA KPNLIQALKKQPFSS 99 HR6979A-111-207-NHT FOXN3 Q00409 PNCKPPYSFSCLIFM PEYRQNLIQALKKTP 97 HR7465A-193-285-NHT FOXN4 Q96NZ1 KPIYSYSCLIAMALK NLARIDKMEEEMHKW 93 HR4552B-151-249-TEV FOXO1 Q12778 KSSSSRRNAWGNLSY SWWMLNPEGGKSGKS 99 HR5548A-14 FOXO1 Q12778 MPPAAAGPLAGQPRK SKFAKSRSRAAKKKA 139 HR5548A-15 FOXO1 Q12778 MPPAAAGPLAGQPRK SKFAKSRSRAAKKKA 139 HR5549A-14 FOXO3 O43524 MLPPPQPGAAGGSGQ NKYTKSRGRAAKKKA 141 HR5549A-15 FOXO3 O43524 MLPPPQPGAAGGSGQ NKYTKSRGRAAKKKA 141 HR4610C-102-197-TEV FOXO4 P98177 GNQSYAELISQAIES EGGKSGKAPRRRAAS 96 HR7590A-462-548-TEV FOXP1 Q9H334 AEVRPPFTYASLIRQ WTVDEVEFQKRRPQK 87 HR7934A-501-587-TEV FOXP2 O15409 DVRPPFTYATLIRQA TVDEVEYQKRRSQKI 87 HR6897A-464-550-TEV FOXP4 Q8IVH2 ADVRPPFTYASLIRQ WTVDEREYQKRRPPK 87 HR8323A-118-217-NHT FOXQ1 Q9C009 PKPPYSYIALIAMAI TFADGVFRRRRKRLS 100 HR8323A-118-217-TEV FOXQ1 Q9C009 PKPPYSYIALIAMAI TFADGVFRRRRKRLS 100 HR7058A-170-271-NHT FOXR1 Q6PIV2 LWSRPPLNYFHLIAL GHRRFAEEARALAST 102 HR8252A-189-311-Av6HT FOXR2 Q6PJQ5 SWQRPPLNCSHLIAL ECMSQPELLTSLFDL 123 HR7804A-20-110-NHT FOXS1 O43638 PYSYIALIAMAIQSS PDCHDMFEHGSFLRR 91 HR8359A-35-121-TEV GABPA Q06546 AECVSQAIDINEPIG KLNILEIVKPADTVE 87 HR7128A-251-311-TEV GATA1 P15976 SKRAGTQCTNCQTTT MRKDGIQTRNRKASG 61 HR4414D-340-402-TEV GATA2 P23769 SAARRAGTCCANCQT MKKEGIQTRNRKMSN 63 HR7641A-308-370-TEV GATA3 P23771 LSAARRAGTSCANCQ TMKKEGIQTRNRKMS 63 HR4783B-262-321-TEV GATA4 P43694 SASRRVGLSCANCQT PLAMRKEGIQTRKRK 60 HR8231A-242-324-15 GBX1 Q14549 MTGAEEGAPVTAGVT QNRRAKWKRIKAGNV 84 HR8231A-242-324-Av6HT GBX1 Q14549 TGAEEGAPVTAGVTA QNRRAKWKRIKAGNV 83 HR6959A-1-173-TEV GCM1 Q9NP62 EPDDFDSEDKEILSW TKLEAEARRAMKKVN 172 HR8430A-6-165-Av6HT GCM2 O75603 VQEAVGVCSYGMQLS FQAKGVHDHPRPESK 160 HR4429D-233-303-14 GFI1 Q99684 MKGAGVKVESELLCT CGKTFGHAVSLEQHK 72 HR4429D-233-315-14 GFI1 Q99684 MKGAGVKVESELLCT QHKAVHSQERSFDCK 84 HR4429D-238-298-14 GFI1 Q99684 MKVESELLCTRLLLG FACEMCGKTFGHAVS 62 HR4429D-238-310-14 GFI1 Q99684 MKVESELLCTRLLLG AVSLEQHKAVHSQER 74 HR4429E-311-392-TEV GFI1 Q99684 SFDCKICGKSFKRSS SQSSNLITHSRKHTG 82 HR7937A-234-388-TEV GLI1 P08151 YVCKLPGCTKRYTDP RLDQLHQLRPIGTRG 155 HR7924A-436-590-TEV GLI2 P10070 ETNCHWEDCTKEYDT TDPSSLRKHVKTVHG 155 HR7118A-479-633-TEV GLI3 P10071 ETNCHWEGCAREFDT TDPSSLRKHVKTVHG 155 HR7155A-189-350-NHT GLIS1 Q8NBF1 RVVAGRQACRWVDCC PSSLRKHVKAHSAKE 162 HR7416A-116-298-Av6HT GLIS2 Q9BZE0 DFQPLRYLDGVPSSF TRTHYVDKPYYCKMP 183 HR7416A-116-318-Av6HT GLIS2 Q9BZE0 DFQPLRYLDGVPSSF YTDPSSLRKHIKAHG 203 HR7416A-150-318-Av6HT GLIS2 Q9BZE0 LTPPKDKCLSPDLPL YTDPSSLRKHIKAHG 169 HR7416A-163-298-Av6HT GLIS2 Q9BZE0 PLPKQLVCRWAKCNQ TRTHYVDKPYYCKMP 136 HR7416A-163-318-Av6HT GLIS2 Q9BZE0 PLPKQLVCRWAKCNQ YTDPSSLRKHIKAHG 156 HR7200A-261-553-TEV GLYR1 Q49A26 GSITPTDKKIGFLGL QSDNDMSAVYRAYIH 293 HR7763A-87-182-TEV GMEB1 Q9Y692 ANEDMEIAYPITCGE YQHDKVCSNTCRSTK 96 HR7418A-64-203-NHT GMEB2 Q9UKD1 AFTASSQLKEAVLVK LSSPTSAEYIPLTPA 140 HR7418A-87-176-Av6HT GMEB2 Q9UKD1 EAEIVYPITCGDSRA LDFYQHDKVCSNTCR 90 HR7418A-87-203-Av6HT GMEB2 Q9UKD1 EAEIVYPITCGDSRA LSSPTSAEYIPLTPA 117 HR7418B-64-179-Av6HT GMEB2 Q9UKD1 AFTASSQLKEAVLVK YQHDKVCSNTCRSTK 116 HR7418B-83-179-Av6HT GMEB2 Q9UKD1 GENLEAEIVYPITCG YQHDKVCSNTCRSTK 97 HR7418B-83-203-Av6HT GMEB2 Q9UKD1 GENLEAEIVYPITCG LSSPTSAEYIPLTPA 121 HR8221A-2125-2211-NHT GON4L Q3T8J9 PEGEQQPKAAEATVC RELMQLFHTACEASS 87 HR7528A-714-848-NHT GPR155 Q7Z3F1 DKHLIILPFKRRLEF LQKSPEQSPPAINAN 135 HR7997A-174-442-Av6HT GRHL1 Q9NZI5 VYHPEPTERVVVFDR KIRDEERKQSKRKVS 269 HR7758A-219-444-Av6HT GRHL2 Q6ISB3 SFKDAATEKFRSASV RKQNRKKGKGQASQT 226 HR7267A-161-223-TEV GSC P56915 RRHRTIFTDEQLEAL KNRRAKWRRQKRSSS 63 HR8103A-123-177-Av6HT GSC2 O15499 QRRTRRHRTIFSEEQ IRLREERVEVWFKNR 55 HR7705A-139-207-NHT GSX1 Q9H4S2 SSSNQLPSSKRMRTA IWFQNRRVKHKKEGK 69 HR8308A-66-146-TEV GTF2E2 P29084 ALSGSSGYKFGVLAK VIDGKYAFKPKYNVR 81 HR8128A-449-517-Av6HT GTF2F1 P35269 SGDVQVTEDAVRRYL ERKMINDKMHFSLKE 69 HR8128A-449-517-TEV GTF2F1 P35269 SGDVQVTEDAVRRYL ERKMINDKMHFSLKE 69 HR7967A-175-243-TEV GTF2F2 P13984 RARADKQHVLDMLFS HKNTWELKPEYRHYQ 69 HR7205-1-238-15 GTF2H2C Q6P1K8 MDEEPERTKRWEGGY DESHYKELLTHHLSP 238 HR7205-1-327-15 GTF2H2C Q6P1K8 MDEEPERTKRWEGGY VSAPHLARSYHHLFP 327 HR7205-1-332-15 GTF2H2C Q6P1K8 MDEEPERTKRWEGGY LARSYHHLFPLDAFQ 332 HR7205-10-327-15 GTF2H2C Q6P1K8 MRWEGGYERTWEILK VSAPHLARSYHHLFP 319 HR7205-10-395-15 GTF2H2C Q6P1K8 MRWEGGYERTWEILK CCPGCIHKIPAPSGV 387 HR7205-15 GTF2H2C Q6P1K8 MDEEPERTKRWEGGY CPGCIHKIPAPSGV* 396 HR7205A-328-386-TEV GTF2H2C Q6P1K8 LDAFQEIPLEEYNGE DVFVHDSLHCCPGCI 59 HR7205B-10-216-15 GTF2H2C Q6P1K8 MRWEGGYERTWEILK SAEVRVCTVLARETG 208 HR7205B-48-220-15 GTF2H2C Q6P1K8 MEHHGQVRLGMMRHL RVCTVLARETGGTYH 174 HR7205B-48-238-15 GTF2H2C Q6P1K8 MEHHGQVRLGMMRHL DESHYKELLTHHLSP 192 HR7205B-53-216-15 GTF2H2C Q6P1K8 MVRLGMMRHLYVVVD SAEVRVCTVLARETG 165 HR7205B-53-236-15 GTF2H2C Q6P1K8 MVRLGMMRHLYVVVD ILDESHYKELLTHHL 185 HR7205C-1-216-TEV GTF2H2C Q6P1K8 DEEPERTKRWEGGYE SAEVRVCTVLARETG 215 HR7205C-1-255-TEV GTF2H2C Q6P1K8 DEEPERTKRWEGGYE ASSSSECSLIRMGFP 254 HR7205C-10-236-TEV GTF2H2C Q6P1K8 RWEGGYERTWEILKE ILDESHYKELLTHHL 227 HR7205C-10-255-TEV GTF2H2C Q6P1K8 RWEGGYERTWEILKE ASSSSECSLIRMGFP 246 HR7820A-107-194-TEV GTF2IRD2 Q86UP8 LRKAVEDYFCFCYGK NRPFLGPESQLGGPG 88 HR7355A-318-415-TEV GTF2IRD2B Q6EKJ0 NEKERLSSIEKIKQL KFTVIRPLPGLELSN 98 HR7357A-130-188-NHT GTF3A Q92664 KQYICSFEDCKKTFK ASPSKLKRHAKAHEG 59 HR7579A-1-143-NHT GZF1 Q9H116 ESGAVLLESKSSPFN KKQMLESVLLELQNF 142 HR7057A-44-107-Av6HT H1FX Q92522 QPGKYSQLVVETIRR IKALVQNDTLLQVKG 64 HR7057A-44-123-Av6HT H1FX Q92522 QPGKYSQLVVETIRR GANGSFKLNRKKLEG 80 HR7057A-61-123-Av6HT H1FX Q92522 ERNGSSLAKIYTEAK GANGSFKLNRKKLEG 63 HR4599B-103-162-14 HAND2 P61296 MTANRKERRRTQSIN IAYLMDLLAKDDQNG 61 HR4798B-422-503-TEV HBP1 Q50381 SSGTVSATSPNKCKR ALAEEQKRLNPDCWK 82 HR7788A-435-507-TEV HDX Q7Z353 KYRLMGIEVPPPRGG SSQEEPNEVVPNDAR 73 HR7299A-109-148-Av6HT HES1 Q14469 KYRAGFSECMNEVTR EVRTRLLGHLANCMT 40 HR7299A-109-153-Av6HT HES1 Q14469 KYRAGFSECMNEVTR LLGHLANCMTQINAM 45 HR7306A-7-75-NHT HES2 Q9Y543 AGDAAELRKSLKPLL EMTVRFLQELPASSW 69 HR8387-1-122-Av6HT HES3 Q5TGS1 EKKRRARINVSLEQL GLGQEAPALFRPCTP 121 HR6986-1-108-TEV HES5 Q5TA89 APSTVAVELLSPKEK WCLQEAVQFLTLHAA 107 HR6986-1-122-TEV HES5 Q5TA89 APSTVAVELLSPKEK ASDTQMKLLYHFQRP 121 HR6986-49-166-TEV HES5 Q5TA89 RHQPNSKLEKADILE AAAAHQPACGLWRPW 118 HR6986A-11-108-Av6HT HES5 Q5TA89 LSPKEKNRLRKPVVE WCLQEAVQFLTLHAA 98 HR6986A-11-80-Av6HT HES5 Q5TA89 LSPKEKNRLRKPVVE VSYLKHSKAFVAAAG 70 HR6986A-21-108-Av6HT HES5 Q5TA89 KPVVEKMRRDRINSS WCLQEAVQFLTLHAA 88 HR6986A-25-108-Av6HT HES5 Q5TA89 EKMRRDRINSSIEQL WCLQEAVQFLTLHAA 84 HR6872A-108-174-TEV HESX1 Q9UBX0 GRRPRTAFTQNQIEV RAKLKRSHRESQFLM 67 HR7863A-111-167-TEV HEY1 Q9Y5J3 AGGKGYFDAHALAMD PLRVRLVSHLNNYAS 57 HR7070A-110-166-TEV HEY2 Q9UBP5 GYFDAHALAMDFMSI RLVSHLSTCATQREA 57 HR7572A-104-158-15 HEYL Q9NQ87 MTGFFDARALAVDFR PVRIRLLSHLNSYAA 56 HR7572A-77-163-15 HEYL Q9NQ87 MQGSSKLEKAEVLQM LLSHLNSYAAEMEPS 88 HR7572A-82-158-15 HEYL Q9NQ87 MLEKAEVLQMTVDHL PVRIRLLSHLNSYAA 78 HR7851A-138-194-Av6HT HHEX Q03014 MKGGQVRFSNDQTIE QVKTWFQNRRAKWRR 58 HR7851A-138-194-TEV HHEX Q03014 KGGQVRFSNDQTIEL QVKTWFQNRRAKWRR 57 HR7402A-1-153-NHT HIC1 Q14526 TFPEADILLKSGECA PDLVALCKKRLKRHG 152 HR7195A-20-139-NHT HIC2 Q96JB3 GPDMELPSHSKQLLL YLQLPELAALCRRKL 120 HR3603B-775-826-TEV HIF1A Q16665 PSDLACRLLGQSMDE LLQGEELLRALDQVN 52 HR7384A-39-112-TEV HIST1H1A Q02339 AGPSVSELIVQAASS QTKGTGASGSFKLNK 74 HR7165A-40-112-TEV HIST1H1B P16401 GPPVSELITKAVAAS QTKGTGASGSFKLNK 73 HR7583A-36-109-TEV HIST1H1C P16403 SGPPVSELITKAVAA QTKGTGASGSFKLNK 74 HR7583A-37-110-TEV HIST1H1C P16403 GPPVSELITKAVAAS TKGTGASGSFKLNKK 74 HR8248A-2087-2143-TEV HIVEP1 P15822 KYICEECGIRCKKPS GNLTKHMKSKAHSKK 57 HR7166A-1798-1854-TEV HIVEP2 P31629 KYICEECGIRCKKPS GNLTKHMKSKAHMKK 57 HR7042A-1753-1809-TEV HIVEP3 Q5T1R4 KYVCEECGIRCKKPS GNLTKHMKSKAHSKK 57 HR7786A-523-577-NHT HKR1 P10072 KPFVCAECGRGFNDK RQKPNLFRHKRAHSG 55 HR7711A-33-224-TEV HLA-DQB1 P01920 RDSPEDFVFQFKGMC PSLQSPITVEWRAQS 192 HR7053A-30-228-TEV HLA-DRB1 P01911 GDTRPRFLWQPKREC TVEWRARSESAQSKM 199 HR8520A-30-221-TEV HLA-DRB1 P04229 GDTRPRFLWQLKFEC PSVTSPLTVEWRARS 192 HR7721A-30-219-TEV HLA-DRB3 P79483 GDTRPRFLELRKSEC EHPSVTSALTVEWRA 190
HR7380A-30-221-TEV HLA-DRB5 Q30154 GDTRPRFLQQDKYEC PSVTSPLTVEWRAQS 192 HR7352A-219-295-TEV HLF Q16534 IPDDLKDDKYWARRR CKNILAKYEARHGPL 77 HR8006A-269-334-Av6HT HLX Q14774 PQTYKRKRSWSRAVF VKVWFQNRRMKWRHS 66 HR7519A-268-352-TEV HMBOX1 Q6NT76 RGSRFTWRKECLAVM KRRANIEAAILESHG 85 HR1506-15 HMG20A Q9NP66 MENLMTSSTLPPLFA SSNAAEGNEQRHEDE 79 HR1506-15.2wt HMG20A Q9NP66 MENLMTSSTLPPLFA SSNAAEGNEQRHEDE 79 HR7093A-68-149-TEV HMG20B Q9P0W2 NGPKAPVTGYVRFLN RAYQQSEAYKMCTEK 82 HR7828-30 HMGB1 P09429 MGKGDPKKPRGKMSS EDEEDEDEEEDDDDE 215 HR7828A-8-78-Av6HT HMGB1 P09429 KPRGKMSSYAFFVQT AKADKARYEREMKTY 71 HR7828A-8-78-TEV HMGB1 P09429 KPRGKMSSYAFFVQT AKADKARYEREMKTY 71 HR7828B-30 HMGB1 P09429 KKKFKDPNAPKRPPS LKEKYEKDIAAYRAK 80 HR8516A-8-78-TEV HMGB1P1 B2RPK0 KPRGKMSSYAFFVQT AKADKTHYERQMKTY 71 HR8015A-1-77-Av6HT HMGB2 P26583 MGKGDPNKPRGKMSS MAKSDKARYDREMKN 77 HR8015A-1-77-TEV HMGB2 P26583 GKGDPNKPRGKMSSY MAKSDKARYDREMKN 76 HR8319A-1-79-TEV HMGB3 Q15347 AKGDPKKPKGKMSAY KADKVRYDREMKDYG 78 HR8540A-11-186-Av6HT HMGB4 Q8WW32 ANVSSYVHFLLNYRN MSARNRCRGKRVRQS 176 HR7956-381-466-Av6HT HMGXB4 Q9UGU5 LHTDGHSEKKKKKEE DKLIWKQKAQYLQHK 86 HR7411A-1-264-TEV HMOX2 P30519 SAEVETSEGVDESEK EDGFPVHDGKGDMRK 263 HR7000A-188-261-NHT HMX1 Q9NP08 AAGETRGGVGVGGGR VKIWFQNRRNKWKRQ 74 HR8029A-131-216-Av6HT HMX2 A2RU54 PGSERPRDGGAERQA NKWKRQLSAELEAAN 86 HR6871A-218-296-NHT HMX3 A6NHT5 SPEKKPACRKKKTRT WKRQLAAELEAANLS 79 HR8251A-233-325-NHT HNF1B P35680 RNRFKWGPASQQILY QKLAMDAYSSNQTHS 93 HR8251A-233-325-TEV HNF1B P35680 RNRFKWGPASQQILY QKLAMDAYSSNQTHS 93 HR7522A-142-391-15 HNF4A P41235 MSSYEDSSLPSINAL GSPSDAPHAHHPLHP 251 HR7522A-142-391-Av6HT HNF4A P41235 SSYEDSSLPSINALL GSPSDAPHAHHPLHP 250 HR7522A-142-391-TEV HNF4A P41235 SSYEDSSLPSINALL GSPSDAPHAHHPLHP 250 HR7522B-142-378-15 HNF4A P41235 MSSYEDSSLPSINAL AKIDNLLQEMLLGGS 238 HR7522B-142-378-Av6HT HNF4A P41235 SSYEDSSLPSINALL AKIDNLLQEMLLGGS 237 HR7522B-142-378-TEV HNF4A P41235 SSYEDSSLPSINALL AKIDNLLQEMLLGGS 237 HR7522C-148-377-15 HNF4A P41235 MSLPSINALLQAEVL MAKIDNLLQEMLLGG 231 HR7522C-148-377-Av6HT HNF4A P41235 SLPSINALLQAEVLS MAKIDNLLQEMLLGG 230 HR7522C-148-377-TEV HNF4A P41235 SLPSINALLQAEVLS MAKIDNLLQEMLLGG 230 HR7522D-58-135-15 HNF4A P41235 MALCAICGDRATGKH FRAGMKKEAVQNERD 79 HR7522D-58-135-Av6HT HNF4A P41235 ALCAICGDRATGKHY FRAGMKKEAVQNERD 78 HR7522D-58-135-TEV HNF4A P41235 ALCAICGDRATGKHY FRAGMKKEAVQNERD 78 HR7469A-9-77-15 HNF4G Q14541 MVLDPTYTTLEFETM ASSCDGCKGFFRRSI 70 HR7469A-9-91-15 HNF4G Q14541 MVLDPTYTTLEFETM IRKSHVYSCRFSRQC 84 HR7469A-9-91-Av6HT HNF4G Q14541 VLDPTYTTLEFETMQ IRKSHVYSCRFSRQC 83 HR7469A-9-91-TEV HNF4G Q14541 VLDPTYTTLEFETMQ IRKSHVYSCRFSRQC 83 HR7469A-9-95-15 HNF4G Q14541 MVLDPTYTTLEFETM HVYSCRFSRQCVVDK 88 HR7469A-9-95-Av6HT HNF4G Q14541 VLDPTYTTLEFETMQ HVYSCRFSRQCVVDK 87 HR7469A-9-95-TEV HNF4G Q14541 VLDPTYTTLEFETMQ HVYSCRFSRQCVVDK 87 HR7469B-103-328-15 HNF4G Q14541 MYCRLRKCFRAGMKK RQYDSRGRFGELLLL 227 HR7469B-103-328-Av6HT HNF4G Q14541 YCRLRKCFRAGMKKE RQYDSRGRFGELLLL 226 HR7469B-103-328-TEV HNF4G Q14541 YCRLRKCFRAGMKKE RQYDSRGRFGELLLL 226 HR8063A-429-485-TEV HOMEZ Q8IX15 SFQDPAIPTPPPSTR AAHQQLRETDIPQLS 57 HR6881-1-73-TEV HOPX Q9BPY8 SAETASGPTEDQVEI RRSEGLPSECRSVTD 72 HR7310A-197-291-TEV HOXA1 P49639 ETSSPAQTFDWMKVK FQNRRMKQKKREKEG 95 HR4742B-299-369-14 HOXA10 P31260 MKDSLGNSKGENAAN SVHLTDRQVKIWFQN 72 HR4742B-299-393-14 HOXA10 P31260 MKDSLGNSKGENAAN RENRIRELTANFNFS 96 HR4742C-309-369-14 HOXA10 P31260 MNAANWLTAKSGRKK SVHLTDRQVKIWFQN 62 HR4742C-314-393-14 HOXA10 P31260 MLTAKSGRKKRCPYT RENRIRELTANFNFS 81 HR4742C-320-393-14 HOXA10 P31260 MRKKRCPYTKHQTLE RENRIRELTANFNFS 75 HR8427A-342-397-Av6HT HOXA10 P31260 PYTKHQTLELEKEFL WFQNRRMKLKKMNRE 56 HR8104A-227-302-Av6HT HOXA11 P31270 GHTEDKAGGSSGQRT WFQNRRMKEKKINRD 76 HR8104A-227-313-Av6HT HOXA11 P31270 GHTEDKAGGSSGQRT INRDRLQYYSANPLL 87 HR8104B-227-296-Av6HT HOXA11 P31270 GHTEDKAGGSSGQRT DRQVKIWFQNRRMKE 70 HR7749A-317-379-TEV HOXA13 P31271 SSYRRGRKKRVPYTK QVTIWFQNRRVKEKK 63 HR7478A-131-205-NHT HOXA2 O43364 ESLEIADGSGGGSRR FQNRRMKHKRQTQCK 75 HR7187A-190-266-Av6HT HOXA3 O43365 SSKRARTAYTSAQLV GKGMLTSSGGQSPSR 77 HR7193A-222-275-TEV HOXA4 Q00056 YTRQQVLELEKEFHF IWFQNRRMKWKKDHK 54 HR7149A-201-257-TEV HOXA5 P20719 AYTRYQTLELEKEFH FQNRRMKWKKDNKLK 57 HR7149A-202-258-TEV HOXA5 P20719 YTRYQTLELEKEFHF QNRRMKWKKDNKLKS 57 HR7925A-156-215-TEV HOXA6 P31267 RRGRQTYTRYQTLEL IWFQNRRMKWKKENK 60 HR4674B-194-270-TEV HOXA9 P31269 NNPAANWLHARSTRK NRRMKMKKINKDRAK 77 HR8367A-171-266-TEV HOXB1 P14653 EPNTPTARTFDWMKV QNRRMKQKKREREEG 96 HR8236A-216-273-TEV HOXB13 Q92826 GRKKRIPYSKGQLRE QITIWFQNRRVKEKK 58 HR7791A-149-216-Av6HT HOXB2 P14652 AYTNTQLLELEKEFH TQHREPPDGEPACPG 68 HR8135A-187-244-Av6HT HOXB3 P14651 ASKRARTAYTSAQLV RQIKIWFQNRRMKYK 58 HR8135A-196-252-Av6HT HOXB3 P14651 TSAQLVELEKEFHFN NRRMKYKKDQKAKGL 57 HR8135B-179-239-Av6HT HOXB3 P14651 DKSPPGSAASKRART LNLSERQIKIWFQNR 61 HR8135B-179-244-Av6HT HOXB3 P14651 DKSPPGSAASKRART RQIKIWFQNRRMKYK 66 HR8335A-169-222-Av6HT HOXB4 P17483 MYTRQQVLELEKEFH IWFQNRRMKWKKDHK 55 HR8335A-169-222-NHT HOXB4 P17483 YTRQQVLELEKEFHY IWFQNRRMKWKKDHK 54 HR8335A-169-222-TEV HOXB4 P17483 YTRQQVLELEKEFHY IWFQNRRMKWKKDHK 54 HR8261-201-257-Av6HT HOXB5 P09067 YTRYQTLELEKEFHF QNRRMKWKKDNKLKS 57 HR7319A-147-206-Av6HT HOXB6 P17509 MRRGRQTYTRYQTLE IWFQNRRMKWKKESK 61 HR7319A-147-206-TEV HOXB6 P17509 RRGRQTYTRYQTLEL IWFQNRRMKWKKESK 60 HR8504A-143-202-TEV HOXB7 P09629 TYTRYQTLELEKEFH RRMKWKKENKTAGPG 60 HR7846A-146-205-TEV HOXB8 P17481 RRRGRQTYSRYQTLE KIWFQNRRMKWKKEN 60 HR7230A-174-249-TEV HOXB9 P17482 NPSANWLHARSSRKK NRRMKMKKMNKEQGK 76 HR4478B-250-312-14 HOXC10 Q9NYD6 MKEEIKAENTTGNWL RLEISKTINLTDRQV 64 HR4478B-255-342-14 HOXC10 Q9NYD6 MAENTTGNWLTAKSG RENRIRELTSNFNFT 89 HR4478B-263-312-14 HOXC10 Q9NYD6 MLTAKSGRKKRCPYT RLEISKTINLTDRQV 51 HR4478B-268-342-14 HOXC10 Q9NYD6 MGRKKRCPYTKHQTL RENRIRELTSNFNFT 76 HR4478C-247-342-14 HOXC10 Q9NYD6 MNEAKEEIKAENTTG RENRIRELTSNFNFT 97 HR7286A-240-304-Av6HT HOXC11 O43248 SKFQIRELEREFFFN LSRDRLQYFSGNPLL 65 HR7847A-205-271-NHT HOXC12 P31275 APWYPINSRSRKKRK QVKIWFQNRRMKKKR 67 HR7251A-255-316-Av6HT HOXC13 P31276 SSYRRGRKKRVPYTK RQVTIWFQNRRVKEK 62 HR8257A-163-216-NHT HOXC4 P09017 YTRQQVLELEKEFHY IWFQNRRMKWKKDHR 54 HR8257A-163-216-TEV HOXC4 P09017 YTRQQVLELEKEFHY IWFQNRRMKWKKDHR 54 HR7011A-156-219-TEV HOXC5 Q00444 KRSRTSYTRYQTLEL NRRMKWKKDSKMKSK 64 HR7839A-148-201-TEV HOXC6 P09630 YSRYQTLELEKEFHF IWFQNRRMKWKKESN 54 HR6394A-149-208-TEV HOXC8 P31273 RRSGRQTYSRYQTLE KIWFQNRRMKWKKEN 60 HR7283A-180-255-TEV HOXC9 P31274 SNPVANWIHARSTRK QNRRMKMKKMNKEKT 76 HR8256A-200-280-Av6HT HOXD1 Q9GZZ0 AAFSTFEWMKVKRNA LHLNDTQVKIWFQNR 81 HR8148A-269-340-15 HOXD10 P28358 MKRCPYTKHQTLELE RENRIRELTANLTFS 73 HR8148A-274-327-Av6HT HOXD10 P28358 TKHQTLELEKEFLFN WFQNRRMKLKKMSRE 54 HR8148A-274-340-15 HOXD10 P28358 MTKHQTLELEKEELF RENRIRELTANLTFS 68 HR8017A-257-326-Av6HT HOXD11 P31277 SSSAVAPQRSRKKRC IWFQNRRMKEKKLNR 70 HR7443A-276-333-TEV HOXD13 P35453 GRKKRVPYTKLQLKE QVTIWFQNRRVKDKK 58 HR7220A-181-257-Av6HT HOXD3 P31249 GESCEDKSPPGPASK QNRRMKYKKDQKAKG 77 HR7700A-161-220-TEV HOXD4 P09016 YTRQQVLELEKEFHF RMKWKKDHKLPNTKG 60 HR7832A-197-256-TEV HOXD8 P13378 RRRGRQTYSRFQTLE KIWFQNRRMKWKKEN 60 HR6999A-263-337-TEV HOXD9 P28356 SQPQQQQLDPNNPAA NLTERQVKIWFQNRR 75 HR7031A-153-237-TEV HP1BP3 Q5SSJ5 ASSPRPKMDAILTEA GASGSFVVVQKSRKT 84 HR7031B-249-3355-15 HP1BP3 Q5SSJ5 MSAVDPEPQVKLEDV GASGTFQLKKSGEKP 88 HR7031B-254-330-15 HP1BP3 Q5SSJ5 MEPQVKLEDVLPLAF QITGKGASGTFQLKK 78 HR7031B-262-330-Av6HT HP1BP3 Q5SSJ5 VLPLAFTRLCEPKEA QITGKGASGTFQLKK 69 HR7031C-332-407-15 HP1BP3 Q5SSJ5 MGEKPLLGGSLMEYA KNGWMEQISGKGFSG 77 HR7031C-332-418-15 HP1BP3 Q5SSJ5 MGEKPLLGGSLMEYA GFSGTFQLCFPYYPS 88 HR7031C-337-403-15 HP1BP3 Q5SSJ5 MLGGSLMEYAILSAI QKCEKNGWMEQISGK 68 HR7031C-337-413-15 HP1BP3 Q5SSJ5 MLGGSLMEYAILSAI QISGKGFSGTFQLCF 78 HR3023-1-506-15 HSF1 Q00613 MDLPVGPGAAGPSNV FELGEGSYFSEGDGF 506 HR3023-1-506-Av6HT HSF1 Q00613 DLPVGPGAAGPSNVP FELGEGSYFSEGDGF 505 HR3023-1-506-TEV HSF1 Q00613 DLPVGPGAAGPSNVP FELGEGSYFSEGDGF 505 HR3023A-14 HSF1 Q00613 MDLPVGPGAAGPSNV PERDDTEFQHPCFLR 106 HR3023A-15 HSF1 Q00613 MDLPVGPGAAGPSNV PERDDTEFQHPCFLR 106 HR3023C-1-123-15 HSF1 Q00613 MDLPVGPGAAGPSNV EQLLENIKRKVTSVS 123 HR3023C-10-123-15 HSF1 Q00613 MAGPSNVPAFLTKLW EQLLENIKRKVTSVS 115 HR3023C-15-118-15 HSF1 Q00613 MVPAFLTKLWTLVSD FLRGQEQLLENIKRK 105 HR3023C-7-118-15 HSF1 Q00613 MPGAAGPSNVPAFLT FLRGQEQLLENIKRK 113 HR8180A-12-124-15 HSF4 Q9ULV5 MPGPSPVPAFLGKLW REQLLERVRRKVPAL 114 HR8180A-12-97-15 HSF4 Q9ULV5 MPGPSPVPAFLGKLW VVSIEQGGLLRPERD 87 HR8180A-17-119-15 HSF4 Q9ULV5 MVPAFLGKLWALVGD SFVRGREQLLERVRR 104
HR8180A-17-93-15 HSF4 Q9ULV5 MVPAFLGKLWALVGD GFRKVVSIEQGGLLR 78 HR8170A-9-94-Av6HT HSF5 Q4G112 INPNNFPAKLWRLVN FIRQLNLYGFRKVVL 86 HR7245A-97-218-NHT HSFX1 Q9UBD0 LPFPQKLWRLVSSNQ LLVRMKRRVGVKSAP 122 HR3123-1-116-15 ID1 P41134 MKVASGSTATAAAGP IRDLQLELNSESEVG 116 HR3123-1-121-15 ID1 P41134 MKVASGSTATAAAGP LELNSESEVGTPGGR 121 HR3123-15 ID1 P41134 MKVASGSTATAAAGP AEAACVPADDRILCR 155 HR3123-21 ID1 P41134 MKVASGSTATAAAGP AEAACVPADDRILCR 155 HR3123A-14 ID1 P41134 ALKAGKTASGAGEVV RDLQLELNSESEVGT 100 HR3123B-14 ID1 P41134 ALKAGKTASGAGEVV AEAACVPADDRILCR 138 HR3123C-14 ID1 P41134 KTASGAGEVVRCLSE RDLQLELNSESEVGT 95 HR3123D-14 ID1 P41134 KTASGAGEVVRCLSE AEAACVPADDRILCR 133 HR3123E-14 ID1 P41134 AGEVVRCLSEQSVAI RDLQLELNSESEVGT 90 HR3123F-14 ID1 P41134 AGEVVRCLSEQSVAI AEAACVPADDRILCR 128 HR3123G-54-145-15 ID1 P41134 MPALLDEQQVNVLLY TLNGEISALTAEAAC 93 HR3123G-59-139-15 ID1 P41134 MEQQVNVLLYDMNGC VRAPLSTLNGEISAL 82 HR2921-14 ID2 Q02363 MKAFSPVRSVRKNSL FPSELMSNDSKALCG 134 HR2921-15 ID2 Q02363 MKAFSPVRSVRKNSL FPSELMSNDSKALCG 134 HR2921-17-85-14 ID2 Q02363 DHSLGISRSKTPVDD YILDLQIALDSHPTI 69 HR2921-21 ID2 Q02363 MKAFSPVRSVRKNSL FPSELMSNDSKALCG 134 HR2921-22-134-15 ID2 Q02363 MISRSKTPVDDPMSL FPSELMSNDSKALCG 114 HR2921-22-85-14 ID2 Q02363 ISRSKTPVDDPMSLL YILDLQIALDSHPTI 64 HR2921-27-124-15 ID2 Q02363 MTPVDDPMSLLYNMN ISILSLQASEFPSEL 99 HR2921-27-134-15 ID2 Q02363 MTPVDDPMSLLYNMN FPSELMSNDSKALCG 109 HR2921-27-85-14 ID2 Q02363 TPVDDPMSLLYNMND YILDLQIALDSHPTI 59 HR2921-40-134-15 ID2 Q02363 MNDCYSKLKELVPSI FPSELMSNDSKALCG 96 HR3111-14 ID3 Q712G9 MKALSPVRGCYEAVC APELVISNDKRSFCH 119 HR3111-15 ID3 Q712G9 MKALSPVRGCYEAVC APELVISNDKRSFCH 119 HR3111-21 ID3 Q712G9 MKALSPVRGCYEAVC APELVISNDKRSFCH 119 HR3111A-27-83-15 ID3 Q712G9 MGRGKGPAAEEPLSL ILQRVIDYILDLQVV 58 HR3111A-32-83-15 ID3 Q712G9 MPAAEEPLSLLDDMN ILQRVIDYILDLQVV 53 HR4584C-53-112-14 ID4 P47928 DEPALCLQCDMNDCY IDYILDLQLALETHP 55 HR4626B IFI16 Q16666 QVTPRRNVLQKRPVI ISEMHSFIQIKKKTN 202 HR3005-100-519-15 IKZF1 Q13422 MGSSALSGVGGIRLP FSSHITRGEHRFHMS 421 HR3005-108-519-15 IKZF1 Q13422 MGGIRLPNGKLKCDI FSSHITRGEHRFHMS 413 HR3005-93-519-15 IKZF1 Q13422 MNGSHRDQGSSALSG FSSHITRGEHRFHMS 428 HR3005A-IDT-14 IKZF1 Q13422 HARNGLSLKEEHRAY FSSHITRGEHRFHMS 99 HR3005B-IDT-14 IKZF1 Q13422 EKMNGSHRDQGSSAL DRLASNVAKRKSSMP 190 HR3064-99-509-15 IKZF3 Q9UKT9 IKLERHVVSFDSSRP FSSHIARGEHRALLK 411 HR6479A-436-509-NHT IKZF3 Q9UKT9 RDSVKVINKEGEVMD FSSHIARGEHRALLK 74 HR7992A-150-221-15 IKZF4 Q9H2S9 MGGIRLPNGKLKCDV KLHSGEKPFKCPFCN 73 HR7992A-150-232-15 IKZF4 Q9H2S9 MGGIRLPNGKLKCDV PFCNYACRRRDALTG 84 HR7992A-150-246-15 IKZF4 Q9H2S9 MGGIRLPNGKLKCDV GHLRTHSVSSPTVGK 98 HR7992A-155-216-15 IKZF4 Q9H2S9 MPNGKLKCDVCGMVC LLRHIKLHSGEKPFK 63 HR7992A-155-216-15.7-15TEV IKZF4 Q9H2S9 MPNGKLKCDVCGMVC LLRHIKLHSGEKPFK 63 HR7992A-155-239-15 IKZF4 Q9H2S9 MPNGKLKCDVCGMVC RRRDALTGHLRTHSV 86 HR7992B-513-585-15 IKZF4 Q9H2S9 MSKEVLRVVGESGEP YEFSSHIVRGEHKVG 74 HR7992B-518-585-15 IKZF4 Q9H2S9 MRVVGESGEPVKAFK YEFSSHIVRGEHKVG 69 HR7992B-523-585-15 IKZF4 Q9H2S9 MSGEPVKAFKCEHCR YEFSSHIVRGEHKVG 64 HR7992B-528-585-15 IKZF4 Q9H2S9 MKAFKCEHCRILFLD YEFSSHIVRGEHKVG 59 HR7992C-155-272-Av6HT IKZF4 Q9H2S9 PNGKLKCDVCGMVCI KQQSTLEEHKERCHN 118 HR7992C-159-272-Av6HT IKZF4 Q9H2S9 LKCDVCGMVCIGPNV KQQSTLEEHKERCHN 114 HR7992C-159-283-Av6HT IKZF4 Q9H2S9 LKCDVCGMVCIGPNV RCHNYLQSLSTEAQA 125 HR7992D-197-260-Av6HT IKZF4 Q9H2S9 TQKGNLLRHIKLHSG KPYKCNYCGRSYKQQ 64 HR7992D-208-283-Av6HT IKZF4 Q9H2S9 LHSGEKPFKCPFCNY RCHNYLQSLSTEAQA 76 HR7992D-210-260-Av6HT IKZF4 Q9H2S9 SGEKPFKCPFCNYAC KPYKCNYCGRSYKQQ 51 HR7630A-358-419-NHT IKZF5 Q9H5V7 QDPQLLHHCQHCDMY YDFACHFARGQHNQH 62 HR7614A-263-300-15 INSM1 Q01101 MPLGEFICQLCKEEY KCSRIVRVEYRCPEC 39 HR7614A-263-319-15 INSM1 Q01101 MPLGEFICQLCKEEY SCPANLASHRRWHKP 58 HR7614B-424-497-15 INSM1 Q01101 MGDGEGAGVLGLSAS GLTRHINKCHPSENR 75 HR7614B-429-493-15 INSM1 Q01101 MAGVLGLSASAECHL YSSPGLTRHINKCHP 66 HR7614B-432-497-15 INSM1 Q01101 MLGLSASAECHLCPV GLTRHINKCHPSENR 67 HR8043A-261-315-Av6HT INSM2 Q96T92 GEFICQLCKEQYADP SCPANLASHRRWHKP 55 HR6405A-1-113-TEV IRF1 P10914 PITRMRMRPWLEMQI RNKGSSAVRVYRMLP 112 HR7043A-1-113-Av6HT IRF2 P14316 MPVERMRMRPWLEEQ IKKGNNAFRVYRMLP 113 HR7043A-1-113-TEV IRF2 P14316 PVERMRMRPWLEEQI IKKGNNAFRVYRMLP 112 HR7278A-1-113-TEV IRF3 Q14653 GTPKPRILPWLVSQL DPHDPHKIYEFVNSG 112 HR7278B-196-386-TEV IRF3 Q14653 LVPGEEWEFEVTAFY LRALVEMARVGGASS 191 HR3173-1-119-14 IRF5 Q13568 MNQSIPVAPTRPRRV DGPRDMPPQPYKIYE 119 HR3173A-14 IRF5 Q13568 MNQSIPVAPTPPRRV PPQPYKIYEVCSNGP 125 HR3173A-15 IRF5 Q13568 MNQSIPVAPTPPRRV PPQPYKIYEVCSNGP 125 HR3173F-8-114-14 IRF5 Q13568 APTPPRRVRLKPWLV FRLIYDGPRDMPPQP 107 HR3173G-232-477-TEV IRF5 Q13568 EQLLPDLLISPHMLP HIWQSQQRLQPVAQA 246 HR7755A-198-455-Av6HT IRF6 O14896 LEMEVPQAPIQPFYS RILQTQESWQPMQPT 258 HR5527A-14 IRF7 Q92985 MALAPERAAPRVLFG RRFVMLRDNSGDPAD 117 HR5527A-15 IRF7 Q92985 MALAPERAAPRVLFG RRFVMLRDNSGDPAD 117 HR8215A-8-154-TEV IRF7 Q92985 AAPRVLFGEWLLGEI EAEAPAAVPPPQGGP 147 HR7337A-9-115-TEV IRF8 Q02556 RLRQWLIEQIDSSMY LDISEPYKVYRIVPE 107 HR7302A-205-393-Av6HT IRF9 Q00978 QRSLEFLLPPEPDYS LEQTPEQQAAILSLV 189 HR7302A-209-393-Av6HT IRF9 Q00978 EFLLPPEPDYSLLLT LEQTPEQQAAILSLV 185 HR7431A-121-188-NHT IRX1 P78414 GQFQYGDPGRPKNAT VSTWFANARRRLKKE 68 HR7304A-126-209-NHT IRX6 P78412 PYERTLGQYQYERYG TWFANARRRLKKENK 84 HR8326A-180-244-TEV ISL1 P61371 KTTRVRTVLNEKQLH QNKRCKDKKRSIMMK 65 HR8291A-190-254-TEV ISL2 Q96A47 KTTRVRTVLNEKQLH QNKRCKDKKKSILMK 65 HR8400A-617-732-TEV JARID2 Q92833 LGRRWGPNVQRLACI RLEKEVLMEKEILEK 116 HR8400B-804-1099-Av6HT JARID2 Q92833 KGVLNDFHKCIYKGR LDELRDTELRQRRQL 296 HR8400B-809-1099-Av6HT JARID2 Q92833 DFHKCIYKGRSVSLT LDELRDTELRQRRQL 291 HR8400B-809-1104-Av6HT JARID2 Q92833 DFHKCIYKGRSVSLT DTELRQRRQLFEAGL 296 HR8400C-900-1086-Av6HT JARID2 Q92833 GSILRHLGAVPGVTI KENGPTLSTISALLD 187 HR8400C-900-1104-Av6HT JARID2 Q92833 GSILRHLGAVPGVTI DTELRQRRQLFEAGL 205 HR7951-28-149-Av6HT IDP2 Q8WYK2 SALTVEELKYADIRN HRPTCIVRTDSVKTP 122 HR4484C-253-308-Av6HT JUN P05412 MIKAERKRMRNRIAA LASTANMLREQVAQL 57 HR4484C-253-308-TEV JUN P05412 IKAERKRMRNRIAAS LASTANMLREQCAQL 56 HR4765B-273-324-TEV JUNB P17275 RKRLRNRLAATKCRK LSSTAGLLREQVAQL 52 HR4754B-269-324-TEV JUND P17535 IKAERKRLRNRIAAS LASTASLLREQVAQL 56 HR2962A-5-79-Av6HT KAT5 Q92993 GEIIEGCRLPVLRRN LKKIQFPKKEAKTPT 75 HR7375A-94-208-TEV KDM5B Q9UGL1 EAQTRVKLNFLDQIA LQKPNLTTDTKDKEY 115 HR7375B-685-750-Av6HT KDM5B Q9UGL1 LPDDERQCVKCKTTC YTLDDLYPMMNALKL 66 HR7375B-696-750-Av6HT KDM5B Q9UGL1 KTTCFMSAISCSCKP YTLDDLYPMMNALKL 55 HR7375C-1487-1544-Av6HT KDM5B Q9UGL1 CPAVSCLQPEGDEVD YICVRCTVKDAPSRK 58 HR7375D-1485-1536-Av6HT KDM5B Q9UGL1 AICPAVSCLQPEGDE PEMAEKEDYICVRCT 52 HR7375E-1123-1227-Av6HT KDM5B Q9UGL1 ESLSDLERALTESKE LRIWLCPHCRRSEKP 105 HR7375E-1123-1241-Av6HT KDM5B Q9UGL1 ESLSDLERALTESKE PPLEKILPLLASLQR 119 HR7375E-1132-1230-Av6HT KDM5B Q9UGL1 LTESKETASAMATLG WLCPHCRRSEKPPLE 99 HR7375E-1134-1241-Av6HT KDM5B Q9UGL1 ESKETASAMATLGEA PPLEKILPLLASLQR 108 HR7375E-1143-1230-Av6HT KDM5B Q9UGL1 ATLGEARLREMEALQ WLCPHCRRSEKPPLE 88 HR7375E-1143-1241-Av6HT KDM5B Q9UGL1 ATLGEARLREMEALQ PPLEKILPLLASLQR 99 HR7188A-306-385-TEV KDM5D Q9BY66 HSSAQFIDSYICQVC EAFGFEQATQEYSLQ 80 HR7714A-77-157-Av6HT KIAA1683 Q9H0B3 RRVPRLRAVVESQAF RHILHSSKSLVKKTR 81 HR7682A-10-80-Av6HT KIAA2018 Q68DE3 PTKKQHRKKNRETHN ITELKRQNDELLLNG 71 HR8201A-51-160-TEV KIN Q60870 QRQLLLASENPQQFM PETIRRQLELEKKKK 110 HR7553A-272-338-15 KLF1 Q13351 MARKRQAAHTCAHPG DELTRHYRKHTGQRP 68 HR7553A-292-335-Av6HT KLF1 Q13351 KSSHLKAHLRTHTGE ARSDELTRHYRKHTG 44 HR7553B-319-362-Av6HT KLF1 Q13351 RFARSDELTRHYRKH FSRSDHLALHMKRHL 44 HR6400A-353-423-TEV KLF10 Q13118 SAAKVTPQIDSSRIR REARSDELSRHRRTH 71 HR6390-1-497-15 KLF11 O14901 MHTPDFAGPDDARAV PGWQAEVGKLNRIAS 497 HR6390-1-501-15 KLF11 O14901 MHTPDFAGPDDARAV AEVGKLNRIASAESP 501 HR6390-12-512-15 KLF11 O14901 ARAVDIMDICESILE AESPGSPLVSMPASA 501 HR6390-123-512-15 KLF11 O14901 VSPQVTDSKACTATD AESPGSPLVSMPASA 390 HR6390-128-512-15 KLF11 O14901 TDSKACTATDVLQSS AESPGSPLVSMPASA 385 HR6390-15 KLF11 O14901 MHTPDEAGPDDARAV AESPGSPLVSMPASA 512 HR6390-7-512-15 KLF11 O14901 AGPDDARAVDIMDIC AESPGSPLVSMPASA 506 HR6390A-379-501-15 KLF11 O14901 SQNCVPQVDFSRRRN AEVGKLNRIASAESP 123 HR6390A-384-497-15 KLF11 O14901 PQVDFSRRRNYVCSF PGWQAEVGKLNRIAS 114 HR6390B-397-462-15 KLF11 O14901 SFPGCRKTYFKSSHL HTGEKKFVCPVCDRR 66 HR6390B-402-457-15 KLF11 O14901 RKTYFKSSHLKAHLR RHRRTHTGEKKFVCP 56
HR7238A-306-400-TEV KLF12 Q9Y4X4 SESPDSRKRRIHRCD FSRSDHLALHRRRHM 95 HR8436A-125-193-Av6HT KLF16 Q9BXK1 KSHRCPFPDCAKAYY RTHTGEKRFSCPLCS 69 HR7123A-272-355-TEV KLF2 Q9Y5W3 HTCSYAGCGKTYTKS FSRSDHLALHMKRHM 84 HR7880A-251-343-TEV KLF3 P57682 PDTQRKRRIHRCDYD FSRSDHLALHRKRHM 93 HR4433-1-347-14 KLF5 Q13887 MATRVLSMSARLGPV ASKLAIHNPNLPTTL 347 HR4433-9-342-14 KLF5 Q13887 MSARLGPVPQPPAPQ YAATIASKLAIHNPN 335 HR4668C-168-283-21 KLF6 Q99612 MELPSPGKVRSGTSG FSRSDHLALHMKRHL 117 HR4668C-173-283-21 KLF6 Q99612 MGKVRSGTSGKPGDK FSRSDHLALHMKRHL 112 HR4668C-173-283-Av6HT KLF6 Q99612 GKVRSGTSGKPGDKG FSRSDHLALHMKRHL 111 HR4668C-173-283-TEV KLF6 Q99612 GKVRSGTSGKPGDKG FSRSDHLALHMKRHL 111 HR4668C-191-283-21 KLF6 Q99612 MASPDGRRRVHRCHF FSRSDHLALHMKRHL 94 HR4668C-196-283-21 KLF6 Q99612 MRRRVHRCHFNGCRK FSRSDHLALHMKRHL 89 HR4668D-205-283-21 KLF6 Q99612 MNGCRKVYTKSSHLK FSRSDHLALHMKRHL 80 HR4668D-210-283-21 KLF6 Q99612 MVYTKSSHLKAHQRT FSRSDHLALHMKRHL 75 HR8165A-231-302-Av6HT KLF7 O75840 TKSSHLKAHQRTHTG FSRSDHLALHMKRHL 72 HR8376A-270-332-Av6HT KLF8 O95600 RRRIHQCDFAGCSKV SDELTRHFRKHTGIK 63 HR7597A-181-244-NHT KLF9 Q13886 LKKFSRSDELTRHYR PSMIKRSKKALANAL 64 HR6918A-877-937-TEV KNL2 Q6P0N0 DKEWNEKELQKLHCA MENPRGKGSQKHVTK 61 HR6489A-15 L3MBTL3 Q96JM7 RRKRRGDSAVLKQGL QPPLSPLELMEASEH 346 HR6489A-Av6HT L3MBTL3 Q96JM7 RRKRRGDSAVLKQGL QPPLSPLELMEASEH 346 HR6489A-TEV L3MBTL3 Q96JM7 RRKRRGDSAVLKQGL QPPLSPLELMEASEH 346 HR6490A-15 L3MBTL4 Q8NA19 MKQPNRKRKLNMDSK SAFGCPYSDMNLKKE 414 HR6490A-30-371-15 L3MBTL4 Q8NA19 MEKKPKDSTTPLSHV TGHPLEVPQRTNDLK 343 HR6490A-30-371-Av6HT L3MBTL4 Q8NA19 EKKPKDSTTPLSHVP TGHPLEVPQRTNDLK 342 HR6490A-30-371-Na6HT L3MBTL4 Q8NA19 EKKPKDSTTPLSHVP TGHPLEVPQRTNDLK 342 HR6490A-30-371-TEV L3MBTL4 Q8NA19 EKKPKDSTTPLSHVP TGHPLEVPQRTNDLK 342 HR6490A-Av6HT L3MBTL4 Q8NA19 KQPNRKRKLNMDSKE SAFGCPYSDMNLKKE 413 HR6490A-TEV L3MBTL4 Q8NA19 KQPNRKRKLNMDSKE SAFGCPYSDMNLKKE 413 HR2473-14 LARP1 Q6PKG0 MNTLFRFWSFFLRDH AKWTSQHSNTQTLGK 185 HR7995A-377-483-Av6HT LARP1 Q6PKG0 ISLIFAALKDSKVVE SASLPDLDSENWIEV 107 HR6994A-210-292-NHT LARP1B Q659C4 VEEALLKEYIKRQIE EVEIVDEKMRKKIEP 83 HR7969A-107-200-TEV LARP4 Q71RC2 SGESNSAVSTEDLKE DEKGEKVRPSHKRCI 94 HR6949A-152-237-NHT LARP4B Q92615 SQEDPREVLKKTLEF DEKGEKVRPNQNRCI 86 HR7099A-69-134-NHT LASS2 Q96G23 NIKEKTRLRAPPNAT RRRNQDRPSLLKKFR 66 HR8001A-86-135-TEV LASS5 Q8N5B7 AQPNAILEKVFISIT KIQCWFRHRRNQDKP 50 HR6906A-77-127-TEV LASS6 Q6ZMG9 APPNAILEKVFTAIT IQRWFRQRRNQEKPS 51 HR6954A-124-198-NHT LBX1 P52954 KRRKSRTAFTNHQIY LEEMKADVESAKKLG 75 HR8118A-343-405-TEV LCOR Q96JN0 RGRYRQYNSEILEEA GTLKNPPKKKMKLMR 63 HR7552A-519-579-TEV LCORL Q8N3X6 RGRYRQYDHEIMEEA RSGTLKTPPKKKLRL 61 HR7767A-314-501-Av6HT LENG9 Q96B70 APCQPRPTHFVALMV RTGGPFQPLAEIRLE 188 HR8129A-23-126-Av6HT LHX1 P48742 AWHVKCVQCCECKCN FVCKEDYLSNSSVAK 104 HR7637A-262-334-TEV LHX2 P50458 SSQKTKRMRTSFKHH KFRRNLLRQENTGVD 73 HR7663A-23-150-TEV LHX3 Q9UBR4 LARRADLRREIPLCA FYLMEDSRLVCKADY 128 HR7789A-16-91-NHT LHX4 Q969G2 LPEMLGVPMQQIPQC CKEDFFKRFGTKCTA 76 HR7587A-24-119-NHT LHX5 Q9H2C1 AWHIKCVQCCECKTN LYVIDENKFVCKDDY 96 HR7172-1-267-TEV LHX9 Q9NQ69 LNGTTLEAAMLFHGI PPSQKTKRMRTSFKH 266 HR7172-1-270-15 LHX9 Q9NQ69 MLNGTTLEAAMLFHG QKTKRMRTSFKHHQL 270 HR7525A-134-187-Av6HT LIN28A Q9H9Z2 MSKGDRCYNCGGLDH SCPLKAQQGPSAQGK 55 HR7525A-134-187-TEV LIN28A Q9H9Z2 SKGDRCYNCGGLDHH SCPLKAQQGPSAQGK 54 HR7198A-25-103-NHT LIN28B Q6ZN17 SQVLRGTGHCKWFNV KSSKGLESIRVTGPG 79 HR7658-1-237-Av6HT LMX1A Q8TE12 LDGLKMEENFQSAID KVRETLAAETGLSVR 236 HR7658-1-247-Av6HT LMX1A Q8TE12 LDGLKMEENFQSAID GLSVRVVQVWFQNQR 246 HR7658-1-257-Av6HT LMX1A Q8TE12 LDGLKMEENFQSAID FQNQRAKMKKLARRQ 256 HR7658-13-303-Av6HT LMX1A Q8TE12 SAIDTSASFSSLLGR PYTALPTPQQLLAIE 291 HR7658A-61-153-NHT LMX1A Q8TE12 QCASCKEPLETTCFY EGQLLCKGDYEKERE 93 HR7658B-13-153-Av6HT LMX1A Q8TE12 SAIDTSASFSSLLGR EGQLLCKGDYEKERE 141 HR7658B-32-153-Av6HT LMX1A Q8TE12 KSVCEGCQRVILDRF EGQLLCKGDYEKERE 122 HR6403A-128-207-15 LYL1 P12980 RLKRRPSHCELDLAE RLAMKYIGFLVRLLR 80 HR6403A-133-207-15 LYL1 P12980 PSHCELDLAEGHQPQ RLAMKYIGFLVRLLR 75 HR6403A-146-207-15 LYL1 P12980 PQKVARRVFTNSRER RLAMKYIGFLVRLLR 62 HR6403A-146-226-15 LYL1 P12980 PQKVARRVFTNSRER ALAAGPTPPGPRKRP 81 HR7569A-1-80-TEV MAEL Q96JY0 PNRKASRNAYYFFVQ GKDPGPSEKQKPVFT 79 HR4779B-255-300-14 MAF O75444 MLHFDDRFSDEQLVT VIRLKQKRRTLKNRG 47 HR7214A-221-319-Av6HT MAFA Q8NHW3 VRLEERFSDDQLVSM KERDLYKEKYEKLAG 99 HR7214A-225-319-Av6HT MAFA Q8NHW3 ERFSDDQLVSMSVRE KERDLYKEKYEKLAG 95 HR7214A-228-313-Av6HT MAFA Q8NHW3 SDDQLVSMSVRELNR EVGRLAKERDLYKEK 86 HR7214A-236-319-Av6HT MAFA Q8NHW3 SVRELNRQLRGFSKE KERDLYKEKYEKLAG 84 HR7214A-246-319-Av6HT MAFA Q8NHW3 GFSKEEVIRLKQKRR KERDLYKEKYEKLAG 74 HR6931A-209-305-Av6HT MAFB Q9Y5Q3 DRFSDDQLVSMSVRE RDAYKVKCEKLANSG 97 HR6931B-210-236-Av6HT MAFB Q9Y5Q3 RFSDDQLVSMSVREL RELNRHLRGFTKDEV 27 HR6931B-210-251-Av6HT MAFB Q9Y5Q3 RFSDDQLVSMSVREL IRLKQKRRTLKNRGY 42 HR8265A-31-74-Av6HT MAFF Q9ULX9 GLSVRELNRHLRGLS KNRGYAASCRVKRVC 44 HR7795A-21-123-TEV MAFG Q15525 GTSLTDEELVTMSVR SKYEALQTFARTVAR 103 HR7958A-24-123-TEV MAFK O60675 LSDDELVSMSVRELN SKYEALQTFARTVAR 100 HR8183A-390-479-Av6HT MATR3 P43243 MQKGRVETSRVVHIM PVRVHLSQKYKRIKK 91 HR8183A-390-479-TEV MATR3 P43243 QKGRVETSRVVHIMD PVRVHLSQKYKRIKK 90 HR8110A-22-107-TEV MAX P61244 ADKRAHHNALERKRR ALLEQQVRALEKARS 86 HR8332A-230-361-NHT MAZ P56270 ACEMCGKAFRDVYHL SRPDHLNSHVRQVHS 82 HR8332A-280-361-TEV MAZ P56270 ACEMCGKAFRDVYHL SRPDHLNSHVRQVHS 82 HR8039A-131-243-TEV MBD1 Q9UIS9 GCCENCGISFSGDGT RGCQTQEDCGHCPIC 113 HR8039A-131-262-TEV MBD1 Q9UIS9 GCCENCGISFSGDGT RPGLRRQWKCVQRRC 132 HR5530A-14 MBD2 Q9UBB5 MEPVPFPSGSAGPGP NDPLNQNKGKPDLNT 118 HR5530A-15 MBD2 Q9UBB5 MEPVPFPSGSAGPGP NDPLNQNKGKPDLNT 118 HR6416-1-220-15 MBD3 O95983 MERKRWECPALPQGW VWLNTTQPLCKAFMV 220 HR6416-1-226-15 MBD3 O95983 MERKRWECPALPQGW QPLCKAFMVTDEDIR 226 HR6416-1-261-15 MBD3 O95983 MERKRWECPALPQGW MLAHVEELARDGEAP 261 HR6416-15 MBD3 O95983 MERKRWECPALPQGW EEEEEPDPDPEMEHV 291 HR6416-33-291-15 MBD3 O95983 VFYYSPSGKKFRSKP EEEEEPDPDPEMEHV 259 HR6416-55-291-15 MBD3 O95983 MGSMDLSTFDFRTGK EEEEEPDPDPEMEHV 238 HR6416A-1-106-15 MBD3 O95983 MERKRWECPALPQGW KPDLNTALPVRQTAS 106 HR6416A-1-111-15 MBD3 O95983 MERKRWECPALPQGW TALPVRQTASIFKQP 111 HR6416A-1-117-15 MBD3 O95983 MERKRWECPALPQGW QTASIFKQPVTKITN 117 HR6416B-1-72-15 MBD3 O95983 MERKRWECPALPQGW DLSTFDFRTGKMLMS 72 HR6416B-1-77-15 MBD3 O95983 MERKRWECPALPQGW DFRTGKMLMSKMNKS 77 HR4635B-14 MBD4 O95243 MTECRKSVPCGWERV VLSKRGIKSRYKDCS 81 HR4635B-15 MBD4 O95243 MTECRKSVPCGWERV VLSKRGIKSRYKDCS 81 HR4635C-14 MBD4 O95243 RSSECNHLLQEPIAS FTVLSKRGIKSRYKD 101 HR4635D-55-161-14 MBD4 O95243 MIKRSSECNPLLQEP SKRGIKSRYKDCSMA 108 HR4635D-55-161-Av6HT MBD4 O95243 IKRSSECNPLLQEPI SKRGIKSRYKDCSMA 107 HR4635D-55-161-TEV MBD4 O95243 IKRSSECNPLLQEPI SKRGIKSRYKDCSMA 107 HR4635D-55-191-14 MBD4 O95243 MIKRSSECNPLLQEP NLRTRSKCKKDVFMP 138 HR4635D-61-156-14 MBD4 O95243 MCNPLLQEPIASAQF DFTVLSKRGIKSRYK 97 HR4635D-61-186-14 MBD4 O95243 MCNPLLQEPIASAQF NNSNWNLRTRSKCKK 127 HR4635E-437-574-TEV MBD4 O95243 KWTPPRSPFNLVQET DHKLNKYHDWLWENH 138 HR8088A-178-246-TEV MBNL1 Q9NR56 RTDRLEVCREYQRGN EKCKYFHPPAHLQAK 69 HR7551A-175-243-TEV MBNL2 Q5VZF2 RTDKLEVCREFQRGN EKCKYFHPPAHLQAK 69 HR7762A-173-241-TEV MBNL3 Q9NUK0 RCSREKCKYFHPPAH NGATPVFNPTVFHCQ 69 HR3168-14 MDS1 Q13465 MRSKGRARKLATNNE QADVYMPGLQCAFLS 169 HR7632A-77-171-TEV MECP2 P51608 SEGSGSAPAVPEASA VGDTSLDPNDFDFTV 95 HR4583C-2-78-TEV MEF2A Q02078 GRKKIQITRIMDERN KVLLKYTEYNEPHES 77 HR8120A-2-94-TEV MEF2B Q02080 GRKKIQISRILDQRN TNTDILETLKRRGIG 93 HR4550C-2-78-TEV MEF2D Q14814 GRKKIQIQRITDERN KVLLKYTEYNEPHES 77 HR8225A-277-341-NHT MEIS1 O00470 GIFPKVATNIMRAWL RRRIVQPMIDQSNRA 65 HR8225A-277-341-TEV MEIS1 O00470 GIFPKVATNIMRAWL RRRIVQPMIDQSNRA 65 HR8514A-250-313-TEV MEIS3P2 A8K058 GIFPKVATNIMRAWL ARRRMVQPMIDQSNR 64 HR7119A-175-247-NHT MEOX2 P50222 QEGNYKSEVNSKPRK VWFQNRRMKWKRVKG 73 HR7798B-1047-1351-Av6HT MET P08581 LQNTVHIDLSALNPE ISAIFSTFIGEHYVH 305 HR8521A-1-179-TEV MGMT P16455 DKDCEMKRTTLDSPL KEWLLAHEGHRLGKP 178 HR7181A-199-243-TEV MIER1 Q8N108 YKENEKVYENDDQLL KDASRRTGDEKGVEA 45 HR7181A-199-265-TEV MIER1 Q8N108 YKENEKVYENDDQLL KDNEQALYELVKCNF 67 HR7181A-203-243-TEV MIER1 Q8N108 EKVYENDDQLLWDPE KDASRRTGDEKGVEA 41 HR7181A-203-265-TEV MIER1 Q8N108 EKVYENDDQLLWDPE KDNEQALYELVKCNF 63 HR7181A-208-260-TEV MIER1 Q8N108 NDDQLLWDPEYLPED EGSHIKDNEQALYEL 53 HR3622D-1-299-14 MINK1 Q8N4C8 MGDPAPARSLDDIDL KFPFIRDQPTERQVR 299 HR3622D-13-294-14 MINK1 Q8N4C8 MIDLSALRDPAGIFE TEQLLKFPFIRDQPT 283 HR3622D-8-299-14 MINK1 Q8N4C8 MRSLDDIDLSALRDP KFPFIRDQPTERQVR 293
HR3622D-9-294-14 MINK1 Q8N4C8 MSLDDIDLSALRDPA TEQLLKFPFIRDQPT 287 HR3622E-1180-1284-14 MINK1 Q8N4C8 MIYGSSAGFHAVDVD GEKAIEIRSVETGHL 106 HR3622E-1185-1282-14 MINK1 Q8N4C8 MAGFHAVDVDSGNSY GWGEKAIEIRSVETG 99 HR7746A-86-152-Av6HT MIXL1 Q9H2W2 QRRKRTSFSAEQLQL RAKSRRQSGKSFQPL 67 HR7244A-248-367-NHT MKRN1 Q9UHC7 DAAQRSQHIKSCIEA EKQKLILKYKEAMSN 120 HR7430A-278-369-NHT MKRN3 Q13064 DAAQREEHMRACIEA NRIVKSCPQCRVTSE 92 HR7905A-54-146-Av6HT MKX Q8IYA7 NLGLRHRRTGARQNG VRQPDLSWALRIKLY 93 HR4516M-1422-1490-15 MLL Q03164 MILTSVPITPRVVCF CRRCKFCHVCGRQHQ 70 HR4516M-1422-1514-15 MLL Q03164 MILTSVPITPRVVCF KCRNSYHPECLGPNY 94 HR4516M-1427-1486-15 MLL Q03164 MPITPRVVCFLCASS ENWCCRRCKFCHVCG 61 HR4516M-1427-1514-15 MLL Q03164 MPITPRVVCFLCASS KCRNSYHPECLGPNY 89 HR4516N-1476-1537-15 MLL Q03164 MCRRCKFCHVCGRQH KVWICTKCVRCKSCG 63 HR4516O-2012-2081-15 MLL Q03164 MNGLEPENIHMMIGS YTCKIVECRPPVVEP 71 HR4516O-2017-2076-15 MLL Q03164 MENIHMMIGSMTIDC RKRCVYTCKIVECRP 61 HR4516O-2017-2082-15 MLL Q03164 MENIHMMIGSMTIDC TCKIVECRPPVVEPD 67 HR8195A-1-143-Av6HT MLLT1 Q03111 DNQCTVQVRLELGHR TEFRYKLLRAGGVMV 142 HR7909A-1-139-Av6HT MLLT3 P42568 ASSCAVQVKLELGHR NNPTEDFRRKLLKAG 138 HR7716A-121-220-NHT MLX Q9UH92 AYKESYKDRRRRAHT TALKIMKVNYEQIVK 100 HR7887A-717-802-Av6HT MLXIP Q9HAP2 LKNRQMKHISAEQKR EELNATIISCQQLLP 86 HR7887A-726-802-Av6HT MLXIP Q9HAP2 SAEQKRRFNIKMCFD EELNATIISCQQLLP 77 HR7434A-647-736-NHT MLXIPL Q9NP71 TENRRITHISAEQKR EELNAAINLCQQQLP 90 HR7223A-347-541-Av6HT MRF Q9Y2G1 NYQSIKWQPHQQNKW IIVRASNPGQFESDS 195 HR7242A-112-186-TEV MRRF Q96E11 ESGMNLNPEVEGTLI DTVSEDTIRLIEKQI 75 HR4485B-103-183-14 MSC O60682 MECKQSQRNAANARE ENGYVHPVNLTWPFV 82 HR4485B-103-188-14 MSC O60682 MECKQSQRNAANARE HPVNLTWPFVVSGRP 87 HR4485B-103-188-Av6HT MSC O60682 ECKQSQRNAANARER HPVNLTWPFVVSGRP 86 HR4485B-103-188-TEV MSC O60682 ECKQSQRNAANARER HPVNLTWPFVVSGRP 86 HR4485B-103-194-14 MSC O60682 MECKQSQRNAANARE WPFVVSGRPDSDTKE 93 HR4485B-103-199-14 MSC O60682 MECKQSQRNAANARE SGRPDSDTKEVSAAN 98 HR4485B-135-194-14 MSC O60682 MPWVPPDTKLSKLDT WPFVVSGRPDSDTKE 61 HR4485C-103-174-14 MSC O60682 MECKQSQRNAANARE RQLLQEDRYENGYVH 73 HR7186A-122-193-NHT MSGN1 A6NI15 SVQRRRKASEREKLR TDLLNRGREPRAQSA 72 HR7207A-25-769-TEV MST1R Q04912 EDWQCPRTPYAASRD GAQVPGSWTFQYRED 745 HR4585B-167-224-TEV MSX1 P28360 RKPRTPFTTAQLLAL VKIWFQNRRAKAKRL 58 HR7691A-143-200-TEV MSX2 P35548 RKPRTPFTTSQLLAL VKIWFQNRRAKAKRL 58 HR4538-1-540-14 MTA1 Q13330 MAANMYRVGDYVYFE LKQAVRKPLEAVLRY 540 HR4538-1-540-15 MTA1 Q13330 MAANMYRVGDYVYFE LKQAVRKPLEAVLRY 540 HR4538-1-545-14 MTA1 Q13330 MAANMYRVGDYVYFE RKPLEAVLRYLETHP 545 HR4538-1-545-15 MTA1 Q13330 MAANMYRVGDYVYFE RKPLEAVLRYLETHP 545 HR4538C-375-438-14 MTA1 Q13330 MGVVNGTGAPGQSPG WKKYGGLKMPTRLDG 65 HR4538C-380-433-14 MTA1 Q13330 MTGAPGQSPGAGRAC SCWTYWKKYGGLKMP 55 HR4538D-15 MTA1 Q13330 ALVPQGGPVLCRDEM QVYIPNYNKPNPNQI 97 HR4538D-Av6HT MTA1 Q13330 ALVPQGGPVLCRDEM QVYIPNYNKPNPNQI 97 HR4538D-TEV MTA1 Q13330 ALVPQGGPVLCRDEM QVYIPNYNKPNPNQI 97 HR4621B-352-412-15 MTA2 O94776 MSKPGMNGAGFQKGL WKKYGGLKTPTQLEG 62 HR4621B-357-407-15 MTA2 O94776 MNGAGFQKGLTCESC SCWIYWKKYGGLKTP 52 HR4621C-1-140-15 MTA2 O94776 MAANMYRVGDYVYFE CFFYSLVFDPVQKTL 140 HR4621C-1-145-15 MTA2 O94776 MAANMYRVGDYVYFE LVFDPVQKTLLADQG 145 HR4621C-1-161-15 MTA2 O94776 MAANMYRVGDYVYFE IRVGCKYQAEIPDRL 161 HR4621C-1-166-15 MTA2 O94776 MAANMYRVGDYVYFE KYQAEIPDRLVEGES 166 HR4468C-268-324-TEV MTA3 Q9BIC8 AISALVPQGGPVLCR IQQDFLPWKSLTSII 57 HR6907A-148-207-NHT MTF1 Q14872 PRTYSTAGNLRTHQK VHTKEKPFECDVQGC 60 HR6878A-57-136-TEV MXD1 Q05195 SRSTHNEMEKNRRAH LQREQRHLKRQLEKL 80 HR6454A-24-140-14 MXD4 Q14582 EHGYASVLPFDGDFA LKRRLEQLSVQSVER 117 HR6454A-44-143-14 MXD4 Q14582 AAGLVRKAPNNRSSH RLEQLSVQSVERVRT 100 HR6454A-44-143-15 MXD4 Q14582 AAGLVRKAPNNRSSH RLEQLSVQSVERVRT 100 HR6454A-49-140-14 MXD4 Q14582 RKAPNNRSSHNELEK LKRRLEQLSVQSVER 92 HR6454A-56-136-14 MXD4 Q14582 SSHNELEKHRRAKLR EHRFLKRRLEQLSVQ 81 HR6454A-56-136-15 MXD4 Q14582 SSHNELEKHRRAKLR EHRFLKRRLEQLSVQ 81 HR6436A-58-155-14 MXI1 P50539 SSGSSNTSTANRSTH KWRLEQLQGPQEMER 98 HR6436A-63-149-14 MXI1 P50539 NTSTANRSTHNELEK REQRFLKWRLEQLQG 87 HR6436A-63-155-14 MXI1 P50539 NTSTANRSTHNELEK KWRLEQLQGPQEMER 93 HR6436A-68-149-14 MXI1 P50539 NRSTHNELEKNRRAH REQRRLKWRLEQLQG 82 HR8112A-85-136-TEV MYBL1 P10243 LIKGPWTKEEDQRVI GKQCRERWHNHLNPE 52 HR3593B-28-78-TEV MYBL2 P10244 SKCKVKWTHEEDEQL RTDQQCQYRWLRVLN 51 HR4620B-353-438-TEV MYC P01106 NVKRRTHNVLERQRR REQLKHKLEQLRNSC 86 HR7184A-279-364-NHT MYCL1 P12524 DVTKRKNHNFLERKR RQQQLQKRIAYLIGY 86 HR8502A-1-111-Av6HT MYCL2 P12525 DRDSYHHYFYDYDGG EPLERAVSDLLAVGA 110 HR6419A-367-464-14 MYCN P04198 AKSLSPRNSDSEDSE RQQQLLKKIEHARTC 98 HR7983A-4-117-TEV MYNN Q9NPC7 SHHCEHLLERLNKQR KVEEVVTKCKIKMED 114 HR4693-66-224-14 MYOG P15173 MLPWACKVCKRKSVS VEDVSVAFPDETMPN 160 HR4693B-71-145-14 MYOG P15173 MKVCKRKSVSVDRRR RLQALLSSLNQEERD 76 HR4693B-73-156-14 MYOG P15173 MCKRKSVSVDRRRAA EERDLRYRGGGGPQP 85 HR4693B-76-138-14 MYOG P15173 MKSVSVDRRRAATLR SAIQYIERLQALLSS 64 HR4693B-78-156-14 MYOG P15173 MVSVDRRRAATLREK EERDLRYRGGGGPQP 80 HR7507A-115-181-TEV MYSM1 Q5VVJ2 ASYSVKWTIEEKELF VKCGLDKETPNQKTG 67 HR7507B-367-470-TEV MYSM1 Q5VVJ2 HEEEELKPPEQEIEI IGAINFGGEQAVYNR 104 HR4437B-298-605-NHT MYST2 O95251 LENLTSEYDLDLFRR RSNSNKTMDPSCLKW 308 HR8033A-559-650-TEV MYT1 Q01538 SYRPNVAPATPRANL LSTRCWEMPENLSTK 92 HR6948A-486-549-TEV MYT1L Q9UL68 HVKKPYYDPSRTEKK PPEILAMHESVLKCP 64 HR7215A-37-128-TEV MZF1 P28698 DPGPEAARLRFRCFR EAAALVDGLRREPGG 92 HR6963A-75-157-TEV NANOG Q9H9S0 KQPTSAEKSVAKKED FQNQRMKSKRWQKNN 83 HR7935-106-160-Av6HT NANOGNB Q7Z5D8 KRLVSKSLMHTLWAK ISQWFCKTRKKYNKE 55 HR8537A-41-101-Av6HT NANOGP1 Q8N7R0 TRTVFSSTQLCVLND QNQRMKSKRWQKNNW 61 HR3639F-24-96-15 NCOA1 Q15788 MCDTLASSTEKRRRE RMEQEKSTTDDDVQK 74 HR3639F-29-91-15 NCOA1 Q15788 MSSTEKRRREQENKY IQLMKRMEQEKSTTD 64 HR3639G-104-174-15 NCOA1 Q15788 MQGVIEKESLGPLLL LHVGDHAEFVKNLLP 72 HR3639G-107-179-15 NCOA1 Q15788 MIEKESLGPLLLEAL HAEFVKNLLPKSLVN 74 HR3639G-112-174-15 NCOA1 Q15788 MLGPLLLEALDGFFF LHVGDHAEFVKNLLP 64 HR3639G-112-203-15 NCOA1 Q15788 MLGPLLLEALDGFFF RRNSHTFNCRMLIHP 93 HR3639G-99-179-15 NCOA1 Q15788 MISSSSQGVIEKESL HAEFVKNLLPKSLVN 82 HR3639H-1185-1441-15 NCOA1 Q15788 MSPFSQLAANPEASL PQAQQKSLLQQLLTE 258 HR3639H-1190-1441-15 NCOA1 Q15788 MLAANPEASLANRNS PQAQQKSLLQQLLTE 253 HR3639H-1205-1441-15 NCOA1 Q15788 MVSRGMTGNIGGQFG PQAQQKSLLQQLLTE 238 HR3639H-1210-1441-15 NCOA1 Q15788 MTGNIGGQFGTGINP PQAQQKSLLQQLLTE 233 HR3639H-1216-1441-15 NCOA1 Q15788 MQFGTGINPQMQQNV PQAQQKSLLQQLLTE 227 HR4453I-100-258-Av6HT NCOA3 Q9Y6Q9 MVSSTGQGVIDKDSL SCMICVARRITTGER 160 HR4453I-100-258-NHT NCOA3 Q9Y6Q9 VSSTGQGVIDKDSLG SCMICVARRITTGER 159 HR7885A-433-486-TEV NCOR1 O75376 DRQFMNVWTDHEKEI PDCVLYYYLTKKNEN 54 HR4636E-602-671-14 NCOR2 Q9Y618 MAELASMELNESSRW KKRQNLDEILQQHKL 71 HR4636E-608-670-14 NCOR2 Q9Y618 MELNESSRWTEEEME YKKRQNLDEILQQHK 64 HR7360A-102-160-TEV NEUROD1 Q13562 RRMKANARERNRMHG AKNYIWALSEILRSG 59 HR7134A-122-180-TEV NEUROD2 Q15784 RRQKANARERNRMHD AKNYIWALSEILRSG 59 HR7078A-87-146-TEV NEUROD4 Q9HD90 ARRVKANARERTRMH ARNYIWALSEVLETG 60 HR8276A-95-153-NHT NEUROD6 Q96NK8 RRQEANARERNRMHG AKNYIWALSEILRIG 59 HR8276A-95-153-TEV NEUROD6 Q96NK8 RRQEANARERNRMHG AKNYIWALSEILRIG 59 HR6971A-104-175-NHT NEUROG2 Q9H2A3 ETVQRIKKTRRLKAN IWALTETLRLADHCG 72 HR7673A-76-167-NHT NEUROG3 Q9Y4Z2 ALSKQRRSRRKKAND APHCGELGSPGGSPG 92 HR7259A-264-544-TEV NFAT5 O94916 KKSPMLCGQYPVKSE AGRSHDVQPFTYTPD 281 HR8282A-416-591-Av6HT NFATC1 O95644 MDWQLPSHSGPYELR SLQVASNPIECSQRS 177 HR8282A-416-591-TEV NFATC1 O95644 DWQLPSHSGPYELRI SLQVASNPIECSQRS 176 HR7889A-421-595-TEV NFATC3 Q12968 DWPLPAHFGQCELKI SLQIASIPVECSQRS 175 HR4653B-214-293-14 NFE2 Q16621 MAKPTARGEAGSRDE AAQNCRKRKLETIVQ 81 HR4653B-218-293-14 NFE2 Q16621 MARGEAGSRDERRAL AAQNCRKRKLETIVQ 77 HR4653B-223-293-14 NFE2 Q16621 MGSRDERRALAMKIP AAQNCRKRKLETIVQ 72 HR4653B-234-293-14 NFE2 Q16621 MKIPFPTDKIVNLPV AAQNCRKRKLETIVQ 61 HR4653C-259-338-14 NFE2 Q16621 MLTESQLALVRDIRR QQLTELYRDIFQHLR 81 HR4653C-274-338-14 NFE2 Q16621 MGKNKVAAQNCRKRK QQLTELYRDIFQHLR 66 HR7672A-605-674-NHT NFE2L1 Q14494 DFLDKQMSRDEHRAR RRGKNKMAAQNCRKR 70 HR3520B-21 NFE2L2 Q16236 MMDLELPPPGLPSQQ TSGSANYSQVAHIPK 110 HR3520F-21 NFE2L2 Q16236 DMDLIDILWRQDIDL TSGSANYSQVAHIPK 95 HR3520L-455-594-14 NFE2L2 Q16236 TRDELRAKALHIPFP EYSLQQTRDGNVFLV 140 HR3520L-455-599-14 NFE2L2 Q16236 TRDELRAKALHIPFP QTRDGNVFLVPKSKK 145 HR3520M-435-523-15 NFE2L2 Q16236 MGHRKTPFTKDKHSS VAAQNCRKRKLENIV 90 HR3520M-440-523-15 NFE2L2 Q16236 MPFTKDKHSSRLEAH VAAQNCRKRKLENIV 85
HR3520N-489-570-15 NFE2L2 Q16236 MQFNEAQLALIRDIR QLSTLYLEVFSMLRD 83 HR3520O-445-523-15 NFE2L2 Q16236 MKHSSRLEAHLTRDE VAAQNCRKRKLENIV 80 HR7720A-530-598-NHT NFE2L3 Q9Y4A8 DTDRNLSRDEQRAKA RRGKNKVAAQNCRKR 69 HR7383A-63-165-TEV NFIA Q12857 EKPEVKQKWASRLLA VKSPQCSNPGLCVQP 103 HR7383A-63-184-TEV NFIA Q12857 EKPEVKQKWASRLLA VSVKELDLYLAYFVH 122 HR7383A-7-165-TEV NFIA Q12857 LTQDEFHPFIEALLP VKSPQCSNPGLCVQP 159 HR7279A-8-166-Av6HT NFIB O00712 LTQDEFHPFIEALLP MKSPHCTNPALCVQP 159 HR7320A-61-170-TEV NFIC P08651 LLGEKPEVKQKWASR QCGHPVLCVQPHHIG 110 HR7320A-61-186-TEV NFIC P08651 LLGEKPEVKQKWASR AVKELDLYLAYFVRE 126 HR7320A-64-166-TEV NFIC P08651 EKPEVKQKWASRLLA VKAAQCGHPVLCVQP 103 HR7320A-8-166-TEV NFIC P08651 LTQDEFHPFIEALLP VKAAQCGHPVLCVQP 159 HR3633C-804-893-TEV NFKB1 P19838 AQGDMKQLAEDVKLQ MGYTEAIEVIQAASS 90 HR3633D-248-354-Av6HT NFKB1 P19838 SNLKIVRMDRTAGCV ETSEPKPFLYYPEIK 107 HR5561A-15 NFKB1 P19838 MQLVRDLLEVTSGLI GADPLVENFEPLYDL 201 HR4541C-445-696-14 NFKB2 Q00653 MEYNARLFGLAQRSA TLTRLLLKAGADIHA 253 HR4541C-477-696-14 NFKB2 Q00653 MQRHLLTAQDENGDT TLTRLLLKAGADIHA 221 HR4541C-482-696-14 NFKB2 Q00653 MTAQDENGDTPLHLA TLTRLLLKAGADIHA 216 HR4541D-37-329-TEV NFKB2 Q00653 GPYLVIVEQPKQRGF GDVSDSKQFTYYPLV 293 HR6920A-980-1063-NHT NFX1 Q12986 SKFSDSLKEDARKDL DSEPKRNVVVTAIRG 84 HR6427-1-270-14 NFYA P23511 MEQYTANSNSSTEQI GAEMLEEEPLYVNAK 270 HR6427-1-290-14 NFYA P23511 MEQYTANSNSSTEQI LKRRQARAKLEAEGK 290 HR6427-1-295-14 NFYA P23511 MEQYTANSNSSTEQI ARAKLEAEGKIPKER 295 HR6427A-38-145-14 NFYA P23511 EAQVASASGQQVQTL QIIIQQPQTAVTAGQ 108 HR6427A-42-150-14 NFYA P23511 ASASGQQVQTLQVVQ QPQTAVTAGQTQTQQ 109 HR6427A-47-145-14 NFYA P23511 QQVQTLQVVQGQPLM QIIIQQPQTAVTAGQ 99 HR4613B-51-143-TEV NFYB P25208 SFREQDIYLPIANVA SYVEPLKLYLQKFRE 93 HR3512B-166-288-15 NKRF O15226 VVAEKQYFIEKLTAT LQKRIEVRVVRRKFK 123 HR3512B-166-293-15 NKRF O15226 VVAEKQYFIEKLTAT EVRVVRRKFKHTFGE 128 HR3512B-188-288-15 NKRF O15226 PEMTSGSDKINYTYM LQKRIEVRVVRRKFK 101 HR4600-161-227-Av6HT NKX2-1 P43699 RRKRVLFSQAQVYE RYKMKRQAKDKAAQQ 67 HR7114A-123-196-TEV NKX2-2 O95096 GDAGKKRKRRVLFSK KMKRARAEKGMEVTP 74 HR4758B-148-211-TEV NKX2-3 Q8TAU0 RRKPRVLFSQAQVFE QNRRYKCKRQRQDKS 64 HR7998A-189-255-TEV NKX2-4 Q9H2Z4 RRKRRVLFSQAQVYE RYKMKRQAKDKAAQQ 67 HR5518A-127-207-14 NKX2-5 P52952 MADNAERPRARRRRK CKRQRQDQTLELVGL 82 HR5518A-127-207-Av6HT NKX2-5 P52952 ADNAERPRARRRRKP CKRQRQDQTLELVGL 81 HR5518A-127-207-TEV NKX2-5 P52952 ADNAERPRARRRRKP CKRQRQDQTLELVGL 81 HR5518A-14 NKX2-5 P52952 MRPRARRRRKPRVLF DQTLELVGLPPPPPP 83 HR5518A-15 NKX2-5 P52952 MRPRARRRRKPRVLF DQTLELVGLPPPPPP 83 HR5518B-128-224-14 NKX2-5 P52952 MDNAERPRARRRRKP PPPPPARRIAVPVLV 98 HR5518B-128-233-14 NKX2-5 P5295 MDNAERPRARRRRKP AVPVLVRDGKPCLGD 107 HR5518B-143-224-14 NKX2-5 P52952 MVLFSQAQVYELERR PPPPPARRIAVPVLV 83 HR5518B-143-228-14 NKX2-5 P52952 MVLFSQAQVYELERR PARRIAVPVLVRDGK 87 HR5518B-143-233-14 NKX2-5 P52952 MVLFSQAQVYELERR AVPVLVRDGKPCLGD 92 HR7861A-83-143-TEV NKX2-8 O15522 KRKKRRVLFSKAQTL KIWFQNHRYKLKRAR 61 HR6470A-127-195-15 NKX3-1 Q99801 SRAAFSHTQVIELER RKQLSSELGDLEKHS 69 HR6470A-132-189-15 NKX3-1 Q99801 SHTQVIELERKFSHQ RRYKTKRKQLSSELG 58 HR6470A-97-163-15 NKX3-1 Q99801 RHLGSYLLDSENTSG LSAPERAHLAKNLKL 67 HR6470A-97-168-15 NKX3-1 Q99801 RHLGSYLLDSENTSG RAHLAKNLKLTETQV 72 HR6470A-97-189-15 NKX3-1 Q99801 RHLGSYLLDSENTSG RRYKTKRKQLSSELG 93 HR6470A-97-195-15 NKX3-1 Q99801 RHLGSYLLDSENTSG RKQLSSELGDLEKHS 99 HR8303A-212-271-Av6HT NKX3-2 P78367 AFSHAQVFELERRFN RRYKTKRRQMAADLL 60 HR6930A-227-296-NHT NKX6-1 P78426 SILLDKDGKRKHTRP VWFQNRRTKWRKKHA 70 HR7948A-199-323-Av6HT NOC3L Q8WTT2 TIEEHLIERKKKLQE LENLEQMVKDWKQRK 125 HR8350A-301-458-Av6HT NOC4L Q9BVI4 GGALSLLALNGLFIL HYHPEVSKAASVINQ 158 HR6913A-130-201-NHT NPAS1 Q99742 VSEVFEQHLGGHILQ HPGDHSEVLEQLGLR 72 HR8115A-87-146-Av6HT NPAS2 Q99743 QLMLEALDGFIIAVT FLPEQEHSEVYKILS 60 HR7386A-142-213-NHT NPAS3 Q8IXF0 AIEVFEAHLGSHILQ HPGDHVEMAEQLGMK 72 HR7814A-184-329-NHT NPAS4 Q8IUM7 GNPVFTAFCAPLEPR SDMEAWSLRQQINSE 146 HR7372A-210-470-15 NR0B1 P51843 MGSTLYCVPTSTNQA VSMDDMMLEMLCTKI 262 HR7372A-210-470-Av6HT NR0B1 P51843 GSTLYCVPTSTNQAQ VSMDDMMLEMLCTKI 261 HR7372A-210-470-TEV NR0B1 P51843 GSTLYCVPTSTNQAQ VSMDDMMLEMLCTKI 261 HR7372A-237-470-15 NR0B1 P51843 MDTSSGALRPVALKS VSMDDMMLEMLCTKI 235 HR7372A-237-470-Av6HT NR0B1 P51843 DTSSGALRPVALKSP VSMDDMMLEMLCTKI 234 HR7372A-237-470-TEV NR0B1 P51843 DTSSGALRPVALKSP VSMDDMMLEMLCTKI 234 HR7372A-245-470-15 NR0B1 P51843 MPVALKSPQVVCEAA VSMDDMMLEMLCTKI 227 HR7372A-245-470-Av6HT NR0B1 P51843 PVALKSPQVVCEAAS VSMDDMMLEMLCTKI 226 HR7372A-245-470-TEV NR0B1 P51843 PVALKSPQVVCEAAS VSMDDMMLEMLCTKI 226 HR8369A-14-257-15 NR0B2 Q15466 MAASRPAILYALLSS DVDIAGLLGDMLLLR 245 HR8278A-123-216-15 NR1D1 P20393 MTKLNGMVLLCKVCG AVRFGRIPKREKQRM 95 HR8278A-123-216-Av6HT NR1D1 P20393 TKLNGMVLLCKVCGD AVRFGRIPKREKQRM 94 HR8278A-123-216-TEV NR1D1 P20393 TKLNGMVLLCKVCGD AVRFGRIPKREKQRM 94 HR8341A-100-171-15 NR1D2 Q14995 MVLLCKVCGDVASGF QQCRFKKCLSVGMSR 73 HR8341A-100-171-Av6HT NR1D2 Q14995 VLLCKVCGDVASGFH QQCRFKKCLSVGMSR 72 HR8341A-100-171-TEV NR1D2 Q14995 VLLCKVCGDVASGFH QQCRFKKCLSVGMSR 72 HR8341A-100-181-15 NR1D2 Q14995 MVLLCKVCGDVASGF VGMSRDAVRFGRIPK 83 HR8341A-100-181-Av6HT NR1D2 Q14995 VLLCKVCGDVASGFH VGMSRDAVRFGRIPK 82 HR8341A-100-181-TEV NR1D2 Q14995 VLLCKVCGDVASGFH VGMSRDAVRFGRIPK 82 HR8341A-100-200-15 NR1D2 Q14995 MVLLCKVCGDVASGF RMLIEMQSAMKTMMN 102 HR8341A-100-200-Av6HT NR1D2 Q14995 VLLCKVCGDVASGFH RMLIEMQSAMKTMMN 101 HR8341A-100-200-TEV NR1D2 Q14995 VLLCKVCGDVASGFH RMLIEMQSAMKTMMN 101 HR8341B-381-579-15 NR1D2 Q14995 MHLVCPMSKSPYVDP NNMHSEELLAFKVHP 200 HR8341B-381-579-Av6HT NR1D2 Q14995 HLVCPMSKSPYVDPH NNMHSEELLAFKVHP 199 HR8341B-381-579-TEV NR1D2 Q14995 HLVCPMSKSPYVDPH NNMHSEELLAFKVHP 199 HR7370A-209-460-15 NR1H2 P55055 MSQGSGEGEGVQLTA DKKLPPLLSEIWDVH 253 HR7370A-209-460-Av6HT NR1H2 P55055 SQGSGEGEGVQLTAA DKKLPPLLSEIWDVH 252 HR7370A-209-460-TEV NR1H2 P55055 SQGSGEGEGVQLTAA DKKLPPLLSEIWDVH 252 HR8107E-205-447-TEV NR1H3 Q13133 QLSPEQLGMIEKLVA KKLPPLLSEIWDVHE 243 HR4469B-125-217-14 NR1H4 Q96RI1 MGASAGRIKGDELCV LAECMYTGLLTEIQC 94 HR4469B-125-234-14 NR1H4 Q96RI1 MGASAGRIKGDELCV KRLRKNVKQHADQTV 111 HR4469B-129-217-14 NR1H4 Q96RI1 MGRIKGDELCVVCGD LAECMYTGLLTEIQC 90 HR4469B-129-234-14 NR1H4 Q96RI1 MGRIKGDELCVVCGD KRLRKNVKQHADQTV 107 HR4469B-130-213-14 NR1H4 Q96RI1 MRIKGDELCVVCGDR EMGMLAECMYTGLLT 85 HR4469B-130-229-14 NR1H4 Q96RI1 MRIKGDELCVVCGDR IQCKSKRLRKNVKQH 101 HR4469B-134-213-14 NR1H4 Q96RI1 MDELCVVCGDRASGY EMGMLAECMYTGLLT 81 HR4469B-134-229-14 NR1H4 Q96RI1 MDELCVVCGDRASGY IQCKSKRLRKNVKQH 97 HR7870A-37-107-15 NR1I2 O75469 MGPQJCRVCGDKATG QCQACRLRKCLESGM 72 HR7870A-37-107-Av6HT NR1I2 O75469 GPQICRVCGDKATGY QCQACRLRKCLESGM 71 HR7870A-37-107-TEV NR1I2 O75469 GPQICRVCGDKATGY QCQACRLRKCLESGM 71 HR7870A-37-130-15 NR1I2 O75469 MGPQICRVCGDKATG EAVEERRALIKRKKS 95 HR7870A-37-130-Av6HT NR1I2 O75469 GPQICRVCGDKATGY EAVEERRALIKRKKS 94 HR7870A-37-130-TEV NR1I2 O75469 GPQICRVCGDKATGY EAVEERRALIKRKKS 94 HR7870B-130-434-15 NR1I2 O75469 MSERTGTQPLGVQGL FATPLMQELFGITGS 306 HR7870B-130-434-Av6HT NR1I2 O75469 SERTGTQPLGVQGLT FATPLMQELFGITGS 305 HR7870B-130-434-TEV NR1I2 O75469 SERTGTQPLGVQGLT FATPLMQELFGITGS 305 HR7870C-142-434-15 NR1I2 O75469 MGLTEEQRMMIRELM FATPLMQELFGITGS 294 HR7870C-142-434-Av6HT NR1I2 O75469 GLTEEQRMMIRELMD FATPLMQELFGITGS 293 HR7870C-142-434-TEV NR1I2 O75469 GLTEEQRMMIRELMD FATPLMQELFGITGS 293 HR7475A-6-105-15 NR1I3 Q14994 MDELRNCVVCGDQAT RAKQAQRRAQQTPVQ 101 HR7475A-6-105-Av6HT NR1I3 Q14994 DELRNCVVCGDQATG RAKQAQRRAQQTPVQ 100 HR7475A-6-105-TEV NR1I3 Q14994 DELRNCVVCGDQATG RAKQAQRRAQQTPVQ 100 HR7475A-6-120-15 NR1I3 Q14994 MDELRNCVVCGDQAT LSKEQEELIRTLLGA 116 HR7475A-6-120-Av6HT NR1I3 Q14994 DELRNCVVCGDQATG LSKEQEELIRTLLGA 115 HR7475A-6-120-TEV NR1I3 Q14994 DELRNCVVCGDQATG LSKEQEELIRTLLGA 115 HR7475B-6-77-15 NR1I3 Q14994 MDELRNCVVCGDQAT CPACRLQKCLDAGMR 73 HR7475B-6-77-Av6HT NR1I3 Q14994 DELRNCVVCGDQATG CPACRLQKCLDAGMR 72 HR7475B-6-77-TEV NR1I3 Q14994 DELRNCVVCGDQATG CPACRLQKCLDAGMR 72 HR7475B-6-82-15 NR1I3 Q14994 MDELRNCVVCGDQAT LQKCLDAGMRKDMIL 78 HR7475B-6-82-Av6HT NR1I3 Q14994 DELRNCVVCGDQATG LQKCLDAGMRKDMIL 77 HR7475B-6-82-TEV NR1I3 Q14994 DELRNCVVCGDQATG LQKCLDAGMRKDMIL 77 HR7475C-103-352-15 NR1I3 Q14994 MPVQLSKEQEELIRT QGLSAMMPLLQEICS 251 HR7475C-103-352-Av6HT NR1I3 Q14994 PVQLSKEQEELIRTL QGLSAMMPLLQEICS 250 HR7475C-103-352-TEV NR1I3 Q14994 PVQLSKEQEELIRTL QGLSAMMPLLQEICS 250 HR8155A-108-196-Av6HT NR2C1 P13056 KVFDLCVVCGDKASG SVQCERKPIEVSREK 89 HR6956-16-584-15 NR2C2 P49116 MAVASPQRIQGSEPA RLMSSNITEELFFTG 570 HR6956-16-584-Av6HT NR2C2 P49116 AVASPQRIQGSEPAS RLMSSNITEELFFTG 569 HR6956-16-584-TEV NR2C2 P49116 AVASPQRIQGSEPAS RLMSSNITEELFFTG 569
HR6956A-115-584-15 NR2C2 P49116 MSASVERLLGKTDVQ RLMSSNITEELFFTG 471 HR6956A-115-584-TEV NR2C2 P49116 SASVERLLGKTDVQR RLMSSNITEELFFTG 470 HR6956B-170-584-15 NR2C2 P49116 MYSCRSNQDCIINKH RLMSSNITEELFFTG 416 HR6956B-170-584-Av6HT NR2C2 P49116 YSCRSNQDCIINKHH RLMSSNITEELFFTG 415 HR6956B-170-584-TEV NR2C2 P49116 YSCRSNQDCIINKHH RLMSSNITEELFFTG 415 HR6956B-181-584-15 NR2C2 P49116 MNKHHRNRCQFCRLK RLMSSNITEELFFTG 405 HR6956B-181-584-Av6HT NR2C2 P49116 NKHHRNRCQFCRLKK RLMSSNITEELFFTG 404 HR6956B-181-584-TEV NR2C2 P49116 NKHHRNRCQFCRLKK RLMSSNITEELFFTG 404 HR6956B-218-584-15 NR2C2 P49116 MEKPSNCAASTEKIY RLMSSNITEELFFTG 368 HR6956B-218-584-Av6HT NR2C2 P49116 EKPSNCAASTEKIYI RLMSSNITEELFFTG 367 HR6956B-218-584-TEV NR2C2 P49116 EKPSNCAASTEKIYI RLMSSNITEELFFTG 367 HR6956C-110-188-15 NR2C2 P49116 MQIVTDSASVERLLG SNQDCIINKHHRNRC 80 HR6956C-110-205-15 NR2C2 P49116 MQIVTDSASVERLLG CRLKKCLEMGMKMES 97 HR6956C-115-183-15 NR2C2 P49116 MSASVERLLGKTDVQ TYSCRSNQDCIINKH 70 HR6956C-115-183-Av6HT NR2C2 P49116 SASVERLLGKTDVQR TYSCRSNQDCIINKH 69 HR6956C-115-183-TEV NR2C2 P49116 SASVERLLGKTDVQR TYSCRSNQDCIINKH 69 HR6956C-115-200-15 NR2C2 P49116 MSASVERLLGKTDVQ NRCQFCRLKKCLEMG 87 HR6956C-115-200-Av6HT NR2C2 P49116 SASVERLLGKTDVQR NRCQFCRLKKCLEMG 86 HR6956C-115-200-TEV NR2C2 P49116 SASVERLLGKTDVQR NRCQFCRLKKCLEMG 86 HR7378A-18-385-15 NR2E1 Q9Y466 MVCGDRSSGKHYGVY PITRLLSDMYKSSDI 369 HR7378A-18-385-Av6HT NR2E1 Q9Y466 VCGDRSSGKHYGVYA PITRLLSDMYKSSDI 368 HR7378A-18-385-TEV NR2E1 Q9Y466 VCGDRSSGKHYGVYA PITRLLSDMYKSSDI 368 HR7378B-134-385-Av6HT NR2E1 Q9Y466 VTQLEPHGLELAAVS PITRLLSDMYKSSDI 252 HR7378B-134-385-TEV NR2E1 Q9Y466 VTQLEPHGLELAAVS PITRLLSDMYKSSDI 252 HR7378B-65-385-15 NR2E1 Q9Y466 MTHRNQCRACRLKKC PITRLLSDMYKSSDI 322 HR7378B-65-385-Av6HT NR2E1 Q9Y466 THRNQCRACRLKKCL PITRLLSDMYKSSDI 321 HR7378B-65-385-TEV NR2E1 Q9Y466 THRNQCRACRLKKCL PITRLLSDMYKSSDI 321 HR7378B-76-385-15 NR2E1 Q9Y466 MKKCLEVNMNKDAVQ PITRLLSDMYKSSDI 311 HR7378B-76-385-Av6HT NR2E1 Q9Y466 KKCLEVNMNKDAVQH PITRLLSDMYKSSDI 310 HR7378B-76-385-TEV NR2E1 Q9Y466 KKCLEVNMNKDAVQH PITRLLSDMYKSSDI 310 HR7378C-18-109-15 NR2E1 Q9Y466 MVCGDRSSGKHYGVY RTSTIRKQVALYFRG 93 HR7378C-18-109-Av6HT NR2E1 Q9Y466 VCGDRSSGKHYGVYA RTSTIRKQVALYFRG 92 HR7378C-18-109-TEV NR2E1 Q9Y466 VCGDRSSGKHYGVYA RTSTIRKQVALYFRG 92 HR7378C-18-83-15 NR2E1 Q9Y466 MVCGDRSSGKHYGVY QCRACRLKKCLEVNM 67 HR7378C-18-83-Av6HT NR2E1 Q9Y466 VCGDRSSGKHYGVYA QCRACRLKKCLEVNM 66 HR7378C-18-83-TEV NR2E1 Q9Y466 VCGDRSSGKHYGVYA QCRACRLKKCLEVNM 66 HR7378C-18-96-15 NR2E1 Q9Y466 MVCGDRSSGKHYGVY NMNKDAVQHERGPRT 80 HR7378C-18-96-Av6HT NR2E1 Q9Y466 VCGDRSSGKHYGVYA NMNKDAVQHERGPRT 79 HR7378C-18-96-TEV NR2E1 Q9Y466 VCGDRSSGKHYGVYA NMNKDAVQHERGPRT 79 HR7906-Av6HT NR2E3 Q9Y5X4 ETRPTALMSSTVAAA NTPMEKLLCDMFKN* 410 HR7906B-101-410-Av6HT NR2E3 Q9Y5X4 QACRLKKCLQAGMNQ GNTPMEKLLCDMFKN 310 HR7906B-101-410-TEV NR2E3 Q9Y5X4 QACRLKKCLQAGMNQ GNTPMEKLLCDMFKN 310 HR7906B-114-410-15 NR2E3 Q9Y5X4 MNQDAVQNERQPRST GNTPMEKLLCDMFKN 298 HR7906B-114-410-Av6HT NR2E3 Q9Y5X4 NQDAVQNERQPRSTA GNTPMEKLLCDMFKN 297 HR7906B-114-410-TEV NR2E3 Q9Y5X4 NQDAVQNERQPRSTA GNTPMEKLLCDMFKN 297 HR7906B-164-410-Av6HT NR2E3 Q9Y5X4 SAARALGHHFMASLI GNIPMEKLLCDMFKN 247 HR7906C-45-119-15 NR2E3 Q9Y5X4 MLQCRVCGDSSSGKH LKKCLQAGMNQDAVQ 76 HR7906C-45-131-15 NR2E3 Q9Y5X4 MLQCRVCGDSSSGKH AVQNERQPRSTAQVH 88 HR7906C-45-142-15 NR2E3 Q9Y5X4 MLQCRVCGDSSSGKH AQVHLDSMESNTESR 99 HR7906C-50-114-15 NR2E3 Q9Y5X4 MCGDSSSGKHYGIYA CQACRLKKCLQAGMN 66 HR7906C-50-126-15 NR2E3 Q9Y5X4 MCGDSSSGKHYGIYA GMNQDAVQNERQPRS 78 HR7906C-50-126-Av6HT NR2E3 Q9Y5X4 CGDSSSGKHYGIYAC GMNQDAVQNERQPRS 77 HR7906C-50-126-TEV NR2E3 Q9Y5X4 CGDSSSGKHYGIYAC GMNQDAVQNERQPRS 77 HR7906C-50-135-Av6HT NR2E3 Q9Y5X4 CGDSSSGKHYGIYAC ERQPRSTAQVHLDSM 86 HR7906C-50-135-TEV NR2E3 Q9Y5X4 CGDSSSGKHYGIYAC ERQPRSTAQVHLDSM 86 HR7906D-36-410-Av6HT NR2E3 Q9Y5X4 EDPTGVSPSLQCRVC GNTPMEKLLCDMFKN 375 HR3061D-77-164-TEV NR2F1 P10589 SGQSQQHIECVVCGD GMRREAVQRGRMPPT 88 HR6377A-77-157-TEV NR2F2 P24468 IECVVCGDKSSGKHY GMRREAVQRGRMPPT 81 HR7636A-56-394-Av6HT NR2F6 P10588 CVVCGDKSSGKHYGV TPIETLIRDMLLSGS 339 HR7636A-56-394-TEV NR2F6 P10588 CVVCGDKSSGKHYGV TPIETLIRDMLLSGS 339 HR7636B-113-394-15 NR2F6 P10588 MLKKCFRVGMRKEAV TPIETLIRDMLLSGS 283 HR7636B-113-394-Av6HT NR2F6 P10588 LKKCFRVGMRKEAVQ TPIETLIRDMLLSGS 282 HR7636B-113-394-TEV NR2F6 P10588 LKKCFRVGMRKEAVQ TPIETLIRDMLLSGS 282 HR7636B-159-394-Av6HT NR2F6 P10588 DLFPGQPVSELIAQL TPIETLIRDMLLSGS 236 HR7636B-159-394-TEV NR2F6 P10588 DLFPGQPVSELIAQL TPIETLIRDMLLSGS 236 HR7636C-56-133-15 NR2F6 P10588 MCVVCGOKSSGKHYG VGMRKEAVQRGRIPH 79 HR4533-15 NR3C1 P04150 MDSKESLTPGREENP KYSNGNIKKLLFHQK 777 HR7785-601-673-15 NR3C2 P08235 MKICLVCGDEASGCH RLQKCLQAGMNLGAR 74 HR7785A-601-673-Av6HT NR3C2 P08235 KICLVCGDEASGCHY RLQKCLQAGMNLGAR 73 HR7785A-601-673-TEV NR3C2 P08235 KICLVCGDEASGCHY RLQKCLQAGMNLGAR 73 HR7785A-601-685-15 NR3C2 P08235 MKICLVCGDEASGCH GARKSKKLGKLKGIH 86 HR7785A-601-685-Av6HT NR3C2 P08235 KICLVCGDEASGCHY GARKSKKLGKLKGIH 85 HR7785A-601-685-TEV NR3C2 P08235 KICLVCGDEASGCHY GARKSKKLGKLKGIH 85 HR7785B-712-984-15 NR3C2 P08235 MAPAKEPSVNTALVP KVESGNAKPLYFHRK 274 HR7785B-712-984-Av6HT NR3C2 P08235 APAKEPSVNTALVPQ KVESGNAKPLYFHRK 273 HR7785B-712-984-TEV NR3C2 P08235 APAKEPSVNTALVPQ KVESGNAKPLYFHRK 273 HR7785C-731-984-Av6HT NR3C2 P08235 SRALTPSPVMVLENI KVESGNAKPLYFHRK 254 HR7785C-731-984-TEV NR3C2 P08235 SRALTPSPVMVLENI KVESGNAKPLYFHRK 254 HR4793B-265-353-TEV NR4A1 P22736 GRCAVCGDNASCQHY TDSLKGRRGRLPSKP 89 HR8241A-261-342-15 NR4A2 P43354 MGLCAVCGDNAACQH MVKEVVRTDSLKGRR 83 HR8241A-261-342-Av6HT NR4A2 P43354 GLCAVCGDNAACQHY MVKEVVRTDSLKGRR 82 HR8241A-261-342-TEV NR4A2 P43354 GLCAVCGDNAACQHY MVKEVVRTDSLKGRR 82 HR8241A-264-328-15 NR4A2 P43354 MAVCGDNAACQHYGV RCQYCRFQKCLAVGM 66 HR8241A-264-328-Av6HT NR4A2 P43354 AVCGDNAACQHYGVR RCQYCRFQKCLAVGM 65 HR8241A-264-328-TEV NR4A2 P43354 AVCGDNAACQHYGVR RCQYCRFQKCLAVGM 65 HR8241B-328-598-15 NR4A2 P43354 MVKEVVRTDSLKGRR PPAIIDKLFLDTLPF 271 HR8241B-328-598-Av6HT NR4A2 P43354 VKEVVRTDSLKGRRG PPAIIDKLFLDTLPF 270 HR8241B-328-598-TEV NR4A2 P43354 VKEVVRTDSLKGRRG PPAIIDKLFLDTLPF 270 HR7224A-291-626-15 NR4A3 Q92570 MTCAVCGDNAACQHY PPSIIDKLFLDTLPF 337 HR7224B-363-626-15 NR4A3 Q92570 MRTDSLKGRRGRLPS PPSIIDKLFLDTLPF 265 HR7224B-363-626-Av6HT NR4A3 Q92570 RTDSLKGRRGRLPSK PPSIIDKLFLDTLPF 264 HR7224B-363-626-TEV NR4A3 Q92570 RTDSLKGRRGRLPSK PPSIIDKLFLDTLPF 264 HR7224B-396-626-15 NR4A3 Q92570 MICMMNALVRALTDS PPSIIDKLFLDTLPF 232 HR7224B-396-626-Av6HT NR4A3 Q92570 ICMMNALVRALTDST PPSIIDKLFLDTLPF 231 HR7224B-396-626-TEV NR4A3 Q92570 ICMMNALVRALTDST PPSIIDKLFLDTLPF 231 HR7224B-411-626-15 NR4A3 Q92570 MPRDLDYSRYCPTDQ PPSIIDKLFLDTLPF 217 HR7224B-411-626-Av6HT NR4A3 Q92570 PRDLDYSRYCPTDQA PPSIIDKLFLDTLPF 216 HR7224B-411-626-TEV NR4A3 Q92570 PRDLDYSRYCPTDQA PPSIIDKLFLDTLPF 216 HR7224C-291-374-15 NR4A3 Q92570 MTCAVCGDNAACQHY EVVRTDSLKGRRGRL 85 HR7224C-291-374-Av6HT NR4A3 Q92570 TCAVCGDNAACQHYG EVVRTDSLKGRRGRL 84 HR7224C-291-374-TEV NR4A3 Q92570 TCAVCGDNAACQHYG EVVRTDSLKGRRGRL 84 HR7224C-294-356-15 NR4A3 Q92570 MVCGDNAACQHYGVR NRCQYCRFQKCLSVG 64 HR7224C-294-356-Av6HT NR4A3 Q92570 VCGDNAACQHYGVRT NRCQYCRFQKCLSVG 63 HR7224C-294-356-TEV NR4A3 Q92570 VCGDNAACQHYGVRT NRCQYCRFQKCLSVG 63 HR7993A-220-461-15 NR5A1 Q13285 MGPNVPELILQLLQL PRNNLLIEMLQAKQT 243 HR7993A-220-461-Av6HT NR5A1 Q13285 GPNVPELILQLLQLE PRNNLLIEMLQAKQT 242 HR7993A-220-461-TEV NR5A1 Q13285 GPNVPELILQLLQLE PRNNLLIEMLQAKQT 242 HR7993B-10-111-15 NR5A1 O13285 MDELCPVCGDKVSGY PMYKRDRALKQQKKA 103 HR7993B-10-111-Av6HT NR5A1 Q13285 DELCPVCGDKVSGYH PMYKRDRALKQQKKA 102 HR7993B-10-111-TEV NR5A1 Q13285 DELCPVCGDKVSGYH PMYKRDRALKQQKKA 102 HR8211A-79-187-Av6HT NR5A2 O00482 MDEDLEELCPVCGDK KRDRALKQQKKALIR 110 HR8211A-79-187-NHT NR5A2 O00482 DEDLEELCPVCGDKV KRDRALKQQKKALIR 109 HR8211A-79-187-TEV NR5A2 O00482 DEDLEELCPVCGDKV KRDRALKQQKKALIR 109 HR7049A-49-474-15 NR6A1 Q15406 MDRAEQRTCLICGDR LFKVVLHSCKTSVGK 427 HR7049A-49-474-Av6HT NR6A1 Q15406 DRAEQRTCLICGDRA LFKVVLHSCKTSVGK 426 HR7049A-49-474-TEV NR6A1 Q15406 DRAEQRTCLICGDRA LFKVVLHSCKTSVGK 426 HR7049B-117-474-15 NR6A1 Q15406 MLQMGMNRKAIREDG LFKVVLHSCKTSVGK 359 HR7049B-117-474-Av6HT NR6A1 Q15406 LQMGMNRKAIREDGM LFKVVLHSCKTSVGK 358 HR7049B-117-474-TEV NR6A1 Q15406 LQMGMNRKAIREDGM LFKVVLHSCKTSVGK 358 HM7049C-49-143-15 NR6A1 Q15406 MDRAEQRTCLICGDR DGMPGGRNKSIGPVQ 96 HR7049C-49-143-Av6HT NR6A1 Q15406 DRAEQRTCLICGDRA DGMPGGRNKSIGPVQ 95 HR7049C-49-143-TEV NR6A1 Q15406 DRAEQRTCLICGDRA DGMPGGRNKSIGPVQ 95 HR7049C-49-159-15 NR6A1 Q15406 MDRAEQRTCLICGDR SEEEIERIMSGQEFE 112 HR7049C-49-159-Av6HT NR6A1 Q15406 DRAEQRTCLICGDRA SEEEIERIMSGQEFE 111 HR7049C-49-159-TEV NR6A1 Q15406 DRAEQRTCLICGDRA SEEEIERIMSGQEFE 111
HR7049C-58-143-15 NR6A1 Q15406 MICGDRATGLHYGII DGMPGGRNKSIGPVQ 87 HR7049C-58-159-15 NR6A1 Q15406 MICGDRATGLHYGII SEEEIERIMSGQEFE 103 HR7049C-58-159-Av6HT NR6A1 Q15406 ICGDRATGLHYGIIS SEEEIERIMSGQEFE 102 HR7049C-58-159-TEV NR6A1 Q15406 ICGDRATGLHYGIIS SEEEIERIMSGQEFE 102 HR8346A-59-490-Av6HT NRF1 Q16656 LNSTAADEVTAHLAA AMAPVTTRISDSAVT 432 HR7765A-130-171-Av6HT NRL P54845 ERFSDAALVSMSVRE ALRLKQRRRTLKNRG 42 HR8036A-96-176-Av6HT OLIG1 Q8TAK6 PDAKEEQQQQLRRKI LLLGSSLQELRRALG 81 HR7010A-102-190-15 OLIG2 Q13516 MTEPELQQLRLKINS IYGGHHAGFHPSACG 90 HR7010A-136-190-15 OLIG2 Q13516 MPYAHGPSVRKLSKI IYGGHHAGFHPSACG 56 HR7010A-97-191-15 OLIG2 Q13516 MDKKQMTEPELQQLR YGGHHAGFHPSACGG 96 HR6912A-76-168-NHT OLIG3 Q7RTU3 LSEQDLQQLRLKING GHHSAFHCGTVGHSA 93 HR4667C-291-437-TEV ONECUT1 Q9UBC0 EINTKEVAQRITTEL GLELSTVSNFFMNAR 147 HR8108A-313-459-TEV ONECUT2 O95948 ERPPSSSSGSQVATS FKENKRPSKEMQITI 147 HR7555A-320-466-TEV ONECUT3 O60422 EINTKEVAQRITAEL GLELNTVSNFFMNAR 147 HR8321A-165-256-TEV OSR1 Q8TAX0 GRLPSKTKKEFVCKF QSRTLAVHKTLHSQV 92 HR6892A-160-254-TEV OSR2 Q8N2R0 SRGRLPSKTKKEFIC SRTLAVHKTLHMQES 95 HR7032A-39-109-TEV OTX1 P32242 RRERTTFTRSQLDVL QQQQSGSGTKSRPAK 71 HR7869A-39-106-TEV OTX2 P32243 PAATPRKQRRERTTF VWFKNRRAKCRQQQQ 68 HR8136A-96-170-Av6HT OVOL1 O14753 RDHGFLRTKMKVTLG NDTFDLKRHVRTHTG 75 HR8149A-102-172-Av6HT OVOL2 Q9BRP0 ARSKIKFTTGTCSDS DTFDLKRHVRTHTGI 71 HR8517-1-166-Av6HT OVOL3 O00110 PRAFLVRSRRPQPPN YRERREKLHVCEDCG 165 HR6980A-489-684-TEV PARP12 Q9H0J9 DSSALPDPGFQKITL YPEYVIQYTTSSKPS 196 HR8222A-355-438-TEV PATZ1 Q9HBE1 VACEICGKIFRDVYH RPDHLNGHIKQVHTS 84 HR7455A-96-228-NHT PAX1 P15863 EQTYGEVNQLGGVFV VSSISRILRNKIGSL 133 HR7856A-1-149-TEV PAX5 Q02548 DLEKNYPTPRTSRTG INRIIRTKVQQPPNQ 148 HR8074A-4-136-TEV PAX6 P26367 SHSGVNQLGGVFVNG SINRVLRNLASEKQQ 133 HR7676A-217-276-TEV PAX7 P23759 QRRSRTTFTAEQLEE QVWFSNRRARWRKQA 60 HR7297A-1-146-TEV PAX8 Q06710 PHNSIRSGHGGLNQL IRTKVQQPFNLPMDS 145 HR7882A-388-494-TEV PBRM1 Q86U86 YYQQIKMPISLQQIR RKSKKNIRKQRMKIL 107 HR7526A-233-295-TEV PBX1 P40424 ARRKRRNFNKQATEI SNWFGNKRIRYKKNI 63 HR7154A-244-306-TEV PBX2 P40425 ARRKRRNFSKQATEV SNWFGNKRIRYKKNI 63 HR7892A-235-297-Av6HT PBX3 P40426 KTAVTAAHAVAAAVQ NGDSYQGSQVGANVQ 63 HR7892A-235-297-TEV PBX3 P40426 KTAVTAAHAVAAAVQ NGDSYQGSQVGANVQ 63 HR7406A-210-272-Av6HT PBX4 Q9BYU1 MARRKRRNFSKQATE SNWFGNKRIRYKKNM 64 HR7406A-210-272-TEV PBX4 Q9BYU1 ARRKRRNFSKQATEV SNWFGNKRIRYKKNM 63 HR7140A-124-182-TEV PCGF6 Q9BYE7 NLSELTPYILCSICK RCPKCNIVVHQTQPL 59 HR7140B-130-350-TEV PCGF6 Q9BYE7 PYILCSICKGYLIDA LVLHYGLVVSPLKIT 221 HR7140B-134-350-TEV PCGF6 Q9BYE7 CSICKGYLIDATTIT LVLHYGLVVSPLKIT 217 HR7140B-143-350-TEV PCGF6 Q9BYE7 DATTITECLHTFCKS LVLHYGLVVSPLKIT 208 HR7140B-182-350-TEV PCGF6 Q9BYE7 LYNIRLDRQLQDIVY LVLHYGLVVSPLKIT 169 HT6303A-249-350-Av6HT PCGF6 Q9BYE7 IPPELDMSLLLEFIG GLLVLHYGLVVSPLK 102 HT6303A-249-350-TEV PCGF6 Q9BYE7 IPPELDMSLLLEFIG GLLVLHYGLVVSPLK 102 HR7628A-146-206-TEV PDX1 P52945 NKRTRTAYTRAQLLE IWFQNRRMKWKKEED 61 HR4675D-563-641-TEV PGR P06401 PQKICLICGDEASGC CCQAGMVLGGRKFKK 79 HR6832-1-194-15 PHB2 Q99623 MAQNLKDLAGRLPAG DDVAITELSFSREYT 194 HR6832-1-194-Av6HT PHB2 Q99623 AQNLKDLAGRLPAGP DDVAITELSFSREYT 193 HR6832-1-291-15 PHB2 Q99623 MAQNLKDLAGRLPAG NLVLNLQDESFTRGS 291 HR6832-1-291-Av6HT PHB2 Q99623 AQNLKDLAGRLPAGP NLVLNLQDESFTRGS 290 HR6832-15 PHB2 Q99623 MAQNLKDLAGRLPAG SFTRGSDSLIKGKK* 300 HR6832-33-299-15 PHB2 Q99623 MAYGVRESVFTVEGG ESFTRGSDSLIKGKK 268 HR6832-33-299-Av6HT PHB2 Q99623 AYGVRESVFTVEGGH ESFTRGSDSLIKGKK 267 HR6832-38-299-15 PHB2 Q99623 MESVFTVEGGHRAIF ESFTRGSDSLIKGKK 263 HR6832-38-299-Av6HT PHB2 Q99623 ESVFTVEGGHRAIFF ESFTRGSDSLIKGKK 262 HR6832-Av6HT PHB2 Q99623 AQNLKDLAGRLPAGP SFTRGSDSLIKGKK* 299 HR6832A-33-194-15 PHB2 Q99623 MAYGVRESVFTVEGG DDVAITELSFSREYT 163 HR6832A-33-194-Av6HT PHB2 Q99623 AYGVRESVFTVEGGH DDVAITELSFSREYT 162 HR6832A-33-207-15 PHB2 Q99623 MAYGVRESVFTVEGG YTAAVEAKQVAQQEA 176 HR6832A-33-207-Av6HT PHB2 Q99623 AYGVRESVFTVEGGH YTAAVEAKQVAQQEA 175 HR6832A-33-207-NHT PHB2 Q99623 AYGVRESVFTVEGGH YTAAVEAKQVAQQEA 175 HR6832A-72-194-15 PHB2 Q99623 MIPWFQYPIIYDIRA DDVAITELSFSREYT 124 HR6832A-72-194-Av6HT PHB2 Q99623 IPWFQYPIIYDIRAR DDVAITELSFSREYT 123 HR6832A-72-207-15 PHB2 Q99623 MIPWFQYPIIYDIRA YTAAVEAKQVAQQEA 137 HR6832A-72-207-Av6HT PHB2 Q99623 IPWFQYPIIYDIRAR YTAAVEAKQVAQQEA 136 HR7710A-641-719-NHT PHF20 Q9BVI0 ELDGDDRYDFEVVRC PGFKYWYDKEWLSRG 79 HR6973A-486-543-TEV PHF21A Q96BD5 IHEDFCSVCRKSGQL CPRCQDQMLKKEEAI 58 HR6412A-62-149-14 PHOX2A O14813 LRDHQPAPYSAVPYK QVWFQNRRAKFRKQE 88 HR6412A-67-144-14 PHOX2A O14813 PAPYSAVPYKFFPEP TEARVQVWFQNRRAK 78 HR6412B-71-149-14 PHOX2A O14813 SAVPYKFFPEPSGLH QVWFQNRRAKFRKQE 79 HR6412B-76-144-14 PHOX2A O14813 KFFPEPSGLHEKRKQ TEARVQVWFQNRRAK 69 HR7334A-91-148-Av6HT PHOX2B Q99453 GLNEKRKQRRIRTTF KIDLTEARVQVWFQN 58 HR8156A-1-65-TEV PIAS1 O75925 ADSAELKQMVMSLRV PAVQMKIKELYRRRP 64 HR7952-96-424-Av6HT PIAS2 O75928 LAVAGIHSLPSTSVT CSDVDEIKFQEDGSW 329 HR3483-121-628-14 PIAS3 Q9Y6X2 MQPVHPDVTMKPLPF GPSLTGCRSDIISLD 509 HR3483-126-628-14 PIAS3 Q9Y6X2 MDVTMKPLPFYEVYG GPSLTGCRSDIISLD 504 HR3042-1-425-14 PIAS4 Q8N2W9 MAAELVEAKNMVMSF KERSCSPQGAILVLG 425 HR3042A-1-55-14 PIAS4 Q8N2W9 MAAELVEAKNMVMSF RALQLVQFDCSPELF 55 HR3042A-1-68-14 PIAS4 Q8N2W9 MAAELVEAKNMVMSF LFKKIKELYETRYAK 68 HR3042A-1-73-14 PIAS4 Q8N2W9 MAAELVEAKNMVMSF KELYETRYAKKNSEP 73 HR7108A-119-222-Av6HT PIKFYVE Q9Y2I7 GHDPRTAVQLRSLST RACTYCRKIALSYAH 104 HR7108A-119-241-Av6HT PIKFYVE Q9Y2I7 GHDPRTAVQLRSLST NSIGEDLNALSDSAC 123 HR7108A-143-222-Av6HT PIKFYVE Q9Y2I7 EGKSQDSDLKQYWMP RACTYCRKIALSYAH 80 HR7108A-143-241-Av6HT PIKFYVE Q9Y2I7 EGKSQDSDLKQYWMP NSIGEDLNALSDSAC 99 HR7108A-150-222-Av6HT PIKFYVE Q9Y2I7 DLKQYWMPDSQCKEC RACTYCRKIALSYAH 73 HR7108A-150-241-NHT PIKFYVE Q9Y2I7 DLKQYWMPDSQCKEC NSIGEDLNALSDSAC 92 HR7108B-586-943-Av6HT PIKFYVE Q9Y2I7 GWHHNNLELLREENG LLELRIVFEKGEQEN 358 HR7108B-610-943-Av6HT PIKFYVE Q9Y2I7 SANHNHMMALLQQLL LLELRIVFEKGEQEN 334 HR7108C-1761-2058-Av6HT PIKFYVE Q9Y2I7 KRETLRGADSAYYQV DKKLEMVVKSTGILG 298 HR7108C-1761-2088-Av6HT PIKFYVE Q9Y2I7 KRETLRGADSAYYQV TRFCEAMDKYFLMVP 328 HR7108C-1807-2058-Av6HT PIKFYVE Q9Y2I7 PHVELQFSDANAKFY DKKLEMVVKSTGILG 252 HR7108C-1807-2088-Av6HT PIKFYVE Q9Y2I7 PHVELQFSDANAKFY TRFCEAMDKYFLMVP 282 HR7108D-342-488-Av6HT PIKFYVE Q9Y2I7 TEDERKILLDSVQLK DSDTEQIAEEGDDNL 147 HR7108D-353-488-Av6HT PIKFYVE Q9Y2I7 VQLKDLWKKICHHSS DSDTEQIAEEGDDNL 136 HR8214A-89-149-TEV PITX1 P78337 QRRQRTHFTSQQLQE VWFKNRRAKWRKRER 61 HR4722B-274-317-Av6HT PITX2 Q99697 RDTCNSSLASLRLKA ASNLSACQYAVDRPV 44 HR7801A-85-145-TEV PITX3 O75364 RYPDMSTREEIAVWT GSFAAPLGGIVPPYE 61 HR7268A-257-319-TEV PKNOX1 P55347 GSSKNKRGVLPKHAT QVNNWFINARRRILQ 63 HR7428A-289-348-TEV PKNOX2 Q96KN3 KNKRGVLPKHATNIM QVNNWFINARRRILQ 60 HR7109-1-351-15 PLAG1 Q6DJT9 MATVIPGDLSEVRDT SSTSYAISIPEKEQP 351 HR7109-1-356-15 PLAG1 Q6DJT9 MATVIPGDLSEVRDT AISIPEKEQPLKGEI 356 HR7109-1-368-15 PLAG1 Q6DJT9 MATVIPGDLSEVRDT GEIESYLMELQGGVP 368 HR7109-1-436-15 PLAG1 Q6DJT9 MATVIPGDLSEVRDT FNFIPLNGPPYNPLS 436 HR7109-1-441-15 PLAG1 Q6DJT9 MATVIPGDLSEVRDT LNGPPYNPLSVGSLG 441 HR7109A-70-128-Av6HT PLAG1 Q6DJT9 TKAFVSKYKLQRHMA HDPNKETFKCEECGK 59 HR7109A-70-163-Av6HT PLAG1 Q6DJT9 TKAFVSKYKLQRHMA DLTCKVCLQTFESTG 94 HR7109A-72-107-Av6HT PLAG1 Q6DJT9 AFVSKYKLQRHMATH KCNYCEKMFHRKDHL 36 HR7109B-36-237-Av6HT PLAG1 Q6DJT9 CQLCDKAFNSVEKLK GRKDHLTRHMKKSHN 202 HR7109C-119-174-Av6HT PLAG1 Q6DJT9 ETFKCEECGKNYNTK ESTGVLLEHLKSHAG 56 HR7109C-122-170-Av6HT PLAG1 Q6DJT9 KCEECGKNYNTKLGF LQTFESTGVLLEHLK 49 HR7109D-183-233-Av6HT PLAG1 Q6DJT9 KKHQCEHCDRRFYTR AQRFGRKDHLTRHMK 51 HR7109D-183-237-Av6HT PLAG1 Q6DJT9 KKHQCEHCDRRFYTR GRKDHLTRHMKKSHN 55 HR7109D-188-233-Av6HT PLAG1 Q6DJT9 EHCDRRFYTRKDVRR AQRFGRKDHLTRHMK 46 HR7895-1-337-Av6HT PLAGL1 Q9UM63 ATFPCQLCGKTFLTL KGFCNISLFEDLPLQ 336 HR7895-1-397-Av6HT PLAGL1 Q9UM63 ATFPCQLCGKTFLTL LLGFWQLPPPATQNT 396 HR7895-159-463-Av6HT PLAGL1 Q9UM63 DHCERCFYTRKDVRR GTGSAILPHFHHAFR 305 HR7895-Av6HT PLAGL1 Q9UM63 ATFPCQLCGKTFLTL TGSAILPHFHHAFR* 463 HR7895A-149-212-15 PLAGL1 Q9UM63 MSGTKEKKHQCDHCE HLTRHTKKTHSQELM 65 HR7895A-154-207-15 PLAGL1 Q9UM63 MKKHQCDHCERLFYT FGRKDHLTRHTKKTH 55 HR7895A-159-199-Av6HT PLAGL1 Q9UM63 DHCERCFYTRKDVRR LCQFCAQRFGRKDHL 41 HR7895B-1-209-AV6HT PLAGL1 Q9UM63 ATFPCQLCGKTFLTL RKDHLTRHTKKTHSQ 208 HR7895C-1-69-Av6HT PLAGL1 Q9UM63 ATFPCQLCGKTFLTL THSPQKSHQCAHCEK 68 HR7895C-12-69-Av6HT PLAGL1 Q9UM63 TFLTLEKFTIHNYSH THSPQKSHQCAHCEK 58 HR7996A-189-243-Av6HT PLAGL2 Q9UPG8 KKHPCDHCDRRFYTR GRKDHLTRHVKKSHS 55 HR8052A-121-223-TEV PLEK P08567 PETIDLGALYLSMKD FLDNPDAFYYFPDSG 103 HR7739A-238-353-TEV PLEK2 Q9NYT0 SLSTVELSGTVVKQG KAERAEWIEAIKKLT 116
HR7495A-45-167-TEV PLEKHA4 Q9H4M7 NALRRDPNLPVHIRG RAEGDDYGQPRSPAR 123 HR8545A-1266-1889-Av6HT PLXNA1 Q9UIW2 AYKRKSRDADRTLKR QARRQRLRSKLEQVV 624 HR7225A-1259-1890-TEV PLXNA2 O75051 AYKRKSRENDLTLKR RQRLAYKVEQLINAM 632 HR8315A-1241-1867-TEV PLXNA3 P51805 AYKRKTQDADRTLKR KHKLRQKLEQIISLV 627 HR7815A-1736-1862-Av6HT PLXNB1 O43157 DNRLLREDVEYRPLT ALVPCLTKHVLRENQ 127 HR7815A-1736-1862-TEV PLXNB1 O43157 DNRLLREDVEYRPLT ALVPCLTKHVLRENQ 127 HR8081A-1224-1827-NHT PLXNB2 O15031 SQQAEREYEKIKSQL PAAQKMQLAFRLQQI 604 HR8081A-1224-1827-TEV PLXNB2 O15031 SQQAEREYEKIKSQL PAAQKMQLAFRLQQI 604 HR6985A-1280-1902-TEV PLXNB3 Q9ULL4 GVGMGAAVLIAAVLL LYNHIHRYYDQIISA 623 HR7941A-1198-1305-TEV PLXNC1 O60486 TVALNVVFEKIPENE HYEISNGSTIKVFKK 108 HR8405A-1553-1678-NHT PLXND1 Q9Y4D7 AKPRNLNVSFQGCGM RVKDLDTEKYFHLVL 126 HR6974A-571-650-TEV PMS1 P54277 IKKPMSASALFVQDH KRAIEQESQMSLKDG 80 HR6952A-34-103-NHT POGK Q9P215 QKVRICSEGGWVPAL PFPKPDMITRLEGEE 70 HR7570A-37-117-NHT POLE4 Q9NR33 RLSRLPLARVKALVK IEAVDEFAFLEGTLD 81 HR7808-15 POLR2L P62875 MIIPVRCFTCGKIVG DLIEKLLNYAPLEK* 68 HR8543A-1-154-Av6HT POTEKP Q9BYX7 DDDTAVLVIDNGSGM LSLYTSGRTTGIVMD 153 HR4466B-129-273-TEV POU1F1 P28069 IRELEKFANEFKVRR RVWFCNRRQREKRVK 145 HR7752A-297-359-TEV POU2F2 P09086 EKSFLANQKPTSEEI APMLPSPGKPASYSP 63 HR7822A-187-257-TEV POU2F3 Q9UKI9 DLEELEKFAKTFKQR CKLKPLLEKWLNDAE 71 HR7177A-250-322-Av6HT POU3F1 Q03052 PSSDDLEQFAKQFKQ KLKPLLNKWLEETDS 73 HR6946A-348-418-15 POU3F2 P20265 MIAAQGRKRKKRTSI NRRQKEKRMTPPGGT 72 HR6946A-353-407-Av6HT POU3F2 P20265 RKRKKRTSIEVSVKG LEKEVVRVWFCNRRQ 55 HR6946A-353-414-15 POU3F2 P20265 MRKRKKRTSIEVSVK VWFCNRRQKEKRMTP 63 HR6946A-356-432-15 POU3F2 P20265 MKKRTSIEVSVKGAL TLPGAEDVYGGSRDT 78 HR6946A-361-427-15 POU3F2 P20265 MIEVSVKGALESHFL TPPGGTLPGAEDVYG 58 HR6946A-377-427-15 POU3F2 P20265 MPKPSAQEITSLADS TPPGGTLPGAEDVYG 52 HR8066A-320-388-TEV POU3F3 P20264 DDLEQFAKQFKQRRI CKLKPLLNKWLEEAD 69 HR8200A-192-260-TEV POU3F4 P49335 DELEQFAKQFKQRRI CKLKPLLNKWLEEAD 69 HR7341A-253-330-NHT POU4F2 Q12837 ADPRDLEAFAERFKQ KPILQAWLEEAEKSH 78 HR7479A-183-261-NHT POU4F3 Q15319 DPRELEAFAERFKQR VLQAWLEEAEAAYRE 79 HR7056A-224-289-TEV POU5F1 Q01860 ETLVQARKRKRTSIE RVWFCNRRQKGKRSS 66 HR8237A-224-288-NHT POU5F1B Q06416 ETLMQARKRKRTSIE RVWFCNRRQKGKRSS 65 HR8392A-142-292-TEV POU6F1 Q14863 INLEEIREFAKNFKI VRVWFCNRRQTLKNT 151 HR7133A-97-168-15 PPARA Q07869 MALNIECRICGDKAS QYCRFHKCLSVGMSH 73 HR7133A-97-168-Av6HT PPARA Q07869 ALNIECRICGDKASG QYCRFHKCLSVGMSH 72 HR7133A-97-168-TEV PPARA Q07869 ALNIECRICGDKASG QYCRFHKCLSVGMSH 72 HR7133A-97-174-15 PPARA Q07869 MALNIECRICGDKAS KCLSVGMSHNAIRFG 79 HR7133A-97-174-Av6HT PPARA Q07869 ALNIECRICGDKASG KCLSVGMSHNAIRFG 78 HR7133A-97-174-TEV PPARA Q07869 ALNIECRICGDKASG KCLSVGMSHNAIRFG 78 HR7133A-97-187-15 PPARA Q07869 MALNIECRICGDKAS FGRMPRSEKAKLKAE 92 HR7133A-97-187-Av6HT PPARA Q07869 ALNIECRICGDKASG FGRMPRSEKAKLKAE 91 HR7133A-97-187-TEV PPARA Q07869 ALNIECRICGDKASG FGRMPRSEKAKLKAE 91 HR7133B-182-468-15 PPARA Q07869 MAKLKAEILTGEHDI AALHPLLQEIYRDMY 288 HR7133B-182-468-Av6HT PPARA Q07869 AKLKAEILTCEHDIE AALHPLLQEIYRDMY 287 HR7133B-182-468-TEV PPARA Q07869 AKLKAEILTCEHDIE AALHPLLQEIYRDMY 287 HR7133C-192-468-15 PPARA Q07869 MEHDIEDSETADLKS AALHPLLQEIYRDMY 278 HR7133C-192-468-Av6HT PPARA Q07869 EHDIEDSETADLKSL AALHPLLQEIYRDMY 277 HR7133C-192-468-TEV PPARA Q07869 EHDIEDSETADLKSL AALHPLLQEIYRDMY 277 HR8028A-67-146-15 PPARD Q03181 MCGSLNMECRVCGDK KCLALGMSHNAIRFG 81 HR8028A-67-146-Av6HT PPARD Q03181 CGSLNMECRVCGDKA KCLALGMSHNAIRFG 80 HR8028A-67-146-TEV PPARD Q03181 CGSLNMECRVCGDKA KCLALGMSHNAIRFG 80 HR8028A-67-156-15 PPARD Q03181 MCGSLNMECRVCGDK AIRFGRMPEAEKRKL 91 HR8028A-67-156-Av6HT PPARD Q03181 CGSLNMECRVCGDKA AIRFGRMPEAEKRKL 90 HR8028A-67-156-TEV PPARD Q03181 CGSLNMECRVCGDKA AIRFGRMPEAEKRKL 90 HR8028A-73-146-15 PPARD Q03181 MECRVCGDKASGFHY KCLALGMSHNAIRFG 75 HR8028A-73-146-Av6HT PPARD Q03181 ECRVCGDKASGFHYG KCLALGMSHNAIRFG 74 HR8028A-73-146-TEV PPARD Q03181 ECRVCGDKASGFHYG KCLALGMSHNAIRFG 74 HR8028A-73-156-15 PPARD Q03181 MECRVCGDKASGFHY AIRFGRMPEAEKRKL 85 HR8028A-73-156-Av6HT PPARD Q03181 ECRVCGDKASGFHYG AIRFGRMPEAEKRKL 84 HR8028A-73-156-TEV PPARD Q03181 ECRVCGDKASGFHYG AIRFGRMPEAEKRKL 84 HR80288-163-441-15 PPARD Q03181 MNEGSQYNPQVADLK TSLHPLLQEIYKDMY 280 HR8028B-163-441-Av6HT PPARD Q03181 NEGSQYNPQVADLKA TSLHPLLQEIYKDMY 279 HR8028B-163-441-TEV PPARD Q03181 NEGSQYNPQVADLKA TSLHPLLQEIYKDMY 279 HR4464B-222-504-TEV PPARG P37231 LAEISSDIDQLNPES DMSLHPLLQEIYKDL 283 HR7373A-58-198-NHT PPP1R10 Q96QC0 RSPEILVKFIDVGGY AEEAPEKKREKPKSL 141 HR7854A-111-311-Av6HT PPP2R3B Q9Y5P8 TRKEEPLPPATSQSI LLEEEADINQLTEFF 201 HR6538A-2-187-TEV PRDM1 O75626 LDICLEKRVGTTLAA LAACQNGMNIYFYTI 186 HR7699A-188-339-TEV PRDM10 Q9NQV6 KHGPLHPIPNRPVLT YAASYAEFVNQKIHD 152 HR6506A-60-229-TEV PRDM12 Q9H4Q4 KTAFTAEVLAQSFSG VPGLEEDQKKNKHED 170 HR7923A-243-372-Av6HT PRDM14 Q9GZV8 DKDSLQLPEGLCLMQ QNQELLVWYGDCYEK 130 HR8160A-72-214-Av6HT PRDM16 Q9HAZ2 VYIPEDIPIPADFEL IEPGEELLVHVKEGV 143 HR4804B-1144-1216-14 PRDM2 Q13029 MLSIKDLTKHLSIHA FLCNLQQHQRDLHPD 74 HR4804B-1144-1221-14 PRDM2 Q13029 MLSIKDLTKHLSIHA QQHQRDLHPDKVCTH 79 HR4804B-1144-1230-14 PRDM2 Q13029 MLSIKDLTKHLSIHA DKVCTHHEFESGTLR 88 HR4804B-1158-1216-14 PRDM2 Q13029 MEEWPFKCEFCVQLF FLCNLQQHQRDLHPD 60 HR4804B-1158-1221-14 PRDM2 Q13029 MEEWPFKCEFCVQLF QQHQRDLHPDKVCTH 65 HR4804B-1158-1230-14 PRDM2 Q13029 MEEWPFKCEFCVQLF DKVCTHHEFESGTLR 74 HR4804C-342-415-14 PRDM2 Q13029 MQIPRTKEEANGDVF TQINRRRHERRHEAG 75 HR4804C-356-417-14 PRDM2 Q13029 METFMFPCQHCERKF INRRRHERRHEAGLK 63 HR4804C-360-422-14 PRDM2 Q13029 MFPCQHCERKFTTKQ HERRHEAGLKRKPSQ 64 HR4804C-367-417-14 PRDM2 Q13029 MRKFTTKQGLERHMH INRRRHERRHEAGLK 52 HR4804D-2-148-TEV PRDM2 Q13029 NQNTTEPVAATETLA EELLVWYNGEDNPEI 147 HR6504A-390-540-TEV PRDM4 Q9UKN5 EHGPVTFVPDTPIES FYYSRDYAQQIGVPE 151 HR7347A-1-135-NHT PRDM5 Q9NQX1 LGMYVPDRFSLKSSR IGYLDSDMEAEEEEQ 134 HR8295A-234-370-Av6HT PRDM6 Q9NQX0 PPELPEWLRDLPREV RGTELLVWYNDSYTS 137 HR7077A-196-395-NHT PRDM7 Q9NQW5 EPQDDDYLYCEMCQN VNCWSGMGMSMARNW 200 HR8098A-623-689-Av6HT PRDM8 Q9NQV8 AQNWCAKCNASFRMT FRERHHLSRHMTSHN 67 HR8069A-197-382-Av6HT PRDM9 Q9NQV7 PQDDDYLYCEMCQNF KWGSKWKKELMAGRE 186 HR7506-125-390-Av6HT PREB Q9HCU5 EKKCGAETQHEGLEL SRCQLHLLPSRRSVP 266 HR7506A-125-312-Av6HT PREB Q9HCU5 EKKCGAETQHEGLEL CGHEVVSCLDVSESG 188 HR7506A-133-315-Av6HT PREB Q9HCU5 QHEGLELRVENLQAV EVVSCLDVSESGTFL 183 HR7506A-155-312-15 PREB Q9HCU5 MPLQKVVCFNHDNTL CGHEVVSCLDVSESG 159 HR8329A-190-376-Av6HT PREX2 Q70Z35 KHSDYAAVMEALQAM RRKGLKLGMEQDTWV 187 HR8329B-674-751-Av6HT PREX2 Q70Z35 PRETVKIPDSADGLG AHVTACRKYRRPTKQ 78 HR8329B-674-760-Av6HT PREX2 Q70Z35 PRETVKIPDSADGLG RRPTKQDSIQWVYNS 87 HR2833A-1-98-NHT PRKRIR O43422 GQLKFNTSEEHHADM ERYENGRKRLKAYLR 97 HR3353-149-736-14 PRKRIR O43422 MDEDILPLTLEEKEN LLNINFDIKHDLDLM 589 HR3353-154-731-14 PRKRIR O43422 MPLTLEEKENKEYLK SSNLALLNINFDIKH 579 HR4564B-74-136-14 PROP1 O75360 MTTFSPVQLEQLESA AKQRKQERSLLQPLA 64 HR4660B-14 PROX1 Q92786 MAMQEGLSPNHLKKA EIFKSPNCLQELLHE 164 HR4660B-15 PROX1 Q92786 MAMQEGLSPNHLKKA EIFKSPNCLQELLHE 164 HR4660C-546-737-14 PROX1 Q92786 MTAEGLSLSLIKSEC EIFKSPNCLQELLHE 193 HR4660C-550-737-14 PROX1 Q92786 MLSISLIKSECGDLQ EIFKSPNCLQELLHE 189 HR4660C-566-737-14 PROX1 Q92786 MSEISPYSGSAMQEG EIFKSPNCLQELLHE 173 HR4660C-572-737-14 PROX1 Q92786 MSGSAMQEGLSPNHL EIFKSPNCLQELLHE 167 HR4660C-577-737-14 PROX1 Q92786 MQEGLSPNHLKKAKL EIFKSPNCLQELLHE 162 HR8100A-409-592-Av6HT PROX2 Q3B8N5 LPLLPSVKMEQRGLH DSDIPEIFKSSSYPQ 184 HR6440-1-237-14 PRRX1 P54821 MTSSYGHVLERQPAL SIANLRLKAKEYSLQ 237 HR6440-14 PRRX1 P54821 MTSSYGHVLERQPAL AKEYSLQRNQVPTVN 245 HR6440-22-245-14 PRRX1 P54821 PGNLDTLQAKKNFSV AKEYSLQRNQVPTVN 224 HR6440-27-245-14 PRRX1 P54821 TLQAKKNFSVSHLLD AKEYSLQRNQVPTVN 219 HR7233A-95-168-Av6HT PRRX2 Q99811 GSAAKRKKKQRRNRT NRRAKFRRNERAMLA 74 HR7750A-100-341-Av6HT PSMD11 O00231 EAATGQEVELCLECI YDNLLEQNLIRVIEP 242 HR7208-1-335-TEV PSMD12 O00232 ADGGSERADGRIVKM VEDYGMELRKGSLES 334 HR7208-11-335-TEV PSMD12 O00232 GRIVKMEVDYSATVD VEDYGMELRKGSLES 325 HR7208-TEV PSMD12 O00232 ADGGSERADGRIVKM THLIAKEEMIHNLQ* 456 HR7208A-338-425-15 PSMD12 O00232 MTDVFGSTEEGEKRW GIINFQRPKDPNNLL 89 HR7208A-338-456-15 PSMD12 O00232 MTDVFGSTEEGEKRW TTHLIAKEEMIHNLQ 120 HR7208A-343-420-15 PSMD12 O00232 MSTEEGEKRWKDLKN VDRLAGIINFQRPKD 79 HR7208A-343-456-15 PSMD12 O00232 MSTEEGEKRWKDLKN TTHLIAKEEMIHNLQ 115 HR7208A-371-456-15 PSMD12 O00232 MTRITMKRMAQLLDL TTHLIAKEEMIHNLQ 87 HR7208B-300-417-TEV PSMD12 O00232 PKYKDLLKLFTTMEL FAKVDRLAGIINFQR 118 HR7208C-300-456-TEV PSMD12 O00232 PKYKDLLKLFTTMEL TTHLIAKEEMIHNLQ 157 HR5110A-437-891-Av6HT Q6ZU11 Q6ZU11 LNKDQATALIQIAQM HCEGREDGLQHANQY 455 HR7981A-147-199-TEV RABGEF1 Q9UJ41 KTFHKTGQEIYKQTK FYHNVAERMQTRGKV 53
HR8212A-1-114-NHT RAD51 Q06609 AMQMQLEANADTSVE IQITTGSKELDKLLQ 113 HR7353A-268-383-TEV RAG1 P15918 NCSKIHLSTKLLAVD LEKYNHHISSHKESK 116 HR7829A-213-923-TEV RAPGEF3 O95398 PDALLTVALRKPPGQ DNQRELSRLSRELEP 711 HR7829B-33-923-TEV RAPGEF3 O95398 DVVPEGTLLNMVLRR DNQRELSRLSRELEP 891 HR8365C-81-160-NHT RARA P10276 LPRIYKPCFVCQDKS QKCFEVGMSKESVRN 80 HR8365C-81-160-TEV RARA P10276 LPRIYKPCFVCQDKS QKCFEVGMSKESVRN 80 HR6970C-82-160-TEV RARB P10826 FVCQDKSSGYHYGVS MSKESVRNDRNKKKK 79 HR7515A-85-154-15 RARG P13631 MRVYKPCFVCNDKSS NRCQYCRLQKCFEVG 71 HR7515A-85-154-Av6HT RARG P13631 RVYKPCFVCNDKSSG NRCQYCRLQKCFEVG 70 HR7515A-85-154-TEV RARG P13631 RVYKPCFVCNDKSSG NRCQYCRLQKCFEVG 70 HR7515A-85-166-15 RARG P13631 MRVYKPCFVCNDKSS EVGMSKEAVRNDRNK 83 HR7515A-85-166-Av6HT RARG P13631 RVYKPCFVCNDKSSG EVGMSKEAVRNDRNK 82 HR7515A-85-166-TEV RARG P13631 RVYKPCFVCNDKSSG EVGMSKEAVRNDRNK 82 HR7515A-97-166-15 RARG P13631 MSSGYHYGVSSCEGC EVGMSKEAVRNDRNK 71 HR7515A-97-166-Av6HT RARG P13631 SSGYHYGVSSCEGCK EVGMSKEAVRNDRNK 70 HR7515A-97-166-TEV RARG P13631 SSGYHYGVSSCEGCK EVGMSKEAVRNDRNK 70 HR7515B-178-423-15 RARG P13631 MDSYELSPQLEELIT PPLIREMLENPEMFE 247 HR7515B-178-423-Av6HT RARG P13631 DSYELSPQLEELITK PPLIREMLENPEMFE 246 HR7515B-178-423-TEV RARG P13631 DSYELSPQLEELITK PPLIREMLENPEMFE 246 HR7515C-183-417-15 RARG P13631 MSPQLEELITKVSKA EIPGPMPPLIREMLE 236 HR7515C-183-417-Av6HT RARG P13631 SPQLEELITKVSKAH EIPGPMPPLIREMLE 235 HR7515C-183-417-TEV RARG P13631 SPQLEELITKVSKAH EIPGPMPPLIREMLE 235 HR7515D-84-169-15 RARG P13631 MPRVYKPCFVCNDKS MSKEAVRNDRNKKKK 87 HR7515D-84-169-Av6HT RARG P13631 PRVYKPCFVCNDKSS MSKEAVRNDRNKKKK 86 HR7515D-84-169-TEV RARG P13631 PRVYKPCFVCNDKSS MSKEAVRNDRNKKKK 86 HR8219A-137-208-Av6HT RAX Q9Y2V3 RRNRTTFTTYQLHEL QEKLEVSSMKLQDSP 72 HR8168A-24-86-Av6HT RAX2 Q96IS3 KKKHRRNRTTFTTYQ QVWFQNRRAKWRRQE 63 HR7540-167-714-15 RBAK Q9NYW8 MECGKTYHGEKMCEF IHRRGNMNVLDVENL 549 HR7540-171-714-15 RBAK Q9NYW8 MTYHGEKMCEFNQNG IHRRGNMNVLDVENL 545 HR7540-183-714-15 RBAK Q9NYW8 MNGDTYSHNEENILQ IHRRGNMNVLDVENL 533 HR7540-188-714-15 RBAK Q9NYW8 MSHNEENILQKISIL IHRRGNMNVLDVENL 528 HR7540-7-562-15 RBAK Q9NYW8 MPVSFKDVAVDFTQE FNELSYYTEHYRSHS 557 HR7540A-397-562-TEV RBAK Q9NYW8 GEKLYKCNECGKSYY FNELSYYTEHYRSHS 166 HR7540A-397-591-TEV RBAK Q9NYW8 GEKLYKCNECGKSYY SHNSSLFRHQRVHTG 195 HR7540A-428-562-TEV RBAK Q9NYW8 PYQCSECGKFFSRVS FNELSYYTEHYRSHS 135 HR7540A-428-591-TEV RBAK Q9NYW8 PYQCSECGKFFSRVS SHNSSLFRHQRVHTG 164 HR7540B-1-64-Av6HT RBAK Q9NYW8 NTLQGPVSFKDVAVD DTTKPNVIIKLEQGE 63 HR7540C-633-701-Av6HT RBAK Q9NYW8 SRMSNLTVHYRSHSG KFHHRSAFNSHQRIH 69 HR7540C-649-701-Av6HT RBAK Q9NYW8 KPYECNECGKVFSQK KFHHRSAFNSHQRIH 53 HR7540C-653-701-Av6HT RBAK Q9NYW8 CNECGKVFSQKSYLT KFHHRSAFNSHQRIH 49 HR8531A-503-605-Av6HT RBM20 Q5T481 LASVGTTFAQRKGAG SKRYKELQLKKPGKA 103 HR7548A-227-304-TEV RBM22 Q9NW64 EDKTITTLYVGGLGD KLIVNGRRLNVKWGR 78 HR7417A-596-679-NHT RBM27 Q9P2N5 QYTNTKLEVKKIPQE RFIRVLWHRENNEQP 84 HR7740A-184-325-NHT RBM5 P52756 DWLCNKCCLNNFRKR DFAKSARKDLVLSDG 142 HR6987A-23-453-TEV RBPJ Q06330 GERPPPKRLTREAMR SLTFTYTPEPGPRPH 431 HR7414A-356-477-NHT RBPJL Q9UBG7 SSCWTIIGTESVEFS DGLFYPSAFSFTYTP 122 HR8038A-1-79-Av6HT RC3H2 Q9HBD1 PVQAAQWTEFLSCPI PVNFALLQLVGAQVP 78 HR7631A-308-440-TEV RCOR1 Q9UKL0 RKPPKGMFLSQEDVE RRFNIDEVLQEWEAE 133 HR7631B-158-237-Av6HT RCOR1 Q9UKL0 GYNMEQALGMLFWHK IASLVKFYYSWKKTR 80 HR7631B-158-247-Av6HT RCOR1 Q9UKL0 GYNMEQALGMLFWHK WKKTRTKTSVMDRHA 90 HR7631B-172-237-Av6HT RCOR1 Q9UKL0 KHNIEKSLADLPNFT IASLVKFYYSWKKTR 66 HR7631B-172-247-Av6HT RCOR1 Q9UKL0 KHNIEKSLADLPNFT WKKTRTKTSVMDRHA 76 HR7640A-292-387-NHT RCOR2 Q8IZ40 ISLKRQVQSMKQTNS YRRRFNLEEVLQEWE 96 HR2844A-14 REL Q04864 EKDTYGNKAKKQKTT NEQLSDSFPYEFFQV 337 HR2844B-14 REL Q04864 EKDTYGNKAKKQKTT NSQGIPPFLRIPVGN 171 HR2844C-14 REL Q04864 DLNASNACIYNNADD NEQLSDSFPYEFFQV 166 HR2845-14 RELA Q04206 MDELFPLIFPAEPAQ IADMDFSALLSQISS 551 HR2845B-14 RELA Q04206 TDDRHRIEEKRKRTY QFDDEDLGALLGNST 167 HR2845C-14 RELA Q04206 DPAVFTDLASVDNSE IADMDFSALLSQISS 93 HR2845D-191-291-Av6HT RELA Q04206 TAELKICRVNRNSGS DRELSEPMEFQYLPD 101 HR2845D-191-291-TEV RELA Q04206 TAELKICRVNRNSGS DRELSEPMEFQYLPD 101 HR2846-40-579-15 RELB Q01201 MLSSLSLAVSRSTDE AAFGGGLLSPGPEAT 541 HR2846B-14 RELB Q01201 DHDSYGVDKKRKRGM AAFGGGLLSPGPEAT 179 HR6006B-21 RERE Q9P2R6 STQGEIRVGPSHQAK LITFYYYWKKTPEAA 165 HR6006C-21 RERE Q9P2R6 STQGEIRVGPSHQAK RLVKKPVPKLIEKCW 116 HR6006D-21 RERE Q9P2R6 STQGEIRVGPSHQAK DLLMYLRAARSMAAF 60 HR6006E-21 RERE Q9P2R6 VTQHEELVWMPGVND ETGELITFYYYWKKT 132 HR6006F-21 RERE Q9P2R6 VTQHEELVWMPGVND RLVKKPVPKLIEKCW 87 HR6006H-21 RERE Q9P2R6 QGEIRVGPSHQAKLP ETGELITFYYYWKKT 159 HR6969A-342-413-NHT REST Q13127 SNQHEVTRHARQVHN SKKCNLQYHFKSKHP 72 HR8119A-438-513-TEV RFX1 P22670 TVQWLLDNYETAEGV RGNSKYHYYGLRIKA 76 HR7754A-200-273-TEV RFX2 P48378 LQWLLDNYETAEGVS TRGNSKYHYYGIRLK 74 HR7471A-184-257-TEV RFX3 P48380 LQWLLDNYETAEGVS TRGNSKYHYYGIRVK 74 HR8007A-101-167-15 RFX5 P48382 MEEHTDTCLPKQSVY GRGQSKYCYSGIRRK 68 HR8007A-76-173-15 RFX5 P48382 MDKSSEPSTLSNEEY YCYSGIRRKTLVSMP 99 HR8007A-81-167-15 RFX5 P48382 MPSTLSNEEYMYAYR GRGQSKYCYSGIRRK 88 HR7289A-107-205-NHT RFX6 Q8HWS3 KKTITQIVKDKKKQT HYYGIGIKESSAYYH 99 HR7790-68-260-TEV RFXANK O14593 KHSTTLTNRQRGNEV ILKLFQSNLVPADPE 193 HR7790-79-260-TEV RFXANK O14593 GNEVSALPATLDSLS ILKLFQSNLVPADPE 182 HR7790A-101-248-15 RFXANK O14593 MGELDQLKEHLRKGD GYRKVQQVIENHILK 149 HR7790A-101-260-15 RFXANK O14593 MGELDQLKEHLRKGD ILKLFQSNLVPADPE 161 HR7790A-79-248-15 RFXANK O14593 MGNEVSALPATLDSL GYRKVQQVIENHILK 171 HR7790A-86-251-15 RFXANK O14593 MPATLDSLSIHQLAA KVQQVIENHILKLFQ 167 HR7790A-91-248-15 RFXANK O14593 MSLSIHQLAAQGELD GYRKVQQVIENHILK 159 HR7790A-91-260-15 RFXANK O14593 MSLSIHQLAAQGELD ILKLFQSNLVPADPE 171 HR7361A-325-470-TEV RGS6 P49758 PSQQRVKRWGFSFDE KGKSLAGKRLTGLMQ 146 HR6895A-323-449-TEV RGS7 P49802 SQQRVKRWGFGMDEA YPRFIRSSAYQELLQ 127 HR6935A-279-424-TEV RGS9 O75916 DLNAKLVEIPTKMRV YKDMLAKAIEPQETT 146 HR7291A-130-236-NHT RHOXF2 Q9BQY4 PGNAQQPNVHAFTPL PLFISGMRDDYFWDH 107 HR7532A-130-236-NHT RHOXF2B P0C7M4 PGNAQQPNVHAFTPL PLFISGMRDDYFWDH 107 HR7189A-91-307-Av6HT RIOK2 Q9BVS4 RQVVESVGNQMGVGK EDTLDVEVSASGYTK 217 HR7201A-769-825-Av6HT RLF Q13129 LRYKCELNGCNIVFS FYYSKIEYQNHLSMH 57 HR7201A-769-837-NHT RLF Q13129 LRYKCELNGCNIVFS SMHNVENSNGDIKKS 69 HR7201B-707-825-Av6HT RLF Q13129 LDMKNRREKCTYCRR FYYSKIEYQNHLSMH 119 HR7201B-707-837-Av6HT RLF Q13129 LDMKNRREKCTYCRR SMHNVENSNGDIKKS 131 HR7201B-716-825-Av6HT RLF Q13129 CTYCRRHFMSAFHLR FYYSKIEYQNHLSMH 110 HR7201B-716-837-Av6HT RLF Q13129 CTYCRRHFMSAFHLR SMHNVENSNGDIKKS 122 HR7201C-707-770-Av6HT RLF Q13129 LDMKNRREKCTYCRR VNELLNHKQKHDDLR 64 HR7201C-725-770-Av6HT RLF Q13129 SAFHLREHEQVHCGP VNELLNHKQKHDDLR 46 HR7461A-26-161-TEV RNASE2 P10153 HVKPPQFTWAQWFET PPQYPVVPVHLDRII 136 HR7865A-252-319-TEV RNF113A O15541 GSDDEEIPFKCFICR GVFNPAKELIAKLEK 68 HR7107A-246-319-TEV RNF113B Q8IZP6 GSEEEEIPFRCFICR KELMAKLQKLQAAEG 74 HR7482A-1-89-NHT RNF114 Q9Y508 AAQQRDCGGAAQLAG VRAVELERQIESTET 88 HR7645A-17-100-NHT RNF125 Q96EQ8 ATARALERRRDPELP ATDVAKRMKSEYKNC 84 HR4563B-87-210-14 RORA P35398 MKEDKEVQTGYMNAQ HRMQQQQRDHQQQPG 125 HR4563B-92-174-14 RORA P35398 MVQTGYMNAQIEIIP HCRLQKCLAVGMSRD 84 HR4563B-92-193-14 RORA P35398 MVQTGYMNAQIEIIP GRMSKKQRDSLYAEV 103 HR4563B-93-210-14 RORA P35398 MQTGYMNAQIEIIPC HRMQQQQRDHQQQPG 119 HR4563B-98-174-14 RORA P35398 MNAQIEIIPCKICGD HCRLQKCLAVGMSRD 78 HR4563B-98-193-14 RORA P35398 MNAQIEIIPCKICGD GRMSKKQRDSLYAEV 97 HR7194B-260-507-TEV RORC P51449 PEAPYASLTEIEHLV VVQAAFPPLYKELFS 248 HR7255A-172-270-Av6HT RPA2 P15927 ANSQPSAGRAPISNP YSTVDDDHFKSTDAE 99 HR7255A-172-270-TEV RPA2 P15927 ANSQPSAGRAPISNP YSTVDDDHFKSTDAE 99 HR7006A-95-145-NHT RREB1 Q92766 ADHSCSICGKSLSSA GQSFTTNGNMHRHMK 51 HR4447C-46-185-Av6HT RUNX1 Q01196 MSGDRSMVEVLADHP DGPREPRRHRQKLDD 141 HR4447C-46-185-TEV RUNX1 Q01196 SGDRSMVEVLADHPG DGPREPRRHRQKLDD 140 HR4568B-112-233-TEV RUNX2 Q13950 ELVRTDSPNFLCSVL VTVDGPREPRRHRQK 122 HR7324A-53-189-TEV RUNX3 Q13761 QAAVGPGGRARPEVR TQVATYHRAIKVTVD 137 HR4643B-135-200-TEV RXRA P19793 CAICGDRSSGKHYGV RCQYCRYQKCLAMGM 66 HR8407C-205-270-NHT RXRB P28702 CAICGDRSSGKHYGV RCQYCRYQKCLATGM 66 HR8407C-205-270-TEV RXRB P28702 CAICGDRSSGKHYGV RCQYCRYQKCLATGM 66 HR47518-139-204-TEV RXRG P48443 CAICGDRSSGKHYGV RCQYCRYQKCLVMGM 66 HR7653A-909-977-NHT SALL2 Q9Y467 SRKACEVCGQAFPSQ HHQVQPFAPHGPQNI 69 HR7433A-975-1045-NHT SALL3 Q9BXA9 PSTVCGVCGKPFACK ELPSQLFDPNFALGP 71 HR6875A-376-433-Av6HT SALL4 Q9UJQ4 MEAALYKHKCKYCSK FTTKGNLKVHFHRHP 59 HR6875A-376-433-NHT SALL4 Q9UJQ4 EAALYKHKCKYCSKV FTTKGNLKVHFHRHP 58
HR4435B-174-250-14 SATB1 Q01826 MPKLEDLPPEQWSHT FGRWYKHFKKTKDMM 78 HR4435B-174-254-14 SATB1 Q01826 MPKLEDLPPEQWSHT YKHFKKTKDMMVEMD 82 HR4435B-179-244-14 SATB1 Q01826 MLPPEQWSHTTVRNA AAKCQEFGRWYKHFK 67 HR4435B-179-250-14 SATB1 Q01826 MLPPEQWSHTTVRNA FGRWYKHFKKTKDMM 73 HR4435C-368-452-TEV SATB1 Q01826 NTEVSSEIYQWVRDE AERDRIYQDERERSL 85 HR4435D-53-254-15 SATB1 Q01826 MQGVPLKHSGHLMKT YKHFKKTKDMMVEMD 203 HR4435D-56-250-15 SATB1 Q01826 MPLKHSGHLMKTNLR FGRWYKHFKKTKDMM 196 HR4435E-53-178-15 SATB1 Q01826 MQGVPLKHSGHLMKT VTLKIQLHSCPKLED 127 HR4435E-56-175-15 SATB1 Q01826 MPLKHSGHLMKTNLR YHVVTLKIQLHSCPK 121 HR7571A-350-437-NHT SATB2 Q9UPW6 KPEPTNSSVEVSPDI NLPEVERDRIYQDER 88 HR7571B-610-674-TEV SATB2 Q9UPW6 SCAKKPRSRTKISLE IKFFQNQRYHVKHHG 65 HR5092A-90-156-Av6HT SCAPER Q9BY12 RHPRKIDLRARYWAF DFKALIDWIQLQEKL 67 HR8394A-256-325-Av6HT SCRT1 Q9BWW7 AFSRPWLLQGHMRSH KSFALKSYLNKHYES 70 HR7196A-158-206-NHT SCRT2 Q9NQ03 ACAECCKTYATSSNL GKAYVSMPALAMHLL 51 HR3583D-653-871-15 SETDB1 Q15047 MLFLEMFCLDPYVLV LDHIESVENFKEGYE 220 HR3583D-658-867-15 SETDB1 Q15047 MFCLDPYVLVDRKFQ YFANLDHIESVENFK 211 HR3583E-555-676-15 SETDB1 Q15047 MLERAPAEPSYRAPM PYVLVDRKFQPYKPF 123 HR3583E-584-681-15 SETDB1 Q15047 MSRVRPMRNEQYRGK DRKFQPYKPFYYILD 99 HR3583E-590-676-15 SETDB1 Q15047 MRNEQYRGKNPLLVP PYVLVDRKFQPYKPF 88 HR8073A-102-182-Av6HT SHOX Q15266 EDVKSEDEDGQTKLK RRAKCRKQENQMHKG 81 HR6933A-647-727-Av6HT SHPRH Q149N8 NTMSPFNTSDYRFEC VSTRATLIISPSSIC 81 HR6933A-647-739-NHT SHPRH Q149N8 NTMSPFNTSDYRFEC SICHQWVDEINRHVR 93 HR6933A-654-727-Av6HT SHPRH Q149N8 TSDYRFECICGELDQ VSTRATLIISPSSIC 74 HR6933A-654-739-Av6HT SHPRH Q149N8 TSDYRFECICGELDQ SICHQWVDEINRHVR 86 HR6933B-437-502-Av6HT SHPRH Q149N8 VQCPPTRVMILTAVK KCLIFEGLVKQIKGH 66 HR6933B-437-512-Av6HT SHPRH Q149N8 VQCPPTRVMILTAVK QIKGHGFSGTFTLGK 76 HR6933C-1495-1636-Av6HT SHPRH Q149N8 KANQEEDIPVKGSHS GQTKPTIVHRFLIKA 142 HR6933C-1495-1646-Av6HT SHPRH Q149N8 KANQEEDIPVKGSHS FLIKATIEERMQAML 152 HR6933C-1495-1659-Av6HT SHPRH Q149N8 KANQEEDIPVKGSHS MLKTAERSHTNSSAK 165 HR7129A-227-345-NHT SIM1 P81133 LHSNMFMFRASLDMK VLTDTEYKGLQLSLD 119 HR8357A-72-194-Av6HT SIM2 Q14190 PLDGVAKELGSHLLQ YKVIHCSGYLKIRQY 123 HR7143A-205-281-Av6HT SIX3 Q95343 DGEQKTHCFKERTRS KNRLQHQAIGPSGMR 77 HR7095A-206-294-Av6HT SIX4 Q9UIU6 DKYRLRRKFPLPRTI NPSETQSKSESDGNP 89 HR8072A-125-195-Av6HT SIX6 O95475 IWDGEQKTHCFKERT QRDRAAAAKNRLQQQ 71 HR4810B-218-313-TEV SKI P12755 VRVYHECFGKCKGLL RLGRCLDDVKEKFDY 96 HR8491B-28-132-Av6HT SKOR2 Q2VWA4 QPRPGHANLKPNQVG ITKREAERLCKSFLG 105 HR8491C-141-237-Av6HT SKOR2 Q2VWA4 DNFAFDVSHECAWGC ELVFAWEDVKAMFNG 97 HR8491C-141-244-Av6HT SKOR2 Q2VWA4 DNFAFDVSHECAWGC DVKAMFNGGSRKRAL 104 HR8491D-43-132-Av6HT SKOR2 Q2VWA4 QVILYGIPIVSLVID ITKREAERLCKSFLG 90 HR7664A-124-217-TEV SLC30A9 Q6PML9 KYTQNNFITGVRAIN ERLFRNQKILREYRD 94 HR8337A-9-132-TEV SMAD1 Q15797 FTSPAVKRLLGWKQG KEVCINPYHYKRVES 124 HR8337B-248-465-TEV SMAD1 Q15797 APPLPSEINRGDVQA LTQMGSPHNPISSVS 218 HR4670B-55-191-14 SMAD2 Q15796 MTGRLDELEKAITTQ PVLVPRHTEILTELP 138 HR4670B-55-196-14 SMAD2 Q15796 MTGRLDELEKAITTQ RHTEILTELPPLDDY 143 HR4670B-55-202-14 SMAD2 Q15796 MTGRLDELEKAITTQ TELPPLDDYTHSIPE 149 HR4670B-55-202-Av6HT SMAD2 Q15796 TGRLDELEKAITTQN TELPPLDDYTHSIPE 148 HR4670B-55-202-TEV SMAD2 Q15796 TGRLDELEKAITTQN TELPPLDDYTHSIPE 148 HR4670B-6-173-14 SMAD2 Q15796 MPFTPPVVKRLLGWK EVCVNPYHYQRVETP 169 HR4670C-101-173-14 SMAD2 Q15796 MLYSFSEQTRSLDGR EVCVNPYHYQRVETP 74 HR4670C-106-173-14 SMAD2 Q15796 MEQTRSLDGRLQVSH EVCVNPYHYQRVETP 69 HR4670D-261-456-Av6HT SMAD2 Q15796 LDLQPVTYSEPAFWC LNGPLQWLDKVLTQM 196 HR4503B-1-148-14 SMAD4 Q13485 MDNMSITNTPTSNDA ERVVSPGIDLSGLTL 148 HR4503B-1-166-14 SMAD4 Q13485 MDNMSITNTPTSNDA APSSMMVKDEYVHDF 166 HR4503B-10-160-14 SMAD4 Q13485 MPTSNDACLSIVHSL LTLQSNAPSSMMVKD 152 HR4503B-11-142-14 SMAD4 Q13485 MTSNDACLSIVHSLM VNPYHYERVVSPGID 133 HR4503C-9-149-14 SMAD4 Q13485 MTPTSNDACLSIVHS RVVSPGIDLSGLTLQ 142 HR4503D-314-552-Av6HT SMAD4 Q13485 ISNHPAPEYWCSIAY EVLHTMPIADPQPLD 239 HR5565A-14 SMAD6 O43541 MRLSPRDEYKPLDLS TSCPCWLEILLNNPR 217 HR5560A-14 SMAD7 O15105 MVPSSAETGGTNYLA ISSCPCWLEVIFNSR 199 HR5560A-15 SMAD7 O15105 MVPSSAETGGTNYLA ISSCPCWLEVIFNSR 199 HR7626A-769-932-TEV SMARCA1 P28370 VSEPKIPKAPRPPKQ QIERGEARIQRRISI 164 HR7914A-754-917-TEV SMARCA5 O60264 VSEPKAPKAPRPPKQ QIERGEARIQRRISI 164 HR7256A-607-680-TEV SMARCC1 Q92922 SKKTLAKSKGASAGR IEDPYLENSDASLGP 74 HR7400B-419-526-Av6HT SMARCC2 Q8TAQ2 EQTHHIIIPSYAAWF YQVDAESRPTPMGPP 108 HR7400B-419-538-Av6HT SMARCC2 Q8TAQ2 EQTHHIIIPSYAAWF GPPPTSHFHVLADTP 120 HR7400B-421-520-Av6HT SMARCC2 Q8TAQ2 THHIIIPSYAAWFDY QWGLINYQVDAESRP 100 HR7400C-421-514-Av6HT SMARCC2 Q8TAQ2 THHIIIPSYAAWFDY VHAFLEQWGLINYQV 94 HR7811A-46-134-NHT SMARCE1 Q969G3 GTNSRVTASSGITIP EAEKIEYNESMKAYH 89 HR7811A-46-146-Av6HT SMARCE1 Q969G3 GTNSRVTASSGITIP AYHNSPAYLAYINAK 101 HR7520-1-242-15 SNAI1 O95863 MPRSELVRKPSDPNR QTHSDVKKYQCQACA 242 HR7520-1-247-15 SNAI1 O95863 MPRSFLVRKPSDPNR VKKYQCQACARTFSR 247 HR7520-1-253-TEV SNAI1 O95863 PRSFLVRKPSDPNRK QACARTFSRMSLLHK 252 HR7520-15 SNAI1 O95863 MPRSFLVRKPSDPNR LHKHQESGCSGCPR* 265 HR7520-34-264-Av6HT SNAI1 O95863 PYDQAHLLAAIPPPE LLHKHQESGCSGCPR 231 HR7520-40-264-15 SNAI1 O95863 MLLAAIPPPEILNPT LLHKHQESGCSGCPR 226 HR7520-45-264-15 SNAI1 O95863 MPPPEILNPTASLPM LLHKHQESGCSGCPR 221 HR7012A-122-179-NHT SNAI2 O43623 AIEAEKFQCNLCNKT DKEYVSLGALKMHIR 58 HR7849A-212-292-NHT SNAI3 Q3KNW1 KICGKAFSRPWLLQG SLLARHEESGCCPGP 81 HR7971A-346-397-Av6HT SNAPC4 Q5SXM2 RKEWTEEEDRMLTQL DSMQLIYRWTKSLDP 52 HR7549A-165-287-NHT SOHLH2 Q9NX45 EHLGYFPTDLFACSE RFCKKQQTPIELSLP 123 HR7723A-49-131-TEV SOX1 O00570 DRVKRPMNAFMVWSR DYKYRPRRKTKTLLK 83 HR7246A-102-183-15 SOX10 P56693 MPHVKRPMNAFMVWA PDYKYQPRRRKNGKA 83 HR7246A-109-178-15 SOX10 P56693 MNAFMVWAQAARRKL HKKDHPDYKYQPRRR 71 HR7246A-127-178-15 SOX10 P56693 MPHLHNAELSKTLGK HKKDHPDYKYQPRRR 53 HR7246A-91-179-15 SOX10 P56693 MPVRVNGASKSKPHV KKDHPDYKYQPRRRK 90 HR7180A-31-110-Av6HT SOX12 O15370 GWCKTPSGHIKRPMN LRLKHMADYPDYKYR 80 HR7313A-421-500-TEV SOX13 Q9UN79 SSHIKRPMNAFMVWA EKYPDYKYKPRPKRT 80 HR7773A-2-88-TEV SOX14 O95416 SKPSDHIKRPMNAFM DYKYRPRRKPKNLLK 87 HR7489A-83-161-TEV SOX18 P35713 SRIRRPMNAFMVWAK RDHPNYKYRPRRKKQ 79 HR8317A-38-121-TEV SOX2 P48431 PDRVKRPMNAFMVWS DYKYRPRRKTKTLMK 84 HR8041A-6-88-TEV SOX21 Q9Y651 DHVKRPMNAFMVWSR DYKYRPRRKPKTLLK 83 HR7838A-132-219-TEV SOX3 P41225 GGTDQDRVKRPMNAF DYKYRPRRKTKTLLK 88 HR8424A-45-130-Av6HT SOX4 Q06945 KADDPSWCKTPSGHI RLKHMADYPDYKYRP 86 HR7351A-554-632-TEV SOX5 P35711 PHIKRPMNAFMVWAK EKYPDYKYKPRPKRT 79 HR7953A-619-697-TEV SOX6 P35712 HNSNISKILGSRWKS YKQLMRSRRQEMRQF 78 HR7275A-43-121-TEV SOX7 Q9BT81 SRIRRPMNAFMVWAK QDYPNYKYRPRRKKQ 79 HR7103A-102-174-Av6HT SOX8 P57073 VKRPMNAFMVWAQAA VQHKKDHPDYKYQPR 73 HR7103A-63-143-Av6HT SOX8 P57073 RFPACIRDAVSQVLK NAELSKTLGKLWRLL 81 HR7103A-63-150-Av6HT SOX8 P57073 RFPACIRDAVSQVLK LGKLWRLLSESEKRP 88 HR7103A-63-173-NHT SOX8 P57073 RFPACIRDAVSQVLK RVQHKKDHPDYKYQP 111 HR7103A-82-143-Av6HT SOX8 P57073 SLVPMPVRGGGGGAL NAELSKTLGKLWRLL 62 HR7103A-82-150-Av6HT SOX8 P57073 SLVPMPVRGGGGGAL LGKLWRLLSESEKRP 69 HR7103A-82-173-Av6HT SOX8 P57073 SLVPMPVRGGGGGAL RVQHKKDHPDYKYQP 92 HR6433-64-495-15 SOX9 P48436 SEEDKFPVCIREAVS ADTSGVPSIPQTHSP 432 HR6433-64-509-14 SOX9 P48436 SEEDKFPVCIREAVS PQHWEQPVYTQLTRP 446 HR6433-68-490-15 SOX9 P48436 KFPVCIREAVSQVLK MYTPIADTSGVPSIP 423 HR6433-68-509-14 SOX9 P48436 KFPVCIREAVSQVLK PQHWEQPVYTQLTRP 442 HR4634C-496-558-14 SP1 P08047 MSSSNTTLTPIASAA VHPIQGLPLAIANAP 64 HR4744B-38-153-14 SP100 P23497 MFTEDQGVDDRLLYD HIYKGFENVIHDKLP 117 HR4744B-42-149-14 SP100 P23497 MQGVDDRLLYDIVFK PDLIHIYKGFENVIH 109 HR4744B-70-135-14 SP100 P23497 MKTFPFLEGLRDRDL VLEALFSDVNMQEYP 67 HR4744B-70-149-14 SP100 P23497 MKTFPFLEGLRDRDL PDLIHIYKGFENVIH 81 HR4744C-595-684-TEV SP100 P23497 DENINFKQSELPVTC MENKFLPEPPSTRKK 90 HR7625A-677-739-NHT SP140 Q13342 SQNNSSVDPCMRNLD RTPWNCIFCRMKESP 63 HR7855A-523-581-Av6HT SP2 Q02086 KKHVCHIPDCGKTFR TRSDELQRHARTHTG 59 HR8154A-336-382-Av6HT SP5 Q6BEB4 SFTRSDELQRHLRTH SDHLAKHVKTHQNKK 47 HR7866A-256-329-Av6HT SP6 Q3SY56 CHIPGCGKAYAKTSH PCAVCSRVFMRSDHL 74 HR7872A-292-352-Av6HT SP7 Q8TDD2 PIHSCHIPGCGKVYG SDELERHVRTHTREK 61 HR7447A-131-213-TEV SPDEF O95238 LKDIETACKLLNITA GDVLHAHLDIWKSAA 83 HR8383A-169-257-NHT SPI1 P17947 KKIRLYQFLLDLLRS KKVKKKLTYQFSGEV 89 HR4679B-130-262-14 SPIB Q01892 MEEEDLPLDSPALEV TYQFDSALLPAVRRA 134 HR4679B-134-262-14 SPIB Q01892 MLPLDSPALEVSDSE TYQFDSALLPAVRRA 130 HR4679B-163-262-14 SPIB Q01892 MAGTRKKLRLYQFLL TYQFDSALLPAVRRA 101 HR4679B-168-262-14 SPIB Q01892 MKLRLYQFLLGLLTR TYQFDSALLPAVRRA 96 HR7954A-111-207-Av6HT SPIC Q8N5J4 LRLFEYLHESLYNPE FSEAILQRLSPSYFL 97
HR7260A-815-910-NHT SRCAP Q6ZRS2 IEGSQEYNEGLVKRL QLRKVCNHPNLFDPR 96 HR7260B-583-850-Av6HT SRCAP Q6ZRS2 EITDIAAAAESLQPK FLLRRVKVDVEKQMP 268 HR7260B-597-840-Av6HT SRCAP Q6ZRS2 KGYTLATTQVKTPIP VKRLHKVLRPFLLRR 244 HR7260B-601-850-Av6HT SRCAP Q6ZRS2 LATTQVKTPIPLLLR FLLRRVKVDVEKQMP 250 HR7260B-607-840-Av6HT SRCAP Q6ZRS2 KTPIPLLLRGQLREY VKRLHKVLRPFLLRR 234 HR7260B-607-850-Av6HT SRCAP Q6ZRS2 KTPIPLLLRGQLREY FLLRRVKVDVEKQMP 244 HR4448F-14 SREBF1 P36956 VLLFVYGEPVTRPHS VVRTSLWRQQQPPAP 432 HR4448G-521-624-14 SREBF1 P36956 MVYHSPGRNVLGTES RALGRPLPTSHLDLA 105 HR4448G-521-643-14 SREBF1 P36956 MVYHSPGRNVLGTES WNLIRHLLQRLWVGR 124 HR4448G-526-619-14 SREBF1 P36956 MGRNVLGTESRDGPG LWLALRALGRPLPTS 95 HR4448G-526-638-14 SREBF1 P36956 MGRNVLGTESRDGPG ACSLLWNLIRHLLQR 114 HR4448G-530-624-14 SREBF1 P36956 MLGTESRDGPGWAQW RALGRPLPTSHLDLA 96 HR4448G-530-643-14 SREBF1 P36956 MLGTESRDGPGWAQW WNLIRHLLQRLWVGR 115 HR4448G-535-619-14 SREBF1 P36956 MRDGPGWAQWLLPPV LWLALRALGRPLPTS 86 HR4448G-535-638-14 SREBF1 P36956 MRDGPGWAQWLLPPV ACSLLWNLIRHLLQR 105 HR4448H-319-400-TEV SREBF1 P36956 QSRGEKRTAHNAIEK SLRTAVHKSKSLKDL 82 HR4448H-TEV SREBF1 P36956 QSRGEKRTAHNAIEK SLRTAVHKSKSLKDL 82 HR6329A-1075-1134-14 SREBF2 Q12772 MPGQRERATAILLAC RSCNDCQQMIVKLGG 61 HR4543C-132-223-TEV SRF P11831 SGAKPGKKTRGRVKI ETGKALIQTCLNSPD 92 HR6924A-56-131-Av6HT SRY Q05066 VQDRVKRPMNAFIVW QAMHREKYPNYKYRP 76 HR6924A-56-131-TEV SRY Q05066 VQDRVKRPMNAFIVW QAMHREKYPNYKYRP 76 HR7075A-105-202-Av6HT SSB P05455 KNDVKNRSVYIKGFP YFAKKNEERKQNKVE 98 HR7075A-105-202-TEV SSB P05455 KNDVKNRSVYIKGFP YFAKKNEERKQNKVE 98 HR7013A-305-448-TEV SSH2 Q76I76 DSPTQIFEHVFLGSE SFMRQLEEYQGILLA 143 HR7844A-287-452-NHT SSH3 Q8TE77 DLESVTSKEIRQALE QALRHVQELRPIARP 166 HR3575-1-551-14 SSRP1 Q08945 MAETLEFNDVYQEVK EVKKGKDPNAPKRPM 551 HR3575-1-556-14 SSRP1 Q08945 MAETLEFNDVYQEVK KDPNAPKRPMSAYML 556 HR3575-1-573-14 SSRP1 Q08945 MAETLEFNDVYQEVK NASREKIKSDHPGIS 573 HR5522A-14 SSRP1 Q08945 MLKKAKMAKDRKSRK RDYEKAMKEYEGGRG 101 HR5522A-15 SSRP1 Q08945 MLKKAKMAKDRKSRK RDYEKAMKEYEGGRG 101 HR7020A-812-906-TEV ST18 O60284 PELKCPVIGCDGQGH GCPLNAQVIKKGKVS 95 HR8389A-136-710-Av6HT STAT1 P42224 LDKQKELDSKVRNVK PKGTGYIKTELISVS 575 HR8389A-136-710-NHT STAT1 P42224 LDKQKELDSKVRNVK PKGTGYIKTELISVS 575 HR8389A-136-710-TEV STAT1 P42224 LDKQKELDSKVRNVK PKGTGYIKTELISVS 575 HR3569D-573-771-14 STAT2 P52630 NDGRIMGFVSRSQER STLEPVIEPTLGMVS 199 HR3569E-1-131-14 STAT2 P52630 MAQWEMLQNLDSPFQ RILIQAQRAQLEQGE 131 HR3569E-1-186-14 STAT2 P52630 MAQWEMLQNLDSPFQ VFCFRYKIQAKGKTP 186 HR5539A-14 STAT2 P52630 MAQWEMLQNLDSPFQ LEEKRILIQAQRAQL 127 HR5539A-15 STAT2 P52630 MAQWEMLQNLDSPFQ LEEKRILIQAQRAQL 127 HR5535A-1-101-14 STAT3 P40763 MAQWNQLQQLDTRYL KQFLQSRYLEKPMEI 101 HR5535A-14 STAT3 P40763 MAQWNQLQQLDTRYL WEESRLLQTAATAAQ 124 HR5535B-1-116-14 STAT3 P40763 MAQWNQLQQLDTRYL ARIVARCLWEESRLL 116 HR5535B-1-133-14 STAT3 P40763 MAQWNQLQQLDTRYL AATAAQQGGQANHPT 133 HR3612-187-748-14 STAT4 Q14765 MNSAMVNQEVLTLQE PTTIETAMKSPYSAE 563 HR5542A-14 STAT5A P42229 MAGWIQAQQLQGDAL NEQRLVREANNCSSP 129 HR5542A-15 STAT5A P42229 MAGWIQAQQLQGDAL NEQRLVREANNCSSP 129 HR55428-128-712-NHT STAT5A P42229 SPAGILVDAMSQKHL QIKQVVPEFVNASAD 585 HR5541A-1-102-14 STAT5B P51692 MAVWIQAQQLQGEAL GHYATQLQNTYDRCP 102 HR5541A-1-106-14 STAT5B P51692 MAVWIQAQQLQGEAL TQLQNTYDRCPMELV 106 HR5541A-14 STAT5B P51692 MAVWIQAQQLQGEAL NEQRLVREANNGSSP 129 HR5541A-15 STAT5B P51692 MAVWIQAQQLQGEAL NEQRLVREANNGSSP 129 HR5541B-1-127-14 STAT5B P51692 MAVWIQAQQLQGEAL LYNEQRLVREANNGS 127 HR5541B-1-135-14 STAT5B P51692 MAVWIQAQQLQGEAL REANNGSSPAGSLAD 135 HR5541C-1-684-TEV STAT5B P51692 AVWIQAQQLQGEALH FPDRPKDEVYSKYYT 683 HR3396C-1-127-14 STAT6 P42226 MSLWGLVSKMPPEKV QFRHLPMPFHWKQEE 127 HR3396C-1-169-14 STAT6 P42226 MSLWGLVSKMPPEKV AEAGQVSLHSLIETP 169 HR3396C-1-174-14 STAT6 P42226 MSLWGLVSKMPPEKV VSLHSLIETPANGTG 174 HR3396D-72-655-14 STAT6 P42226 GEGSTILQHISTLES YVPATIKMTVERDQP 584 HR3396E-90-279-14 STAT6 P42226 RDPLKLVATFRQILQ LRTLVTSCFLVEKQP 190 HR3396E-90-327-14 STAT6 P42226 RDPLKLVATFRQILQ ADMVTEKQARELSVP 238 HR3396F-1-630-TEV STAT6 P42226 SLWGLVSKMPPEKVQ YPKKPKDEAFRSHYK 629 HR7864A-355-443-TEV TADA2A O75478 SNSGRRSAPPLNLTG KIYDFLIREGYITKG 89 HR8503A-244-333-Av6HT TADA2B B3KX99 KEDGKDSEFAAIENL LNSLTESGWISRDAS 90 HR4753B-177-253-14 TAL1 P17542 MEITDGPHTKVVRRI LAKLLNDQEEEGTQR 78 HR4753B-177-280-14 TAL1 P17542 MEITDGPHTKVVRRI GGGGGGGGGAPPDDL 105 HR4753B-182-247-14 TAL1 P17542 MPHTKVVRRIFTNSR MKYINFLAKLLNDQE 67 HR4753B-182-262-14 TAL1 P17542 MPHTKVVRRIFTNSR EEGTQRAKTGKDPVV 82 HR4753B-182-262-Av6HT TAL1 P17542 PHTKVVRRIFTNSRE EEGTQRAKTGKDPVV 81 HR4753B-182-262-TEV TAL1 P17542 PHTKVVRRIFTNSRE EEGTQRAKTGKDPVV 81 HR4753B-182-287-14 TAL1 P17542 MPHTKVVRRIFTNSR GGAPPDDLLQDVLSP 107 HR6460A-1-79-15 TAL2 Q16559 MTRKIFTNTRERWRQ QTGVAAQGNILGLFP 79 HR6460A-1-84-15 TAL2 Q16559 MTRKIFTNTRERWRQ AQGNILGLFPQGPHL 84 HR6460A-1-96-15 TAL2 Q16559 MTRKIFTNTRERWRQ PHLPGLEDRTLLENY 96 HR6460A-34-96-15 TAL2 Q16559 PDKKLSKNETLRLAM PHLPGLEDRTLLENY 63 HR464-14 TAX1BP1 Q86VP1 MTSFQEVPLQTSNFA NSDMLVVTTKAGLLE 151 HR464-21 TAX1BP1 Q86VP1 MTSFQEVPLQTSNFA NSDMLVVTTKAGLLE 151 HR7030-1-512-TEV TAX1BP1 Q86VP1 TSFQEVPLQTSNFAH TSASTVDVKPSPSAA 511 HR7030-1-529-TEV TAX1BP1 Q86VP1 TSFQEVPLQTSNFAH DFDIVTKGQVCEMTK 528 HR7030-1-588-TEV TAX1BP1 Q86VP1 TSFQEVPLQTSNFAH ENVKLELAEVQDNYK 587 HR7030-1-597-TEV TAX1BP1 Q86VP1 TSFQEVPLQTSNFAH VQDNYKELKRSLENP 596 HR7030A-15-466-TEV TAX1BP1 Q86VP1 AHVIFQNVAKSYLPN KFKECQRLQKQINKL 452 HR7030A-15-470-TEV TAX1BP1 Q86VP1 AHVIFQNVAKSYLPN CQRLQKQINKLSDQS 456 HR8311A-205-394-Av6HT TBR1 Q16650 QVYLCNRPLWLKFHR LKIDHNPFAKGFRDN 190 HR8240A-138-330-Av6HT TBX18 O95935 APRVDLQGAELWKRF RLKIDRNPFAKGFRD 193 HR7379A-91-283-TEV TBX2 Q13207 SLKSLEPEDEVEDDP DKITQLKIDNNPFAK 193 HR7868A-127-326-Av6HT TBX21 Q9UL17 LPAGLEVSGKLRVAL QLKIDNNPFAKGFRE 200 HR7452A-91-277-NHT TBX22 Q9Y458 DIQMELQGSELWKRF QNQQITKLKIERNPF 187 HR7369A-99-311-TEV TBX3 O15119 VEDDPKVHLEAKELW LTLQSMRVFDERHKK 213 HR7232A-61-248-Av6HT TBX4 P57082 EQTIENIKVGLHEKE KITQLKIENNPFAKG 188 HR8313A-52-232-Av6HT TBX5 Q99593 EGIKVFLHERELWLK QNHKITQLKIENNPF 181 HR7389A-90-273-NHT TBX6 O95947 GVSLSLENRELWKEF QLKIAANPFAKGFRE 184 HR6334A-578-637-TEV TCF12 Q99081 RRMANNARERLRVRD AVAVILSLEQQVRER 60 HR6965A-1-144-Av6HT TCF19 Q9Y242 MLPCFQLLRIGGGRG DFAAITIPRSRGEAR 144 HR6965A-1-144-NHT TCF19 Q9Y242 LPCFQLLRIGGGRGG DFAAITIPRSRGEAR 143 HR8141A-73-167-Av6HT TCF21 O43680 SQEGKQVQRNAANAR PFMVAGKPESDLKEV 95 HR7160A-75-162-NHT TCF23 Q7RTU1 SEASPENAARERSRV LRYLHPLKKWPMRSR 88 HR7366A-178-588-Av6HT TCF25 Q9BQ70 LYVEHRHLNPDTELK DVTTQSVMGFDPLPP 411 HR4404E-550-609-TEV TCF3 P15923 RRVANNARERLRVRD AVSVILNLEQQVRER 60 HR4645C-565-624-TEV TCF4 P15884 RRMANNARERLRVRD AVAVILSLEQQVRER 60 HR8064A-27-105-TEV TEAD1 P28347 IDNDAEGVWSPDIEQ SSHIQVLARRKSRDF 79 HR7830A-40-115-TEV TEAD2 Q15562 DAEGVWSPDIEQSFD SSHIQVLARRKSREI 76 HR7697A-27-104-TEV TEAD3 Q99594 LDNDAEGVWSPDIEQ VSSHIQVLARKKVRE 78 HR6976A-217-434-TEV TEAD4 Q15561 RSVASSKLWMLEFSA SEHGAQHHIYRLVKE 218 HR7931A-446-500-Av6HT TERF2 Q15554 KKQKWTVEESEWVKA MIKDRWRTMKRLGMN 55 HR7931A-446-500-TEV TERF2 Q15554 KKQKWTVEESEWVKA MIKDRWRTMKRLGMN 55 HR7939A-132-190-Av6HT TERF2IP Q9NYB0 GRIAFTDADDVAILT SWQSLKDRYLKHLRG 59 HR7939A-132-190-TEV TERF2IP Q9NYB0 GRIAFTDADDVAILT SWQSLKDRYLKHLRG 59 HR8166A-153-218-TEV TFAM Q00059 GKPKRPRSAYNVYVA AKEDETRYHNEMKSW 66 HR3078B-202-418-14 TFAP2A P05549 GGVVNPNEVFCSVPG EALKAMDKMYLSNNP 217 HR3078B-207-414-14 TFAP2A P05549 PNEVFCSVPGRLSLL NYLTEALKAMDKMYL 208 HR3162-15 TFAP2B Q92481 MHSPPRDQAAIMLWK GPGSKTGDKEEKHRK 460 HR7501-139-450-15 TFAP2C Q92754 MRRDAYRRSDLLLPH ADSNKTLEKMEKHRK 313 HR7501A-206-427-TEV TFAP2C Q92754 NLPCQKELVGAVMNP QNYIKEALIVIDKSY 222 HR7501A-219-427-TEV TFAP2C Q92754 NPTEVFCSVPGRLSL QNYIKEALIVIDKSY 209 HR7501B-128-430-Av6HT TFAP2C Q92754 LSGLEAGAVSARRDA IKEALIVIDKSYMNP 303 HR7501B-128-450-Av6HT TFAP2C Q92754 LSGLEAGAVSARRDA ADSNKTLEKMEKHRK 323 HR7501B-139-430-TEV TFAP2C Q92754 RRDAYRRSDLLLPHA IKEALIVIDKSYMNP 292 HR7501B-206-450-TEV TFAP2C Q92754 NLPCQKELVGAVMNP ADSNKTLEKMEKHRK 245 HR7501B-219-450-TEV TFAP2C Q92754 NPTEVFCSVPGRLSL ADSNKTLEKMEKHRK 232 HR7272A-212-422-Av6HT TFAP2E Q6VUC0 TNPGEVFCSVPGRLS YLLESLKGLDKMFLS 211 HR7122A-21-122-Av6HT TFAP4 Q01664 EKEVIGGLCSLANIP QQNTQLKRFIQELSG 102 HR7110A-303-394-15 TFCP2 Q12800 MLGEGNGSPNHQPEP ALKGRMVRPRLTIYV 93 HR7110A-303-400-15 TFCP2 Q12800 MLGEGNGSPNHQPEP VRPRLTIYVCQESLQ 99 HR7110A-303-404-15 TFCP2 Q12800 MLGEGNGSPNHQPEP LTIYVCQESLQLREQ 103 HR7110A-332-395-15 TFCP2 Q12800 MEAQQWLHRNRFSTF LKGRMVRPRLTIYVC 65 HR7110A-332-399-15 TFCP2 Q12800 MEAQQWLHRNRFSTF MVRPRLTIYVCQESL 69
HR7110A-332-404-15 TFCP2 Q12800 MEAQQWLHRNRFSTF LTIYVCQESLQEREQ 74 HR7022A-278-385-NHT TFCP2L1 Q9NZI6 SPNSFGLGEGNASPT MTIYVCQELEQNRVP 108 HR4671B-105-200-TEV TFDP1 Q14186 RNRKGEKNGKGLRHF KKEIKWIGLPTNSAQ 96 HR7048A-121-215-TEV TFDP2 Q14188 RRRVYDALNVLMAMN QNQGPPALNSTIQLP 95 HR7261A-244-347-NHT TFDP3 Q5H9I0 QRPLPNSVIHVPFII AQGTFGGVFTTAGSR 104 HR4665C-333-388-14 TFE3 P19532 MISETEAKALLKERQ PKSSDPEMRWNKGTI 57 HR4665C-333-443-14 TFE3 P19532 MISETEAKALLKERQ ELELQAQIHGLPVPP 112 HR4665C-338-383-14 TFE3 P19532 MAKALLKERQKKDNH LGTLIPKSSDPEMRW 47 HR665C-338-438-14 TFE3 P19532 MAKALLKERQKKDNH QLRIQELELQAQIHG 102 HR7480A-223-309-NHT TFEB P19484 TDAESRALAKERQKK SRELENHSRRLEMTN 87 HR4411B-151-237-14 TGIF1 Q15583 MDIPLDLSSSAGSGK LPDMLRKDGKDPNQF 88 HR4411B-170-232-14 TGIF1 Q15583 MNLPKESVQILRDWL ARRRLLPDMLRKDGK 64 HR4411B-170-252-14 TGIF1 Q15583 MNLPKESVQILRDWL TISRRGAKISETSSV 84 HR4411B-171-248-14 TGIF1 Q15583 MLPKESVQILRDWLY PNQFTISRRGAKISE 79 HR4411B-189-248-14 TGIF1 Q15583 MNAYPSEQEKALLSQ PNQFTISRRGAKISE 61 HR4411C-171-241-14 TGIF1 Q15583 MLPKESVQILRDWLY LRKDGKDPNQFTISR 72 HR4393-12-199-15 TGIF2 Q9GZN2 LLSLAGKRKRRGNLP PTPPEQDKEDFSSFQ 188 HR4393-12-223-15 TGIF2 Q9GZN2 LLSLAGKRKRRGNLP AAEMELQKQQDPSLP 212 HR4393-17-220-15 TGIF2 Q9GZN2 GKRKRRGNLPKESVK LQRAAEMELQKQQDP 204 HR4393-6-199-15 TGIF2 Q9GZN2 LGEDEGLLSLAGKRK PTPPEQDKEDFSSFQ 194 HR4393-6-223-15 TGIF2 Q9GZN2 LGEDEGLLSLAGKRK AAEMELQKQQDPSLP 218 HR7881A-51-127-TEV TGIF2LX Q8IUE1 KKRKGNLPAESVKIL LQQRRNDPIIGHKTG 77 HR8232A-51-127-NHT TGIF2LY Q8IUE0 KKRKGNLPAESVKIL LQQRRNDPIIGHKTG 77 HR8232A-51-127-TEV TGIF2LY Q8IUE0 KKRKGNLPAESVKIL LQQRRNDPIIGHKTG 77 HR7047A-1-87-TEV THAP1 Q9NVV9 VQSCSAYGCKNRYDK KENAVPTIFLCTEPH 86 HR1517A-1-91-Av6HT THAP10 Q9P2Z0 PARCVAAHCGNTTKS QRLRLVAGAVPTLHR 90 HR7799A-1-67-TEV THAP11 Q96EK4 PGFTCCVPGCYNNSH QPTTGHRLCSVHFQG 66 HR7799A-1-72-TEV THAP11 Q96EK4 PGFTCCVPGCYNNSH HRLCSVHFQGGRKTY 71 HR7799A-1-82-Av6HT THAP11 Q96EK4 PGFTCCVPGCYNNSH GRKTYTVRVPTIFPL 81 HR8301A-1-87-TEV THAP2 Q9H0W7 PTNCAAAGCATTYNK MDAVPTIFDFCTHIK 86 HR7028A-1-83-NHT THAP5 Q7Z6K1 PRYCAAICCKNRRGR RWGIRYLKQTAVPTI 82 HR8415A-1-149-Av6HT THAP6 Q8TBB0 VKCCSAIGCASRCLP QFIFEHSYSVMDSPK 148 HR7818A-1-92-Av6HT THAP7 Q9BT49 PRHCSAAGCCTRDTR ISGYHRLKEGAVPTI 91 HR6978A-1-82-15 THAP8 Q8NA92 MPKYCRAPNCSNTAG QWRWGVRYLRPDAVP 82 HR6978A-1-87-15 THAP8 Q8NA92 MPKYCRAPNCSNTAG VRYLRPDAVPSIFSR 87 HR6978A-16-87-15 THAP8 Q8NA92 MRLGADNRPVSFYKF VRYLRPDAVPSIFSR 73 HR6978A-21-82-15 THAP8 Q8NA92 MNRPVSFYKFPLKDG QWRWGVRYLRPDAVP 63 HR7271A-1-88-NHT THAP9 Q9H5L6 TRSCSAVGCSTRDTV YGIRRKLKKGAVPSV 87 HR7130A-202-461-15 THRB P10828 MEELQKSIGHKPEPT PTELFPPLFLEVFED 261 HR7130A-202-461-Av6HT THRB P10828 EELQKSIGHKPEPTD PTELFPPLFLEVFED 260 HR7130A-202-461-TEV THRB P10828 EELQKSIGHKPEPTD PTELFPPLFLEVFED 260 HR7130B-104-206-15 THRB P10828 MDELCVVCGDKATGY IEENREKRRREELQK 104 HR7130B-104-206-Av6HT THRB P10828 DELCVVCGDKATGYH IEENREKRRREELQK 103 HR7130B-104-206-TEV THRB P10828 DELCVVCGDKATGYH IEENREKRRREELQK 103 HR6921A-74-139-NHT TIGD3 Q6B0B8 SKYSGIDEALLCWYH VRWKRRNNVGFGARH 66 HR7457A-14-77-NHT TIGD4 Q8IY51 TVKKKKSLSIEEKID VLEAFESLRFDPKRK 64 HR7206A-68-132-NHT TIGD6 Q17RP2 KRMRSALYDDIDKAV QASVGWLNRFRDRHG 65 HR7729A-62-136-NHT TIGD7 Q6NT04 PLVGAEKRKRTTGAK STGWLFRFRNRHAIG 75 HR7316A-298-418-NHT TIPARP Q7Z3E1 NDRMRMKYGGQEFWA LFRSCFILLPYLQTL 121 HR7535A-206-260-TEV TLX1 P31314 TSFTRLQICELEKRF KTWFQNRRTKWRRQT 55 HR6480A-162-216-TEV TLX2 O43763 TSFSRSQVLELERRF KTWFQNRRTKWRRQT 55 HR7241A-171-225-TEV TLX3 O43711 TSFSRVQICELEKRF KTWFQNRRTKWRRQT 55 HR3551B-1-370-TEV TNFAIP3 P21580 AEQVLPQALYLSNMR WQENSEQGRREGHAQ 369 HR8218A-34-319-Av6HT TOE1 Q96GM8 VPVVDVQSNNFKEMW AYGWCPLGPQCPQSH 286 HR5174A-643-706-NHT TOP3A Q13472 QQEDIYPAMPEPIRK PDSVLEASRDSSVCP 64 HR8243A-244-339-NHT TOX O94900 GKKPKTPKKKKKKDP LAAYRASLVSKSYSE 96 HR8243A-244-339-TEV TOX O94900 GKKPKTPKKKKKKDP LAAYRASLVSKSYSE 96 HR7258A-238-302-TEV TOX2 Q96NM4 VASMWDSLGEEQKQA STQANPPAKMLPPKQ 65 HR7680A-238-335-TEV TOX3 O15405 GKKPKTPKKKKKKDP AYRASLVSKAAAESA 98 HR7250A-206-291-TEV TOX4 O94842 GKKQKAPKKRKKKDP EAAKKEYLKALAAYK 86 HR6989-94-312-15-TEV TP53 P04637 MLSSSVPSQKTYQGS ELPPGSTKRALPNNT 221 HR6989-94-312-R175H-15-TEV TP53 P04637 MLSSSVPSQKTYQGS ELPPGSTKRALPNNT 221 HR6989A-20-73-Av6HT TP53 P04637 SDLWKLLPENNVLSP GPDEAPRMPEAAPPV 54 HR6989A-20-73-TEV TP53 P04637 SDLWKLLPENNVLSP GPDEAPRMPEAAPPV 54 HR3500C-14 TP63 Q9H3D4 MDALSPSPAIPSNTD GTKRPFRQNTHGIQM 232 HR3500C-15 TP63 Q9H3D4 MDALSPSPAIPSNTD GTKRPFRQNTHGIQM 232 HR3500D-540-614-TEV TP63 Q9H3D4 PPPYPTDCSIVSFLA GILDHRQLHEFSSPS 75 HR3466-110-636-14 TP73 O15350 MSPAPVIPSNTDYPG RKQPIKEEFTEAEIH 528 HR3466-114-636-14 TP73 O15350 MVIPSNTDYPGPHHF RKQPIKEEFTEAEIH 524 HR3466D-14 TP73 O15350 MSPAPVIPSNTDYPG KADEDHYREQQALNE 209 HR3466D-15 TP73 O15350 MSPAPVIPSNTDYPG KADEDHYREQQALNE 209 HR3466E-487-554-TEV TP73 O15350 YHADPSLVSFLTGLG TIWRGLQDLKQGHDY 68 HR8230A-78-139-TEV TRAFD1 O14545 HEETECPLRLAVCQH VKDLKTHPEVCGREG 62 HR4455D-876-951-14 TRERF1 Q96PN7 MCHPLANYHYAGSDK LGRKHRTRLAEIIDD 77 HR4455D-881-945-14 TRERF1 Q96PN7 MNYHYAGSDKWTSLE WKKIMRLGRKHRTRL 66 HR4455E-773-1200-15 TRERF1 Q96PN7 MQTVDVEPRINIGLR LDDQDSVLLQGDAEL 429 HR4455E-778-1200-15 TRERF1 Q96PN7 MEPRINIGLRFQAEI LDDQDSVLLQGDAEL 424 HR4455F-773-841-15 TRERF1 Q96PN7 MQTVDVEPRINIGLR ENLLNLCCSSALPGG 70 HR4455F-778-836-15 TRERF1 Q96PN7 MEPRINIGLRFQAEI LQQRVENLLNLCCSS 60 HR7441A-22-107-NHT TRIM23 P36406 GTAVVKVLECGVCED FALLELLERLQNGPI 86 HR7486A-13-98-NHT TRIM3 O75382 QPMDKQFLVCSICLD SLMEAMQQAPDGAHD 86 HR7466A-4-90-TEV TRIM32 Q13049 AAASHLNLDALREVL LTDNLTVLKIIDTAG 87 HR5056A-48-428-Av6HT TRIT1 Q9H3H1 GGEIVSADSMQVYEG IKSKSHLNQLKKRRR 381 HR7683A-316-386-15 TSC22D4 Q9Y3Q8 MVGIDNKIEQAMDLV EQLAQLPSSGVPRLG 72 HR7683A-320-381-15 TSC22D4 Q9Y3Q8 MNKIEQAMDLVKSHL ALASPEQLAQLPSSG 63 HR7683A-320-395-Av6HT TSC22D4 Q9Y3Q8 NKIEQAMDLVKSHLM GVPRLGPPAPNGPSV 76 HR8019A-232-335-TEV TSHZ1 Q6ZSZ6 DKDSEKTKRWSKPRK EPAGMAAEVALSESA 104 HR7516A-824-951-NHT TSHZ2 Q9NRE2 DVRRFEDVSSEVSTL TPSTYISHLESHLGF 128 HR6901A-200-303-TEV TSHZ3 Q63HK5 SSKLYGSIFTGASKF DLSVHMIKTKHYQKV 104 HR7321A-615-661-Av6HT TTF1 Q15361 NYKGRYSEGDTEKLK ARSSLSVALKFSQIS 47 HR7321A-615-669-Av6HT TTF1 Q15361 NYKGRYSEGDTEKLK LKFSQISSQRNRGAW 55 HR7321A-615-678-Av6HT TTF1 Q15361 NYKGRYSEGDTEKLK RNRGAWSKSETRKLI 64 HR7321A-615-687-Av6HT TTF1 Q15361 NYKGRYSEGDTEKLK ETRKLIKAVEEVILK 73 HR7321A-615-697-NHT TTF1 Q15361 NYKGRYSEGDTEKLK EVILKKMSPQELKEV 83 HR8382A-244-506-NHT TUB P50607 GISSSMSFDEDEEDE QSYVLNFHGRVTQAS 263 HR8277A-291-536-TEV TULP1 O00294 PREFVLRPAPQGRTV LCALQAFAIALSSFD 246 HR7732A-263-520-Av6HT TULP2 O00295 SPCPGLEEDMEAYVL FSPLQAFSICLSSFN 258 HR6409A-100-175-14 TWIST1 Q15672 PQSYEELQTQRVMAN QVLQSDELDSKMASC 76 HR6409A-102-171-14 TWIST1 Q15672 SYEELQTQRVMANVR DFLYQVLQSDELDSK 70 HR7529A-43-146-TEV U2AF1 Q01081 SQTIALLNIYRNPQN NRWFNGQPIHAELSP 104 HR7415B-479-562-Av6HT UBTF P17480 MEMTWNNMEKKEKLM NGELNHLPLKERMVE 85 HR7415B-479-562-TEV UBTF P17480 EMTWNNMEKKEKLMW NGELNHLPLKERMVE 84 HR8089A-89-170-Av6HT UNCX A6NJT0 KLSDSGDPDKESPGC RRAKWRKKENTKKGP 82 HR7768A-197-260-TEV USF1 P22415 DEKRRAQHNEVERRR KACDYIQELRQSNHR 64 HR6458A-220-346-15 USF2 Q15853 PYSPKIDGTRTPRDE LQQHNLEMVGEGTRQ 127 HR6458A-226-279-15 USF2 Q15853 DGTRTPRDERRRAQH CNADNSKTGASKGGI 54 HR6458A-226-346-15 USF2 Q15853 DGTRTPRDERRRAQH LQQHNLEMVGEGTRQ 121 HR6458B-226-330-15 USF2 Q15853 DGTRTPRDERRRAQH QQIEELKNENALLRA 105 HR6458B-231-325-15 USF2 Q15853 PRDERRRAQHNEVER NELLRQQIEELKNEN 95 HR8005-106-565-15 USP39 Q53GS9 MPYLDTINRSVLDFD IWKRRDNDETNQQGA 461 HR8005A-189-554-15 USP39 Q53GS9 MITYVLKPTFTKQQI QMITLSEAYIQIWKR 367 HR8005A-189-565-15 USP39 Q53GS9 MITYVLKPTFTKQQI IWKRRDNDETNQQGA 378 HR8005A-194-549-15 USP39 Q53GS9 MKPTFTKQQIANLDK TDILPQMITLSEAYI 357 HR8005A-194-555-15 USP39 Q53GS9 MKPTFTKQQIANLDK MITLSEAYIQIWKRR 363 HR8005B-210-555-15 USP39 Q53GS9 MKLSRAYDGTTYLPG MITLSEAYIQIWKRR 347 HR8005C-106-183-TEV USP39 Q53GS9 PYLDTINRSVLDFDF TLKFYCLPDNYEIID 78 HR8005C-129-183-TEV USP39 Q53GS9 SHINAYACLVCGKYF TLKFYCLPDNYEIID 55 HR6997A-81-167-NHT VAX1 Q5SQQ9 DAKGSIREIILPKGL TKQKKDQGKDSELRS 87 HR8032A-81-165-Av6HT VAX2 Q9UIW0 VRDAKGTIREIVLPK QNRRTKQKKDQSRDL 85 HR7564A-21-427-Av6HT VDR P11473 PRICGVCGDRATGFH KLTPLVLEVFGNEIS 407 HR7564A-21-427-TEV VDR P11473 PRICGVCGDRATGFH KLTPLVLEVFGNEIS 407 HR7564B-16-125-15 VDR P11473 MFDRNVPRICGVCGD KEEEALKDSLRPKLS 111 HR7564B-16-125-Av6HT VDR P11473 FDRNVPRICGVCGDR KEEEALKDSLRPKLS 110 HR7564B-16-125-TEV VDR P11473 FDRNVPRICGVCGDR KEEEALKDSLRPKLS 110 HR7703A-97-158-Av6HT VENTX O95231 AFTMEQVRTLEGVFQ MKHKRQMQDPQLHSP 62
HR7928A-148-214-Av6HT VSX1 Q9NZR4 EDRNDLKASPTLGKR KTELPEDRIQVWFQN 67 HR8065A-153-211-Av6HT VSX2 P58304 TIFTSYQLEELEKAF QNRRAKWRKREKCWG 59 HR7106A-20-70-TEV VTN P04004 DQESCKGRCTEGFNV AECKPQVTRGDVFTM 51 HR7713A-1017-1084-TEV WDHD1 O75717 RPKTGFQMWLEENRS KGETASEGTEAKKRK 68 HR7541A-815-891-NHT WHSC1 O96028 AHFTARKGKRHHAHV KLHFQDIIWVKLGNY 77 HR8130A-318-438-TEV WT1 P19544 HSTGYESDNHTTPII HQRRHTGVKPFQCKT 121 HR3172-15 XBP1 P17861 MVVVAAAPNPADGTP CQWGRHQPSWKPLMN 261 HR8228A-98-208-TEV XPA P23025 EFDYVICEECGKEFM WGSQEALEEAKEVRQ 110 HR8027A-52-129-TEV YBX1 P67809 KKVIATKVLGTVKWF EGEKGAEAANVTGPG 78 HR7254A-87-164-TEV YBX2 Q9Y2T7 KPVLAIQVLGTVKWF EGEKGAEATNVTGPG 78 HR7538A-193-335-NHT YEATS2 Q9ULM3 RNADLTDETSRLFVK AETVVDVELHRHSLG 143 HR8298A-14-147-15 YEATS4 O95619 MGRVKGVTIVKPIVY TVVSEFYDEMIFQDP 135 HR8137A-293-414-TEV YY1 P25490 PRTIACPHKGCTKMF LKSHILTHAKAKNNQ 122 HR8207A-251-371-TEV YY2 O15391 PKTVPCSYSGCEKMF NLKTHILTHVKTKNN 121 HR7328A-23-83-TEV ZBED1 O96006 SKVWKYFGFDTNAEG KNHPEEFCEFVKSNT 61 HR7606A-49-119-TEV ZBED2 Q9BTP6 NKGTRFSEAWFYFHL MHREELEKSGHGQAG 71 HR8174A-11-69-15 ZBP1 Q9H171 MEGHLEQRILQVLTE ELKVSLTSPATWCLG 60 HR8174A-26-69-15 ZBP1 Q9H171 MGSPVKLAQLVKECQ ELKVSLTSPATWCLG 45 HR8174A-6-74-15 ZBP1 Q9H171 MADPGREGHLEQRIL LTSPATWCLGGTDPE 70 HR7903A-21-117-Av6HT ZBTB1 Q9Y2K1 GFLCDCCIAIDDIYF YLQLYNVPDCLEDIQ 97 HR6940A-194-334-NHT ZBTB11 O95625 PKHCQAVLKQLNEQR KKGEVQTVASTQDLR 141 HR6940B-764-842-Av6HT ZBTB11 O95625 RGYHCTQCEKSFFEA GKEFYEKALFRRHVK 79 HR4454-14 ZBTB12 Q9Y330 MASGVEVLRFQLPGH NVLEASVAEINVLIR 459 HR4454C-1-132-14 ZBTB12 Q9Y330 MASGVEVLRFQLPGH KCRNALSQFIEPKIG 132 HR4454C-1-137-14 ZBTB12 Q9Y330 MASGVEVLRFQLPGH LSQFIEPKIGLKEDG 137 HR4454C-1-151-14 ZBTB12 Q9Y330 MASGVEVLRFQLPGH GVSEASLVSSISATK 151 HR4454D-328-447-14 ZBTB12 Q9Y330 MNPLKNIKCTKCPEV HLKEQHGKTTAENVL 121 HR4454E-361-421-14 ZBTB12 Q9Y330 MCPRCGKQFNHSSNI NLHSGARPYRCSYCD 62 HR4454E-366-418-14 ZBTB12 Q9Y330 MKQFNHSSNLNRHMN DHLNLHSGARPYRCS 54 HR3471-145-673-15 ZBTB16 Q05516 MEEDRKARYLKNIFI PDWRIEKTYLYLCYV 530 HR3471-150-673-15 ZBTB16 Q05516 MARYLKNIFISKHSS PDWRIEKTYLYLCYV 525 HR3471-189-673-15 ZBTB16 Q05516 MTSFGLSAMSPTKAA PDWRIEKTYLYLCYV 486 HR3471-194-673-15 ZBTB16 Q05516 MSAMSPTKAAVDSLM PDWRIEKTYLYLCYV 481 HR4581F-2-115-TEV ZBTB17 Q03105 DFPQHSQHVLEQLNQ MQDIITACHALKSLA 114 HR7182A-1-107-15 ZBTB2 Q8N680 MDLANHGLILLQQLN VRLEQGIKFLHAYPL 107 HR7182A-1-113-15 ZBTB2 Q8N680 MDLANHGLILLQQLN IKFLHAYPLIQEASL 113 HR7182B-1-117-15 ZBTB2 Q8N680 MDLANHGLILLQQLN HAYPLIQEASLASQG 117 HR7182B-1-147-15 ZBTB2 Q8N680 MDLANHGLILLQQLN YGIQIADHQLRQATK 147 HR7182B-1-152-15 ZBTB2 Q8N680 MDLANHGLILLQQLN ADHQLRQATKIASAP 152 HR7182C-227-390-15 ZBTB2 Q8N680 MSDEQPASLTIAHVK SHWREHMYIHTGKPF 165 HR7182C-232-385-15 ZBTB2 Q8N680 MASLTIAHVKPSIMK KFIQKSHWREHMYIH 155 HR7182C-245-390-15 ZBTB2 Q8N680 MKRNGSFPKYYACHL SHWREHMYIHTGKPF 147 HR7182C-248-385-15 ZBTB2 Q8N680 MGSFPKYYACHLCGR KFIQKSHWREHMYIH 139 HR7182C-252-385-15 ZBTB2 Q8N680 MKYYACHLCGRRFTL KFIQKSHWREHMYIH 135 HR8336A-81-201-Av6HT ZBTB20 Q9HC78 INLHNFSNSVLETLN DECTRIVSQNVGDVF 121 HR7741A-30-151-NHT Z8T822 O15209 AVVHVSFPEVTSALL WHIVDKCTELLREGR 122 HR7877A-1-114-15 ZBTB25 P24278 MDTASHSLVLLQQLN RFLHADYLSHIATEM 114 HR7877A-1-120-15 ZBTB25 P24278 MDTASHSLVLLQQLN YLSHIATEMNQVFSP 120 HR7877A-1-138-15 ZBTB25 P24278 MDTASHSLVLLQQLN QSSNLYGIQISTTQK 138 HR7877A-1-144-15 ZBTB25 P24278 MDTASHSLVLLQQLN GIQISTTQKTVVKQG 144 HR7877B-231-376-15 ZBTB25 P24278 MTENSVKIHLCHYCG SQLLEHMYTHKGKSY 147 HR7877B-236-373-15 ZBTB25 P24278 MKIHLCHYCGERFDS PRKSQLLEHMYTHKG 139 HR7877B-244-373-15 ZBTB25 P24278 MGERFDSRSNLRQHL PRKSQLLEHMYTHKG 131 HR7877B-261-373-15 ZBTB25 P24278 MVSGSLPFGVPASII PRKSQLLEHMYTHKG 114 HR7877B-270-376-15 ZBTB25 P24278 MPASILESNDLGEVH SQLLEHMYTHKGKSY 108 HR7877B-275-373-15 ZBTB25 P24278 MESNDLGEVHPLNEN PRKSQLLEHMYTHKG 100 HR7422A-1-129-NHT ZBTB26 Q9HCK0 SERSDLLHFKFENYG IVERCTQALWKFIKP 128 HR6960A-46-179-NHT ZBTB3 Q9H5J0 PSWGTMEFPEHSQQL CKRRLQARALAEADS 134 HR7977A-1-110-Av6HT ZBTB32 Q9Y2Y4 SLPPIRLPSPYGSDR AARALGVQSLEEACW 109 HR7008A-1-116-TEV ZBTB33 Q86T24 ESRKLISATDIQYSG IKSGQLLGVKFIAEL 115 HR6893A-1-124-NHT ZBTB34 Q8NCN2 DSSSFIQFDVPEYSS QMQCVIDKCTQILES 123 HR7018A-1-125-NHT ZBTB37 Q5TC79 EKGGNIQLEIPDFSN MQHIIDKCTQILEGI 124 HR7837A-13-139-NHT ZBTB38 Q8NAP3 DFHSDTVLSILNEQR RNFSNSPGPYVFCIT 127 HR7896A-1-125-Av6HT ZBTB39 O15060 GMRIKLQSTNHPNNL MEDLLQACHSTFPDL 124 HR7527A-1-112-NHT ZBTB40 Q9NUA8 ELPNYSRQLLQQLYT DSLQMFDVAVSCKNL 111 HR8293A-24-183-Av6HT ZBTB41 Q5SVQ8 EGNVAVECDQVTYTH DAVKLLNNENVAPFH 160 HR7772A-367-467-TEV ZBTB43 O43298 SATDKLYPCQCGKSF SYEAAKAEQNTTEAN 101 HR7772B-1-126-15 ZBTB43 O43298 MEPGTNSFRVEFPDF MWHVVDKCTEVLEGN 126 HR7772B-1-137-15 ZBTB43 O43298 MEPGTNSFRVEFPDF LEGNPTVLCQKLNHG 137 HR7772C-10-126-Av6HT ZBTB43 O43298 VEFPDFSSTILQKLN MWHVVDKCTEVLEGN 117 HR7772C-10-131-Av6HT ZBTB43 O43298 VEFPDFSSTILQKLN DKCTEVLEGNPTVLC 122 HR7772C-29-131-15 ZBTB43 O43298 MQGQLCDVSIVVQGH DKCTEVLEGNPTVLC 104 HR7772C-29-137-15 ZBTB43 O43298 MQGQLCDVSIVVQGH LEGNPTVLCQKLNHG 110 HR7772C-33-126-15 ZBTB43 O43298 MCDVSIVVQGHIFRA MWHVVDKCTEVLEGN 95 HR7772C-33-131-15 ZBTB43 O43298 MCDVSIVVQGHIFRA DKCTEVLEGNPTVLC 100 HR8333A-1-128-15 ZBTB44 Q8NCP5 MGVKTFTHSSSSHSQ FSVASTCSEFMKSSI 128 HR8333A-1-133-15 ZBTB44 Q8NCP5 MGVKTFTHSSSSHSQ TCSEFMKSSILWNTP 133 HR7817A-6-125-NHT ZBTB45 Q96K62 AVHHIHLQNFSRSLL IQTVIDECTQIIARA 120 HR7445A-291-405-NHT ZBTB48 P10074 VECPTCHKKFLSKYY KDLQSHMIKLHGAPK 115 HR7445A-291-405-TEV ZBTB48 P10074 VECPTCHKKFLSKYY KDLQSHMIKLHGAPK 115 HR7445B-4-120-TEV ZBTB48 P10074 SFVQHSVRVLQELNK EAVELCQSFKPKTSV 117 HR7910A-1-125-Av6HT ZBTB49 Q6ZSB9 DPVATHSCHLLQQLH SLCHTFLKSATVVQP 124 HR7620A-1-125-NHT ZBTB5 O15062 DFPGHFEQIFQQLNY VVKACKHYLTTRTLP 124 HR8300A-1-134-Av6HT ZBTB6 Q15916 AAESDVLHFQFEQQG TEALSKYLEIDLSMK 133 HR4695C-9-128-TEV ZBTB7A O95365 IGIPFPDHSSDILSG LEIPAVSHVCADLLD 120 HR8347A-1-143-Av6HT ZBTB7B O15156 GSPEDDLIGIPFPDH EIPCVIAACMEILQG 142 HR7365A-6-129-NHT ZBTB7C A1YPR0 DELIGIPFPNHSSEV EIQCIVNVCLEIMEP 124 HR8095A-1-121-Av6HT ZBTB8A Q96BR9 EISSHQSHLLQQLNE MTDVISVCKTFIKSS 120 HR7919A-1-131-Av6HT ZBTB8B Q8NAP8 EMQSYYAKLLGELNE YIRSSLDICRKMEKE 130 HR7153A-20-140-NHT ZBTB9 Q96C00 PRTIQIEFPQHSSSL QMMQVVDQCSEILRE 121 HR6996-34-350-Av6HT ZC3H15 Q8WU90 KKGAKQQKFIKAVTH LYIPRDVDETGITVA 317 HR7121A-417-503-TEV ZC3H4 Q9UPT8 ELPKKRELCKFYITG GAEDEKEVEELKKQG 87 HR7136A-892-947-TEV ZC3H7A Q8IWR0 QYCWQHRFPTGYFSI WEERRDALKMKLNKA 56 HR6981A-218-283-NHT ZC3H8 Q8N5P1 EIEKKKEMCKFYVQG APLTPETQELLAKVI 66 HR8421A-724-896-TEV ZC3HAV1 Q7Z2W4 SSKKYKLSEIHHLHP KDQVYPQYVIEYTED 173 HR4840-1-454-14 ZCCHC4 Q9H5U6 MAASRNGFEAVEAEG GPKHGCFICGELDHK 454 HR4840-1-459-14 ZCCHC4 Q9H5U6 MAASRNGFEAVEAEG CFICGELDHKRSTCP 459 HR4840-102-513-14 ZCCHC4 Q9H5U6 MLSRTQCVERYLKFI RRKKRRERAHQYLGS 413 HR4840-41-454-14 ZCCHC4 Q9H5U6 MPHGPTLLFVKVTQG GPKHGCFICGELDHK 415 HR4840-41-459-14 ZCCHC4 Q9H5U6 MPHGPTLLFVKVTQG CFICGELDHKRSTCP 420 HR4840-41-513-14 ZCCHC4 Q9H5U6 MPHGPTLLFVKVTQG RRKKRRERAHQYLGS 474 HR7192A-927-1161-Av6HT ZCCHC6 Q5VYS8 SLKEENVCEEKNSPV LCYTMKVFTKMCDIG 235 HR4656C-161-443-14 ZEB1 P37275 MGTPDAFSQLLTCPY LENNQANLASKEQET 284 HR4656C-165-439-14 ZEB1 P37275 MAFSQLLTCPYCDRG IRQVLENNQANLASK 276 HR4656D-885-1017-14 ZEB1 P37275 MNDSDSTPPKKKMRK PEILSNEHVGARASP 134 HR4656D-885-992-14 ZEB1 P37275 MNDSDSTPPKKKMRK HMNHRYSYCKREAEE 109 HR4656D-890-1014-14 ZEB1 P37275 MTPPKKKMRKTENGM EAGPEILSNEHVGAR 126 HR4656D-890-987-14 ZEB1 P37275 MTPPKKKMRKTENGM GSYSQHMNHRYSYCK 99 HR4656D-897-1014-14 ZEB1 P37275 MRKTENGMYACDLCD EAGPEILSNEHVGAR 119 HR4656D-897-987-14 ZEB1 P37275 MRKTENGMYACDLCD GSYSQHMNHRYSYCK 92 HR4656E-583-642-TEV ZEB1 P37275 SPSQPPLKNLLSLLK WFEKMQAGQISVQSS 60 HR4589D-647-707-Av6HT ZEB2 O60315 MSPINPYKDHMSVLK EQRKVYQYSNSRSPS 62 HR4589D-647-707-TEV ZEB2 O60315 SPINPYKDHMSVLKA EQRKVYQYSNSRSPS 61 HR6404A-128-196-TEV ZFAND5 O76080 SPSVSQPSTSQSEEK DKHNCPYDYKAEAAA 69 HR8363-123-190-Av6HT ZFAND6 Q6FIF0 SVSDTAQQPSEEQSK SDVHNCSYNYKADAA 68 HR7859A-697-770-TEV ZFHX2 Q9C0A1 LGSSSDSLPTSPPPD DTHRCKLCCYGTQLK 74 HR7545B-1398-1452-Av6HT ZFHX3 Q15911 VYKYRCNQCSLAFKT FRTFQALKKHLETSH 55 HR7545B-1398-1483-Av6HT ZFHX3 Q15911 VYKYRCNQCSLAFKT ANGDLLAMGDPTLAE 86 HR7545C-2943-2998-Av6HT ZFHX3 Q15911 RPGQKRFRTQMTNLQ GLPKRVVQVWFQNAR 56 HR7545C-2943-3010-Av6HT ZFHX3 Q15911 RPGQKRFRTQMTNLQ NARAKEKKSKLSMAK 68 HR7545C-2954-3005-Av6HT ZFHX3 Q15911 TNLQLKVLKSCFNDY QVWFQNARAKEKKSK 52 HR7545C-2954-3010-Av6HT ZFHX3 Q15911 TNLQLKVLKSCFNDY NARAKEKKSKLSMAK 57 HR7573D-2953-3003-TEV ZFHX4 Q86UP3 TPTMQECEMLGNEIG INIGKPFMINQGGTE 51 HR8105A-360-407-Av6HT ZFP1 Q6P2D0 TFSQRSTLRLHCRIH SRLSVHQRVHIGEKP 48 HR7592A-1509-1806-Av6HT ZFP106 Q9H2Y7 SSEISSEPGDDDEPT MIYTGCYDGSIQAVR 298
HR7696A-237-310-NHT ZFP112 Q9UJU3 GEDIMKVSLLNQESI NYSSLLHIHQNIERE 74 HR7929A-142-207-Av6HT ZFP14 Q9HCL3 QVKITSEKMTTYKRH IHTGEKPYKCKECGQ 66 HR7835A-8-127-NHT ZFP161 O43829 SETIKYNDDDHKTLF QILGIRFLDKLCSQK 120 HR7794A-82-137-NHT ZFP2 Q6ZN57 DATQNSELIKTQRMF IHTGEKPYKCNVCGK 56 HR7491A-224-296-NHT ZFP28 Q8NHY6 LFETQPGLVTIKNLA QEKEPWWVKRELTGS 73 HR7037A-395-483-Av6HT ZFP3 Q96NJ6 CFECGKAFRRTSHLI RIHTGEKPYECQECQ 89 HR7776A-306-378-NHT ZFP30 Q9Y2G7 AFLCSTGLRLHHKLH SRGYHLTLHQRIHTG 73 HR4743B-99-171-TEV ZFP36 P26651 TTPSRYKTELCRTFS PYGSRCHFIHNPSED 73 HR7685A-112-181-TEV ZFP36L1 Q07352 SSRYKTELCRPFEEN CPYGPRCHFIHNAEE 70 HR7167A-151-220-TEV ZFP36L2 P47974 STRYKTELCRPFEES CPYGPRCHFIHNADE 70 HR7105A-571-623-NHT ZFP37 Q9Y6Q3 KPYECNECEKAFNAK TFKQNASLTKHVKTH 53 HR7063A-83-140-NHT ZFP41 Q8N8Y5 RKKPYECSECGRIFK HSSDVTKHQRTHTGE 58 HR7124A-165-241-NHT ZFP42 Q96MM3 PKQLAEFARKKPPIN VESSKLKRHFLVHTG 77 HR7554A-339-397-TEV ZFP64 Q9NPA5 SEHPEKCSECSYSCS PSNLSKHMKKFHGDM 59 HR7612A-318-373-Av6HT ZFP82 Q8N141 AFLCGSGLRVHHKLH THTGFKPYECKECGK 56 HR7734A-483-537-NHT ZFP90 Q8TF47 AFSQQAISHPGEKPY KPYECNECGEAFSRR 55 HR7784A-364-427-15 ZFP91 Q96JP5 MKHHTDQRDYICEYC ASLNWHMKKHDADSF 65 HR7784A-370-422-15 ZFP91 Q96JP5 MRDYICEYCARAFKS TCRQKASLNWHMKKH 54 HR7784A-370-456-15 ZFP91 Q96JP5 MRDYICEYCARAFKS KDSVVAHKAKSHPEV 88 HR7784B-313-454-Av6HT ZFP91 Q96JP5 CEMEGCGTVLAHPRY EKKDSVVAHKAKSHP 142 HR7665A-150-204-NHT ZFP92 A6NM28 KRYLCQQCGKAFSRS RRSFALLEHQRIHSG 55 HR7876A-297-383-Av6HT ZFPM1 Q8IX07 CRKSCPSASSLEIHM TNHMVCQPGSKGEIY 87 HR7512A-673-743-NHT ZFX P17010 RPSELKKHVAAHKGK RQQSELKKHMKTHSG 71 HR8053A-728-784-Av6HT ZFYVE20 Q9H1K0 PEAEEPIEEELLLQQ RELKHTLAKQKGGTD 57 HR8053B-133-256-15 ZFYVE20 Q9H1K0 MAFDRTNTESAKIRA DEKDDDRIRCCTHCK 125 HR8053B-133-276-15 ZFYVE20 Q9H1K0 MAFDRTNTESAKIRA REQQIDEKEHTPDIV 145 HR8053B-139-251-15 ZFYVE20 Q9H1K0 MTESAKIRAIEKSVV VSSVLDEKDDDRIRC 114 HR8053B-139-273-15 ZFYVE20 Q9H1K0 MTESAKIRAIEKSVV LLKREQQIDEKEHTP 136 HR8053B-150-251-15 ZFYVE20 Q9H1K0 MSVVPWVNDQDVPFC VSSVLDEKDDDRIRC 103 HR8053B-150-273-15 ZFYVE20 Q9H1K0 MSVVPWVNDQDVPFC LLKREQQIDEKEHTP 125 HR7907A-60-153-TEV ZHX1 Q9UKY1 NQQNKKVEGGYECKY NNQTIFEQTINQLTF 94 HR7907B-565-641-TEV ZHX1 Q9UKY1 PDFTPQKFKEKTAEQ EEKMEIDESNAGSSK 77 HR7907C-295-358-Av6HT ZHX1 Q9UKY1 NNPLLLNTYNKFPYP TPEEVEEARRKQFNG 64 HR7907D-768-820-Av6HT ZHX1 Q9UKY1 NWDRGPSLIKFKTGT KSHMGYEQVREWFAE 53 HR7907D-768-830-Av6HT ZHX1 Q9UKY1 NWDRGPSLIKFKTGT EWFAERQRRSELGIE 63 HR7907E-658-720-Av6HT ZHX1 Q9UKY1 SGSTGKICKKTPEQL SWFGDTRYAWKNGNL 63 HR7907E-658-728-Av6HT ZHX1 Q9UKY1 SGSTGKICKKTPEQL AWKNGNLKWYYYYQS 71 HR7907F-462-532-Av6HT ZHX1 Q9UKY1 PDSFGIRAKKTKEQL NQRNSKSNQCLHLNN 71 HR7907F-468-513-Av6HT ZHX1 Q9UKY1 RAKKTKEQLAELKVS KITGLTKGEIKKWFS 46 HR7907F-468-532-Av6HT ZHX1 Q9UKY1 RAKKTKEQLAELKVS NQRNSKSNQCLHLNN 65 HR8292A-524-605-NHT ZHX2 Q9Y6X8 AYPDFAPQKFKEKTQ VLDSMGSGKKGQDVG 82 HR8292A-524-605-TEV ZHX2 Q9Y6X8 AYPDFAPQKFKEKTQ VLDSMGSGKKGQDVG 82 HR7743A-613-675-TEV ZHX3 Q9H4I2 PTKYKERAPEQLRAL SERRKKVNAEETKKA 63 HR7728A-219-303-TEV ZIC1 Q15915 QPIKQELICKWIEPE LVNHIRVHTGEKPFP 85 HR7748A-250-334-TEV ZIC2 O95409 QCIKQELICKWIDPE LVNHIRVHTGEKPFP 85 HR8404A-122-262-NHT ZIC4 Q8N9L1 QPIKQELICKWLAAD SSDRKKHSHVHTSDK 141 HR8404A-122-262-TEV ZIC4 Q8N9L1 QPIKQELICKWLAAD SSDRKKHSHVHTSDK 141 HR7356A-417-487-NHT ZIK1 Q3SY52 SQSSILIQHRRIHTG SQCSSLIHHQKCHNT 71 HR7796A-474-527-NHT ZIM2 Q9NZV7 VFSRNSYLIQHYRTH YQLHSQAEKTVECDHC 54 HR8306A-277-331-Av6HT ZIM3 Q96PE6 KSYQCNECEKSFRQN IYKSDLVKHQRIHTG 55 HR8102A-61-140-Av6HT ZKSCAN1 P17029 RFCYQNTFGPREALS RAVTLLEDLELDLSG 80 HR8296A-7-131-Av6HT ZKSCAN2 Q63HK3 SQIDAPLEVEGCLIM VALVVHLEKETGRLR 125 HR7446A-37-132-NHT ZKSCAN3 Q9BRR0 SPDLGSEGSRERFRG VVLLEYLERQLDERA 96 HR7362A-49-138-NHT ZKSCAN4 Q969J2 PERSRQRFRGFRYPE VVVLLEYLERQLDEP 90 HR7407A-25-130-NHT ZKSCAN5 Q9Y2L8 LFIVKVEEEDCTWMQ ESGEEAVAVIENIQR 106 HR7288A-62-140-NHT ZMAT2 Q96NC0 LGKTIVITKTTPQSE FEVNKKKMEEKQKDY 79 HR7490-503-839-Av6HT ZMIZ1 Q9ULI6 PVANYPHSPVPGNPT VPIKSDLHIKDDPDG 337 HR7144A-212-289-NHT ZNF10 P21506 SNECGQTFCQNIHLI SWRSNLTRHQLIHTG 78 HR8024A-409-481-Av6HT ZNF100 Q8IYN0 GFNWSSALTKHKRIH NRSSQLTAHKMIHTG 73 HR8250A-364-426-Av6HT ZNF101 Q8IZC7 KPYECTRCGKAFGWC HERTHLAGRSQCFGR 63 HR7096A-555-620-15 ZNF107 Q9UII5 MEEHGKVFNQSSNLT KPHKCEECGKAYNRF 67 HR7096A-560-615-15 ZNF107 Q9UII5 MVFNQSSNLTTQKII IYTGEKPHKCEECGK 57 HR7096B-607-719-Av6HT ZNF107 Q9UII5 PHKCEECGKAYNRFS SNLTTHKKIHTSEKP 113 HR7096C-611-676-15 ZNF107 Q9UII5 MEECGKAYNRFSNLT KPYKCKECGKAFNLS 67 HR7096C-616-671-15 ZNF107 Q9UII5 MAYNRFSNLTIHKRI IHTGEKPYKCKECGK 57 HR7096D-53-131-15 ZNF107 Q9UII5 MECTGHKGGHNTVNQ SQLTQHRRIHTRVNS 80 HR7096D-58-126-15 ZNF107 Q9UII5 MKGGHNTVNQCLTAT SFCVLSQLTQHRRIH 70 HR7096D-69-131-15 ZNF107 Q9UII5 MTATPSKIFQCNKYV SQLTQHRRIHTRVNS 64 HR7096D-71-126-15 ZNF107 Q9UII5 MTPSKIFQCNKYVKV SFCVLSQLTQHRRIH 57 HR7096E-158-269-TEV ZNF107 Q9UII5 KPYKCEECGKAFNQS LFSNLTNHKRIHAGE 112 HR7096F-672-727-Av6HT ZNF107 Q9UII5 AFNLSSTLTAHKKIH IHTSEKPYKCEECGK 56 HR7096G-659-772-Av6HT ZNF107 Q9UII5 TGEKPYKCKECGKAF NLSSNLTTHKKIHTG 114 HR7096H-702-776-Av6HT ZNF107 Q9UII5 NQSSNLTTHKKIHTS NLTTHKKIHTGEKPY 75 HR7096H-716-776-Av6HT ZNF107 Q9UII5 SEKPYKCEECGKSFN NLTTHKKIHTGEKPY 61 HR8087A-338-417-Av6HT ZNF114 Q8NC26 GKAFRYSLHLNKHLR LKKHLKTHKDEKPCE 80 HR7502A-318-390-NHT ZNF121 P58317 GKAFATSSQLIEHIR AYNRFYLLTKHLKTH 73 HR7834A-112-166-NHT ZNF132 P52740 KANSCDMCGPFLKDI WLNANLHQHQKEHSG 55 HR7007A-48-120-NHT ZNF134 P52741 TALPCDICGPILKDI LRRDKSEASIVKNCT 73 HR8079A-458-526-Av6HT ZNF136 P52737 SYLNSFRTHEMIHTG AYSCRASFQRHMLTH 69 HR8523A-1-79-Av6HT ZNF137P P52743 NVARFLVEKHTLHVI IHGVGKLCKCNDCHK 78 HR6957A-157-213-NHT ZNF140 P52738 VERPYGCHECGKTFG SQISNLVKHQMIHTG 57 HR7470A-405-474-NHT ZNF141 Q15928 RRSTDRSQHKKIHSA FKRFSHLNKHKKIHT 70 HR8111A-337-394-Av6HT ZNF143 P52747 SFTTSNIRKVHVRTH IHTGEKPYVCTVPGC 58 HR8395A-1-68-Av6HT ZNF146 Q15072 SHLSQQRIYSGENPF SQKQYVIKHQNTHTG 67 HR3636C-225-287-NHT ZNF148 Q9UQR1 KPFRCDECGMRFIQK HKRMCHENHDKKLNR 63 HR7492A-160-244-NHT ZNF154 Q13106 CYICSECGKSFSKSY SNLIKHRRVHTGERP 85 HR8099A-408-497-Av6HT ZNF155 Q12901 GFYTNSQLSSHQRSH PFKCEDCGKRLVHRT 90 HR7481A-394-466-NHT ZNF157 P51786 AFYVKARLIEHQRMH YVKVRLIEHQRIHTG 73 HR7426A-245-317-NHT ZNF16 P17020 TFSQNSVLKNRHRSH SQNSSLKKHQKSHMS 73 HR7677A-461-550-Av6HT ZNF160 Q9HCG1 AFSMHSNLATHQVIH PYKCIECGKSFTQKS 90 HR8279A-12-131-Av6HT ZNF165 P49910 NSPEDEGLLIVKIEE GEEAVTILEDLERGT 120 HR7169A-37-134-NHT ZNF167 Q9P0L1 GQGSSLQKNYPPVCE ESGEEAVAVVEDFQR 98 HR7079A-232-286-NHT ZNF169 Q14929 KHHVCPECGRGFCQR SQKASLSIHQRKHSG 55 HR8144A-504-578-Av6HT ZNF17 P17021 GKSFRCRSTLDTHQR SQNSHLIRHQKVHTR 75 HR7831A-37-132-TEV ZNF174 Q15697 KNCPDPELCRQSFRR VTLVEDFHRASKKPK 96 HR7382A-565-650-NHT ZNF175 Q9Y473 GKAFTSKSQFKEHQR THMGEKPYECLDCGK 86 HR8386A-246-321-Av6HT ZNF177 Q13360 STGSYLIVHKRTHTG LIMHKRIHNGQKLHE 76 HR8047A-6-143-Av6HT ZNF18 P17022 GQALGLLPSLAKAED WISIQVLGQDILSEK 138 HR7449A-517-602-Av6HT ZNF180 Q9UIW8 GEKPFECNQCGKSFS QSYVLVVHQRTHTGE 86 HR7508A-221-306-NHT ZNF181 Q2M3W8 QGKSLTLPQTCNREK PYKCIECGKAFSHVS 86 HR7707A-292-396-NHT ZNF189 Q75820 KCKKSFSRNSLLVEH SQLCNLTRHQRIHTG 105 HR8281A-147-213-Av6HT ZNF19 P17023 IQGKVPRIPCARKPF NGNSSLIRHQRIHTG 67 HR8500A-45-132-Av6HT ZNF192 Q15776 LGQEVFRLRFRQLRY NGEEVVTLLEDLERQ 88 HR7141A-10-132-NHT ZNF193 O15535 SLGVQVPEAWEELLT ESGEEAVILLEDLER 123 HR7496A-33-148-NHT ZNF197 O14709 SSSSVWETSHLHFRQ LVKDQDTLQKVVSAP 116 HR7949A-310-387-Av6HT ZNF20 P17024 PYECKQCGKAFRCGS GKGFRCASQLQIHER 78 HR7725A-16-132-NHT NF202 O95125 EGILMVKLEDDFTCR VTLVEGLQKQPRRPR 117 HR7127A-305-371-NHT ZNF205 O95201 RKSYRCEQCGKGFSW IHTGEKPYTCPACRK 67 HR8501A-1095-1149-Av6HT ZNF208 O43345 KPYKCEECGKAFSTF SWLSVFSKHKKIHTG 55 HR7117A-425-476-NHT ZNF212 Q9UDV6 SSLICGYCGKSFSHP KSFVQKQHLLQHQKI 52 HR7052A-50-129-NHT ZNF213 O14771 QFCYGDVHGPHEAFS EAVALVEDLQKQPVK 80 HR6908A-201-269-NHT ZNF214 Q9UL59 VGVICQEDLLRDSME CFSQRSDLYRHPRNH 69 HR8316A-49-130-Av6HT ZNF215 Q9UL58 QKFRHFQYLKVSGPH SKDMVTLIEDVIEML 82 HR7041A-127-180-NHT ZNF217 O75362 EFSCEVCGQTFRVAF KEPWFLKNHMRTHNG 54 HR7381A-39-109-NHT ZNF219 Q9P2Y4 SLGMGAVSWSESRAG AQRALLRSHLRTHQP 71 HR7603A-137-200-Av6HT ZNF22 P17026 MKPYQCDECGRCFSQ MKVHKEEKPRKTRGK 65 HR7603A-137-200-NHT ZNF22 P17026 KPYQCDECGRCFSQS MKVHKEEKPRKTRGK 64 HR7848A-283-341-Av6HT ZNF222 Q9UK12 KLYKSEKYGRGFIDR YLLVHQRVHTGEKPY 59 HR8507A-136-200-Av6HT ZNF223 Q9UK11 EGLSIMHTGQKPSNC CYISALHIHQRVHLG 65 HR7500A-576-665-Av6HT ZNF225 Q9UK10 SFSRASSILNHKRLH LLQCEDCGKSIVHSS 90 HR7826A-501-553-NHT ZNF226 Q9NYT6 KPYKCNECGKSFRRN GFSQSSYLQIHQKAH 53 HR7039-1-527-TEV ZNF227 Q86WZ6 PSQNYDLPQKKQEKM VHTGEKRFKCETCGK 526 HR7039-255-799-Av6HT ZNF227 Q86WZ6 CGRGFSYSPRLPLHP SRLTYHQKVHTGKKL 545 HR7039-320-799-Av6HT ZNF227 Q86WZ6 GEKSYRCDSCGKGFS SRLTYHQKVHTGKKL 480 HR7039A-21-80-Av6HT ZNF227 Q86WZ6 EAVTFKDVAVVFSRE PFQPDMVSQLEAEEK 60 HR7249A-552-607-NHT ZNF229 Q9UJW7 SFGRSSDLHIHQRVH VHTGERPYVCDVCGK 56
HR8056A-178-248-Av6HT ZNF23 P17027 RCDSQLIQHQENNTE SYSSHYITHQTIHSG 71 HR7277A-201-287-Av6HT ZNF230 Q9UIE0 RGKEFSQSSCLQTRE IHTGEKPFKCEICGK 87 HR7779A-56-136-Av6HT ZNF232 Q9UNY5 EEEQSCEYETRLPGN LVLEQFLTILPEELQ 81 HR7083A-324-410-NHT ZNF233 A6NK53 SQGSHLQPHQRVSTG RACKCDVYDKGFSQT 87 HR7425A-606-678-Av6HT ZNF234 Q14588 SQASSLQLHQSVHTG RSNLVSHHKIHAAGT 73 HR6869A-681-738-NHT ZNF235 Q14590 KPYTCQQCGKGFSQA SHLIYHQRVHTGGNL 58 HR7932A-71-144-Av6HT ZNF236 Q9UL36 CPQTFNVEFNLTLHK FTLQSQLAVHMEEHR 74 HR7756A-1-111-NHT ZNF238 Q99592 EFPDHSRHLLQCLSE VLAAASYLHMYDIVK 110 HR7813A-381-458-NHT ZNF239 Q16600 GKGFSQSSDLRIHLR SNLHIHQRVHKKDPR 78 HR7147A-272-331-TEV ZNF24 P17028 IHSGEKPYGCVECGK SQNSGLINHQRIHTG 60 HR7147B-49-112-Av6HT ZNF24 P17028 EIFRQRFRQFGYQDS EQFVAILPKELQTWV 64 HR7147B-49-117-Av6HT ZNF24 P17028 EIFRQRFRQFGYQDS ILPKELQTWVRDHHP 69 HR7147B-49-138-Av6HT ZNF24 P17028 EIFRQRFRQFGYQDS VTVLEDLESELDDPG 90 HR7111A-518-570-Av6HT ZNF248 Q8NDW4 KPYKCNECGKTFCEK TFSQRSVLTKHQRIH 53 HR7751A-154-226-NHT ZNF25 P17030 SESKNEDLIRHQKIH YQKPHLTEHQKTHTG 73 HR6988A-235-324-NHT ZNF250 P15622 AFSQSSVLSKHRRIH PYVCPLCGKAFNHST 90 HR7737A-208-296-Av6HT ZNF253 O75346 AFNQSANLTTHKRIH KPYKCEECGKAFKHP 89 HR6990A-584-652-NHT ZNF254 O75437 NRSSTFTKHKVIHTG AFNRSSHLTTDKITH 69 HR8384A-202-266-Av6HT ZNF256 Q9Y2P7 VAFHSVKNHYNWGEC CSLSDHLRVHTSEKP 65 HR7634A-464-533-Av6HT ZNF26 P17031 PRKASLQIHQKTHSG FCWNSGLRIHRKTHK 70 HR8255A-134-188-Av6HT ZNF260 Q3ZCT1 KPYACKECGKAFNGK SQKQYLIKHQNIHTG 55 HR7211A-35-111-NHT ZNF263 O14978 PSPEASHLRFRRFRF IQSRVQELHPESGEE 77 HR6888A-230-294-Av6HT ZNF264 O43296 PYECTECGKTFIKST IHSGEKPYKCNECGK 65 HR8327A-276-365-Av6HT ZNF266 Q14584 AFTVSSCLSQHMKIH PYKCKDCGKAFTQNS 90 HR6896A-62-137-NHT ZFN268 Q14587 LEWLFISQEQPKITK QHTKPDIIFKLEQGE 76 HR8262A-158-241-Av6HT ZNF273 Q14593 VHKRGYNGLNQCLTT TATRVNFYKCKTCGK 84 HR7968A-77-161-Av6HT ZNF276 Q8N554 GHCRLCHGKFSSRSL HSLLKSFLQRVNASP 85 HR8173A-209-282-Av6HT ZNF277 Q9NRM2 NCNEFLCTLQKKLDN ELGKSWEEVQLEDDR 74 HR8391A-440-492-Av6HT ZNF280C Q8ND82 KNLLCPFCLKVSKMA QFLTSKEKAEHKAQH 53 HR7724A-288-373-NHT ZNF281 Q9Y2X9 PFQCSQCSMGFIQKY RLLKHRRTCGEVIVK 86 HR7574A-86-180-Av6HT ZNF282 Q9UDV7 REPQLPTAEISLWTV RRLENLENLLRNRNF 94 HR6982A-569-623-NHT ZNF283 Q8N7M2 KPFKCKECGKAFSWG GSGYQLSVHQRFHTG 55 HR8101A-140-217-Av6HT ZNF284 Q2VY69 IHIGETPSEHGKCKK YKCDVCSKAFSQNSQ 78 HR7778A-510-590-NHT ZNF285 Q96NJ3 KPYKCDECGKGFSRN DLLTHQRLHEQRETL 81 HR7046A-197-250-NHT ZNF286A Q9HBT8 SFNQKSVLITEDRVP TYKEKKPHKCNDCGE 54 HR7764A-1340-1400-TEV ZNF292 O60281 PEKVKKDRGRGPNGK NPRSLGGHLSKRSYC 61 HR7764B-550-593-Av6HT ZNF292 O60281 EFLGHRIVRHAQKHY NSKETFVPHVTLHVK 44 HR7764C-779-824-Av6HT ZNF292 O60281 AKCMFPKCGRIFSEA KFTGCGKVYRSQGEL 46 HR7764C-779-829-Av6HT ZNF292 O60281 AKCMFPKCGRIFSEA GKVYRSQGELEKHLD 51 HR7401A-713-806-TEV ZNF295 Q9ULJ3 ASPVENKEVYQCRLC RHQVEVHNQNNMAPT 94 HR7401B-907-958-Av6HT ZNF295 Q9ULJ3 SLWPCEKCGKMFTVH KAFRTNFRLWSHFQS 52 HR7401B-907-963-Av6HT ZNF295 Q9ULJ3 SLWPCEKCGKMFTVH NFRLWSHFQSHMSQA 57 HR7401C-1-125-Av6HT ZNF295 Q9ULJ3 EGLLHYINPAHAISL ISFLTNIVSKTPQAP 124 HR7401C-1-133-Av6HT ZNF295 Q9ULJ3 EGLLHYINPAHAISL SKTPQAPFPTCPNRK 132 HR7401D-1-110-Av6HT ZNF295 Q9ULJ3 EGLLHYINPAHAISL KSSLAAVQELGYSLG 109 HR7401D-1-114-Av6HT ZNF295 Q9ULJ3 EGLLHYINPAHAISL AAVQELGYSLGISFL 113 HR7438A-396-451-NHT ZNF296 Q8WUU4 TNSSNLTVHRRSHTG GMTPGSTRFECPHCH 56 HR7980A-567-639-Av6HT ZNF304 Q9HCX3 AYISSSHLVQHKKVH SRSSHLVRHQKAHTG 73 HR6886A-245-327-NHT ZNF311 Q5JNZ3 KLHECARCGKNFSWH NSRSALCRHKKTHSG 83 HR8348A-456-510-Av6HT ZNF319 Q9P2F9 KPLRCTLCERRFFSS KYASDLQRHRRVHTG 55 HR7649A-335-419-Av6HT ZNF320 A2RRD8 DKVFSRKSHLERHRR KLHTGEKLYECEECD 85 HR7116A-290-344-NHT ZNF322A Q6U7Q0 THTFKCLEYEKSFNC FLLGMDFVAQQKMRT 55 HR7719-1-389-Av6HT ZNF322B Q5SYY0 YTSEEKCNQRTQKRK GEKPFVCNVSEKGLE 388 HR7473A-7-125-NHT ZNF323 Q96LW9 QYDLKIVKVEEDPIW VAVVEDLEQELSEPG 119 HR7209A-240-309-NHT ZNF324 O75467 PSTWDELGEALHAGE SQTSHLTQHQRIHSG 70 HR7920A-181-255-Av6HT ZNF329 Q86UD4 ENIFTLSSSLNENQR SKNYNLIVHQRIHTG 75 HR8085A-409-463-Av6HT ZNF331 Q9NQX6 KPYGCTECGKSFSHG NHLNHLREHQRIHNS 55 HR8071A-542-633-Av6HT ZNF333 Q96JL9 VLSRLSTLKSHMRTH QCNQCEKAFRHSSSL 92 HR7926A-511-568-Av6HT ZNF334 Q9HCZ1 NTKENLYECSEHGHA CRKSALTHHQRTHTG 58 HR8140A-560-612-Av6HT ZNF335 Q9H4Z2 SSFPCPVCGRVYPMQ SFKKRYTFKMHLLTH 53 HR7962A-683-744-Av6HT ZNF337 Q9Y3M9 KPFVCQECKRGYTSK KHLKRHLREKRFCTG 62 HR7329A-327-374-NHT ZNF33B Q06732 KHFECNECGKAFWEK NQCGKTFWEKSNLTK 48 HR7973A-53-101-Av6HT ZNF343 Q6P1L6 EGKAQIVVPVTFRDV YKEVMLENYRNLLSL 49 HR7782A-408-478-NHT ZNF345 Q14585 SSGSALNRHQRIHTG GRDSEFQQHKKSHNG 71 HR8375A-52-156-Av6HT ZNF346 Q9UL40 QPVGREEVEHMIQKN TFSSPVVAQSHYLGK 105 HR7359A-200-274-NHT ZNF35 P13682 GGKYSLNSGAVKNPK IQSANLVVHQRIHTG 75 HR7521A-532-605-NHT ZNF354A O60765 GQSSALIQHRRIHTG SSLTNHYKIHIEEDP 74 HR8204A-166-238-Av6HT ZNF354B Q96LW1 NFYLKSVFIKQQRFA IHNSSLRKHQKNHTG 73 HR7986A-494-548-Av6HT ZNF354C Q86Y25 KLYKCMECGKAYSYR ICSSSLTQYQRFFKG 55 HR8492A-212-270-Av6HT ZNF355P Q9NSJ1 CKCEECGKACKQSLG IHAGEKPYNCEKCGK 59 HR7559A-189-242-NHT ZNF358 Q9NW07 SHGATLAQHRGIHTG SHSGEKPHHCPVCGK 54 HR8534A-152-309-Av6HT ZNF365 Q70YC5 DTKASFEAHVREKFN QQASGFVRDLSGHVL 158 HR7222A-233-319-NHT ZNF366 Q8N895 DVNVQIDDSYYVDVG GTRPHKCQVCHKAFT 87 HR7913A-160-248-Av6HT ZNF367 Q7RTV3 GEHSSSRIRCNICNR SRFTHANRHCPKHPY 89 HR8143A-116-153-Av6HT ZNF37A P17032 EPSEYNKNGNSFWLN IKNWEQSFEYNECGK 38 HR7024A-370-456-NHT ZNF383 Q8NA42 ECGKAFTQSSQLRQH RIHTGEKPYNCKECG 87 HR8244A-91-161-Av6HT ZNF391 Q9UJN7 KDNSDLIKHQRLFSQ SRSTHLIEHQRTHTG 71 HR7003A-55-179-NHT ZNF394 Q53GI3 AASPDPETSRLHFRQ TWEEWERLDPARRDF 125 HR7436A-17-136-NHT ZNF397 Q8NF99 PEQELILVKVEDNFS VTLLEDLEREFDDPG 120 HR6874A-291-375-Av6HT ZNF41 P51814 RIHAGEKSRECDKSN GKAFFQRSDLFRHLR 85 HR7062-124-478-15 ZNF410 Q86VK4 MLNLTRAGLGSSAEH PQELLNQGDLTERRT 356 HR7062-129-478-15 ZNF410 Q86VK4 MAGLGSSAEHLVFVQ PQELLNQGDLTERRT 351 HR7062-150-478-15 ZNF410 Q86VK4 MNDFLSSESTDSSIP PQELLNQGDLTERRT 330 HR7062-155-478-15 ZNF410 Q86VK4 MSESTDSSIPWFLRV PQELLNQGDLTERRT 325 HR7338A-520-571-NHT ZNF416 Q9BWM5 RPYDCGQCGKSFIQK KSFTQHSGLILHRKS 52 HR6922A-214-292-NHT ZNF417 Q8TAU3 CGKRTKAFSTKHSVI SRKSSLIQHQRVHTG 79 HR7936A-544-625-AV6HT ZNF418 Q8TF45 GKSFHQSSSLLRHQK RLHTRGKPYECSECG 82 HR8286A-145-234-Av6HT ZNF420 Q8TAQ5 GKAFRRASHLTQHQS EKPYKCEECGKAFIR 90 HR7298A-293-344-NHT ZNF423 Q2M1K9 ADLQCIHCPEVFVDE EQFSSVEGVYCHLDS 52 HR7298B-1204-1284-Av6HT ZNF423 Q2M1K9 NQMFDSPAKLLCHLI FQTELQNHTMSQHAQ 81 HR7298C-136-178-Av6HT ZNF423 Q2M1K9 LPYPCQFCDKSFIRL LPFKCTYCSRLFKHK 43 HR7298D-627-684-Av6HT ZNF423 Q2M1K9 ISNGEYPCNQCDLKF DFDSQESLLQHLTVH 58 HR7298E-750-803-Av6HT ZNF423 Q2M1K9 YRCTACNWDFRKEAD TFSTEVELQCHITTH 54 HR7298F-923-981-Av6HT ZNF423 Q2M1K9 AEFIKGSHKCNVCSR RFPSLLTLTEHKVTH 59 HR7298F-928-981-Av6HT ZNF423 Q2M1K9 GSHKCNVCSRTFFSE RFPSLLTLTEHKVTH 54 HR8124A-692-742-Av6HT ZNF425 Q6IV72 RPFQCPECGKGFLQK GRSFTYVGALKTHIA 51 HR7371A-502-554-NHT ZNF426 Q9BUY5 KPYECKECGKAFTCS AYSHPRSLRRHEQIH 53 HR7017A-571-643-NHT ZNF429 Q86V71 DKAFTHSSNLSSHKK AFTRSSRLTQHKKIH 73 HR8161A-229-300-Av6HT ZNF43 P17038 PYTCEECGKVFNWSS YKCKECAKAFNQSSN 72 HR8378A-511-570-Av6HT ZNF430 Q9H8G1 TSYKYLECDKAFSQS LIEQSNSYWRETLQM 60 HR6876A-159-226-NHT ZNF431 Q8TF32 EGYNELNQCLTTTQS SFCMLLHLSQHKRIH 68 HR7979A-260-324-Av6HT ZNF432 O94892 SFICSECGKVFTMKS NHTGEKSYICSECGK 65 HR7340A-616-673-NHT ZNF433 Q8N7K0 KPYKCKQCGKAFGCP SQLQVHGRAHCIDTP 58 HR7145A-271-333-NHT ZNF434 Q9NX65 SHQSFCARDKACTHI SRSSYLVRHQRIHTG 63 HR6863A-401-470-NHT ZNF436 Q9C0F3 ERSDLIKHQRTHTGE SRSSALIKHKRVHTD 70 HR6993A-498-598-NHT ZNF438 Q7Z4V0 GFSGIKKPWHRCHVC GHLKEVHRVVISTEP 101 HR7001A-19-66-NHT ZNF439 Q8NDP4 VAFKDVAVNFTQEEW FWNLTSIGKKWKDQN 48 HR7627A-522-574-NHT ZNF44 P15621 EPYECKECGKAFSSF AFSRFSYLKTHERTH 53 HR7091A-1-51-NHT ZNF440 Q8IYI8 DPVAFKDVAVNFTQE FRNLTSLGKRWKDQN 50 HR6977A-625-693-NHT ZNF441 Q8N8Z8 SHSSYLRIHERVHTG AFHCISSFHKHEMTH 69 HR7410A-570-627-NHT ZNF442 Q9H7R0 KSYECQQCGKAFTRS SSLHRHKRTHWRDTL 58 HR7294A-14-105-NHT ZNF444 Q8N0Y2 LALDSPWHRFRRFHL AVALLEELWGPAASP 92 HR8025A-61-139-Av6HT ZNF445 P59923 LRYHESSGPLETLSR EAVALLEELQRDLDG 79 HR8393A-22-126-Av6HT ZNE446 Q9NWS9 PETARLRFRGFCYQE LGWITAHVLKQEVLP 105 HR7503A-25-115-NHT ZNF449 Q6P9G9 DCEVFRQRFRQFQYR VVSLIEDLQRELEIP 91 HR7588A-340-406-Av6HT ZNF45 Q02386 SFSYSSHLNIHCRIH ECGKGFCRASNLLDH 67 HR7276A-252-326-Av6HT ZNF454 Q8N9P8 AFSVSSSLTYHQKIH RAHLTKHQNIHSGEK 75 HR7023A-306-367-NHT ZNF460 Q14592 KPFACSECGKGFYES QHERIHTGEKPFVCS 62 HR7305A-244-297-NHT ZNF461 Q8TAF7 KCNECKECWKAFVHC NYGSELTLHQRIHTG 54 HR8320A-1871-1948-TEV ZNF462 Q96JM2 SRDLKRDFIILGNGP KQKYADGAFADFKQE 78 HR8302A-424-504-15 NF467 Q7Z7K2 MAPSGERSFFCPDCG AQCGRRFSRKSHLGR 82 HR8302A-429-499-15 ZNF467 Q7Z7K2 MRSFFCPDCGRGFSH RPFACAQCGRRFSRK 72 HR8302B-485-539-15 ZNF467 Q7Z7K2 MRPFACAQCGRRFSR SSKTNLVRHQAIHTG 56 HR8302C-540-595-TEV ZNF467 Q7Z7K2 SRPFSCPQCGKSFSR AWSAPPEVAPPPLFF 56 HR8962C-551-595-TEV ZNF467 Q7Z7K2 SFSRKTHLVRHQLIH AWSAPPEVAPPPLFF 45
HR8121A-1-49-Av6HT ZNF468 Q5VIY5 ALPQGLLTFRDVAIE DVMLENYRNLVSLDI 48 HR8083A-181-258-Av6HT ZNF471 Q9BX82 TSDKKSFSKNSMVIK KQRQHLAQHHRTHTG 78 HR7760A-205-288-Av6HT ZNF473 Q8WTR7 GEKPYQCSECGKSFS FSQSTYLWHQKTHTG 84 HR8431-87-906-Av6HT ZNF474 Q6S9Z5 IPARRPGFRVCYICG RIFTSDRLLVHQRSC 220 HR6879A-445-517-NHT ZNF479 Q96JC4 AFSLSSTLTDHKRIH KWHSSLAKHKIIHTG 73 HR8210A-201-255-Av6HT ZNF480 Q8WV37 KPYECNEHSKVFRVS SRNSHLAEHCRIHTG 55 HR7266A-379-438-TEV ZNF483 Q8TF39 KRQKIHLGDRSQKCS AALNKDEGNESGEKT 60 HR7735A-326-380-NHT ZNF484 Q5JVG2 NYYKCSDYGRAPIQK PQNSNLNIHKKIHTG 55 HR7735B-1-66-Av6HT ZNF484 Q5JVG2 TKSLESVSFKDVTVD PKPEVIFSLEQEEPC 65 HR7735B-6-66-Av6HT ZNF484 Q5JVG2 ESVSFKDVTVDFSRD PKPEVIFSLEQEEPC 61 HR7735C-259-310-Av6HT ZNF484 Q5JVG2 VFSPKSHAFAHESIC GSQRVYAGICTEYEK 52 HR7735C-259-316-Av6HT ZNF484 Q5JVG2 VFSPKSHAFAHESIC AGICTEYEKDFSLKS 58 HR8114A-115-182-Av6HT ZNF485 Q8NCK3 EKGLDWEGRSSTEKN MNSSSLLNHHKVHAG 68 HR8541A-108-234-Av6HT ZNF486 Q96H40 ILRKFEKCGHGNLHF NRSSHLTTHKITHTR 127 HR7094A-456-529-NHT ZNF490 Q9ULM2 IYFSHLRRHERSHTG KSLHVHERTHSRQKP 74 HR8126A-332-407-Av6HT ZNF491 Q8N8L2 CGKAFRSAKYIRIHG TCSIYIRIHERIHTG 76 HR7429A-33-114-NHT ZNF496 Q96IT1 GELPSPESSRRLFRR SWVRAQEPESGEQAV 82 HR7643A-35-118-NHT ZNF498 Q6NSZ9 DPSPETFRLRFRQFR EHGPESGKALAAMVE 84 HR7635A-55-136-NHT ZNF500 O60304 LFCYQEVAGPREALS VVLVEGLQRKPRKHR 82 HR7681A-163-225-NHT ZNF501 Q96CX3 KCNECGKAFNQSACL THTGEKLYKCSECEK 63 HR8192A-151-240-Av6HT ZNF502 Q8TBZ5 QKKSWKCNECGKTFT LTQHQRIHTGEKPYK 90 HR7474A-5-630-TEV ZNF503 Q96F45 PSLSALRSSKHSGGG PVPVPAATGPYYSPY 626 HR7624A-141-197-NHT ZNF506 Q5JVG8 QRKIFQCDEYVKFLH NQSSTRTTYKKIDAG 57 HR7678A-639-723-NHT ZNF507 Q8TCN5 RPYRCRLCHYTSGNK KSQLRNHEREQHSLP 85 HR7670A-34-107-15 ZNF510 Q9Y2H8 MQEQQKMNISQASVS EVIFKLEQGEEPWFS 75 HR7670A-40-102-15 ZNF510 Q9Y2H8 MNISQASVSPKDVTI CCFKPEVIFKLEQGE 64 HR7670B-515-683-Av6HT ZNF510 Q9Y2H8 SFQCNQCGKTFGQKS TLSLYQKIQGEGNPY 169 HR7670B-521-652-Av6HT ZNF510 Q9Y2H8 CGKTFGQKSNLRIHQ GQKSNLRIHQRTHSG 132 HR7670B-521-683-Av6HT ZNF510 Q9Y2H8 CGKTFGQKSNLRIHQ TLSLYQKIQGEGNPY 163 HR7670C-582-683-Av6HT ZNF510 Q9Y2H8 ARTSTLRVHQRIHTG TLSLYQKIQGEGNPY 102 HR7670C-595-683-Av6HT ZNF510 Q9Y2H8 TGEKPFKCNECGKKF TLSLYQKIQGEGNPY 89 HR7670D-552-607-Av6HT ZNF510 Q9Y2H8 SFWRKDHLIQHQKTH IHTGEKPFKCNECGK 56 HR8051A-493-578-TEV ZNF512B Q96KM6 PGGPEEQWQRAIHER SAKPSDAEASEGGEQ 86 HR7686A-202-256-NHT ZNF514 Q96K75 KSCKCNECGKSFHFQ GHISSLIKHQRTHTG 55 HR7203A-240-299-NHT ZNF516 Q92618 KPELSPGEFPCEVCG FKEPWFLKNHMKAHG 60 HR8163A-367-458-Av6HT ZNF517 Q6ZMY9 PHECPVCGRPFRHNS RLHSGERPYRCRACG 92 HR6938A-228-328-NHT ZNF518A Q6AHZ1 RHNEIHYKCGKCHHV ILKRYKIGASRKTFW 101 HR8175A-141-213-Av6HT ZNF518B Q9C0D4 RFSTKDPLQYKKHTL AIRNDYIVKHTKRVH 73 HR8275A-281-327-Av6HT ZNF519 Q8TB69 GHQKIHTGEKPYKCK IHTGEKPFKCKECGK 47 HR8035A-114-170-Av6HT ZNF521 Q96K83 PGLPYPCQFCDKSFS KHKRSRDRHIKLHTG 57 HR8035B-928-981-Av6HT ZNF521 Q96K83 GNYKCNVCSRTFFSE RFPSLLTLTEHKVTH 54 HR8035C-1253-1292-Av6HT ZNF521 Q96K83 GGTFKCPVCFTVFVQ AHGQEDKIYDCTQCP 40 HR8035C-1253-1311-Av6HT ZNF521 Q96K83 GGTFKCPVCFTVFVQ FQTELQNHTMTQHSS 59 HR8035D-1177-1247-Av6HT ZNF521 Q96K83 QVSPMPRISPSQSDE TFDSPAKLQCHLIEH 71 HR8035D-1187-1247-Av6HT ZNF521 Q96K83 SQSDEKKTYQCIKCQ TFDSPAKLQCHLIEH 61 HR8035D-1193-1247-Av6HT ZNF521 Q96K83 KTYQCIKCQMVFYNE TFDSPAKLQCHLIEH 55 HR8035E-750-805-Av6HT ZNF521 Q96K83 KVYRCTSCNWDFRNE SFGTEVELQCHITTH 56 HR8035E-750-841-Av6HT ZNF521 Q96K83 KVYRCTSCNWDFRNE HLREKHCVFETKTPN 92 HR8035F-632-686-Av6HT ZNF521 Q96K83 GEYICNQCGAKYTSL EFPNQESLLKHVTIH 55 HR8035G-692-745-Av6HT ZNF521 Q96K83 TYYICESCDKQFTSV FDSKVSIQLHLAVKH 54 HR7651A-311-395-NHT ZNF526 Q8TF50 QRSFSSANRLQAHGR AHTANPLHRCRCGKT 85 HR8497A-368-478-Av6HT ZNF527 Q8NB42 SRYAFLVEHQRIHTG HTGEKPYECIKCGKF 111 HR7761A-499-570-NHT ZNF528 Q3MIS6 GKVFSRSSNLVCHQK KAFRGCSGLTAHLAI 72 HR6966A-331-386-NHT ZNF530 Q6P9A1 SFSHSTNLYRHRSAH VHTGVRPYECSECGK 56 HR7961A-840-894-Av6HT ZNF532 Q9HCE3 VGFRCVHCNVVYSDV KSAPSTHSHAYTQHP 55 HR6910A-112-178-NHT ZNF536 O15090 GIMSQMSDIEDDARK DHRAAQKGNLKIHLR 67 HR7987A-333-390-Av6HT ZNF540 Q8NDQ6 GKAFSVCGQLTRHQK THAGKKPYECKECGK 58 HR8055A-1071-1130-Av6HT ZNF541 Q9H0D2 EPHINIGSRFQAEIP TQDRVTELCNVACSS 60 HR8506A-95-170-Av6HT ZNF542 Q5EBM4 CTRNVCKECGNLYCH CNECIKTFNQRAHLT 76 HR7303A-250-315-NHT ZNF544 Q6NX49 SLNYGSSLCFHGRTF DECRETCSESLCLVQ 66 HR7708A-449-521-NHT ZNF546 Q86UE3 AFRLQTELTRHHRTH SSRYHLTQHYRIHTG 73 HR8224A-347-390-Av6HT ZNF547 Q8IVP9 TGERPYECSECGKAF AAKQCSECGKFERYN 44 HR7308A-303-359-NHT ZNF552 Q9H707 KFFRHKYHLIAHQRV VHTGQKPYECSECGK 57 HR7586A-351-442-Av6HT ZNF554 Q86TJ5 PYECQECGRAFTHSS RTHTGFKPYECSECG 92 HR6864A-534-597-NHT ZNF555 Q8NEP9 KPYECKECGKVFKWP VRIHTTEKQYKCNVG 64 HR7560A-285-337-NHT ZNF556 Q9HAH1 RPYECKQCGKAYCWA AFGWRSSLHKHARTH 53 HR7484-16-423-TEV ZNF557 Q8N988 FPASQREGHTEGGEL CGKSFTSNSYLSVHT 408 HR7484-32-423-TEV ZNF557 Q8N988 NELLKSWLKGLVTFE CGKSFTSNSYLSVHT 392 HR7484-TEV ZNF557 Q8N988 AAVVLPPTAALSSLF SYLSVHTRMHNRQM* 430 HR7484A-12-94-15 ZNF557 Q8N988 MLSSLFPASQREGHT ASLGNQVDKPRLISQ 84 HR7484A-16-89-15 ZNF557 Q8N988 MFPASQREGHTEGGE NCRNLASLGNQVDKP 75 HR7484A-32-89-15 ZNF557 Q8N988 MNELLKSWLKGLVTF NCRNLASLGNQVDKP 59 HR7484B-344-408-15 ZNF557 Q8N988 MGEKPYTCNECGKSF HMRTHTGKKPYECNY 66 HR7484B-344-423-15 ZNF557 Q8N988 MGEKPYTCNECGKSF CGKSFTSNSYLSVHT 81 HR7484B-349-403-15 ZNF557 Q8N988 MTCNECGKSFTNSFS SSVKKHMRTHTGKKP 56 HR7484B-349-423-15 ZNF557 Q8N988 MTCNECGKSFTNSFS CGKSFTSNSYLSVHT 76 HR8385A-150-204-Av6HT ZNF558 Q96NG5 KLNECNQCFKVFSTK SSRSYLTIHKRIHNG 55 HR7908A-195-264-Av6HT ZNF559 Q9BR84 PSSSHLRECVRIYGG FTESSYLTQHLRTHS 70 HR8030A-275-342-Av6HT ZNF560 Q96MR9 RLILNVQVQRKCTQD AFTHSTSHAVNVETH 68 HR8284A-153-246-Av6HT ZNF561 Q8N587 KDTLSVHKEASTGQE RAVTASSHLKQCVAV 94 HR7120A-315-406-NHT ZNF562 Q6V9R5 PHKCTECGKAFTRST RIHTGEKPYECVECG 92 HR7101A-177-267-NHT ZNF563 Q8TA94 TFSSRRNLRRHMVVQ YECKQCSKALPDSSS 91 HR7852A-258-333-NHT ZNF564 Q8TBZ8 CGKAFDRPSLFRIHE IFPSYVRKHERTHTG 76 HR7421A-165-219-Av6HT ZNF565 Q8N9K5 KLMECHECGKAFSRG SRASHLVQHQRIHTG 55 HR6953A-201-290-Av6HT ZNF566 Q969W8 CKECGKSFRHPSRLT IHTGEKPYECKECGK 90 HR7055-1-501-TEV ZNF567 Q8N184 DVMLENYCHLISVGC TNLNLHQRIHTGEKP 500 HR7055A-1-62-15 ZNF567 Q8N184 MDVMLENYCHLISVG KAEDFLVKFKEHQEK 62 HR7055A-1-67-15 ZNF567 Q8N184 MDVMLENYCHLISVG LVKFKEHQEKYSRSV 67 HR7513A-584-636-NHT ZNF568 Q3ZCX4 KPYECNKCGKAFSQC AFSQRASLSIHKRGH 53 HR8322A-184-236-Av6HT ZNF569 Q5MGW4 TPFKCNHCGKGFNQT AFSHKEKLIKHYKIH 53 HR7036A-222-276-NHT ZNF57 Q68EA5 KTYKCEQCRMAFNGF IYPSTFQRHMTTHTG 55 HR8437A-468-518-Av6HT ZNF570 Q96NI8 KPYECTVCGKAFSYC KKTFRQHAHLAHHQR 51 HR7898A-556-609-Av6HT ZNF571 Q7Z3V5 KPYECKECGRAFSRG FRCPSQLTQHTRLHN 54 HR7069A-130-212-NHT ZNF572 Q7Z3I7 RPYKCSECWKSFSNS SNTSHLIIHERTHTG 83 HR7339A-346-414-NHT ZNF574 Q6ZN55 PSPSSLDQHLGDHSS FVNLTKFLYHRRTHG 69 HR7766A-185-234-NHT ZNF575 Q86XF7 AFSFPSKLAAHRLCH QAFGQRRLLLLHQRS 50 HR7135A-110-164-NHT ZNF576 Q9H609 PTFPCPDCGKTFGQA QDFAQEAGLHQHYIR 55 HR7392A-95-172-Av6HT ZNF580 Q9UK33 PECARVFASPLRLQS RFQDAAELAQHVRLH 78 HR7332A-85-197-NHT ZNF581 Q9P0T4 KCYSCPVCSRVFEYM MEQNTLQKHTRWKHP 113 HR7613A-143-226-NHT ZNF582 Q96NG8 IIRHEEMPTFDQHAS SRLIQHENIHSGKKP 84 HR8213A-490-546-Av6HT ZNF583 Q96ND8 KPYECNVCGKAFSYS RAHLAHHERIHTMES 57 HR7005A-111-199-NHT ZNF584 Q8IVC4 EHLKSYRVIQHQDTH RPFRCPTGRSAFKKS 89 HR7126A-700-769-NHT ZNF585B Q52M93 TKKSQLQVHQRIHTG FVQKSVFSVHQSSHA 70 HR7959A-294-369-Av6HT ZNF586 Q9NXT0 ECGKSFSLRSNLIHH AENSSLIKHLRVHTG 76 HR8398-1-385-15 ZNF587 Q96SQ5 MAAAVPRRPTQQGTV QRVHTGERPYKCGEC 385 HR8398A-13-68-15 ZNF587 Q96SQ5 MGTVTFEDVAVNFSQ LGCWCGSKDEEAPCK 57 HR8398A-8-73-15 ZNF587 Q96SQ5 MRPTQQGTVTFEDVA GSKDEEAPCKQRISV 67 HR8398B-85-147-15 ZNF587 Q96SQ5 MGVSPKKAHPCEMCG AYLHQHQKQHIGEKF 64 HR8398B-90-144-15 ZNF587 Q96SQ5 MKAHPCEMCGLILED DDTAYLHQHQKQHIG 56 HR7374A-223-283-NHT ZNF589 Q86UQ0 AFNQKSNLFRQKAVT THTGEKPYVCGECGR 61 HR7253A-8-134-TEV ZNF593 O00488 GAHRAHSLARQMKAK PTEVSTEVPEMDTST 127 HR7622A-658-732-NHT ZNF594 Q96JF6 GKAFSQRSHLATHQK MWHTAFLKHQRLHAG 75 HR8535A-211-338-Av6HT ZNF595 Q8IYB9 RSTSLSKHKRIHTGE SRSLNEHKNIHTGEK 128 HR7605A-165-247-NHT ZNF596 Q8TC21 KSYGSHLFDYAFIQN THCSDLRKHERTHTG 83 HR6958A-339-424-NHT ZNF597 Q96LX8 KPLQCPDCDMTFPCF LHLITHKRTHIKNTT 86 HR7504A-200-279-NHT ZNF599 Q96NL3 TCTECGKGFSKKWAL KRRFHLTEHQRIHTG 80 HR7536A-401-473-NHT ZNF605 Q86T29 AFFKKSELIRHQKIH TQKSSLISHQRTHTG 73 HR7780A-327-407-NHT ZNF606 Q8WXB4 NQSPSFNEHPRLHVG TYTAEKPYDYNECGT 81 HR7972A-628-696-Av6HT ZNF607 Q96SK3 CASYLVRHESVHADG FRLRSILEVHQRIHI 69 HR7618A-201-256-NHT ZNF610 Q8N9Z0 SYEYECSEDGEVFRV SRNSHLVEHWRIHTG 56 HR7693A-602-688-NHT ZNF611 Q8N823 TFSRRSSLHCHRRLH AEKPYKCNECGKAFN 87 HR8205A-466-535-Av6HT ZNF613 Q6PF04 SHKSGLINHQRIHTG FSHLSCLVYHKGMLH 70 HR7312A-239-311-NHT ZNF614 Q8N883 KLSRSVLFTKHLKTN TMKRYLIAHQRTHSG 73 HR7638A-239-293-NHT ZNF616 Q08AN1 KSYQCDVCGKIFRKN SKSSHLAVHQRIHTG 55 HR8536A-215-271-Av6HT ZNF619 Q8N2I2 PYTCKECGKTFRYNS SHLLQHQKLHGGQRP 57 HR7004A-342-416-Av6HT ZNF620 Q6ZNG0 GKRLSSNTALTQHQR SWCGRFILHQKLHTQ 75
HR6865A-260-345-NHT ZNF621 Q6ZSS3 EKLYKCKECWKAFGC YGSFVQHQKLHPVEK 86 HR7076A-233-357-Av6HT ZNF622 Q969S3 MQDAEEEEAEEGPPL FADFYDFRSSYPDHK 126 HR7076A-233-357-NHT ZNF622 Q969S3 QDAEEEEAEEGPPLG FADFYDFRSSYPDHK 125 HR8004A-806-858-Av6HT ZNF624 Q9P2J8 RPYKCEECGKAFRTN AFRSSSSLTVHQRIH 53 HR8159A-235-289-Av6HT ZNF625 Q96I27 KPYECKQCGKAFRSA GCASSVKIHERTHTG 55 HR8312A-451-505-Av6HT ZNF626 Q68DY1 KFYKCEECGKAFKCS NQSSIDTTHERIILE 55 HR7221A-166-234-Av6HT ZNF627 Q7L945 PYDCKECGETFISLV EKPYECKQCGKAFSC 69 HR7999A-830-869-Av6HT ZNF629 Q9UEG4 GQNPKTLVEEKPYLC AALLLHRSCHPGVSL 40 HR7098A-597-651-NHT ZNF630 Q2M218 KTPECAESGMTFFWK CQHVYFTGHQNPYRK 55 HR7646-132-485-Av6HT ZNF639 Q9UID6 VHTAEDVPIAVEVHA NERELISHLPVHETT 354 HR7646-158-485-Av6HT ZNF639 Q9UID6 NSSESLQDQTDEEPP NERELISHLPVHETT 328 HR7646-168-485-Av6HT ZNF639 Q9UID6 DEEPPAKLCKILDKS NERELISHLPVHETT 318 HR7646-24-485-Av6HT ZNF639 Q9UID6 ISRIADGFNGIFSDH NERELISHLPVHETT 462 HR7646-80-485-Av6HT ZNF639 Q9UID6 RNQNYLVPSPVLRIL NERELISHLPVHETT 406 HR7646-Av6HT ZNF639 Q9UID6 NEYPKKRKRKTLHPS ERELISHLPVHETT* 485 HR7646A-406-471-15 ZNF639 Q9UID6 MDDCGKGFSSMLEYC DLPHKCSDCLMRFGN 67 HR7646A-406-485-15 ZNF639 Q9UID6 MDDCGKGFSSMLEYC NERELISHLPVHETT 81 HR7646A-411-466-15 ZNF639 Q9UID6 MGFSSMLEYCKHLNS FKHSADLPHKCSDCL 57 HR7646A-411-485-15 ZNF639 Q9UID6 MGFSSMLEYCKHLNS NERELISHLPVHETT 76 HR7646B-233-313-Av6HT ZNF639 Q9UID6 NVCRVCKESFSTNML SSSSELYLHFQEHSC 81 HR7646B-256-313-Av6HT ZNF639 Q9UID6 EEDPYICKYCDYKTV SSSSELYLHFQEHSC 58 HR7646C-372-425-Av6HT ZNF639 Q9UID6 NFFVCQVCGFRSRLH GFSSMLEYCKHLNSH 54 HR7646D-202-255-Av6HT ZNF639 Q9UID6 GLYKCELCEFNSKYF SFSTNMLLIEHAKLH 54 HR7858A-251-323-Av6HT ZNF642 Q49AA0 RNTYKLDLINHPTSY SQSASLSTHQRIHTG 73 HR7770A-52-130-NHT ZNF645 Q8N7E2 LPIHFCDKCDLPIKI IVQQCKRTYLSQKSL 79 HR7348A-260-345-NHT ZNF648 Q5T619 QKPSKPLSPAETRGG GEKPYPCPDCGKAFV 86 HR7533A-232-314-NHT ZNF649 Q9BS31 KPHGCSLCGKAFYKR SRKSLLVVHQRTHTG 83 HR7463A-365-451-NHT ZNF652 Q9Y209 SFKRSMSLKVHSLQH GEKPFICETCGKSFT 87 HR8324A-526-605-Av6HT ZNF653 Q96CK0 REFTCETCGKSFKRK CGKRFEKLDSVKFHT 80 HR8422A-173-222-15 ZNF655 Q8N720 MGKHEHLNLTEDFQS TEKSYKCDVCGKIFH 51 HR8422A-173-239-15 ZNF655 Q8N720 MGKHEHLNLTEDFQS SALTRHQRIHTREKP 68 HR8422A-178-218-15 ZNF655 Q8N720 MLNLTEDFQSSECKE SIPNTEKSYKCDVCG 42 HR8422A-178-234-15 ZNF655 Q8N720 MLNLTEDFQSSECKE IFHQSSALTRHQRIH 58 HR8422A-182-234-TEV ZNF655 Q8N720 EDFQSSECKESLMDL IFHQSSALTRHQRIH 53 HR8422B-430-491-TEV ZNF655 Q8N720 HRKEKSYECNEYEGS AHLVQHQSIHTKENS 62 HR8422B-434-491-TEV ZNF655 Q8N720 KSYECNEYEGSFSHS AHLVQHQSIHTKENS 58 HR8422C-372-430-Av6HT ZNF655 Q8N720 GIHFREKPYTCSECG AFSQTSCLIQHHKMH 59 HR8422C-372-470-Av6HT ZNF655 Q8N720 GIHFREKPYTCSECG EVLTROKAFDCDVWE 99 HR8422C-372-475-Av6HT ZNF655 Q8N720 GIHFREKPYTCSECG QKAFDCDVWEKNSSQ 104 HR8422D-378-430-Av6HT ZNF655 Q8N720 KPYTCSECGKDFRLN AFSQTSCLIQHHKMH 53 HR7623A-279-349-NHT ZNF658 Q5TYW1 CDKTTAVEYNKVHMA SQSSAHIVHQKTQAG 71 HR8538A-1-85-Av6HT ZNF663 Q8NDT4 YTGEKPDECKENEKA VLKESCLTPNQRIKT 84 HR6955A-195-249-NHT ZNF664 Q8N3J9 GEKPYRCCGCGKAFS AFSQSTSLCIHQRVH 55 HR7621A-171-230-NHT ZNF667 Q5HYK9 PFECSNCRKAFRQIS ILHMRIHDGKEILDC 60 HR6925A-83-165-NHT ZNF670 Q9BS34 TFSQDSNLNLNKKVS ISLTSVDRHMVTHTS 83 HR7883A-267-337-Av6HT ZNF671 Q8TAW3 KPHKSTKLVSGFLMG SQSYDLFKHQTVHTG 71 HR7237A-1-66-NHT ZNF672 Q499Z4 FATSGAVAAGKPYSC ARAADLRAHRRTHAG 65 HR7236A-365-437-NHT ZNF674 Q2M3X9 ASDEKPSPTKHWRTH SGKSHLSVHHRTHTG 73 HR7578A-1-67-15 ZNF675 Q8TD23 MGLLTFRDVAIEFSL ITCLEQEKEPLTVKR 67 HR7578A-1-74-15 ZNF675 Q8TD23 MGLLTFRDVAIEFSL KEPLTVKRHEMVNEP 74 HR7578B-131-199-15 ZNF675 Q8TD23 MGLNQCLPTMQSKMF SHLTRHERNYTKVNF 70 HR7578B-136-194-15 ZNF675 Q8TD23 MLPTMQSKMFQCDKY SFCMLSHLTRHERNY 60 HR7578B-137-199-15 ZNF675 Q8TD23 MPTMQSKMFQCDKYV SHLTRHERNYTKVNF 64 HR7578B-140-194-15 ZNF675 Q8TD23 MQSKMFQCDKYVKVF SFCMLSHLTRHERNY 56 HR7891A-111-163-Av6HT ZNF676 Q8N7Q3 KVFQCGKYANVFHKC SFCMLSHLSQHERIY 53 HR7577A-497-565-NHT ZNF677 Q86XU0 TERSNLTQHKKIHTG ALFQSSNIGDHQKSY 69 HR8530A-404-487-Av6HT ZNF678 Q5SXM1 PYKCEECGKVFKQCS FSSLTRHKRIHTGEK 84 HR7709A-360-411-NHT ZNF679 Q8IYX0 AFAFSSTLNTHKRIH NHKSMHTGEKPYKCE 52 HR8058A-136-204-Av6HT ZNF680 Q8NEM1 KEGYNELNQCLRTTQ SFCMLSHLTQHIRIH 69 HR7323A-486-575-NHT ZNF681 Q96N22 AFNQSSILTTHKRIH PYQCEECGKAFNOSS 90 HR7060A-141-225-NHT ZNF682 O95780 PSKIFPYNKCVKVFS KWFSYLTKHKRIHTG 85 HR7991A-155-211-Av6HT ZNF684 Q5T5D7 VENAYECSECGKAFK SRKAHLATHQKIHNG 57 HR7327A-962-1050-Na6HT ZNF687 Q8N1G0 GWTCGLCHSWFPERD SSRLILEKHVQVRHG 89 HR7862A-150-239-Av6HT ZNF689 Q96CS4 ICPDCGCTFPDHOAL VIHTGEKPYHCPDCG 90 HR8314A-328-397-NHT ZNF692 Q9BU19 KKHLKEHMKLHSDTR QKASLNWHQRKHAET 70 HR7296A-298-376-NHT ZNF695 Q8IW36 CEECGKSFKLFPYLT NQSSHLTEHRRIHTG 79 HR8310A-158-221-Av6HT ZNF696 Q9H7X3 CGKAFIHSSHVVRHQ KPYACADCGKAFGQR 64 HR7368A-352-405-NHT ZNF697 Q5TEC3 PFACGECGKGFVRRS SWRSDLVKHQRVHTG 54 HR8203A-585-642-Av6HT ZNF699 Q32M78 KPFECLECGKAFSCP AYFRRHVKTHTRENI 58 HR7900-118-686-15 ZNF7 P17097 MQNPGFGDVSDSEVW NRSSRLTQHQKIHMG 570 HR7900-123-686-15 ZNF7 P17097 MGDVSDSEVWLDSHL NRSSRLTQHQKIHMG 565 HR7900-176-686-15 ZNF7 P17097 MSSGLDCQPLESQGE NRSSRLTQHQKIHMG 512 HR7900-181-686-15 ZNF7 P17097 MCQPLESQGESAEGM NRSSRLTQHQKIHMG 507 HR7900-192-686-15 ZNF7 P17097 MEGMSQRCEECGKGI NRSSRLTQHQKIHMG 496 HR7900-98-686-Av6HT ZNF7 P17097 DILKSESYGTVVRIS NRSSRLTQHQKIHMG 589 HR7900A-632-686-Av6HT ZNF7 P17097 KLHQCEDCEKIFRWR NRSSRLTQHQKIHMG 55 HR7964A-390-437-Av6HT ZNF70 Q9UC06 KPYTCECGKAFRHRS LCGKSFRGSSHLIRH 48 HR8076A-25-71-Av6HT ZNF700 Q9H0M5 AFEDVAVNFTQEEWT FRNLTSIGKKWSDQN 47 HR7819A-251-341-Av6HT ZNF701 Q9NV72 DFHQKRYLACHRCHT KCEECDKVFSRKSHL 91 HR6936A-105-196-NHT ZNF705A Q6ZN79 TMENSLILEDPFECN TNCFRLRRHKMTHTG 92 HR7717A-105-196-NHT ZNF705G A8MUZ8 TMENSLILEDPFECN TNCFHLRRHKMTHTG 92 HR7599A-294-364-NHT ZNF707 Q96C28 GKAFRTKENLSHHQR GKGFRHLGFFTRHQR 71 HR8522A-127-192-Av6HT ZNF708 P17019 GLNRCVTTTQSKIVQ CMLSQLTQHEIIHTG 66 HR8299A-146-232-Av6HT ZNF709 Q8N972 GKRFSFRSSFRIHER HTGEKPYKCKECGKT 87 HR7598A-420-489-NHT ZNF71 Q9NQZ8 SQSAYLIEHQRIHTG FSRNTNLTRHLRIHT 70 HR7994A-273-347-Av6HT ZNF710 Q8N1W2 RLDINVQIDDSYLVE KQPSHLQTHLLTHQG 75 HR7730A-640-700-NHT ZNF711 Q9Y462 IHKGRKIHQCRHCDF RQQNELKKHMKTHTG 61 HR7315A-202-251-NHT ZNF713 Q8N859 SIKHNSDLIYYQGNY LTDHIHTAEKPSECG 50 HR8420A-149-221-Av6HT ZNF718 Q3SXZ3 VKVFHKFSNSNKDKI AFNWSSILTKHKRIH 73 HR7757A-1-62-NHT ZNF720 Q7Z2F6 GLLTFRDVAIEFSRE SKPOLITFLEQRKEP 61 HR8490A-143-197-Av6HT ZNF730 Q6ZMV8 KIFQCDKYVKVFHKF CILSHLAQHKKIHTG 55 HR8539A-16-91-Av6HT ZNF738 Q8NE65 GYPGAERNLLEYSYF DVSKPDLITCLEQGK 76 HR8339A-526-578-Av6HT ZNF74 Q16587 KPYKCSECGRAFSQN MFNWSSHLTEHQRLH 53 HR8360A-96-181-Av6HT ZNF740 Q8NDX6 KIPKNFVCEHCFGAF SRTDRLLRHKRMCQG 86 HR8176A-34-86-Av6HT ZNF747 Q9BV97 PGAVSFADVAVYFSR HLGALGESPTCLPGP 53 HR8509A-408-460-Av6HT ZNF749 Q43361 RLYKCSECGKAFSLK AFVRKSHLVQHQKIH 53 HR7901A-1-56-Av6HT ZNF75A Q96N20 YFSQEEWELLDPTQK KVISCLEQGEEPWVQ 55 HR6964-1-358-Av6HT ZNF76 P36508 ESLGLHTVTLSDGTT PYTCSTCGKTYRQTS 357 HR6964-1-369-Av6HT ZNF76 P36508 ESLGLHTVTLSDGTT RQTSTLAMHKRSAHG 368 HR6964A-173-267-NHT ZNF76 P36508 GRLYTTAHHLKVHER RPFQCPFEGCGRSFT 95 HR6964B-161-251-Av6HT ZNF76 P36508 GDRAFRCGYKGCGRL KTSGDLQKHVRTHTG 91 HR6964C-227-276-Av6HT ZNF76 P36508 CPEELCSKAFKTSGD CGRSFTTSNIRKVHV 50 HR6964C-227-294-Av6HT ZNF76 P36508 CPEELCSKAFKTSGD TGERPYTCPEPHCGR 68 HR6964C-232-294-Av6HT ZNF76 P36508 CSKAFKTSGDLQKHV TGERPYTCPEPHCGR 63 HR6964C-235-276-Av6HT ZNF76 P36508 AFKTSGDLQKHVRTH CGRSFTTSNIRKVHV 42 HR7027A-389-461-NHT ZNF765 Q7L2R6 SKTFSHKSSLTYHRR YSFKSNLFIHQKIHT 73 HR7836A-303-379-NHT ZNF766 Q5HY98 KCGKVYSSSSYLAQH RHKFSLTVHQRNHNG 77 HR7774A-291-355-NHT ZNF768 Q9H5H4 CEVCSKAFSQSSDLI GQKPYKCPHCGKAFG 65 HR8123A-304-359-Av6HT ZNF77 Q15935 SFSCYSSFRDHVRTH THSGEKPYECKECGK 56 HR7610A-1-82-NHT ZNF770 Q6IQ21 AENNLKMLKIQQCVV VHLERHQLTHSLPFK 80 HR7742A-127-216-NHT ZNF771 Q7L3S4 RFSAASNLRQHRRRH PYACADCGTRFAQSS 90 HR7325A-385-458-NHT ZNF772 Q68DY9 KYFGHKYRLIKHWSV SHKHVLVQHHRIHTG 74 HR8057A-186-243-Av6HT ZNF773 Q6PK81 AGKRHYKCSECGKAF SHKSNLFIHQIVHTG 58 HR7824A-105-159-Av6HT ZNF775 Q96BV0 GHFVCLDCGKRFSWW SQKPNLARHQRHHTG 55 HR7510A-199-288-NHT ZNF776 Q68DI1 IPLQGGKTHYICGES WYKAHLTEHQRVHTG 90 HR7596A-583-631-NHT ZNF780A Q75290 KPFECKECGKAFRLH ECGKVFSLPTQLNRH 49 HR7666A-735-815-NHT ZNF780B Q9Y6R6 GLLTQLAQHQIIHTG KLVQVRNPLNVRNVG 81 HR7344A-514-586-NHT ZNF782 Q6ZMW2 AFKLKSGLRKHHRTH SQKSNLRVHHRTHTG 73 HR8508A-34-122-Av6HT ZNF783 Q6ZMS7 SYLYSTEITLWTVVA LLQRRLENVENLLRN 89 HR8227A-257-318-Av6HT ZNF785 A8K8V0 ACSDCKSRFTYPYLL RIHTGEKPYPCPDCG 62 HR7825A-407-473-NHT ZNF786 Q8N393 RLRRLLQVHQHAHGG GRNFRQRGQLLRHQR 67 HR7915A-65-146-Av6HT ZNF787 Q6DD87 PYICNECGKSFSHWS SWSSNLMQHQRIHTG 82 HR8290A-153-225-Av6HT ZNF789 Q5FWF6 GFLQNLNLIQDQNAQ RRKAWFDQHQRIHFL 73 HR7454A-424-498-NHT ZNF79 Q15937 KFFSESSALIRHHII CSSAFVRHQRLHAGE 75 HR7139A-544-636-NHT ZNF790 Q6PG37 IWGSQLTRHKKIHTD FEKAFSSSSHFISLL 93
HR8412A-506-576-Av6HT ZNF791 Q3KP31 IYPTSFQGHMRMHTG SVSTSLKKHMRMHNR 71 HR8238A-532-599-15 ZNF792 Q3KQV3 MRPYECSECGKTFRQ IRERSMENVLLPCSQ 69 HR8238A-532-599-Av6HT ZNF792 Q3KQV3 RPYECSECGKTFRQR IRERSMENVLLPCSQ 68 HR7343A-484-570-NHT ZNF799 Q96GE5 AFSCFQYLSQHRRTH REKPYECQQCGKAFT 87 HR4794D-252-417-15 ZNF8 P17098 VQDKPYKCTDCGKSF GKGFRHSSSLAQHQR 166 HR4794D-256-412-15 ZNF8 P17098 PYKCTDCGKSFNHNA ECNHCGKGFRHSSSL 157 HR4794D-274-417-15 ZNF8 P17098 VHKRIHTGERPYMCK GKGFRHSSSLAQHQR 144 HR4794D-280-412-15 ZNF8 P17098 TGERPYMCKECGKAF ECNHCGKGFRHSSSL 133 HR4794E-339-417-15 ZNF8 P17098 KPYECQDCGRAFNQN GKGFRHSSSLAQHQR 79 HR4794E-344-411-15 ZNF8 P17098 QDCGRAFNQNSSLGR YECNHCGKGFRHSSS 68 HR7462A-85-152-NHT ZNF80 P51504 AFPEKVDFVRPMRIH CGKTFSYHSVFIQHR 68 HR8132A-480-640-Av6HT ZNF800 Q2TB10 GFDFKQLYCKLCKRQ AFAKKTYLEHHKKTH 161 HR7014A-407-492-NHT ZNF808 Q8N4W9 AFNHQSSLARHHILH TGEKTYKCNECRKTF 86 HR7427A-226-281-NHT ZNF81 P51508 VFTQNSSYSHHENTH FPIGEKANTCTEFGK 56 HR8002A-591-645-Av6HT ZNF816A Q0VGE8 KPYKCNECGKVFNQK TGQSTLIHHQAIHGC 55 HR8532A-59-113-Av6HT ZNF818P Q6ZRF7 KRSLTNVCGKVLSQN TQGSRFINHQIVHTG 55 HR7648A-533-610-NHT ZNF823 P16415 GKAFSWLTCLLRHER RSLHRHKRTHWKDTL 78 HR7405A-703-761-NHT ZNF828 Q96JM3 KRGKGKYYCKICCCR FLLESLLKNHVAAHG 59 HR8193A-154-208-Av6HT ZNF829 Q3KNS6 KPWECKICGKTFNQN SRGSLVTRHQRIHTG 55 HR7002A-127-184-15 ZNF83 P51522 MGKIFNKKSNLASHQ IHTGEKPYKCNECGK 59 HR7002B-70-148-15 ZNF83 P51522 MTYECNFVDSLFTQK SNLASHQRIHTGEKP 80 HR7002B-74-145-15 ZNF83 P51522 MNFVDSLFTQKEKAN NKKSNLASHQRIHTG 73 HR7002B-89-145-15 ZNF83 P51522 MGTEHYKCSERGKAF NKKSNLASHQRIHTG 58 HR8533A-9-176-Av6HT ZNF833P Q6ZTB9 PYKCKFCGKAFDNLH FSSFHSHEGVHTGEK 168 HR8077A-450-518-Av6HT ZNF835 Q9Y2P0 SQGSSLALHQRTHTG AFSFSSALIRHQRTH 69 HR8234A-206-287-15 ZNF836 Q6ZNA1 MTQLEKTHIREKPYM PYQCGVCGKIFRQNS 83 HR8234A-206-287-Av6HT ZNF836 Q6ZNA1 TQLEKTHIREKPYMC PYQCGVCGKIFRQNS 82 HR7704A-427-482-NHT ZNF837 Q96EG3 AFKGRSGLVQHQRAH LHSGEKPYICRDCGK 56 HR8403A-681-738-Av6HT ZNF84 P51523 KPYGCSECRKAFSQK SQLINHQRTHTVKKS 58 HR8489A-197-283-Av6HT ZNF841 Q6ZN19 RGKPYQCDVCGRIFR SSSLATHQTVHTGDK 87 HR8361A-897-970-Av6HT ZNF845 Q96IR2 NQQAHLACHHRIHTG AKLARHHRIHTGKKH 74 HR7777A-476-525-NHT ZNF846 Q147U1 KPYACKECGKAFRYS CGKNFTQSSALAKHL 50 HR7585A-536-595-NHT ZNF85 Q03923 KPYTCEECGKAFNQS LTKHKIIHTGEKLQI 60 HR8493A-449-519-Av6HT ZNF852 B4DLD7 SYNSSLMVHQRTHTG SQRSTFNHHQRTHAG 71 HR8177-500-555-Av6HT ZNF880 Q6PD84 VFSHNSHLARHRQIH IHTGEKPYRCHECGK 56 HR6923A-519-589-NHT ZNF90 Q03938 KRSSVLSKHKIIHTG NLSSDLNTHKRIHIG 71 HR8498A-486-572-Av6HT ZNF98 A6NK75 GEKPYKCEECGKAFN IAKISKYKRNCAGEK 87 HR8425A-706-753-Av6HT ZNF99 A8MXY4 AFNNSSTLRKHEIIH IHTGKKPYKCEECGK 48 HR7451A-925-1258-Av6HT ZNFX1 Q9P2E3 LDLSSRWQLYRLWLQ SKIIHTLRENNQIGP 334 HR6880A-166-356-NHT ZRSR2 Q15696 EKDRANCPFYSKTGA ANRDIYLSPDRTGSS 191 HR7933A-24-120-Av6HT ZSCAN1 Q8NBBT ADPGPASPRDTEAQR CREAASLVEDLTQMC 97 HR7806A-1-70-NHT ZSCAN10 Q96SZ4 GPRASLSRLRELCGH DGEEVVLLLEGIHRE 69 HR8495A-9-132-Av6HT ZSCAN12 O43309 AHMDQDEPLEVKIEE VTVLEDLERELDEPG 124 HR7081A-224-291-TEV ZSCAN16 Q9H4T2 GRSEWQQRERRRYKC SHLIGHHRVHTGVKP 68 HR7530A-36-127-NHT ZSCAN21 Q9Y5A6 KYLPSLEMFRQRFRQ AEEAVTLLEDLEREL 92 HR7904A-40-135-Av6HT ZSCAN22 P10073 DHIAHSEAARLRFRH AVLVEDLTQVLDKRG 96 HR7247A-37-133-NHT ZSCAN23 Q3MJ62 SRNNPHTREIFRRRF AVTVLEDLERELDDP 97 HR8429A-9-104-Av6HT ZSCAN29 Q8IWY8 ENGTNSETFRQRFRR VTLVEDLEREPGRPR 96 HR6932A-12-134-NHT ZSCAN80 Q86W11 APEEQEGLLVVKVEE VTMLEELEKELEEPR 123 HR7089A-35-123-Av6HT ZSCAN4 Q8NAM6 REEGISEFSRMVLNS KSSGKNLERFIEDLT 89 HR7089A-35-130-NHT ZSCAN4 Q8NAM6 REEGISEFSRMVLNS ERFIEDLTDDSINPP 96 HR7089A-46-123-Av6HT ZSCAN4 Q8NAM6 VLNSFQDSNNSYARQ KSSGKNLERFIEDLT 78 HR7089A-46-130-Av6HT ZSCAN4 Q8NAM6 VLNSFQDSNNSYARQ ERPIEDLTDDSINPP 85 HR6950A-39-129-NHT ZSCAN5A Q9BUG6 DPEISHVNFRMFSCP LEDLLRNNRRPKKWS 91 HR8432A-35-142-Av6HT ZSCAN5B A6NJL1 NHDRNPETWHMNFRM WSIVNLLGKEYLMLN 108 HR7759A-37-130-NHT ZSCAN5C A6NGD5 DSDPETCHVNFRMFS EDLLRNNRRPKKWSV 94 HR8021A-314-557-Av6HT ZUFSP Q96AP4 DGKTKTSGIIEALHR LKHKQYDILAVEGAL 244 HR7812A-358-413-NHT ZXDA P98168 NSFKCEVCEESFPTQ TFITVSALFSHNRAH 56 HR7168A-360-417-NHT ZXDB P98169 QENSFKCEVCEESFP TFITVSALFSHNRAH 58 HR7131A-652-715-TEV ZZZ3 Q8IYH5 NQLWTVEEQKKLEQL KYFIKLTKAGIPVPG 64
REFERENCES
[0194] Acton, T. B., et al., 2011. Preparation of protein samples for NMR structure, function, and small-molecule screening studies. Methods Enzymol. 493, 21-60.
[0195] Agaton et al., Molecular & Cellular Proteomics 2:405-414, 2003.
[0196] Bindewald, E., et al., CyloFold: secondary structure prediction including pseudoknots. Nucleic Acids Res. 38, W368-72.
[0197] Brodskii, L. I., et al., 1995. [GeneBee-NET: An Internet based server for biopolymer structure analysis]. Biokhimiia. 60, 1221-30.
[0198] Crowe, J., et al., 1994. 6×His-Ni-NTA chromatography as a superior technique in recombinant protein expression/purification. Methods Mol Biol. 31, 371-87.
[0199] Ding, Y., et al., 2004. Sfold web server for statistical folding and rational design of nucleic acids. Nucleic Acids Res. 32, W135-41.
[0200] Do, C. B., et al., 2006. CONTRAfold: RNA secondary structure prediction without physics-based models. Bioinformatics. 22, e90-8.
[0201] Gonzalez de Valdivia, E. I., Isaksson, L. A., 2004. A codon window in mRNA downstream of the initiation codon where NGG codons give strongly reduced gene expression in Escherichia coli. Nucleic Acids Res. 32, 5198-205.
[0202] Gruber, A. R., et al., 2008. The Vienna RNA websuite. Nucleic Acids Res. 36, W70-4.
[0203] Hamada, M., et al., 2009. Predictions of RNA secondary structure by combining homologous sequence information. Bioinformatics. 25, i330-8.
[0204] Jansson, M.; et al., 1996. High-level production of uniformly 15N- and 13C-enriched fusion proteins in Escherichia coli. B. J. Biomol. NMR. 7, 131-141.
[0205] Kapust, R. B., et al., 2002. The P1' specificity of tobacco etch virus protease. Biochem Biophys Res Commun. 294, 949-55.
[0206] Kudla, G., et al., 2009. Coding-sequence determinants of gene expression in Escherichia coli. Science. 324, 255-8.
[0207] Lamla, T., Erdmann, V. A., 2004. The Nano-tag, a streptavidin-binding peptide for the purification and detection of recombinant proteins. Protein Expr Purif. 33, 39-47.
[0208] Lui et al., 2002, Loopy proteins appear conserved in evolution. J Mol Biol. 322-53-64)
[0209] Markham, N. R., Zuker, M., 2008. UNAFold: software for nucleic acid folding and hybridization. Methods Mol Biol. 453, 3-31.
[0210] Mathews, D. H., et al., 2004. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc Natl Acad Sci USA. 101, 7287-92.
[0211] Netzer and Hartl, 1997. Recombination of protein domains facilitated by co-translational folding in eukaryotes. Nature. 358-343-9.
[0212] Nomura, M., et al., 1984. Influence of messenger RNA secondary structure on translation efficiency. Nucleic Acids Symp Ser. 173-6.
[0213] Quan, J., et al., 2011. Parallel on-chip gene synthesis and application to optimization of protein expression. Nat Biotechnol. 29, 449-52.
[0214] Reeder, J., et al., 2007. pknotsRG: RNA pseudoknot folding including near-optimal structures and sliding windows. Nucleic Acids Res. 35, W320-4.
[0215] Rivas, E., Eddy, S. R., 1999. A dynamic programming algorithm for RNA structure prediction including pseudoknots. J Mol Biol. 285, 2053-68.
[0216] Rocha, E. P., et al., 1999. Translation in Bacillus subtilis: roles and trends of initiation and termination, insights from a genome analysis. Nucleic Acids Res. 27, 3567-76.
[0217] Sharp, P. M., Li, W. H., 1987. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15, 1281-95.
[0218] Scholle, M. D., et al., 2004. In vivo biotinylated proteins as targets for phage-display selection experiments. Protein Expr Purif. 37, 243-52.
[0219] Schroeder, S. J., et al., 2011. Ensemble of secondary structures for encapsidated satellite tobacco mosaic virus RNA consistent with chemical probing and crystallography constraints. Biophys J. 101, 167-75.
[0220] Voss, B., et al., 2006. Complete probabilistic analysis of RNA shapes. BMC Biol. 4, 5.
[0221] Xayaphoummine, A., et al., 2005. Kinefold web server for RNA/DNA folding path and structure prediction including pseudoknots and knots. Nucleic Acids Res. 33, W605-10.
[0222] Xayaphoummine, A., et al., 2003. Prediction and statistics of pseudoknots in RNA structures using exactly clustered stochastic simulations. Proc Natl Acad Sci USA. 100, 15310-5.
[0223] Zuker, M., Stiegler, P., 1981. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 9, 133-48.
[0224] The foregoing examples and description of the preferred embodiments should be taken as illustrating, rather than as limiting the present invention as defined by the claims. As will be readily appreciated, numerous variations and combinations of the features set forth above can be utilized without departing from the present invention as set forth in the claims. Such variations are not regarded as a departure from the scope of the invention, and all such variations are intended to be included within the scope of the following claims. All references cited herein are incorporated herein in their entireties.
User Contributions:
Comment about this patent or add new information about this topic: