Patent application title: Method for yield improvement in glyphosate-resistent legumes
Inventors:
Peter Oakley (Neustadt, DE)
Annette Freund (Limburgerhof, DE)
Klaus Schelberger (Gonnheim, DE)
IPC8 Class: AA01N5720FI
USPC Class:
504128
Class name: Plural active ingredients phosphorus containing active ingredient wherein the phosphorus is other than solely as part of an inorganic ion in an addition salt with an active heterocyclic compound
Publication date: 2011-02-10
Patent application number: 20110034333
yield in glyphosate-resistant legumes, which
comprises treating the plants or the seed with a mixture comprising a)
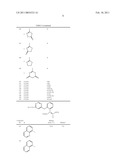
azoxystrobinwhere X, m, Q, A have the meaning given in the description
and b) a glyphosate derivative IIin a synergistically active amount.Claims:
1. A method for synergistically increasing the yield in
glyphosate-resistant legumes, which comprises treating the plants with a
mixture comprising(a) azoxystrobinand(b) a glyphosate derivative II
selected from the group consisting of N-(phosphonomethyl)glycine as a
free acid or a salt thereofin a synergistically active amount, wherein
the weight ratio of the compound of the formula Ia to the glyphosate
derivative II is from 1:1 to 0.01:1.
2. The method as claimed in claim 1, wherein the salt of N-(phosphonomethyl)glycine is selected from the group consisting of the isopropylammonium salt, sodium salt, ammonium salt and trimethylsulfenium salt.
3. The method as claimed in claim 1, wherein the mixture comprises:(a) azoxystrobin and(b) a glyphosate derivative II.
4. The method as claimed in claim 1, wherein component (b) is N-(phosphonomethyl)glycine as a free acid.
5. A method as claimed in claim 1, wherein a fungicidal azole selected from the group consisting of: fluquinconazole, metconazole, prochloraz, propiconazole, prothioconazole, tebuconazole, epoxiconazole or myclobutanil is employed as component a) in addition to the active compound of the formula Ia.
6. A synergistic mixture comprising(a) azoxystrobin and(b) a glyphosate derivative II selected from the group consisting of N-(phosphonomethyl)glycine as a free acid or a salt thereofwherein the weight ratio of the compound of the formula Ia to the glyphosate derivative II is from 1:1 to 0.01:1.
7. A mixture as claimed in claim 6, wherein the mixture comprises:(a) azoxystrobin and(b) a glyphosate derivative II.
8. A mixture as claimed in claim 7, wherein component a) comprises an azole selected from the group consisting of: metconazole, myclobutanil, epoxiconazole, propiconazole, prothioconazole and tebuconazole in addition to the active compound pyraclostrobin.
9. A mixture as claimed in claim 7, wherein component (b) is a salt of N-(phosphonomethyl)glycine selected from the group consisting of the isopropylammonium salt, sodium salt, ammonium salt and trimethylsulfenium salt.
10. The method as claimed in claim 3, wherein the weight ratio of the compound pyraclostrobin to the glyphosate derivative II is 1:1 to 0.1:1.
11. A mixture as claimed in claim 7, wherein the weight ratio of the compound pyraclostrobin to the glyphosate derivative II is 1:1 to 0.1:1.Description:
[0001]The present application is a 37 C.F.R. §1.53(b) divisional of
Ser. No. 10/534,637, and claims priority to, U.S. application Ser. No.
10/534,637 filed May 12, 2005. application Ser. No. 10/534,634 is the
national phase under 35 U.S.C. §371 of International Application No.
PCT/EP2003/012483, filed on Nov. 8, 2003. Priority is also claimed to
German application 10252881.0 filed on Nov. 12, 2002. The entire contents
of each of these applications is hereby incorporated by reference.
[0002]The present invention relates to a method for increasing the yield in glyphosate-resistant legumes, which comprises treating the plants or the seed with a mixture comprising
a) compound of formula I
##STR00001##
in which [0003]X is halogen, C1-C4-alkyl or trifluoromethyl, [0004]m is 0 or 1, [0005]Q is C(═CH--CH3)--COOCH3, C(═CH--OCH3)--COOCH3, C(═N--OCH3)--CONHCH3, C(═N--OCH3)--COOCH3 or N(--OCH3)--COOCH3, [0006]A is --O--B, --CH2O--B, --OCH2--B, --CH═CH--B, --C≡C--B, --CH2O--N═C(R1)--B or --CH2O--N═C(R1)--C(R2)═N--OR3, where [0007]B is phenyl, naphthyl, 5-membered or 6-membered hetaryl or 5-membered or 6-membered heterocyclyl, comprising one to three N atoms and/or one O or S atom or one or two O and/or S atoms, the ring systems being unsubstituted or substituted by one to three radicals Ra: [0008]Ra being cyano, nitro, amino, aminocarbonyl, aminothiocarbonyl, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkylcarbonyl, C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl, C3-C6-cycloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkyloxycarbonyl, C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino, C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl, C2-C6-alkenyloxy, phenyl, phenoxy, benzyl, benzyloxy, 5- or 6-membered heterocyclyl, 5- or 6-membered hetaryl, 5- or 6-membered hetaryloxy, C(═NOR')--OR'' or OC(R')2--C(R'')═NOR'' the cyclic radicals, in turn, being unsubstituted or substituted by one to three radicals Rb: [0009]Rb being cyano, nitro, halogen, amino, aminocarbonyl, aminothiocarbonyl, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl, C3-C6-cycloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkoxycarbonyl, C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino, C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl, C2-C6-alkenyloxy, C3-C6-cycloalkyl, cycloalkenyl, phenyl, phenoxy, phenylthio, benzyl, benzyloxy, 5- or 6-membered heterocyclyl, 5- or 6-membered hetaryl, 5- or 6-membered hetaryloxy or C(═NOR')--OR'', [0010]R' is hydrogen, cyano, C1-C6-alkyl, C3-C6-cycloalkyl or C1-C4-haloalkyl, [0011]R'' is hydrogen, C1-C6-alkyl, C3-C6-alkenyl, C3-C6-alkynyl, C1-C4-haloalkyl, C3-C6-haloalkenyl or haloalkynyl, [0012]R1 is hydrogen, cyano, C1-C4-alkyl, C1-C4-haloalkyl, C3-C6-cycloalkyl or C1-C4-alkoxy, [0013]R2 is phenyl, phenylcarbonyl, phenylsulfonyl, 5- or 6-membered hetaryl, 5- or 6-membered hetarylcarbonyl or 5- or 6-membered hetarylsulfonyl, the ring systems being unsubstituted or substituted by one to three radicals Ra, [0014]C1-C10-alkyl, C3-C6-cycloalkyl, C2-C10-alkenyl, C2-C10-alkynyl, C1-C10-alkylcarbonyl, C2-C10-alkenylcarbonyl, C3-C10-alkynylcarbonyl, C1-C10-alkylsulfonyl or C(R')═NOR'', the hydrocarbon radicals of these groups being unsubstituted or substituted by one to three radicals Rc: [0015]Rc being cyano, nitro, amino, aminocarbonyl, aminothiocarbonyl, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkoxycarbonyl, C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino, C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl, C2-C6-alkenyloxy, C3-C6-cycloalkyl, C3-C6-cycloalkyloxy, 5- or 6-membered heterocyclyl, 5- or 6-membered heterocyclyloxy, benzyl, benzyloxy, phenyl, phenoxy, phenylthio, 5- or 6-membered hetaryl, 5- or 6-membered hetaryloxy and hetarylthio, it being possible for the cyclic groups, in turn, to be partially or fully halogenated or to have attached to them one to three radicals Ra, and [0016]R3 is hydrogen, C1-C6-alkyl, C2-C6-alkenyl or C2-C6-alkynyl, the hydrocarbon radicals of these groups being unsubstituted or substituted by one to three radicals Rc,andb) a glyphosate derivativein a synergistically active amount.
[0017]It is already known from the literature that active ingredients of the formula I, which are generally referred to as strobilurins, are capable of bringing about increased yields in crop plants in addition to their fungicidal action (Koehle H. et al. in Gesunde Pflanzen 49 (1997), pages 267-271; Glaab J. et al. Planta 207 (1999), 442-448).
[0018]Furthermore, it is known from WO-A 97/36488 that the application of glyphosate derivatives in glyphosate-tolerant plants selected from the group consisting of sugar beet, fodder beet, maize, oilseed rape and cotton may bring about increased yields. Furthermore, it is known from U.S. Pat. No. 3,988,142 that the sublethal application of glyphosate in plants such as sugar cane increases starch and sugar production and thus the overall yield of the plant.
[0019]Surprisingly, it has now been found that the application of glyphosate and strobilurins such as, in particular, pyraclostrobin results in a synergistic effect in legumes. This means that the purely additive (in mathematical terms) yield-increasing effect of strobilurin and of the glyphosate derivative is surpassed by application of the mixture according to the invention. This synergistic effect is more than surprising, since normally it can be assumed that a fungicide and herbicide have completely different mechanisms of action.
[0020]Accordingly, the method defined at the outset has been found. The active ingredients of the formula I which are used are known as fungicides and in some cases also as insecticides (EP-A 253 213; WO-A 95/18789; WO-A 95/24396; WO-A 96/01256; WO-A 97/15552). However, there has been no suggestion to date that these active ingredients in combination with glyphosate derivatives might possibly bring about an increased yield in legumes.
[0021]The good tolerance of the active ingredients of the formula I by plants, at the concentrations required for controlling plant diseases, permits the treatment of aerial plant parts.
[0022]In the method according to the invention, the active ingredient I is preferably taken up by the leaves and distributed throughout the entire plant in the plant sap.
[0023]In a preferred embodiment of the method, the above-ground plant parts of genetically modified legumes are treated with a mixture according to the invention comprising a) a strobilurin derivative I and b) a glyphosate derivative. The application of glyphosate reduces the competition of the crop plant and the weed plants for nutrients and light and thus increases the yield of the crop plant. The mixture according to the invention is especially preferably applied to the above-ground part of the plant.
[0024]Methods for generating plants which are resistant to the effect of glyphosate are described in the more recent literature (EP-A 218 571, EP-A 293 358, WO-A 92/00377 and WO-A 92/04449). Chemical Abstracts, 123, No. 21 (1995) A.N. 281158c describes the generation of glyphosate-resistant soybean plants. Other glyphosate-resistant legumes can be generated in a similar manner. Methods for the transformation of legumes are known in the literature and can be used--as outlined further above--for generating, for example, glyphosate-resistant beans, peas, lentils, peanuts and lupins: Plant Science (Shannon) 150(1) Jan. 14, 2000, 41-49; J. of Plant Biochemistry & Biotechnology 9(2) July, 2000, 107-110; Acta Physiologiae Plantarum 22(2), 2000, 111-119; Molecular Breeding 5(1) 1999, 43-51; In Vitro Cellular & Developmental Biology, Animal 34 (3 Part 2) March, 1998, 53A; Plant Cell Reports 16(8), 1997, 513-519 and 541-544; Theoretical & Applied Genetics 94(2), 1997, 151-158; Plant Science, 117 (1-2), 1996, 131-138; Plant Cell Reports 16(1-2), 1996, 32-37.
[0025]For example soya varieties such as NIDERA AX 4919® which are resistant to numerous fungal diseases and the herbicide glyphosate can be used.
[0026]The preparation of the active ingredients used in the method according to the invention is known from the literature cited at the outset.
[0027]Active ingredients with the following meanings of the substituents, in each case on their own or in combination, are especially preferred for the method according to the invention:
[0028]Especially preferred active ingredients for the method according to the invention are, in particular, those of the formulae Ia to Ig in which
V is OCH3 or NHCH3 and Y is CH or N.
[0029]Preferred active ingredients of the formula I in which Q is C(═N--OCH3)--COOCH3 are the compounds described in the publications EP-A 253 213 and EP-A 254 426.
[0030]Preferred active ingredients of the formula I in which Q is C(═N--OCH3)--CONHCH3 are the compounds described in the publications EP-A 398 692, EP-A 477 631 and EP-A 628 540.
[0031]Preferred active ingredients of the formula I in which Q is N(--OCH3)--COOCH3 are the compounds described in the publications WO-A 93/15046 and WO-A 96/01256.
[0032]Preferred active ingredients of the formula I in which Q is C(═CH--OCH3)--COOCH3 are the compounds described in the publications EP-A 178 826 and EP-A 278 595.
[0033]Preferred active ingredients of the formula I in which Q is C(═CH--CH3)--COOCH3 are the compounds described in the publications EP-A 280 185 and EP-A 350 691.
[0034]Preferred active ingredients of the formula I in which A is --CH2O--N═C(R1)--B are the compounds described in the publications EP-A 460 575 and EP-A 463 488.
[0035]Preferred active ingredients of the formula I in which A is --O--B are the compounds described in the publications EP-A 382 375 and EP-A 398 692.
[0036]EP-A 382 375 describes pyrimidines having the formula (I):
##STR00002##
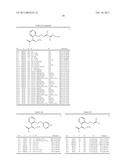
in which any two of K, L and M are nitrogen and the other is CE; X and Y are independently hydrogen, halogen, C1-4 alkyl, C3-6 cycloalkyl, C2-4 alkenyl, C2-4 alkynyl, C2-4 alkynyloxy, phenyl, benzyloxy, cyano, isocyano, isothiocyanato, nitro, NR1R2, NR1OR2, N3, NHCOR1, NR1CO2R2, NHCONR1R2, N═CHNR1R2, NHSO2R1, OR1, OCOR1, OSO2R1, SR1, SOR1, SO2R1, SO2OR1, SO2NR1R2, COR1, CR1═NOR2, CHR1CO2R2, CO2R1, CONR1R2, CSNR1R2, CH3O2C.C:CH.OCH3, 1-(imidazol-1-yl) vinyl, a 5-membered heterocyclic ring containing one, two or three nitrogen heteroatoms, or a 5- or 6-membered heterocyclic ring containing one or two oxygen or sulphur heteroatoms, optionally a nitrogen heteroatom and optionally one or two oxo or thioxo substituents; or X and Y, when ortho to one another, join to form a 5- or 6-membered aliphatic or aromatic ring optionally containing one or two oxygen, sulphur or nitrogen atoms or one, two or three nitrogen atoms; A, B, D, E, G, U and V are independently hydrogen, halogen (especially fluorine and chlorine), C1-4 alkyl (especially methyl), C1-4 alkoxy (especially methoxy), cyano, nitro or trifluoromethyl; and R1 and R2 are independently hydrogen, C1-4 alkyl, C2-4 alkenyl or phenyl; the aliphatic moieties of any of the foregoing being optionally substituted with one or more of halogen, cyano, OR1, SR1, NR1R2, SiR13 or OCOR1 and the phenyl moieties of any of the foregoing being optionally substituted with one or more of halogen, C1-4 alkyl, C1-4 alkoxy, nitro or cyano.
[0037]Because of the unsymmetrically substituted double bond of the propenoate group, the compounds of the invention may be obtained in the form of mixtures of (E) and (Z) geometric isomers. However, these mixtures can be separated into individual isomers, and this invention embraces such isomers and mixtures thereof in all proportions including those which consist substantially of the (Z)-isomer and those which consist substantially of the (E)-isomer.
[0038]The (E)-isomer, in which the groups --CO2CH3 and --OCH3 are on opposite sides of the olefinic bond of the propenoate group, are the more fungicidally active and form a preferred embodiment of the invention.
[0039]Alkyl groups contain from 1 to 4 carbon atoms and may be in the form of straight or branched chains. Examples are methyl, ethyl, iso-propyl, n-butyl and t-butyl. Cycloalkyl groups contain from 3 to 6 carbon atoms and include cyclopropyl and cyclohexyl.
[0040]Alkenyl and alkynyl groups contain from 2 to 4 carbon atoms and may be in the form of straight or branched chains. Examples are ethenyl, allyl, methylallyl and propargyl.
[0041]Halogen is typically fluorine, chlorine or bromine.
[0042]Substituted aliphatic moieties include, in particular, halo(C1-4) alkyl, halo(C1-4alkoxy, halo(C1-4)alkylthio, CH2OR1, CH2SR1 and CH2NR1R2, wherein R1 and R2 are H, C1-4 alkyl or phenyl.
[0043]Typical optional substituents of phenyl moieties are fluorine, chlorine, methyl, methoxy, nitro and cyano.
[0044]The ring
##STR00003##
in formula (I) is a pyrimidine ring which may be joined to the phenoxy groups by any two of its ring carbon atoms adjacent to a ring nitrogen atom. Of particular interest are those compounds of formula (I) in which K and L are both nitrogen and M is CH. Typically, one or both of X and Y are hydrogen. When one of X and Y is not hydrogen it is preferably attached to the 2-position of the phenyl ring.
[0045]Thus, in one aspect, EP-A 382 375 describes compounds of formula (I) in which K, L and M have the meanings previously given; X, which is preferably attached to the 2-position of the phenyl ring, is hydrogen, halogen (e.g. fluorine, chlorine or bromine), C1-4 alkyl (e.g. methyl or ethyl), C1-4 alkyl (especially methyl) substituted with halogen (e.g. fluorine, chlorine or bromine), hydroxy, cyano, C1-4 alkoxy (e.g. methoxy) or C1-4 alkanoyloxy (e.g. acetoxy), C2-4 alkenyl (e.g. ethenyl, allyl or methylallyl), C2-4 alkynyl (e.g. ethynyl or propargyl), C2-4 alkenyloxy (e.g. allyloxy), C2-4 alkynyloxy (e.g. propargyloxy), phenyl, benzyl, cyano, isocyano, isothiocyanato, nitro, amino, mono- or di(C1-4)alkylamino (e.g. methylamino or dimethylamino), formylamino, C1-4 alkanoylamino (e.g. acetamido), benzylamino, ureido, phenylureido, C1-4 alkylsulphonylamino (e.g. mesylamino), phenylsulphonylamino, hydroxy, C1-4 alkoxy (e.g. methoxy or ethoxy), phenoxy, C1-4 alkanoyloxy (e.g. acetoxy), C1-4 alkylsulphonyloxy (e.g. mesyloxy), phenylsulphonyloxy, C1-4 alkylthio (e.g. methylthio), C1-4 alkylsulphinyl (e.g. methylsulphinyl), C1-4 alkylsulphonyl (e.g. mesyl and n-butylsulphonyl), formyl, C1-4 alkanoyl (e.g. acetyl), benzoyl, hydroxyimino(C1-4)alkyl (e.g. hydroxyiminomethyl), C1-4 alkoxyimino(C1-4)alkyl (e.g. methoxyiminomethyl), carbamoyl, C1-4 alkylcarbamoyl (e.g. methylcarbamoyl), thiocarbamoyl or C1-4 alkylthiocarbamoyl (e.g. methylthiocarbamoyl), the phenyl ring of any of the foregoing being optionally substituted with halogen (e.g. fluorine or chlorine), C1-4 alkyl (e.g. methyl), C1-4 alkoxy (e.g. methoxy), nitro or cyano; and Y is halogen (e.g. fluorine or chlorine), C1-4 alkyl (e.g. methyl), C1-4 alkoxy (e.g. methoxy), nitro, cyano or preferably, hydrogen, or X and Y, when ortho to one another, together form methylenedioxy, or together with the phenyl ring to which they are attached form a naphthalene, quinoline, benzimidazole or benzothienyl ring.
[0046]In another aspect EP-A 382 375 describes compounds of the formula (I.1):
##STR00004##
in which X is hydrogen, halogen (especially chlorine), C1-4 alkyl (especially methyl), C1-4 alkoxy (especially methoxy), trifluoromethyl, cyano, thiocarbamoyl or nitro, and Y is hydrogen or fluoro.
[0047]EP-A 382 375 also discloses compounds listed in Tables I to III which follow. Throughout these Tables the methyl 3-methoxypropenoate group has the (E)-configuration and the substituents E, G, U and in are all hydrogen.
TABLE-US-00001 TABLE I ##STR00005## Compound Melting No. X Y point (° C.) Olefinic* 1 H H glass 7.46 2 2-F H gum 7.47 3 3-F H gum 7.47 4 4-F H 87-9 7.46 5 2-Cl H glass 7.38 6 3-Cl H 7 4-Cl H 8 2-Br H glass 7.42 9 2-Cyano H 118-119 7.50 10 3-Cyano H gum 7.49 11 4-Cyano H gum 7.49 12 2-Isocyano H 13 2-NO2 H 120-121 7.52 14 3-NO2 H gum 7.49 15 4-NO2 H gum 7.48 16 2-NH2 H gum 7.46 17 3-NH(CH3) H 18 2-N(CH3)2 H 19 2-NH•CHO H 20 2-NH•COCH3 H 21 3-NH•COC6H5 H 22 2-NH•CONH2 H 23 3-NH•CONH(C2H5) H 24 2-NH•SO2CH3 H 25 3-NH•SO2C6H5 H 26 2-OH H 159-161 7.45 27 3-OH H 28 4-OH H 29 2-OCH3 H gum 7.49 30 3-OCH3 H gum 7.47 31 4-OCH3 H 88-90 7.45 32 2-OC2H5 H glass 7.46 33 3-(2-F--C6H4O) H 34 2-OCOCH3 H gum 7.47 35 2-OSO2CH3 H foam 7.47 36 3-(4-CH3--C6H4SO2O) H 37 2-SCN H 38 3-SCN H 39 4-SCN H 40 2-SCH3 H gum 7.48 41 3-SCH3 H 42 4-SCH3 H 43 2-S(O)CH3 H 135-6 7.48 44 2-SO2CH3 H 61-4 7.49 45 4-SO2(CH2)3CH3 H 46 2-CHO H foam 7.50 47 3-CHO H 48 4-CHO H 49 2-COCH3 H 99-101 7.42 50 3-COC6H5 H 51 2-(E)--CH═NOH H 146-7 7.45 52 3-(E)--CH═NOH H 53 4-(E)--CH═NOH H 54 2-(E)--CH═NOCH3 H 55 2-(E)--C(CH3)═NOH H 56 2-CONH2 H 57 3-CONH(CH3) H 58 4-CON(CH3)2 H 59 2-CSNH2 H 131-3 7.49 60 2-CSNH(CH3) H 61 2-CH3 H gum 7.48 62 3-CH3 H 92-5 7.45 63 4-CH3 H gum 7.46 64 2-C2H5 H 60-2 7.47 65 2-CH2F H 66 2-CH2Br H 67 2-CH2Cl H 68 2-CH2CN H 69 2-CH2OH H 70 2-CH2OCH3 H 71 2-CH2OCOCH3 H 72 3-CH2CN H 73 4-CH2OH H 74 3-CH2OCH3 H 75 2-CH═CH2 H 76 2-CH2CH═CH2 H gum 7.47 77 2-C≡CH H 66-8 7.46 78 2-CH2C≡CH H 79 3-CH2C(CH3)═CH2 H 80 2-OCH2CH═CH2 H glass 7.47 81 2-OCH2C≡CH H gum 7.47 82 2-C6H5 H 55 7.40 83 3-C6H5 H 84 4-C6H5 H 85 2-C6H5O H 86 3-C6H5O H 87 4-C6H5O H 88 2-(4-Cl--C6H4O) H 89 2-C6H5CH2O H 90 2-Cyano 4-Cl 91 2-NO2 4-F 92 2-Cl 4-Cl 93 2-OCH3 3-OCH3 94 2-Cyano 5-Cl 95 2-Cyano 6-Cyano 96 2-F 5-Cl 97 3-OCH3 5-OCH3 98 3-Cyano 4-F 99 2-NO2 3-OCH3 100 3-OCH3 5-Cyano 101 2-CO2CH3 H glass 7.50 102 2-I H glass 7.48 103 2-CF3 H 99-101 7.48 104 2-i-C3H7 H 63-5 7.47 105 2-i-C3H7O H glass 7.47 106 2-F 6-F 87-8 7.49 107 2-F 4-F 92-4 7.48 108 2-F 3-F gum 7.48 109 2-n-C3H7O H gum 7.46 110 2-n-C4H9O H gum 7.47 111 2-CH(OH)CH3 H 50-3 7.46 112 2-t-C4H9 H gum 7.47 113 2-s-C4H9 H gum 7.47 114 2-n-C3H7 H gum 7.47 115 2-(E/Z)--CH═CH(CH3) H glass 7.461 116 2-Cyano 4-OCH3 gum 7.50 117 2-Cyano 5-OCH3 oil 7.50 118 2-Cyano 4-Cl 78-82 7.50 119 2-Cyano 5-N(C2H5)2 oil 7.50 120 2-CONH2 H 138-141 7.46 121 2-C≡CSi(CH3)3 H gum 7.46 122 2-F 5-F 100-101 7.48 123 2-(E)--CH3O2C•C═CH•OCH3 H 130-131 7.45 124 3-F 5-F 68-70 7.47 125 2-NHOH H 126 2-CH2OCH3 H 127 2-CH2CN H 128 2-N3 H 129 2-Cyano 6-F 130 2-NO2 6-F 131 2-CSNH2 6-F 132 2-Cyano 3-F 133 2-Cyano 5-F 134 2-Cyano 3-OCH3 135 2-Cyano 6-OCH3 136 2-NO2 4-OCH3 137 2-NO2 5-OCH3 138 2-NO2 6-OCH3 139 2-CSNH2 3-OCH3 140 2-CSNH2 4-OCH3 141 2-CSNH2 5-OCH3 142 2-CSNH2 6-OCH3 143 2-Cyano 3-Cyano 144 2-F 3-Cyano 144 2-OCH3 3-Cyano 145 3-Cyano 6-F 146 ##STR00006## H 147 ##STR00007## H 148 ##STR00008## H 149 ##STR00009## H 150 2-Cyano 4-Br 151 2-Cyano 6-Br 152 2-Cyano 4-NO2 153 2-Cyano 6-NO2 154 2-Cyano 6-OC2H5 155 2-Cyano 4-CO2CH3 156 2-Cyano 6-CO2C2H5 157 2-Cyano 6-CH3 158 2-Cyano 5-CH2C6H5 159 2-Cyano 4-OCF3 160 2-Cyano 4-Cyano ##STR00010## Compound Melting No. Ar point (° C.) Olefinic* 161 ##STR00011## 133-5 7.52 162 ##STR00012## 163 ##STR00013## 164 ##STR00014## 165 ##STR00015## 166 ##STR00016## 167 Pentafluorophenyl 168 2,4,6-Tri-F--C6H2 169 2,3,5,6-Tetra-F--C6H 170 2,3,6-Tri-F--C6H2 171 2,3-Di-cyano-6-F--C6H2 172 2,6-Di-F-3-CH3O--C6H2 173 2,6-Di-F-4-CH3O--C6H2 174 2,6-Di-F-3-NO2--C6H2 175 2,6-Di-F-4-NO2--C6H2 176 2,6-Di-F-3,5-di-CH3O--C6H 177 4,6-Di-Br-2-cyano-C6H2 178 3-Cyano-2,6-di-F--C6H2 179 6-Br-2-cyano-4-CH3O--C6H2 180 6-Br-4-Cl-2-cyano-C6H2 181 6-Br-2-cyano-4-NO2--C6H2 182 3-Br-2-cyano-6-CH3O--C6H2 183 3,5-Di-Cl-2-cyano-C6H2 184 4,6-Di-Cl-2-cyano-C6H2 185 3-Br-2-cyano-4-CH3O--C6H2 186 4-Br-2-cyano-6-NO2--C6H2 187 4-Br-2-cyano-6-CH3O--C6H2 188 2-Cyano-4-I-6-CH3O--C6H2 189 2-Cyano-6-CH3O-4-NO2--C6H2 190 2-Cyano-4,6-di-NO2--C6H2 191 2-Cyano-4-CH3-6-NO2--C6H2 192 2-Cyano-4-CH3O-6-NO2--C6H2 193 2-Cyano-5,6-di-CH3O--C6H2 194 2-Cyano-5,6-di-CH3O-3-CH3--C6H 195 3,4-Di-Br-2-cyano-6-CH3O--C6H 196 3-Br-2-cyano-6-CH3O-4-NO2--C6H 197 2-Cyano-6-CH3CH2O-4-NO2--C6H2 198 ##STR00017## 199 ##STR00018## *Chemical shift of singlet from olefinic proton on beta-methoxypropenoate group (ppm from tetramethyl-silane). Solvent: CDCl3 unless otherwise stated. 1The ratio of the (E)- and (Z)-isomers of the prop-1-enyl group of compound No. 115 is either 2:1 or 1:2.
TABLE-US-00002 TABLE II ##STR00019## Compound Melting No X Y Point (° C.) Olefinic* 1 H H 114-115 7.46 123 2-(E)--CH3O2C•C═CH•OCH3 H 60-70 7.44 and 7.47 *Chemical shift of singlet from olefinic proton on beta-methoxypropenoate group (ppm from tetramethylsilane). Solvent: CDCl3 unless otherwise stated.
[0048]Table II comprises 199 compounds of the general structure above with all the values of X and Y listed in Table I. That is, compounds numbers 1 to 199 of Table II are the same as those of Table I except that the pyrimidine ring is 4,6-disubstituted in Table 1 and 2,4-disubstituted as shown in Table II.
TABLE-US-00003 TABLE III ##STR00020## Compound Melting No X Y Point (° C.) Olefinic* 1 H H 96-97 7.42 9 2-Cyano H foam 7.43 *Chemical shift of singlet from olefinic proton on beta-methoxypropenoate group (ppm from tetramethylsilane). Solvent: CDCl3 unless otherwise stated.
[0049]Table III comprises 199 compounds of the general structure above with all the values of X and Y listed in Table I. That is, compounds numbers 1 to 199 of Table III are the same as those of Table I except that the pyrimidine ring is 4,6-disubstituted in Table I and 2,4-disubstituted as shown in Table III.
EXAMPLE
[0050]This example illustrates the preparation of (E)-methyl 2-[2-(6-(2-cyanophenoxy)pyrimidin-4-yloxy)phenyl]-3-methoxypropenoate (compound No. 9 of Table I or "azoxystrobin").
[0051]To a solution of 4,6-dichloropyrimidine (0.76 g, 5.10 mmol) in dry DMF (4 ml) at 0° C. was added anhydrous potassium carbonate (0.70 g, 5.10 mmol). A solution of (E)-methyl 2-(2-hydroxyphenyl)-3-methoxypropenoate (0.53 g, 2.55 mmol, prepared as described in Example 3 of EP-A-0242081) in dry DMF (2 ml) was then added dropwise with stirring. After the addition was complete, the reaction mixture was allowed to warm to room temperature and stirring continued over the weekend. The reaction mixture was then diluted with water (15 ml) and extracted with ether (3×20 ml). The combined ether extracts were washed with brine and dried. Evaporation afforded a brown liquid (1.10 g) which was chromatographed (eluent ether:n-hexane, 3:2) to give (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxypropenoate as a thick, pale yellow oil (0.58, 71 percent yield) which crystallised on standing. Recrystallisation from ether/dichloromethane (trace)/n-hexane at -78° C. gave the product as a white powder (0.25 g), mp 94-5° C. In a separate preparation, 15 g of product was obtained from 4,6-dichloropyrimidine (15.90 g), (E)-methyl 2-(2-hydroxyphenyl)-3-methoxypropenoate (14.80 g) and anhydrous potassium carbonate (19.64 g).
[0052](E)-Methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxypropenoate (1.50 g, 4.68 mmol) was heated overnight at 95-100° C. with 2-cyanophenol (0.61 g, 5.15 mmol) and potassium carbonate (0.71 g, 5.15 mmol) in DMF (35 ml) in the presence of a catalytic amount of copper(I) chloride. The reaction mixture was cooled, diluted with water and then extracted with ether. The combined ether layers were washed with 2M sodium hydroxide solution and brine and then dried. Evaporation of the solvent gave a pale yellow oil (1.52 g). Recrystallisation from ether/dichloromethane/n-hexane gave the title compound as a pale yellow powder (1.20 g, 64% yield), mp 110-111° C.; 1H NMR δ: 3.63 (3H, s); 3.74 (3H, s); 6.42 (1H, s); 7.19-7.47 (6H, m); 7.50 (1H, s); 7.62-7.75 (2H, m); 8.40 (1H, s) ppm. In a subsequent preparation of the title compound, recrystallisation gave a white crystalline solid, mp 118-119° C.
[0053]Preferred active ingredients of the formula I in which A is --CH2O--N═C(R1)--C(R2)═N--OR3 are the compounds described in the publications WO-A 95/18789, WO-A 95/21153, WO-A 95/21154, WO-A 97/05103, WO-A 97/06133 and WO-A 97/15552.
[0054]Especially preferred are active ingredients of the formula I in which [0055]Q is C(═N--OCH2)--COOCH3 or C(═N--OCH2)--CONHCH2, [0056]A is CH2--O-- and [0057]B is --N═C(R1)--C(R2)═N--OR2, where [0058]R1 is hydrogen, cyano, cyclopropyl, C1-C4-alkyl or C1-C2-haloalkyl, in particular methyl, ethyl, 1-methylethyl or trifluoromethyl, and [0059]R2 is C1-C4-alkyl, C2-C5-alkenyl, phenyl which is substituted by one or two halogen atoms, or is C(R')═NOR'', where [0060]R' is one of the groups mentioned above under R1 and [0061]R'' is hydrogen, cyclopropyl or C1-C4-alkyl, in particular methyl, ethyl or isopropyl, and [0062]R3 is one of the groups mentioned under R'';these active ingredients are described by the formula Ib
##STR00021##
[0062]in which the variables have the abovementioned meanings.
[0063]Active ingredients of the formula Ib'
##STR00022##
in which the variables have the abovementioned meanings are particularly preferred.
[0064]In addition, other compounds which are especially preferred are those of the formula Ia where T is CH or N and R' and Rb are halogen or C1-C4-alkyl and x is 0, 1 or 2 and y is 0 or 1.
##STR00023##
[0065]The active ingredients compiled in the tables which follow are especially preferred with regard to their use in increasing yield.
TABLE-US-00004 TABLE I Ia ##STR00024## Position of the No. T (Ra')y group phenyl-(Rb)x (Rb)x Reference Ia-1 N -- 1 2,4-Cl2 WO-A 96/01256 Ia-2 N -- 1 4-Cl WO-A 96/01256 Ia-3 CH -- 1 2-Cl WO-A 96/01256 Ia-4 CH -- 1 3-Cl WO-A 96/01256 Ia-5 CH -- 1 4-Cl WO-A 96/01256 Ia-6 CH -- 1 4-CH3 WO-A 96/01256 Ia-7 CH -- 1 H WO-A 96/01256 Ia-8 CH -- 1 3-CH3 WO-A 96/01256 Ia-9 CH 5-CH3 1 3-CF3 WO-A 96/01256 Ia-10 CH 1-CH3 5 3-CF3 WO-A 99/33812 Ia-11 CH 1-CH3 5 4-Cl WO-A 99/33812 Ia-12 CH 1-CH3 5 -- WO-A 99/33812
[0066]The active ingredient Ia-5 (common name: pyraclostroblin) is especially preferred.
TABLE-US-00005 TABLE II II ##STR00025## No. V R1 R2 R3 Reference Ib-1 OCH3 CH3 CH3 CH3 WO-A 95/18789 Ib-2 OCH3 CH3 CH(CH3)2 CH3 WO-A 95/18789 Ib-3 OCH3 CH3 CH2CH3 CH3 WO-A 95/18789 Ib-4 NHCH3 CH3 CH3 CH3 WO-A 95/18789 Ib-5 NHCH3 CH3 4-F--C6H4 CH3 WO-A 95/18789 Ib-6 NHCH3 CH3 4-Cl--C6H4 CH3 WO-A 95/18789 Ib-7 NHCH3 CH3 2,4-C6H3 CH3 WO-A 95/18789 Ib-8 NHCH3 Cl 4-F--C6H4 CH3 WO-A 98/38857 Ib-9 NHCH3 Cl 4-Cl--C6H4 CH2CH3 WO-A 98/38857 Ib-10 NHCH3 CH3 CH2C(═CH2)CH3 CH3 WO-A 97/05103 Ib-11 NHCH3 CH3 CH═C(CH3)2 CH3 WO-A 97/05103 Ib-12 NHCH3 CH3 CH═C(CH3)2 CH2CH3 WO-A 97/05103 Ib-13 NHCH3 CH3 CH═C(CH3) CH2CH3 CH3 WO-A 97/05103 Ib-14 NHCH3 CH3 O--CH(CH3)2 CH3 WO-A 97/06133 Ib-15 NHCH3 CH3 O--CH2CH(CH3)2 CH3 WO-A 97/06133 Ib-16 NHCH3 CH3 C(CH3)═NOCH3 CH3 WO-A 97/15552 Ib-17 NHCH3 CH3 C(CH3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-18 NHCH3 CH3 C(CH3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-19 NHCH3 CH3 C(CH3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-20 NHCH3 CH3 C(CH3)═NOCH2CH═CH2 CH2CH═CH2 WO-A 97/15552 Ib-21 NHCH3 CF3 C(CF3)═NOCH3 CH3 WO-A 97/15552 Ib-22 NHCH3 CF3 C(CF3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-23 NHCH3 CF3 C(CF3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-24 NHCH3 CF3 C(CF3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-25 NHCH3 CF3 C(CF3)═NOCH2CH═CH2 CH2CH═CH2 WO-A 97/15552 Ib-26 OCH3 CH3 C(CH3)═NOCH3 CH3 WO-A 97/15552 Ib-27 OCH3 CH3 C(CH3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-28 OCH3 CH3 C(CH3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-29 OCH3 CH3 C(CH3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-30 OCH3 CH3 C(CH3)═NOCH2CH═CH2 CH2CH═CH2 WO-A 97/15552 Ib-31 OCH3 CF3 C(CF3)═NOCH3 CH3 WO-A 97/15552 Ib-32 OCH3 CF3 C(CF3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-33 OCH3 CF3 C(CF3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-34 OCH3 CF3 C(CF3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-35 OCH3 CF3 C(CF3)═NOCH2CH═CH3 CH2CH═CH2 WO-A 97/15552
TABLE-US-00006 TABLE III Ic ##STR00026## No. V Y T Ra Reference Ic-1 OCH3 CH N 2-OCH3, 6-CF3 WO-A 96/16047 Ic-2 OCH3 CH N 2-OCH(CH3)2, 6-CF3 WO-A 96/16047 Ic-3 OCH3 CH CH 5-CF3 EP-A 278 595 Ic-4 OCH3 CH CH 6-CF3 EP-A 278 595 Ic-5 NHCH3 N CH 3-Cl EP-A 398 692 Ic-6 NHCH3 N CH 3-CF3 EP-A 398 692 Ic-7 NHCH3 N CH 3-CF3, 5-Cl EP-A 398 692 Ic-8 NHCH3 N CH 3-Cl, 5-CF3 EP-A 398 692
TABLE-US-00007 TABLE IV Id ##STR00027## No. V Y R1 B Reference Id-1 OCH3 CH CH3 (3-CF3)C6H4 EP-A 370 629 Id-2 OCH3 CH CH3 (3,5-Cl2)C6H3 EP-A 370 629 Id-3 NHCH3 N CH3 (3-CF3)C6H4 WO-A 92/13830 Id-4 NHCH3 N CH3 (3-OCF3)C6H4 WO-A 92/13830 Id-5 OCH3 N CH3 (3-OCF3)C6H4 EP-A 460 575 Id-6 OCH3 N CH3 (3-CF3)C6H4 EP-A 460 575 Id-7 OCH3 N CH3 (3,4-Cl2)C6H3 EP-A 460 575 Id-8 OCH3 N CH3 (3,5-Cl2)C6H3 EP-A 463 488
TABLE-US-00008 TABLE V Ie ##STR00028## No. V Y Ra Reference Ie-1 OCH3 N 2-CH3 EP-A 253 213 Ie-2 OCH3 N 2,5-(CH3)2 EP-A 253 213 Ie-3 NHCH3 N 2,5-(CH3)2 EP-A 477 631 Ie-4 NHCH3 N 2-Cl EP-A 477 631 Ie-5 NHCH3 N 2-CH3 EP-A 477 631 Ie-6 NHCH3 N 2-CH3, 4-OCF3 EP-A 628 540 Ie-7 NHCH3 N 2-Cl, 4-OCF3 EP-A 628 540 Ie-8 NHCH3 N 2-CH3, 4-OCH(CH3)--C(CH3)═NOCH3 EP-A 11 18 609 Ie-9 NHCH3 N 2-Cl, 4-OCH(CH3)--C(CH3)═NOCH3 EP-A 11 18 609 Ie-10 NHCH3 N 2-CH3, 4-OCH(CH3)--C(CH2CH3)═NOCH3 EP-A 11 18 609 Ie-11 NHCH3 N 2-Cl, 4-OCH(CH3)--C(CH3)═NOCH2CH3 EP-A 11 18 609
TABLE-US-00009 TABLE VI If ##STR00029## No. V Y Ra Reference If-1 NHCH3 N H EP-A 398 692 If-2 NHCH3 N 3-CH3 EP-A 398 692 If-3 NHCH3 N 2-NO2 EP-A 398 692 If-4 NHCH3 N 4-NO2 EP-A 398 692 If-5 NHCH3 N 4-Cl EP-A 398 692 If-6 NHCH3 N 4-Br EP-A 398 692
TABLE-US-00010 TABLE VII Ig ##STR00030## No. V Y T Ra Reference Ig-1 OCH3 CH N 6-O--(2-CN--C6H4) EP-A 382 375 Ig-2 OCH3 CH N 6-O--(2-Cl--C6H4) EP-A 382 375 Ig-3 OCH3 CH N 6-O--(2-CH3--C6H4) EP-A 382 375 Ig-4 NHCH3 N N 6-O--(2-Cl--C6H4) GB-A 22 53 624 Ig-5 NHCH3 N N 6-O--(2, 4-Cl2--C6H3) GB-A 22 53 624 Ig-6 NHCH3 N N 6-O--(2-CH3--C6H3) GB-A 22 53 624 Ig-7 NHCH3 N N 6-O--(2-CH3, 3-Cl--C6H3) GB-A 22 53 624 Ig-8 NHCH3 N N 2-F, 6-O--(2-CH3--C6H4) WO-A 98/21189 Ig-9 NHCH3 N N 2-F, 6-O--(2-Cl--C6H4) WO-A 98/21189 Ig-10 NHCH3 N N 2-F, 6-O--(2-CH3, WO-A 98/21189 3-Cl--C6H3)
[0067]Fungicidal active ingredients which can be employed are the strobilurins I alone or in mixture with other fungicidal active ingredients, in particular those from the class of the azoles Ix.
[0068]Azole active ingredients which are suitable for this purpose are: [0069]fluquinconazole, Proc. Br. Crop Prot. Conf.--Pests Dis., 5-3, 411 (1992); [0070]metconazole, Proc. Br. Crop Prot. Conf.--Pests Dis., 5-4, 419 (1992); [0071]prochloraz, U.S. Pat. No. 3,991,071; [0072]propiconazole, GB-A 1,522,657; [0073]prothioconazole, WO-A 96/016048; [0074]tebuconazole, U.S. Pat. No. 4,723,984; [0075]epoxiconazole, EP-A 196038; [0076]myclobutanil, CAS RN [88671-89-0];
[0077]Azoles which are especially suitable are: metconazole, myclobutanil, epoxiconazole, propiconazole, prothioconazole or tebuconazole.
[0078]If fungicide mixtures of, for example, strobilurins I and azoles Ix are employed, they are generally employed in a weight ratio I to Ix of 20:1 to 0.05:1, preferably 10:1 to 0.1:1.
[0079]Glyphosate derivatives II are essentially understood as meaning the following compounds, which are mentioned in The Pesticide Manual: for example, glyphosate may be employed as the free acid or in the form of salts such as the isopropylammonium salt, the sodium salt, the ammonium salt or the trimesium (trimethylsulfenium) salt. Mixtures of the salts may also be employed. Moreover, the glyphosate derivatives II include the compound N-(phosphonomethyl)glycine. The preparation of the glyphosate derivatives II can be found in the literature cited in The Pesticide Manual (12th edition).
[0080]The compounds I in combination with glyphosate derivatives raise the yield potential in legumes. They are especially important for the treatment of various glyphosate-resistant crop plants such as peas, beans, lentils, peanuts, lupins and in particular soybeans. The synergistic effect is demonstrated independently of the generation of the glyphosate-resistant legumes.
[0081]Specifically, they are suitable for controlling the following symptoms: [0082]signs of wilting despite the availability of sufficient nutrients, [0083]discolorations of the green leaf tissue such as, for example bleaching of soybeans.
[0084]The compounds I are applied by treating the plants to be protected with an effective amount of the active ingredients. Application can be effected both before and after application of the glyphosate derivatives II to the plants.
[0085]In a preferred embodiment of the method, the treatment of the plant is effected jointly with the application of the fungicide I and the herbicide II. The synergistic effect is particularly pronounced in this case.
[0086]When using an active ingredient I, the application rates are in the range of from 0.01 to 2.0 kg of active ingredient per hectare, depending on the weather conditions and the plant species.
[0087]When using a glyphosate derivative II, the application rates are in the range of from 0.1 to 6.0 kg of active ingredient (acid equivalent) per hectare, depending on the weather conditions and the plant species.
[0088]As a rule, the fungicide I, or the fungicidal mixture I and Ix, is employed in a weight ratio to the herbicide II of 5:1 to 0.01:1, preferably 1:1 to 0.1:1.
[0089]The compounds I and the glyphosate derivatives II may be converted into the formulations conventionally used for crop protection products, for example solutions, emulsions, suspensions, dusts, powders, pastes and granules. The use form depends on the application in question; in any case, it should ensure uniform and even distribution of the mixture according to the invention.
[0090]The formulations are prepared in the known manner, for example by extending the active ingredient with solvents and/or carriers, if desired using emulsifiers and dispersants, it also being possible to use other organic solvents as cosolvents if water is used as the diluent. Auxiliaries are essentially those also conventionally used for fungicides.
[0091]In general, the formulations comprise between 0.01 and 95% by weight, preferably between 0.1 and 90% by weight, of the active ingredient. The active ingredients are employed in a purity of from 90% to 100%, preferably 95% to 100% (according to NMR spectrum).
[0092]Examples of formulations are known from the publications cited at the outset.
[0093]Aqueous use forms can usually be prepared from emulsion concentrates, pastes or wettable powders (sprayable powders, oil dispersions) by addition of water. To prepare emulsions, pastes or oil dispersions, the substances, as such or dissolved in an oil or solvent, may be homogenized in water by means of wetter, sticker, dispersant or emulsifier. Alternatively, it is possible to prepare concentrates consisting of active substance, wetter, sticker, dispersant or emulsifier and, if appropriate, solvent or oil, and such concentrates are suitable for dilution with water.
[0094]The active ingredient concentrations in the ready-to-use products may be varied within substantial ranges. In general, they are between 0.0001 and 10%, preferably between 0.01 and 1%.
[0095]The active ingredients may also be used successfully by the ultra-low-volume (ULV) method, it being possible to apply formulations comprising more than 95% by weight of active ingredient, or indeed the active ingredient without additions.
[0096]Various types of oils or herbicides, other fungicides, other pesticides or bactericides may be added to the active ingredients, if appropriate just prior to use (tank mix). These agents can be admixed with the compositions according to the invention in a weight ratio of from 1:10 to 10:1.
[0097]The active ingredients I are preferably applied to the plant jointly or separately with the glyphosate II.
[0098]In general, the compounds I and II are applied within a period of weeks to 3 months, preferably within 1 to 2 months, after planting the legume seeds. It may be advantageous to carry out the fungicide or herbicide treatment repeatedly, preferably twice.
[0099]In the case of separate use, it may be advantageous to apply the herbicide II for example 3-6 weeks after planting the legume seeds and then to apply either the fungicide I alone or a mixture of fungicide I and herbicide II in a second application 4-8 weeks after planting.
[0100]In the case of joint application, a mixture of the compounds I and II is generally applied once to twice within a period of 1 to 3 months after planting the legume seeds.
[0101]The abovementioned application methods are understood as meaning foliar treatment of the legumes. In comparison to, for example, a seed treatment, these methods have pronounced advantages.
[0102]The use examples demonstrate the increased yield achieved by the use of pyraclostrobin and glyphosate in soya plantations.
[0103]It must be added that the increased yield is not connected to a successful control of harmful fungi. In the experiments, the experimental fields were free from disease. Naturally, in such a case the yield would be increased even more since the fungicidal active ingredients I (strobilurins) and Ix (azoles) or their mixtures constitute extremely efficient fungicides. Yield losses caused by harmful fungi can be counteracted effectively by the methods according to the invention.
[0104]Mention of the use according to the invention of the active ingredients I may be made in the form of an imprint on the packaging or else in product data sheets. Such mention may also be made in the case of products which can be used in combination with the active ingredients I.
[0105]Use examples for the increased yield in legumes
Use Example
[0106]The results shown hereinbelow were obtained in experiments in the open which were carried out during the winter season in the Argentinian northern pampas. The plots used were arranged randomly relative to one another. Each treatment variant was replicated four times. The crop plant used was the soya variety NIDERA AX 4910, which is resistant to numerous fungal diseases and to the herbicide glyphosate.
[0107]In all 5 experiments, two foliar treatments with glyphosate were carried out 30 or 60 days after planting the soya seeds, using equipment conventionally used under practice conditions. In the experiments 2 and 3, pyraclostrobin was added at "30 days after planting", while pyraclostrobin was added at "60 days after planting" in the experiments 4 and 5. As demonstrated by the results, the addition of pyraclostrobin in amounts of 50 or 100 g of a.s./ha at both the early and the late treatment times markedly increased the yield in comparison with the conventional use of glyphosate alone.
TABLE-US-00011 Treatment 30 Treatment 60 Experiment days after a.s. days after a.s. number planting g/ha planting g/ha Yield 1 glyphosate 360 glyphosate 360 100% 2 glyphosate 360 glyphosate 360 116% pyra- 50 clostrobin 3 glyphosate 360 glyphosate 360 129% pyra- 100 clostrobin 4 glyphosate 360 glyphosate 360 122% pyra- 50 clostrobin 5 glyphosate 360 glyphosate 360 135% pyra- 100 clostrobin a.s. = active substance
Claims:
1. A method for synergistically increasing the yield in
glyphosate-resistant legumes, which comprises treating the plants with a
mixture comprising(a) azoxystrobinand(b) a glyphosate derivative II
selected from the group consisting of N-(phosphonomethyl)glycine as a
free acid or a salt thereofin a synergistically active amount, wherein
the weight ratio of the compound of the formula Ia to the glyphosate
derivative II is from 1:1 to 0.01:1.
2. The method as claimed in claim 1, wherein the salt of N-(phosphonomethyl)glycine is selected from the group consisting of the isopropylammonium salt, sodium salt, ammonium salt and trimethylsulfenium salt.
3. The method as claimed in claim 1, wherein the mixture comprises:(a) azoxystrobin and(b) a glyphosate derivative II.
4. The method as claimed in claim 1, wherein component (b) is N-(phosphonomethyl)glycine as a free acid.
5. A method as claimed in claim 1, wherein a fungicidal azole selected from the group consisting of: fluquinconazole, metconazole, prochloraz, propiconazole, prothioconazole, tebuconazole, epoxiconazole or myclobutanil is employed as component a) in addition to the active compound of the formula Ia.
6. A synergistic mixture comprising(a) azoxystrobin and(b) a glyphosate derivative II selected from the group consisting of N-(phosphonomethyl)glycine as a free acid or a salt thereofwherein the weight ratio of the compound of the formula Ia to the glyphosate derivative II is from 1:1 to 0.01:1.
7. A mixture as claimed in claim 6, wherein the mixture comprises:(a) azoxystrobin and(b) a glyphosate derivative II.
8. A mixture as claimed in claim 7, wherein component a) comprises an azole selected from the group consisting of: metconazole, myclobutanil, epoxiconazole, propiconazole, prothioconazole and tebuconazole in addition to the active compound pyraclostrobin.
9. A mixture as claimed in claim 7, wherein component (b) is a salt of N-(phosphonomethyl)glycine selected from the group consisting of the isopropylammonium salt, sodium salt, ammonium salt and trimethylsulfenium salt.
10. The method as claimed in claim 3, wherein the weight ratio of the compound pyraclostrobin to the glyphosate derivative II is 1:1 to 0.1:1.
11. A mixture as claimed in claim 7, wherein the weight ratio of the compound pyraclostrobin to the glyphosate derivative II is 1:1 to 0.1:1.
Description:
[0001]The present application is a 37 C.F.R. §1.53(b) divisional of
Ser. No. 10/534,637, and claims priority to, U.S. application Ser. No.
10/534,637 filed May 12, 2005. application Ser. No. 10/534,634 is the
national phase under 35 U.S.C. §371 of International Application No.
PCT/EP2003/012483, filed on Nov. 8, 2003. Priority is also claimed to
German application 10252881.0 filed on Nov. 12, 2002. The entire contents
of each of these applications is hereby incorporated by reference.
[0002]The present invention relates to a method for increasing the yield in glyphosate-resistant legumes, which comprises treating the plants or the seed with a mixture comprising
a) compound of formula I
##STR00001##
in which [0003]X is halogen, C1-C4-alkyl or trifluoromethyl, [0004]m is 0 or 1, [0005]Q is C(═CH--CH3)--COOCH3, C(═CH--OCH3)--COOCH3, C(═N--OCH3)--CONHCH3, C(═N--OCH3)--COOCH3 or N(--OCH3)--COOCH3, [0006]A is --O--B, --CH2O--B, --OCH2--B, --CH═CH--B, --C≡C--B, --CH2O--N═C(R1)--B or --CH2O--N═C(R1)--C(R2)═N--OR3, where [0007]B is phenyl, naphthyl, 5-membered or 6-membered hetaryl or 5-membered or 6-membered heterocyclyl, comprising one to three N atoms and/or one O or S atom or one or two O and/or S atoms, the ring systems being unsubstituted or substituted by one to three radicals Ra: [0008]Ra being cyano, nitro, amino, aminocarbonyl, aminothiocarbonyl, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkylcarbonyl, C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl, C3-C6-cycloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkyloxycarbonyl, C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino, C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl, C2-C6-alkenyloxy, phenyl, phenoxy, benzyl, benzyloxy, 5- or 6-membered heterocyclyl, 5- or 6-membered hetaryl, 5- or 6-membered hetaryloxy, C(═NOR')--OR'' or OC(R')2--C(R'')═NOR'' the cyclic radicals, in turn, being unsubstituted or substituted by one to three radicals Rb: [0009]Rb being cyano, nitro, halogen, amino, aminocarbonyl, aminothiocarbonyl, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl, C3-C6-cycloalkyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkoxycarbonyl, C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino, C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl, C2-C6-alkenyloxy, C3-C6-cycloalkyl, cycloalkenyl, phenyl, phenoxy, phenylthio, benzyl, benzyloxy, 5- or 6-membered heterocyclyl, 5- or 6-membered hetaryl, 5- or 6-membered hetaryloxy or C(═NOR')--OR'', [0010]R' is hydrogen, cyano, C1-C6-alkyl, C3-C6-cycloalkyl or C1-C4-haloalkyl, [0011]R'' is hydrogen, C1-C6-alkyl, C3-C6-alkenyl, C3-C6-alkynyl, C1-C4-haloalkyl, C3-C6-haloalkenyl or haloalkynyl, [0012]R1 is hydrogen, cyano, C1-C4-alkyl, C1-C4-haloalkyl, C3-C6-cycloalkyl or C1-C4-alkoxy, [0013]R2 is phenyl, phenylcarbonyl, phenylsulfonyl, 5- or 6-membered hetaryl, 5- or 6-membered hetarylcarbonyl or 5- or 6-membered hetarylsulfonyl, the ring systems being unsubstituted or substituted by one to three radicals Ra, [0014]C1-C10-alkyl, C3-C6-cycloalkyl, C2-C10-alkenyl, C2-C10-alkynyl, C1-C10-alkylcarbonyl, C2-C10-alkenylcarbonyl, C3-C10-alkynylcarbonyl, C1-C10-alkylsulfonyl or C(R')═NOR'', the hydrocarbon radicals of these groups being unsubstituted or substituted by one to three radicals Rc: [0015]Rc being cyano, nitro, amino, aminocarbonyl, aminothiocarbonyl, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl, C1-C6-alkoxy, C1-C6-haloalkoxy, C1-C6-alkoxycarbonyl, C1-C6-alkylthio, C1-C6-alkylamino, di-C1-C6-alkylamino, C1-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl, C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkylaminothiocarbonyl, C2-C6-alkenyl, C2-C6-alkenyloxy, C3-C6-cycloalkyl, C3-C6-cycloalkyloxy, 5- or 6-membered heterocyclyl, 5- or 6-membered heterocyclyloxy, benzyl, benzyloxy, phenyl, phenoxy, phenylthio, 5- or 6-membered hetaryl, 5- or 6-membered hetaryloxy and hetarylthio, it being possible for the cyclic groups, in turn, to be partially or fully halogenated or to have attached to them one to three radicals Ra, and [0016]R3 is hydrogen, C1-C6-alkyl, C2-C6-alkenyl or C2-C6-alkynyl, the hydrocarbon radicals of these groups being unsubstituted or substituted by one to three radicals Rc,andb) a glyphosate derivativein a synergistically active amount.
[0017]It is already known from the literature that active ingredients of the formula I, which are generally referred to as strobilurins, are capable of bringing about increased yields in crop plants in addition to their fungicidal action (Koehle H. et al. in Gesunde Pflanzen 49 (1997), pages 267-271; Glaab J. et al. Planta 207 (1999), 442-448).
[0018]Furthermore, it is known from WO-A 97/36488 that the application of glyphosate derivatives in glyphosate-tolerant plants selected from the group consisting of sugar beet, fodder beet, maize, oilseed rape and cotton may bring about increased yields. Furthermore, it is known from U.S. Pat. No. 3,988,142 that the sublethal application of glyphosate in plants such as sugar cane increases starch and sugar production and thus the overall yield of the plant.
[0019]Surprisingly, it has now been found that the application of glyphosate and strobilurins such as, in particular, pyraclostrobin results in a synergistic effect in legumes. This means that the purely additive (in mathematical terms) yield-increasing effect of strobilurin and of the glyphosate derivative is surpassed by application of the mixture according to the invention. This synergistic effect is more than surprising, since normally it can be assumed that a fungicide and herbicide have completely different mechanisms of action.
[0020]Accordingly, the method defined at the outset has been found. The active ingredients of the formula I which are used are known as fungicides and in some cases also as insecticides (EP-A 253 213; WO-A 95/18789; WO-A 95/24396; WO-A 96/01256; WO-A 97/15552). However, there has been no suggestion to date that these active ingredients in combination with glyphosate derivatives might possibly bring about an increased yield in legumes.
[0021]The good tolerance of the active ingredients of the formula I by plants, at the concentrations required for controlling plant diseases, permits the treatment of aerial plant parts.
[0022]In the method according to the invention, the active ingredient I is preferably taken up by the leaves and distributed throughout the entire plant in the plant sap.
[0023]In a preferred embodiment of the method, the above-ground plant parts of genetically modified legumes are treated with a mixture according to the invention comprising a) a strobilurin derivative I and b) a glyphosate derivative. The application of glyphosate reduces the competition of the crop plant and the weed plants for nutrients and light and thus increases the yield of the crop plant. The mixture according to the invention is especially preferably applied to the above-ground part of the plant.
[0024]Methods for generating plants which are resistant to the effect of glyphosate are described in the more recent literature (EP-A 218 571, EP-A 293 358, WO-A 92/00377 and WO-A 92/04449). Chemical Abstracts, 123, No. 21 (1995) A.N. 281158c describes the generation of glyphosate-resistant soybean plants. Other glyphosate-resistant legumes can be generated in a similar manner. Methods for the transformation of legumes are known in the literature and can be used--as outlined further above--for generating, for example, glyphosate-resistant beans, peas, lentils, peanuts and lupins: Plant Science (Shannon) 150(1) Jan. 14, 2000, 41-49; J. of Plant Biochemistry & Biotechnology 9(2) July, 2000, 107-110; Acta Physiologiae Plantarum 22(2), 2000, 111-119; Molecular Breeding 5(1) 1999, 43-51; In Vitro Cellular & Developmental Biology, Animal 34 (3 Part 2) March, 1998, 53A; Plant Cell Reports 16(8), 1997, 513-519 and 541-544; Theoretical & Applied Genetics 94(2), 1997, 151-158; Plant Science, 117 (1-2), 1996, 131-138; Plant Cell Reports 16(1-2), 1996, 32-37.
[0025]For example soya varieties such as NIDERA AX 4919® which are resistant to numerous fungal diseases and the herbicide glyphosate can be used.
[0026]The preparation of the active ingredients used in the method according to the invention is known from the literature cited at the outset.
[0027]Active ingredients with the following meanings of the substituents, in each case on their own or in combination, are especially preferred for the method according to the invention:
[0028]Especially preferred active ingredients for the method according to the invention are, in particular, those of the formulae Ia to Ig in which
V is OCH3 or NHCH3 and Y is CH or N.
[0029]Preferred active ingredients of the formula I in which Q is C(═N--OCH3)--COOCH3 are the compounds described in the publications EP-A 253 213 and EP-A 254 426.
[0030]Preferred active ingredients of the formula I in which Q is C(═N--OCH3)--CONHCH3 are the compounds described in the publications EP-A 398 692, EP-A 477 631 and EP-A 628 540.
[0031]Preferred active ingredients of the formula I in which Q is N(--OCH3)--COOCH3 are the compounds described in the publications WO-A 93/15046 and WO-A 96/01256.
[0032]Preferred active ingredients of the formula I in which Q is C(═CH--OCH3)--COOCH3 are the compounds described in the publications EP-A 178 826 and EP-A 278 595.
[0033]Preferred active ingredients of the formula I in which Q is C(═CH--CH3)--COOCH3 are the compounds described in the publications EP-A 280 185 and EP-A 350 691.
[0034]Preferred active ingredients of the formula I in which A is --CH2O--N═C(R1)--B are the compounds described in the publications EP-A 460 575 and EP-A 463 488.
[0035]Preferred active ingredients of the formula I in which A is --O--B are the compounds described in the publications EP-A 382 375 and EP-A 398 692.
[0036]EP-A 382 375 describes pyrimidines having the formula (I):
##STR00002##
in which any two of K, L and M are nitrogen and the other is CE; X and Y are independently hydrogen, halogen, C1-4 alkyl, C3-6 cycloalkyl, C2-4 alkenyl, C2-4 alkynyl, C2-4 alkynyloxy, phenyl, benzyloxy, cyano, isocyano, isothiocyanato, nitro, NR1R2, NR1OR2, N3, NHCOR1, NR1CO2R2, NHCONR1R2, N═CHNR1R2, NHSO2R1, OR1, OCOR1, OSO2R1, SR1, SOR1, SO2R1, SO2OR1, SO2NR1R2, COR1, CR1═NOR2, CHR1CO2R2, CO2R1, CONR1R2, CSNR1R2, CH3O2C.C:CH.OCH3, 1-(imidazol-1-yl) vinyl, a 5-membered heterocyclic ring containing one, two or three nitrogen heteroatoms, or a 5- or 6-membered heterocyclic ring containing one or two oxygen or sulphur heteroatoms, optionally a nitrogen heteroatom and optionally one or two oxo or thioxo substituents; or X and Y, when ortho to one another, join to form a 5- or 6-membered aliphatic or aromatic ring optionally containing one or two oxygen, sulphur or nitrogen atoms or one, two or three nitrogen atoms; A, B, D, E, G, U and V are independently hydrogen, halogen (especially fluorine and chlorine), C1-4 alkyl (especially methyl), C1-4 alkoxy (especially methoxy), cyano, nitro or trifluoromethyl; and R1 and R2 are independently hydrogen, C1-4 alkyl, C2-4 alkenyl or phenyl; the aliphatic moieties of any of the foregoing being optionally substituted with one or more of halogen, cyano, OR1, SR1, NR1R2, SiR13 or OCOR1 and the phenyl moieties of any of the foregoing being optionally substituted with one or more of halogen, C1-4 alkyl, C1-4 alkoxy, nitro or cyano.
[0037]Because of the unsymmetrically substituted double bond of the propenoate group, the compounds of the invention may be obtained in the form of mixtures of (E) and (Z) geometric isomers. However, these mixtures can be separated into individual isomers, and this invention embraces such isomers and mixtures thereof in all proportions including those which consist substantially of the (Z)-isomer and those which consist substantially of the (E)-isomer.
[0038]The (E)-isomer, in which the groups --CO2CH3 and --OCH3 are on opposite sides of the olefinic bond of the propenoate group, are the more fungicidally active and form a preferred embodiment of the invention.
[0039]Alkyl groups contain from 1 to 4 carbon atoms and may be in the form of straight or branched chains. Examples are methyl, ethyl, iso-propyl, n-butyl and t-butyl. Cycloalkyl groups contain from 3 to 6 carbon atoms and include cyclopropyl and cyclohexyl.
[0040]Alkenyl and alkynyl groups contain from 2 to 4 carbon atoms and may be in the form of straight or branched chains. Examples are ethenyl, allyl, methylallyl and propargyl.
[0041]Halogen is typically fluorine, chlorine or bromine.
[0042]Substituted aliphatic moieties include, in particular, halo(C1-4) alkyl, halo(C1-4alkoxy, halo(C1-4)alkylthio, CH2OR1, CH2SR1 and CH2NR1R2, wherein R1 and R2 are H, C1-4 alkyl or phenyl.
[0043]Typical optional substituents of phenyl moieties are fluorine, chlorine, methyl, methoxy, nitro and cyano.
[0044]The ring
##STR00003##
in formula (I) is a pyrimidine ring which may be joined to the phenoxy groups by any two of its ring carbon atoms adjacent to a ring nitrogen atom. Of particular interest are those compounds of formula (I) in which K and L are both nitrogen and M is CH. Typically, one or both of X and Y are hydrogen. When one of X and Y is not hydrogen it is preferably attached to the 2-position of the phenyl ring.
[0045]Thus, in one aspect, EP-A 382 375 describes compounds of formula (I) in which K, L and M have the meanings previously given; X, which is preferably attached to the 2-position of the phenyl ring, is hydrogen, halogen (e.g. fluorine, chlorine or bromine), C1-4 alkyl (e.g. methyl or ethyl), C1-4 alkyl (especially methyl) substituted with halogen (e.g. fluorine, chlorine or bromine), hydroxy, cyano, C1-4 alkoxy (e.g. methoxy) or C1-4 alkanoyloxy (e.g. acetoxy), C2-4 alkenyl (e.g. ethenyl, allyl or methylallyl), C2-4 alkynyl (e.g. ethynyl or propargyl), C2-4 alkenyloxy (e.g. allyloxy), C2-4 alkynyloxy (e.g. propargyloxy), phenyl, benzyl, cyano, isocyano, isothiocyanato, nitro, amino, mono- or di(C1-4)alkylamino (e.g. methylamino or dimethylamino), formylamino, C1-4 alkanoylamino (e.g. acetamido), benzylamino, ureido, phenylureido, C1-4 alkylsulphonylamino (e.g. mesylamino), phenylsulphonylamino, hydroxy, C1-4 alkoxy (e.g. methoxy or ethoxy), phenoxy, C1-4 alkanoyloxy (e.g. acetoxy), C1-4 alkylsulphonyloxy (e.g. mesyloxy), phenylsulphonyloxy, C1-4 alkylthio (e.g. methylthio), C1-4 alkylsulphinyl (e.g. methylsulphinyl), C1-4 alkylsulphonyl (e.g. mesyl and n-butylsulphonyl), formyl, C1-4 alkanoyl (e.g. acetyl), benzoyl, hydroxyimino(C1-4)alkyl (e.g. hydroxyiminomethyl), C1-4 alkoxyimino(C1-4)alkyl (e.g. methoxyiminomethyl), carbamoyl, C1-4 alkylcarbamoyl (e.g. methylcarbamoyl), thiocarbamoyl or C1-4 alkylthiocarbamoyl (e.g. methylthiocarbamoyl), the phenyl ring of any of the foregoing being optionally substituted with halogen (e.g. fluorine or chlorine), C1-4 alkyl (e.g. methyl), C1-4 alkoxy (e.g. methoxy), nitro or cyano; and Y is halogen (e.g. fluorine or chlorine), C1-4 alkyl (e.g. methyl), C1-4 alkoxy (e.g. methoxy), nitro, cyano or preferably, hydrogen, or X and Y, when ortho to one another, together form methylenedioxy, or together with the phenyl ring to which they are attached form a naphthalene, quinoline, benzimidazole or benzothienyl ring.
[0046]In another aspect EP-A 382 375 describes compounds of the formula (I.1):
##STR00004##
in which X is hydrogen, halogen (especially chlorine), C1-4 alkyl (especially methyl), C1-4 alkoxy (especially methoxy), trifluoromethyl, cyano, thiocarbamoyl or nitro, and Y is hydrogen or fluoro.
[0047]EP-A 382 375 also discloses compounds listed in Tables I to III which follow. Throughout these Tables the methyl 3-methoxypropenoate group has the (E)-configuration and the substituents E, G, U and in are all hydrogen.
TABLE-US-00001 TABLE I ##STR00005## Compound Melting No. X Y point (° C.) Olefinic* 1 H H glass 7.46 2 2-F H gum 7.47 3 3-F H gum 7.47 4 4-F H 87-9 7.46 5 2-Cl H glass 7.38 6 3-Cl H 7 4-Cl H 8 2-Br H glass 7.42 9 2-Cyano H 118-119 7.50 10 3-Cyano H gum 7.49 11 4-Cyano H gum 7.49 12 2-Isocyano H 13 2-NO2 H 120-121 7.52 14 3-NO2 H gum 7.49 15 4-NO2 H gum 7.48 16 2-NH2 H gum 7.46 17 3-NH(CH3) H 18 2-N(CH3)2 H 19 2-NH•CHO H 20 2-NH•COCH3 H 21 3-NH•COC6H5 H 22 2-NH•CONH2 H 23 3-NH•CONH(C2H5) H 24 2-NH•SO2CH3 H 25 3-NH•SO2C6H5 H 26 2-OH H 159-161 7.45 27 3-OH H 28 4-OH H 29 2-OCH3 H gum 7.49 30 3-OCH3 H gum 7.47 31 4-OCH3 H 88-90 7.45 32 2-OC2H5 H glass 7.46 33 3-(2-F--C6H4O) H 34 2-OCOCH3 H gum 7.47 35 2-OSO2CH3 H foam 7.47 36 3-(4-CH3--C6H4SO2O) H 37 2-SCN H 38 3-SCN H 39 4-SCN H 40 2-SCH3 H gum 7.48 41 3-SCH3 H 42 4-SCH3 H 43 2-S(O)CH3 H 135-6 7.48 44 2-SO2CH3 H 61-4 7.49 45 4-SO2(CH2)3CH3 H 46 2-CHO H foam 7.50 47 3-CHO H 48 4-CHO H 49 2-COCH3 H 99-101 7.42 50 3-COC6H5 H 51 2-(E)--CH═NOH H 146-7 7.45 52 3-(E)--CH═NOH H 53 4-(E)--CH═NOH H 54 2-(E)--CH═NOCH3 H 55 2-(E)--C(CH3)═NOH H 56 2-CONH2 H 57 3-CONH(CH3) H 58 4-CON(CH3)2 H 59 2-CSNH2 H 131-3 7.49 60 2-CSNH(CH3) H 61 2-CH3 H gum 7.48 62 3-CH3 H 92-5 7.45 63 4-CH3 H gum 7.46 64 2-C2H5 H 60-2 7.47 65 2-CH2F H 66 2-CH2Br H 67 2-CH2Cl H 68 2-CH2CN H 69 2-CH2OH H 70 2-CH2OCH3 H 71 2-CH2OCOCH3 H 72 3-CH2CN H 73 4-CH2OH H 74 3-CH2OCH3 H 75 2-CH═CH2 H 76 2-CH2CH═CH2 H gum 7.47 77 2-C≡CH H 66-8 7.46 78 2-CH2C≡CH H 79 3-CH2C(CH3)═CH2 H 80 2-OCH2CH═CH2 H glass 7.47 81 2-OCH2C≡CH H gum 7.47 82 2-C6H5 H 55 7.40 83 3-C6H5 H 84 4-C6H5 H 85 2-C6H5O H 86 3-C6H5O H 87 4-C6H5O H 88 2-(4-Cl--C6H4O) H 89 2-C6H5CH2O H 90 2-Cyano 4-Cl 91 2-NO2 4-F 92 2-Cl 4-Cl 93 2-OCH3 3-OCH3 94 2-Cyano 5-Cl 95 2-Cyano 6-Cyano 96 2-F 5-Cl 97 3-OCH3 5-OCH3 98 3-Cyano 4-F 99 2-NO2 3-OCH3 100 3-OCH3 5-Cyano 101 2-CO2CH3 H glass 7.50 102 2-I H glass 7.48 103 2-CF3 H 99-101 7.48 104 2-i-C3H7 H 63-5 7.47 105 2-i-C3H7O H glass 7.47 106 2-F 6-F 87-8 7.49 107 2-F 4-F 92-4 7.48 108 2-F 3-F gum 7.48 109 2-n-C3H7O H gum 7.46 110 2-n-C4H9O H gum 7.47 111 2-CH(OH)CH3 H 50-3 7.46 112 2-t-C4H9 H gum 7.47 113 2-s-C4H9 H gum 7.47 114 2-n-C3H7 H gum 7.47 115 2-(E/Z)--CH═CH(CH3) H glass 7.461 116 2-Cyano 4-OCH3 gum 7.50 117 2-Cyano 5-OCH3 oil 7.50 118 2-Cyano 4-Cl 78-82 7.50 119 2-Cyano 5-N(C2H5)2 oil 7.50 120 2-CONH2 H 138-141 7.46 121 2-C≡CSi(CH3)3 H gum 7.46 122 2-F 5-F 100-101 7.48 123 2-(E)--CH3O2C•C═CH•OCH3 H 130-131 7.45 124 3-F 5-F 68-70 7.47 125 2-NHOH H 126 2-CH2OCH3 H 127 2-CH2CN H 128 2-N3 H 129 2-Cyano 6-F 130 2-NO2 6-F 131 2-CSNH2 6-F 132 2-Cyano 3-F 133 2-Cyano 5-F 134 2-Cyano 3-OCH3 135 2-Cyano 6-OCH3 136 2-NO2 4-OCH3 137 2-NO2 5-OCH3 138 2-NO2 6-OCH3 139 2-CSNH2 3-OCH3 140 2-CSNH2 4-OCH3 141 2-CSNH2 5-OCH3 142 2-CSNH2 6-OCH3 143 2-Cyano 3-Cyano 144 2-F 3-Cyano 144 2-OCH3 3-Cyano 145 3-Cyano 6-F 146 ##STR00006## H 147 ##STR00007## H 148 ##STR00008## H 149 ##STR00009## H 150 2-Cyano 4-Br 151 2-Cyano 6-Br 152 2-Cyano 4-NO2 153 2-Cyano 6-NO2 154 2-Cyano 6-OC2H5 155 2-Cyano 4-CO2CH3 156 2-Cyano 6-CO2C2H5 157 2-Cyano 6-CH3 158 2-Cyano 5-CH2C6H5 159 2-Cyano 4-OCF3 160 2-Cyano 4-Cyano ##STR00010## Compound Melting No. Ar point (° C.) Olefinic* 161 ##STR00011## 133-5 7.52 162 ##STR00012## 163 ##STR00013## 164 ##STR00014## 165 ##STR00015## 166 ##STR00016## 167 Pentafluorophenyl 168 2,4,6-Tri-F--C6H2 169 2,3,5,6-Tetra-F--C6H 170 2,3,6-Tri-F--C6H2 171 2,3-Di-cyano-6-F--C6H2 172 2,6-Di-F-3-CH3O--C6H2 173 2,6-Di-F-4-CH3O--C6H2 174 2,6-Di-F-3-NO2--C6H2 175 2,6-Di-F-4-NO2--C6H2 176 2,6-Di-F-3,5-di-CH3O--C6H 177 4,6-Di-Br-2-cyano-C6H2 178 3-Cyano-2,6-di-F--C6H2 179 6-Br-2-cyano-4-CH3O--C6H2 180 6-Br-4-Cl-2-cyano-C6H2 181 6-Br-2-cyano-4-NO2--C6H2 182 3-Br-2-cyano-6-CH3O--C6H2 183 3,5-Di-Cl-2-cyano-C6H2 184 4,6-Di-Cl-2-cyano-C6H2 185 3-Br-2-cyano-4-CH3O--C6H2 186 4-Br-2-cyano-6-NO2--C6H2 187 4-Br-2-cyano-6-CH3O--C6H2 188 2-Cyano-4-I-6-CH3O--C6H2 189 2-Cyano-6-CH3O-4-NO2--C6H2 190 2-Cyano-4,6-di-NO2--C6H2 191 2-Cyano-4-CH3-6-NO2--C6H2 192 2-Cyano-4-CH3O-6-NO2--C6H2 193 2-Cyano-5,6-di-CH3O--C6H2 194 2-Cyano-5,6-di-CH3O-3-CH3--C6H 195 3,4-Di-Br-2-cyano-6-CH3O--C6H 196 3-Br-2-cyano-6-CH3O-4-NO2--C6H 197 2-Cyano-6-CH3CH2O-4-NO2--C6H2 198 ##STR00017## 199 ##STR00018## *Chemical shift of singlet from olefinic proton on beta-methoxypropenoate group (ppm from tetramethyl-silane). Solvent: CDCl3 unless otherwise stated. 1The ratio of the (E)- and (Z)-isomers of the prop-1-enyl group of compound No. 115 is either 2:1 or 1:2.
TABLE-US-00002 TABLE II ##STR00019## Compound Melting No X Y Point (° C.) Olefinic* 1 H H 114-115 7.46 123 2-(E)--CH3O2C•C═CH•OCH3 H 60-70 7.44 and 7.47 *Chemical shift of singlet from olefinic proton on beta-methoxypropenoate group (ppm from tetramethylsilane). Solvent: CDCl3 unless otherwise stated.
[0048]Table II comprises 199 compounds of the general structure above with all the values of X and Y listed in Table I. That is, compounds numbers 1 to 199 of Table II are the same as those of Table I except that the pyrimidine ring is 4,6-disubstituted in Table 1 and 2,4-disubstituted as shown in Table II.
TABLE-US-00003 TABLE III ##STR00020## Compound Melting No X Y Point (° C.) Olefinic* 1 H H 96-97 7.42 9 2-Cyano H foam 7.43 *Chemical shift of singlet from olefinic proton on beta-methoxypropenoate group (ppm from tetramethylsilane). Solvent: CDCl3 unless otherwise stated.
[0049]Table III comprises 199 compounds of the general structure above with all the values of X and Y listed in Table I. That is, compounds numbers 1 to 199 of Table III are the same as those of Table I except that the pyrimidine ring is 4,6-disubstituted in Table I and 2,4-disubstituted as shown in Table III.
EXAMPLE
[0050]This example illustrates the preparation of (E)-methyl 2-[2-(6-(2-cyanophenoxy)pyrimidin-4-yloxy)phenyl]-3-methoxypropenoate (compound No. 9 of Table I or "azoxystrobin").
[0051]To a solution of 4,6-dichloropyrimidine (0.76 g, 5.10 mmol) in dry DMF (4 ml) at 0° C. was added anhydrous potassium carbonate (0.70 g, 5.10 mmol). A solution of (E)-methyl 2-(2-hydroxyphenyl)-3-methoxypropenoate (0.53 g, 2.55 mmol, prepared as described in Example 3 of EP-A-0242081) in dry DMF (2 ml) was then added dropwise with stirring. After the addition was complete, the reaction mixture was allowed to warm to room temperature and stirring continued over the weekend. The reaction mixture was then diluted with water (15 ml) and extracted with ether (3×20 ml). The combined ether extracts were washed with brine and dried. Evaporation afforded a brown liquid (1.10 g) which was chromatographed (eluent ether:n-hexane, 3:2) to give (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxypropenoate as a thick, pale yellow oil (0.58, 71 percent yield) which crystallised on standing. Recrystallisation from ether/dichloromethane (trace)/n-hexane at -78° C. gave the product as a white powder (0.25 g), mp 94-5° C. In a separate preparation, 15 g of product was obtained from 4,6-dichloropyrimidine (15.90 g), (E)-methyl 2-(2-hydroxyphenyl)-3-methoxypropenoate (14.80 g) and anhydrous potassium carbonate (19.64 g).
[0052](E)-Methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxypropenoate (1.50 g, 4.68 mmol) was heated overnight at 95-100° C. with 2-cyanophenol (0.61 g, 5.15 mmol) and potassium carbonate (0.71 g, 5.15 mmol) in DMF (35 ml) in the presence of a catalytic amount of copper(I) chloride. The reaction mixture was cooled, diluted with water and then extracted with ether. The combined ether layers were washed with 2M sodium hydroxide solution and brine and then dried. Evaporation of the solvent gave a pale yellow oil (1.52 g). Recrystallisation from ether/dichloromethane/n-hexane gave the title compound as a pale yellow powder (1.20 g, 64% yield), mp 110-111° C.; 1H NMR δ: 3.63 (3H, s); 3.74 (3H, s); 6.42 (1H, s); 7.19-7.47 (6H, m); 7.50 (1H, s); 7.62-7.75 (2H, m); 8.40 (1H, s) ppm. In a subsequent preparation of the title compound, recrystallisation gave a white crystalline solid, mp 118-119° C.
[0053]Preferred active ingredients of the formula I in which A is --CH2O--N═C(R1)--C(R2)═N--OR3 are the compounds described in the publications WO-A 95/18789, WO-A 95/21153, WO-A 95/21154, WO-A 97/05103, WO-A 97/06133 and WO-A 97/15552.
[0054]Especially preferred are active ingredients of the formula I in which [0055]Q is C(═N--OCH2)--COOCH3 or C(═N--OCH2)--CONHCH2, [0056]A is CH2--O-- and [0057]B is --N═C(R1)--C(R2)═N--OR2, where [0058]R1 is hydrogen, cyano, cyclopropyl, C1-C4-alkyl or C1-C2-haloalkyl, in particular methyl, ethyl, 1-methylethyl or trifluoromethyl, and [0059]R2 is C1-C4-alkyl, C2-C5-alkenyl, phenyl which is substituted by one or two halogen atoms, or is C(R')═NOR'', where [0060]R' is one of the groups mentioned above under R1 and [0061]R'' is hydrogen, cyclopropyl or C1-C4-alkyl, in particular methyl, ethyl or isopropyl, and [0062]R3 is one of the groups mentioned under R'';these active ingredients are described by the formula Ib
##STR00021##
[0062]in which the variables have the abovementioned meanings.
[0063]Active ingredients of the formula Ib'
##STR00022##
in which the variables have the abovementioned meanings are particularly preferred.
[0064]In addition, other compounds which are especially preferred are those of the formula Ia where T is CH or N and R' and Rb are halogen or C1-C4-alkyl and x is 0, 1 or 2 and y is 0 or 1.
##STR00023##
[0065]The active ingredients compiled in the tables which follow are especially preferred with regard to their use in increasing yield.
TABLE-US-00004 TABLE I Ia ##STR00024## Position of the No. T (Ra')y group phenyl-(Rb)x (Rb)x Reference Ia-1 N -- 1 2,4-Cl2 WO-A 96/01256 Ia-2 N -- 1 4-Cl WO-A 96/01256 Ia-3 CH -- 1 2-Cl WO-A 96/01256 Ia-4 CH -- 1 3-Cl WO-A 96/01256 Ia-5 CH -- 1 4-Cl WO-A 96/01256 Ia-6 CH -- 1 4-CH3 WO-A 96/01256 Ia-7 CH -- 1 H WO-A 96/01256 Ia-8 CH -- 1 3-CH3 WO-A 96/01256 Ia-9 CH 5-CH3 1 3-CF3 WO-A 96/01256 Ia-10 CH 1-CH3 5 3-CF3 WO-A 99/33812 Ia-11 CH 1-CH3 5 4-Cl WO-A 99/33812 Ia-12 CH 1-CH3 5 -- WO-A 99/33812
[0066]The active ingredient Ia-5 (common name: pyraclostroblin) is especially preferred.
TABLE-US-00005 TABLE II II ##STR00025## No. V R1 R2 R3 Reference Ib-1 OCH3 CH3 CH3 CH3 WO-A 95/18789 Ib-2 OCH3 CH3 CH(CH3)2 CH3 WO-A 95/18789 Ib-3 OCH3 CH3 CH2CH3 CH3 WO-A 95/18789 Ib-4 NHCH3 CH3 CH3 CH3 WO-A 95/18789 Ib-5 NHCH3 CH3 4-F--C6H4 CH3 WO-A 95/18789 Ib-6 NHCH3 CH3 4-Cl--C6H4 CH3 WO-A 95/18789 Ib-7 NHCH3 CH3 2,4-C6H3 CH3 WO-A 95/18789 Ib-8 NHCH3 Cl 4-F--C6H4 CH3 WO-A 98/38857 Ib-9 NHCH3 Cl 4-Cl--C6H4 CH2CH3 WO-A 98/38857 Ib-10 NHCH3 CH3 CH2C(═CH2)CH3 CH3 WO-A 97/05103 Ib-11 NHCH3 CH3 CH═C(CH3)2 CH3 WO-A 97/05103 Ib-12 NHCH3 CH3 CH═C(CH3)2 CH2CH3 WO-A 97/05103 Ib-13 NHCH3 CH3 CH═C(CH3) CH2CH3 CH3 WO-A 97/05103 Ib-14 NHCH3 CH3 O--CH(CH3)2 CH3 WO-A 97/06133 Ib-15 NHCH3 CH3 O--CH2CH(CH3)2 CH3 WO-A 97/06133 Ib-16 NHCH3 CH3 C(CH3)═NOCH3 CH3 WO-A 97/15552 Ib-17 NHCH3 CH3 C(CH3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-18 NHCH3 CH3 C(CH3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-19 NHCH3 CH3 C(CH3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-20 NHCH3 CH3 C(CH3)═NOCH2CH═CH2 CH2CH═CH2 WO-A 97/15552 Ib-21 NHCH3 CF3 C(CF3)═NOCH3 CH3 WO-A 97/15552 Ib-22 NHCH3 CF3 C(CF3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-23 NHCH3 CF3 C(CF3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-24 NHCH3 CF3 C(CF3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-25 NHCH3 CF3 C(CF3)═NOCH2CH═CH2 CH2CH═CH2 WO-A 97/15552 Ib-26 OCH3 CH3 C(CH3)═NOCH3 CH3 WO-A 97/15552 Ib-27 OCH3 CH3 C(CH3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-28 OCH3 CH3 C(CH3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-29 OCH3 CH3 C(CH3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-30 OCH3 CH3 C(CH3)═NOCH2CH═CH2 CH2CH═CH2 WO-A 97/15552 Ib-31 OCH3 CF3 C(CF3)═NOCH3 CH3 WO-A 97/15552 Ib-32 OCH3 CF3 C(CF3)═NOCH2CH3 CH2CH3 WO-A 97/15552 Ib-33 OCH3 CF3 C(CF3)═NOCH(CH3)2 CH(CH3)2 WO-A 97/15552 Ib-34 OCH3 CF3 C(CF3)═NO(c-C3H5) c-C3H5 WO-A 97/15552 Ib-35 OCH3 CF3 C(CF3)═NOCH2CH═CH3 CH2CH═CH2 WO-A 97/15552
TABLE-US-00006 TABLE III Ic ##STR00026## No. V Y T Ra Reference Ic-1 OCH3 CH N 2-OCH3, 6-CF3 WO-A 96/16047 Ic-2 OCH3 CH N 2-OCH(CH3)2, 6-CF3 WO-A 96/16047 Ic-3 OCH3 CH CH 5-CF3 EP-A 278 595 Ic-4 OCH3 CH CH 6-CF3 EP-A 278 595 Ic-5 NHCH3 N CH 3-Cl EP-A 398 692 Ic-6 NHCH3 N CH 3-CF3 EP-A 398 692 Ic-7 NHCH3 N CH 3-CF3, 5-Cl EP-A 398 692 Ic-8 NHCH3 N CH 3-Cl, 5-CF3 EP-A 398 692
TABLE-US-00007 TABLE IV Id ##STR00027## No. V Y R1 B Reference Id-1 OCH3 CH CH3 (3-CF3)C6H4 EP-A 370 629 Id-2 OCH3 CH CH3 (3,5-Cl2)C6H3 EP-A 370 629 Id-3 NHCH3 N CH3 (3-CF3)C6H4 WO-A 92/13830 Id-4 NHCH3 N CH3 (3-OCF3)C6H4 WO-A 92/13830 Id-5 OCH3 N CH3 (3-OCF3)C6H4 EP-A 460 575 Id-6 OCH3 N CH3 (3-CF3)C6H4 EP-A 460 575 Id-7 OCH3 N CH3 (3,4-Cl2)C6H3 EP-A 460 575 Id-8 OCH3 N CH3 (3,5-Cl2)C6H3 EP-A 463 488
TABLE-US-00008 TABLE V Ie ##STR00028## No. V Y Ra Reference Ie-1 OCH3 N 2-CH3 EP-A 253 213 Ie-2 OCH3 N 2,5-(CH3)2 EP-A 253 213 Ie-3 NHCH3 N 2,5-(CH3)2 EP-A 477 631 Ie-4 NHCH3 N 2-Cl EP-A 477 631 Ie-5 NHCH3 N 2-CH3 EP-A 477 631 Ie-6 NHCH3 N 2-CH3, 4-OCF3 EP-A 628 540 Ie-7 NHCH3 N 2-Cl, 4-OCF3 EP-A 628 540 Ie-8 NHCH3 N 2-CH3, 4-OCH(CH3)--C(CH3)═NOCH3 EP-A 11 18 609 Ie-9 NHCH3 N 2-Cl, 4-OCH(CH3)--C(CH3)═NOCH3 EP-A 11 18 609 Ie-10 NHCH3 N 2-CH3, 4-OCH(CH3)--C(CH2CH3)═NOCH3 EP-A 11 18 609 Ie-11 NHCH3 N 2-Cl, 4-OCH(CH3)--C(CH3)═NOCH2CH3 EP-A 11 18 609
TABLE-US-00009 TABLE VI If ##STR00029## No. V Y Ra Reference If-1 NHCH3 N H EP-A 398 692 If-2 NHCH3 N 3-CH3 EP-A 398 692 If-3 NHCH3 N 2-NO2 EP-A 398 692 If-4 NHCH3 N 4-NO2 EP-A 398 692 If-5 NHCH3 N 4-Cl EP-A 398 692 If-6 NHCH3 N 4-Br EP-A 398 692
TABLE-US-00010 TABLE VII Ig ##STR00030## No. V Y T Ra Reference Ig-1 OCH3 CH N 6-O--(2-CN--C6H4) EP-A 382 375 Ig-2 OCH3 CH N 6-O--(2-Cl--C6H4) EP-A 382 375 Ig-3 OCH3 CH N 6-O--(2-CH3--C6H4) EP-A 382 375 Ig-4 NHCH3 N N 6-O--(2-Cl--C6H4) GB-A 22 53 624 Ig-5 NHCH3 N N 6-O--(2, 4-Cl2--C6H3) GB-A 22 53 624 Ig-6 NHCH3 N N 6-O--(2-CH3--C6H3) GB-A 22 53 624 Ig-7 NHCH3 N N 6-O--(2-CH3, 3-Cl--C6H3) GB-A 22 53 624 Ig-8 NHCH3 N N 2-F, 6-O--(2-CH3--C6H4) WO-A 98/21189 Ig-9 NHCH3 N N 2-F, 6-O--(2-Cl--C6H4) WO-A 98/21189 Ig-10 NHCH3 N N 2-F, 6-O--(2-CH3, WO-A 98/21189 3-Cl--C6H3)
[0067]Fungicidal active ingredients which can be employed are the strobilurins I alone or in mixture with other fungicidal active ingredients, in particular those from the class of the azoles Ix.
[0068]Azole active ingredients which are suitable for this purpose are: [0069]fluquinconazole, Proc. Br. Crop Prot. Conf.--Pests Dis., 5-3, 411 (1992); [0070]metconazole, Proc. Br. Crop Prot. Conf.--Pests Dis., 5-4, 419 (1992); [0071]prochloraz, U.S. Pat. No. 3,991,071; [0072]propiconazole, GB-A 1,522,657; [0073]prothioconazole, WO-A 96/016048; [0074]tebuconazole, U.S. Pat. No. 4,723,984; [0075]epoxiconazole, EP-A 196038; [0076]myclobutanil, CAS RN [88671-89-0];
[0077]Azoles which are especially suitable are: metconazole, myclobutanil, epoxiconazole, propiconazole, prothioconazole or tebuconazole.
[0078]If fungicide mixtures of, for example, strobilurins I and azoles Ix are employed, they are generally employed in a weight ratio I to Ix of 20:1 to 0.05:1, preferably 10:1 to 0.1:1.
[0079]Glyphosate derivatives II are essentially understood as meaning the following compounds, which are mentioned in The Pesticide Manual: for example, glyphosate may be employed as the free acid or in the form of salts such as the isopropylammonium salt, the sodium salt, the ammonium salt or the trimesium (trimethylsulfenium) salt. Mixtures of the salts may also be employed. Moreover, the glyphosate derivatives II include the compound N-(phosphonomethyl)glycine. The preparation of the glyphosate derivatives II can be found in the literature cited in The Pesticide Manual (12th edition).
[0080]The compounds I in combination with glyphosate derivatives raise the yield potential in legumes. They are especially important for the treatment of various glyphosate-resistant crop plants such as peas, beans, lentils, peanuts, lupins and in particular soybeans. The synergistic effect is demonstrated independently of the generation of the glyphosate-resistant legumes.
[0081]Specifically, they are suitable for controlling the following symptoms: [0082]signs of wilting despite the availability of sufficient nutrients, [0083]discolorations of the green leaf tissue such as, for example bleaching of soybeans.
[0084]The compounds I are applied by treating the plants to be protected with an effective amount of the active ingredients. Application can be effected both before and after application of the glyphosate derivatives II to the plants.
[0085]In a preferred embodiment of the method, the treatment of the plant is effected jointly with the application of the fungicide I and the herbicide II. The synergistic effect is particularly pronounced in this case.
[0086]When using an active ingredient I, the application rates are in the range of from 0.01 to 2.0 kg of active ingredient per hectare, depending on the weather conditions and the plant species.
[0087]When using a glyphosate derivative II, the application rates are in the range of from 0.1 to 6.0 kg of active ingredient (acid equivalent) per hectare, depending on the weather conditions and the plant species.
[0088]As a rule, the fungicide I, or the fungicidal mixture I and Ix, is employed in a weight ratio to the herbicide II of 5:1 to 0.01:1, preferably 1:1 to 0.1:1.
[0089]The compounds I and the glyphosate derivatives II may be converted into the formulations conventionally used for crop protection products, for example solutions, emulsions, suspensions, dusts, powders, pastes and granules. The use form depends on the application in question; in any case, it should ensure uniform and even distribution of the mixture according to the invention.
[0090]The formulations are prepared in the known manner, for example by extending the active ingredient with solvents and/or carriers, if desired using emulsifiers and dispersants, it also being possible to use other organic solvents as cosolvents if water is used as the diluent. Auxiliaries are essentially those also conventionally used for fungicides.
[0091]In general, the formulations comprise between 0.01 and 95% by weight, preferably between 0.1 and 90% by weight, of the active ingredient. The active ingredients are employed in a purity of from 90% to 100%, preferably 95% to 100% (according to NMR spectrum).
[0092]Examples of formulations are known from the publications cited at the outset.
[0093]Aqueous use forms can usually be prepared from emulsion concentrates, pastes or wettable powders (sprayable powders, oil dispersions) by addition of water. To prepare emulsions, pastes or oil dispersions, the substances, as such or dissolved in an oil or solvent, may be homogenized in water by means of wetter, sticker, dispersant or emulsifier. Alternatively, it is possible to prepare concentrates consisting of active substance, wetter, sticker, dispersant or emulsifier and, if appropriate, solvent or oil, and such concentrates are suitable for dilution with water.
[0094]The active ingredient concentrations in the ready-to-use products may be varied within substantial ranges. In general, they are between 0.0001 and 10%, preferably between 0.01 and 1%.
[0095]The active ingredients may also be used successfully by the ultra-low-volume (ULV) method, it being possible to apply formulations comprising more than 95% by weight of active ingredient, or indeed the active ingredient without additions.
[0096]Various types of oils or herbicides, other fungicides, other pesticides or bactericides may be added to the active ingredients, if appropriate just prior to use (tank mix). These agents can be admixed with the compositions according to the invention in a weight ratio of from 1:10 to 10:1.
[0097]The active ingredients I are preferably applied to the plant jointly or separately with the glyphosate II.
[0098]In general, the compounds I and II are applied within a period of weeks to 3 months, preferably within 1 to 2 months, after planting the legume seeds. It may be advantageous to carry out the fungicide or herbicide treatment repeatedly, preferably twice.
[0099]In the case of separate use, it may be advantageous to apply the herbicide II for example 3-6 weeks after planting the legume seeds and then to apply either the fungicide I alone or a mixture of fungicide I and herbicide II in a second application 4-8 weeks after planting.
[0100]In the case of joint application, a mixture of the compounds I and II is generally applied once to twice within a period of 1 to 3 months after planting the legume seeds.
[0101]The abovementioned application methods are understood as meaning foliar treatment of the legumes. In comparison to, for example, a seed treatment, these methods have pronounced advantages.
[0102]The use examples demonstrate the increased yield achieved by the use of pyraclostrobin and glyphosate in soya plantations.
[0103]It must be added that the increased yield is not connected to a successful control of harmful fungi. In the experiments, the experimental fields were free from disease. Naturally, in such a case the yield would be increased even more since the fungicidal active ingredients I (strobilurins) and Ix (azoles) or their mixtures constitute extremely efficient fungicides. Yield losses caused by harmful fungi can be counteracted effectively by the methods according to the invention.
[0104]Mention of the use according to the invention of the active ingredients I may be made in the form of an imprint on the packaging or else in product data sheets. Such mention may also be made in the case of products which can be used in combination with the active ingredients I.
[0105]Use examples for the increased yield in legumes
Use Example
[0106]The results shown hereinbelow were obtained in experiments in the open which were carried out during the winter season in the Argentinian northern pampas. The plots used were arranged randomly relative to one another. Each treatment variant was replicated four times. The crop plant used was the soya variety NIDERA AX 4910, which is resistant to numerous fungal diseases and to the herbicide glyphosate.
[0107]In all 5 experiments, two foliar treatments with glyphosate were carried out 30 or 60 days after planting the soya seeds, using equipment conventionally used under practice conditions. In the experiments 2 and 3, pyraclostrobin was added at "30 days after planting", while pyraclostrobin was added at "60 days after planting" in the experiments 4 and 5. As demonstrated by the results, the addition of pyraclostrobin in amounts of 50 or 100 g of a.s./ha at both the early and the late treatment times markedly increased the yield in comparison with the conventional use of glyphosate alone.
TABLE-US-00011 Treatment 30 Treatment 60 Experiment days after a.s. days after a.s. number planting g/ha planting g/ha Yield 1 glyphosate 360 glyphosate 360 100% 2 glyphosate 360 glyphosate 360 116% pyra- 50 clostrobin 3 glyphosate 360 glyphosate 360 129% pyra- 100 clostrobin 4 glyphosate 360 glyphosate 360 122% pyra- 50 clostrobin 5 glyphosate 360 glyphosate 360 135% pyra- 100 clostrobin a.s. = active substance
User Contributions:
Comment about this patent or add new information about this topic: