Patent application title: PHARMACEUTICAL SOLUTION HAVING ANTI-TUMOR EFFECT-ENHANCING AND TOXICITY-REDUCING EFFECT, AND PHARMACEUTICAL COMPOSITION COMPRISING SAME
Inventors:
IPC8 Class: AA61K3300FI
USPC Class:
1 1
Class name:
Publication date: 2017-03-16
Patent application number: 20170071976
Abstract:
The present disclosure relates to a pharmaceutical solution having an
anti-tumor efficacy-enhancing and toxicity-reducing effect, and a
pharmaceutical composition comprising the solution as well as a method
for using them. The pharmaceutical solution uses deuterium oxide as a
solvent.Claims:
1. A pharmaceutical solution, comprising deuterium oxide as a solvent,
wherein in the deuterium oxide, the isotope abundance of deuterium is
50.0% to 99.9%.
2. The pharmaceutical solution according to claim 1, wherein the isotope abundance of deuterium is 99.0% to 99.9%.
3. The pharmaceutical solution according to claim 1, further comprising sodium chloride, the pharmaceutical solution containing 0.1 g to 5 g sodium chloride per 100 ml solution.
4. The pharmaceutical solution according to claim 1, further comprising glucose, the pharmaceutical solution comprising 0.1 g to 50 g glucose per 100 ml solution.
5. The pharmaceutical solution according to claim 1, further comprising sodium bicarbonate, the pharmaceutical solution comprising 1 g to 10 g sodium bicarbonate per 100 ml solution.
6. The pharmaceutical solution according to claim 1, further comprising glucose and sodium chloride, the pharmaceutical solution comprising 5 g glucose and 0.9 g sodium chloride per 100 ml solution and having a pH adjusted to 3.5 to 5.5.
7. The pharmaceutical solution according to claim 1, wherein the pharmaceutical solution is a Ringer's deuterium oxide solution comprising 0.9 g sodium chloride, 0.012 g potassium chloride and 0.024 g calcium chloride per 100 ml solution and having a pH adjusted to 4.5 to 7.5.
8. The pharmaceutical solution according to claim 1, further comprising sodium hyaluronate, the pharmaceutical solution comprising 0.04 g to 3 g sodium hyaluronate per 100 ml solution.
9. The pharmaceutical solution according to claim 1, further comprising hydroxyethyl starch, the pharmaceutical solution comprising 3 g to 8 g hydroxyethyl starch per 100 ml solution.
10. The pharmaceutical solution according to claim 1, further comprising hydroxypropyl-.beta.-cyclodextrin (HP-.beta.-CD), the pharmaceutical solution comprising 0.4 g to 10 g HP-.beta.-CD per 100 ml solution.
11. The pharmaceutical solution according to claim 1, wherein the pharmaceutical solution is a solution for lavage and perfusion, a solution for injection, a suspension, an emulsion, or a solution for embolization.
12. An anti-tumor medicament for combinational administration, characterized in that the medicament comprises the pharmaceutical solution according to claim 1, and at least one anti-tumor drug, and optionally one or more pharmaceutically acceptable excipients.
13. The medicament for combinational administration according to claim 12, wherein the anti-tumor drug includes antimetabolites, phytogenic anti-tumor drugs, tumor antibiotics, alkylating agents, platinum preparations, monoclonal antibody anti-tumor drugs, or mixtures thereof.
14. The medicament for combinational administration according to claim 13, wherein the antimetabolites include 5-fluorouracil, gemcitabine, floxuridine, pemetrexed, raltitrexed, fludarabine, cytarabine, or mixtures thereof; the tumor antibiotics include mitomycin, epirubicin, peplomycin, daunorubicin, adriamycin, pirarubicin, aclarubicin, or mixtures thereof; the platinum preparations include cisplatin, oxaliplatin, carboplatin, nedaplatin, or mixtures thereof; the phytogenic anti-tumor drugs include paclitaxel, paclitaxel liposomes, paclitaxel albumin, docetaxel, etoposide, hydroxycamptothecin, or mixtures thereof; the alkylating agents include thiotepa, carmustine, nimustine, fotemustine, estramustine, cyclophosphamide, myleran, or mixtures thereof; the monoclonal antibody anti-tumor drugs include bevacizumab, cetuximab, trastuzumab, panitumumab, nimotuzumab, recombinant human endostatin, or mixtures thereof.
15. An anti-tumor pharmaceutical composition, wherein the pharmaceutical composition comprises the pharmaceutical solution according to claim 1, at least one anti-tumor drug, and optionally one or more pharmaceutically excipients.
16. The pharmaceutical composition according to claim 15, wherein the anti-tumor drug includes antimetabolites, phytogenic anti-tumor drugs, tumor antibiotics, alkylating agents, platinum preparations, monoclonal antibody anti-tumor drugs, or mixtures thereof.
17. The pharmaceutical composition according to claim 16, wherein the antimetabolites include 5-fluorouracil, gemcitabine, floxuridine, pemetrexed, raltitrexed, fludarabine, cytarabine, or mixtures thereof; the tumor antibiotics include mitomycin, epirubicin, peplomycin, daunorubicin, adriamycin, pirarubicin, aclarubicin, or mixtures thereof; the platinum preparations include cisplatin, oxaliplatin, carboplatin, nedaplatin, or mixtures thereof; the phytogenic anti-tumor drugs include paclitaxel, paclitaxel liposomes, paclitaxel albumin, docetaxel, etoposide, hydroxycamptothecin, or mixtures thereof; the alkylating agents include thiotepa, carmustine, nimustine, fotemustine, estramustine, cyclophosphamide, myleran, or mixtures thereof; the monoclonal antibody anti-tumor drugs include bevacizumab, cetuximab, trastuzumab, panitumumab, nimotuzumab, recombinant human endostatin, or mixtures thereof.
18. A method for treating tumors, comprising administering a therapeutically effective amount of the pharmaceutical solution according to claim 1, to a mammal having a tumor.
19. The method according to claim 18, wherein the pharmaceutical solution is administered by perfusion and lavage, hyperthermic perfusion and lavage, intravenous injection, intra-arterial injection, topical intrathecal injection, or intratumoral and peritumoral injection.
20. The method according to claim 18, wherein the pharmaceutical solution is a solution for lavage and perfusion, a solution for injection, a solution for suspension, an solution for emulsion, or a solution for embolization.
21. The method according to claim 20, wherein the administration by perfusion and lavage or by hyperthermic perfusion and lavage is performed by perfusing and lavaging thoracic cavity, peritoneal cavity, pelvic cavity, bladder cavity, buccal cavity, nasal cavity, enteric cavity, uterine cavity, or skin of the mammal having a tumor, with a solution for lavage and perfusion.
22. The method according to claim 21, wherein in the administration by hyperthermic perfusion and lavage, the temperature of the solution for lavage and perfusion is 40.degree. C. to 48.+-.1.degree. C.
23. The method according to claim 21, wherein in the administration by perfusion and lavage or by hyperthermic perfusion and lavage, the dose of a solution for lavage and perfusion is 5 to 6,000 ml/administration.
24. The method according to claim 20, wherein in the administration by intravenous injection, intra-arterial injection, intrathecal injection, or intratumoral and peritumoral injection, the dose of a solution for injection is 1 ml/kg to 20 ml/kg.
25. The method according to claim 18, wherein the tumor is selected from lung cancer, colorectal cancer, primary liver cancer, esophageal cancer, gastric cancer and cardiac cancer, pancreatic cancer, renal cell carcinoma, bladder cancer, prostate cancer, head and neck cancer, nasopharyngeal cancer, cervical cancer, ovarian cancer, breast cancer, brain tumor, bone and joint sarcoma, thyroid cancer, skin cancer, malignant melanoma, malignant lymphoma, leukemia, and complications and recurrence of various malignant tumors, such as thoracic, peritoneal and/or pelvic cavity metastasis and implantation of tumor cells, and malignant effusion in thoracic, peritoneal and/or pelvic cavity.
26. The method according to claim 25, wherein the lung cancer includes small cell lung cancer (SCLC) and non-small cell lung cancer; the colorectal cancer includes early stage colorectal cancer and advanced stage colorectal cancer; the primary liver cancer includes a hepatic cell type, a hepatic duct cell type, and a mixed type; the esophageal cancer includes adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and small cell carcinoma; the gastric cancer and cardiac cancer include adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, small cell carcinoma, and malignant gastrointestinal stromal tumor; the pancreatic cancer includes ductal cell carcinoma and osteoclast-like giant cell carcinoma; the renal cell carcinoma includes clear cell carcinoma and papillary renal cell carcinoma; the bladder cancer includes urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma; the prostate cancer includes adenocarcinoma, ductal adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma; the head and neck cancer includes squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma; the nasopharyngeal carcinoma includes squamous cell carcinoma and adenocarcinoma; the cervical cancer includes squamous cell carcinoma, adenocarcinoma, and sarcoma; the ovarian cancer includes ovarian epithelial cancer, germ cell tumors, and ovarian sex cord-stromal tumors; the breast cancer includes epithelial tumors, mesenchymal tumors, and myoepitheliomas; the brain tumors include primary brain tumors and brain tumor metastases; the bone and joint sarcomas includes chondrosarcoma, osteosarcoma, Ewing's sarcoma, and soft tissue sarcoma; the thyroid cancer includes papillary carcinoma, follicular carcinoma, and medullary carcinoma; the skin cancer includes basal cell carcinoma and squamous cell carcinoma; the malignant melanoma includes superficial diffusive melanoma, nodular melanoma, and acral-lentiginous melanoma; the malignant lymphomas include Hodgkin's lymphoma and non-Hodgkin's lymphoma; and the leukemia includes acute leukemia and chronic leukemia.
27. The method according to claim 18, wherein the mammal is selected from a rodent and a human.
Description:
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application is a continuation-in-part application of PCT/CN2015/079686 filed May 25, 2015, which claims priority to Chinese application number 201410227737.7 filed May 26, 2014.
TECHNICAL FIELD
[0002] The present disclosure belongs in the field of medicine, and relates to a pharmaceutical solution having an anti-tumor efficacy-enhancing and toxicity-reducing effect, and a pharmaceutical composition comprising the solution, as well as a method for using them.
BACKGROUND ART
[0003] According to a report by the World Health Organization (WHO), in 2014 there were 12 million cases diagnosed with malignancy tumors, and 8.5 million deaths from malignant tumors. Therefore, effective control of malignant tumors is a very urgent and enormous task for people practicing in medicine.
[0004] At present, the main therapies against malignant tumors are surgery, radiotherapy, and chemotherapy with various anti-tumor drugs, and these methods have their own characteristics but also certain limitations, especially the unremarkable efficacy often shown in chemotherapy with anti-tumor drugs. For example, a commonly used anti-tumor drug 5-fluorouracil (5-FU) shows an overall response rate of only 10% as a single drug in clinical settings. And many anti-tumor drugs have considerable toxicity which is proportional to their dose. In order to improve efficacy, an increase in dosage of a drug is needed but also results in an increase in toxicity caused by the drug, which poses a dilemma. Therefore, to increase the efficacy of anti-tumor drugs while reducing their toxicity is an important strategy towards improvement of clinical efficacy of anti-tumor drugs.
[0005] Many existing anti-tumor drugs must be systemically or topically administered by injection because of their physical or chemical properties, pharmacokinetics and pharmacodynamics. These are commonly used as carrier solutions such as a sodium chloride physiological solution and a 5% glucose solution. These solutions only serve as carriers for anti-tumor drugs, and they neither have any anti-tumor efficacy nor can enhance the efficacy of the anti-tumor drugs.
[0006] Deuterium oxide (heavy water, D.sub.2O, Mw: 20.03, CAS No. 7789-20-0) can be isolated from seawater. A normal body of adult human contains about 5 g deuterium oxide. It has been demonstrated by experiments using dogs as an animal model that exogenous deuterium oxide that reaches a concentration of 30% in the body causes a toxic effect. Based on this experimental result, theoretically, assuming the body weight of an adult human is 60 kg, deuterium oxide that reaches 18 kg in the body starts to cause toxicity. However, it is actually impossible for a human body to have 18 kg deuterium oxide. Furthermore, the state government of California, USA clearly indicates in the list of chemicals known to the State that deuterium oxide does not cause cancer or defect in reproductive development. Therefore, deuterium oxide should be a non-toxic substance [D. M. Czajka: Am. J. Physiol. 201(1961): 357-362; California Proposition 65]. In 1960s, Gross P. R. et al. found that deuterium oxide may inhibit DNA synthase in cells (Science 133(1961): 1131-1133). Afterwards, Laissue et al. studied the anti-tumor effect of deuterium oxide using mice as a model (Cancer Res. 42 (1982) 1125-1129), and Alterman et al. studied the anti-tumor effect of drinking deuterium oxide in animals using nude mice bearing a human tumor cells as a model (Cancer 62(1988): 462-466. Intl. Cancer. 45(1990): 475-480). In the recent 10 years, Hartmann et al. demonstrated that deuterium oxide can inhibit in vitro proliferation of pancreatic cancer cells (Anticancer Res. 25(2005): 3407-3412), and Bahk et al. demonstrated that deuterium oxide can inhibit in vitro proliferation of bladder cancer cells (J. Ind. Eng. Chem. 13(2007):501-507).
[0007] The aforementioned studies on the anti-tumor effect of deuterium oxide focus on in vitro cell-based experiments. It is well acknowledged through research and development of new drugs that drug candidates showing efficacy in in vitro cell-based experiments have a chance of only 1 in 100,000 to still show efficacy in vivo. Therefore, based on the experimental results using in vitro cell culture as a model, whether a drug candidate is clinically efficacious and approvable is poorly predictable. The only two animal studies that have been carried out both use a simple drinking liquid of deuterium oxide, which is a hypotonic solution. The mechanism of action is to increase in the in vivo deuterium content to combat tumors. In order to have an effective in vivo therapeutic dosage, the animals need to orally take a large dose of the drinking liquid of deuterium oxide. Specifically, in the animal experiments, a drinking liquid of deuterium oxide at a concentration of 30% was prepared by mixing deuterium oxide with drinking water, and used to completely replace drinking water for the animals to drink freely and arbitrarily. Several days to weeks later, the animals reached a deuteration state with a very high level of deuterium, which starts to exert an anti-tumor effect. However, such an drinking liquid of deuterium oxide has serious drawbacks: 1) currently most of the clinically efficacious anti-tumor therapies are a combinational chemotherapy of multiple anti-tumor drugs, while most anti-tumor drugs cannot be orally administered for various reasons; therefore, it is difficult to use deuterium oxide in combination with many anti-tumor drugs, and in turn it is impossible to carry out an effective combinational chemotherapy for patients; 2) the drinking amount of water varies between individuals, and if deuterium oxide is drunk arbitrarily, the dosage therefore cannot be quantified, and the therapeutic effects among different individuals cannot be compared; and under the influence of pharmacokinetic factors like digestion, assimilation, and metabolism, deuterium oxide needs to be taken in a large amount, and is less likely to be approved; 3) the patients need to drink a large amount drinking liquid of deuterium oxide in a short time, which is difficult to implement, results in poor clinical compliance, and also incurs high cost; 4) many mid-stage or late stage cancer patients have dysphagia or have taken a gastrointestinal surgery, and cannot orally take food or water, let alone deuterium oxide.
[0008] Moreover, about 50% of mid-stage or late stage cancer patients have tumor metastasis and recurrence at topical sites (such as thoracic cavity, peritoneal cavity, pelvic cavity, bladder cavity, rectal lumen, buccal and nasal cavity, uterine cavity, and skin), which is the direct cause of death of most patients. Recently developed topical perfusion chemotherapy has become an effective therapy in prevention or treatment of tumor metastasis and recurrence. However, in this method anti-tumor drugs are dissolved or diluted in the sodium chloride physiological solution and administered by direct perfusion and lavage, and also have the problem of unsatisfactory efficacy. In addition, the sodium chloride physiological solution does not have any anti-tumor efficacy-enhancing action.
[0009] Therefore, in the art there is still a demand for a pharmaceutical composition having anti-tumor efficacy, especially a pharmaceutical composition employing a solution that enhances the efficacy.
SUMMARY OF THE DISCLOSURE
[0010] The present disclosure is made based on the following finding of the inventor:
1) use of deuterium oxide to dissolve or dilute other anti-tumor drugs to prepare various pharmaceutical solutions, avoids oral administration, and can apply various administration routes such as intravenous injection or instillation, intra-arterial injection, transcatheter embolization, topical (such as thoracic cavity, peritoneal cavity, pelvic cavity, bladder cavity, rectal lumen, buccal and nasal cavity, uterine cavity, and skin) perfusion and lavage and 40.degree. C.-48.degree. C. hyperthermic perfusion and lavage; 2) direct injection of small doses of deuterium oxide changes the cell cycle of tumor cells and kills tumor cells; 3) efficacy-enhancing and toxicity reducing effect is obtained by directly dissolving or diluting a small dose of anti-tumors drugs in deuterium oxide, and administering the solution by injection, or by topical (such as thoracic cavity, peritoneal cavity, pelvic cavity, bladder cavity, rectal lumen, buccal and nasal cavity, uterine cavity, articular cavity, and skin) perfusion and lavage and 40.degree. C.-48.degree. C. hyperthermic perfusion and lavage, which can achieve the same efficacy as a large dose of the anti-tumor drugs but avoid the toxicity therefore; the application of deuterium oxide with hyperthermia exhibiting an unexpected anti-tumor effect of enhancing, thereby also improves quality of life of the mammal having a tumor; 4) drug approvability is increased: administration of deuterium oxide by injection allows dosage quantification, which overcomes the drawback of short of drug approvability due to arbitrary drinking without a quantified dose; 5) safety is achieved: administration of a certain dose of deuterium oxide by direct injection, or by topical (such as thoracic cavity, peritoneal cavity, pelvic cavity, bladder cavity, rectal lumen, buccal and nasal cavity, uterine cavity, articular cavity, and skin) perfusion and lavage and 40.degree. C.-48.degree. C. hyperthermic perfusion and lavage is safe to mammals.
[0011] In summary, deuterium oxide per se has anti-tumor efficacy, and when administered in combination with various different anti-tumor drugs, it can significantly enhance the anti-tumor efficacy in a synergistic manner and also reduce toxicity of these anti-tumor drugs, thereby useful for treatment of malignant tumors, which is an unexpected effect.
[0012] An aspect of the present disclosure is to provide a pharmaceutical solution characterized by using deuterium oxide as a solvent.
[0013] Another aspect of the present disclosure is to provide a medicament for combinational administration, characterized in that the medicament comprises the pharmaceutical solution of the present disclosure, at least one anti-tumor drug, and optionally one or more pharmaceutically acceptable excipients.
[0014] Another aspect of the present disclosure is to provide a pharmaceutical composition, characterized in that the pharmaceutical composition comprises the pharmaceutical solution of the present disclosure, at least one anti-tumor drug, and optionally one or more pharmaceutically acceptable excipients.
[0015] Another aspect of the present disclosure is to provide use of the pharmaceutical solution, the pharmaceutical composition, or the medicament for combinational administration according to the present disclosure in the manufacture of an anti-tumor agent.
[0016] Another aspect of the present disclosure is to provide a method for preventing or treating tumors, comprising administering a therapeutically effective amount of the pharmaceutical solution, the pharmaceutical composition, or the medicament for combinational administration according to the present disclosure, to a mammal having a tumor.
[0017] Another aspect of the present disclosure is to provide a method for preventing or treating tumors, characterized in that the medicament for combinational administration comprises deuterium oxide and at least one anti-tumor drug, wherein the deuterium oxide and the anti-tumor drug may be administered separately or concomitantly.
[0018] Another aspect of the present disclosure is to provide a method for preventing or treating tumors, wherein the pharmaceutical solution, the medicament for combinational administration, and the pharmaceutical composition are a solution for lavage and perfusion, a solution for injection, a suspension, an emulsion, or an embolization.
[0019] Another aspect of the present disclosure is to provide a method for preventing or treating tumors, comprising administering a therapeutically effective amount of the pharmaceutical solution, the pharmaceutical composition, or the medicament for combinational administration according to the present disclosure to a mammal having a tumor by various administration routes, excluding oral administration, including intravenous injection or instillation, intra-arterial injection, topical perfusion and lavage, topical hyperthermic perfusion and lavage, trascatheter embolization, topical intratumoral and peritumoral administration.
[0020] Another aspect of the present disclosure is to provide a method for preventing or treating malignant tumors, wherein administration by perfusion and lavage is performed by perfusing and lavaging topical sites of the mammal having a tumor, such as thoracic cavity, peritoneal cavity, pelvic cavity, bladder cavity, buccal cavity, nasal cavity, enteric cavity, uterine cavity, articular cavity, and skin, with a solution for perfusion and lavage.
[0021] Another aspect of the present disclosure is to provide a method for preventing or treating malignant tumors, wherein in administration by hyperthermic perfusion and lavage, the temperature of the solution for perfusion and lavage is 40.degree. C. to 48.degree. C., preferably 42.degree. C. to 44.5.degree. C., and more preferably 42.degree. C.
[0022] Another aspect of the present disclosure is to provide a method for preventing or treating malignant tumors, wherein in administration by perfusion and lavage or by hyperthermic perfusion and lavage, the dose of the solution for perfusion and lavage are 5 to 6,000 ml/administration to the mammal having a tumor.
[0023] Another aspect of the present disclosure is to provide a method for preventing or treating malignant tumors, wherein in administration by intravenous injection, intra-arterial injection, intrathecal injection, or intratumoral and peritumoral injection, the dose of the solution for injection is 1 ml/kg to 20 ml/kg to the mammal having a tumor.
[0024] In the method according to the present disclosure, the mammal is selected from a rodent and a human.
[0025] Another aspect of the present disclosure is to provide use of deuterium oxide in the manufacture of a pharmaceutical solution.
[0026] The pharmaceutical composition or the medicament for combinational administration according to the present disclosure shows a marked anti-tumor effect and lower toxicity than current anti-tumor drugs. The pharmaceutical composition or the medicament for combinational administration according to the present disclosure is useful for treatment or prevention of malignant tumors. Deuterium oxide shows an effect of synergistically enhancing the anti-tumor efficacy of anti-tumor drugs, such that a small dose of anti-tumor drugs can achieve the same efficacy as a large dose of the anti-tumor drugs, thereby reducing the amount and toxicity of anti-tumor drugs to be used. The pharmaceutical composition or the medicament for combinational administration according to the present disclosure is useful for preventing or reducing invasion, metastasis, recurrence, and drug-resistance of tumors.
DESCRIPTION OF FIGURES
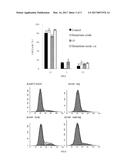
[0027] FIG. 1. Effect of deuterium oxide on the cell cycle of human colon cancer HCT-166 cells.
[0028] FIG. 2. Effect of deuterium oxide on the cell cycle of human colon cancer HCT-166 cells, as measured by the FCM method.
[0029] FIG. 3. Effect of deuterium oxide on the cell cycle of human lung cancer A549 cells.
[0030] FIG. 4. Effect of deuterium oxide on the cell cycle of human lung cancer A549 cells, as measured by the FCM method.
[0031] FIG. 5. Effect of the glucose deuterium oxide solution in combination with 5-FU on the tumor size of xenograft tumors from human colon cancer HCT-166 cells in nude mice.
[0032] FIG. 6. Effect of the glucose deuterium oxide solution in combination with 5-FU on the tumor weight of xenograft tumors from human colon cancer HCT-166 cells in nude mice.
[0033] FIG. 7. Effect of the glucose deuterium oxide solution in combination with 5-FU on the body weight of nude mice bearing xenograft tumors from human colon cancer HCT-166 cells.
[0034] FIG. 8. Effect of the sodium chloride deuterium oxide solution in combination with Gemcitabine on the tumor size of xenograft tumors from human breast cancer MCF-7 cells in nude mice.
[0035] FIG. 9. Effect of the sodium chloride deuterium oxide solution in combination with Gemcitabine on the tumor weight of xenograft tumors from human breast cancer MCF-7 cells in nude mice.
[0036] FIG. 10. Effect of the sodium chloride deuterium oxide solution in combination with Gemcitabine on the body weight of nude mice bearing xenograft tumors from human breast cancer MCF-7 cells.
[0037] FIG. 11. Effect of the sodium chloride deuterium oxide solution in combination with Gemcitabine on the tumor size of xenograft tumors from human lung cancer A549 cells in nude mice.
[0038] FIG. 12. Effect of the sodium chloride deuterium oxide solution in combination with Gemcitabine on the tumor weight of xenograft tumors from human lung cancer A549 cells in nude mice.
[0039] FIG. 13. Effect of the sodium chloride deuterium oxide solution in combination with Gemcitabine on the body weight of nude mice bearing xenograft tumors from human lung cancer A549 cells.
[0040] In the figures, both DJ and HW refer to deuterium oxide.
DETAILED DESCRIPTION
[0041] The present application relates to the following embodiments.
Embodiment 1
[0042] A pharmaceutical solution, characterized by using deuterium oxide as a solvent.
Embodiment 2
[0043] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises sodium chloride and deuterium oxide, being a sodium chloride deuterium oxide solution containing 0.1 g to 5 g sodium chloride, preferably 0.5 g to 2.5 g sodium chloride per 100 ml solution.
Embodiment 3
[0044] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises sodium chloride and deuterium oxide, being a sodium chloride deuterium oxide solution containing 0.9 g sodium chloride per 100 ml solution, which is prepared with deuterium oxide and sodium chloride and has a pH of 4.5 to 7.0.
Embodiment 4
[0045] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises glucose and deuterium oxide, being a glucose deuterium oxide solution containing 0.1 g to 50 g glucose, preferably 5 to 10 g, most preferably 5 g glucose per 100 ml pharmaceutical solution, which is prepared with deuterium oxide and glucose and has a pH of 3.2 to 6.5.
Embodiment 5
[0046] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises glucose and sodium chloride and deuterium oxide, being a glucose sodium deuterium oxide solution containing 0.9 g sodium chloride and 5 g glucose per 100 ml pharmaceutical solution, which is prepared with deuterium oxide, sodium chloride and glucose and has a pH of 3.5 to 5.5.
Embodiment 6
[0047] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises sodium bicarbonate and deuterium oxide, being a sodium bicarbonate deuterium oxide solution containing 1 to 10 g, preferably 5 to 10 g, most preferably 5 g sodium bicarbonate per 100 ml pharmaceutical solution, which is prepared with deuterium oxide and sodium bicarbonate and has a pH of 7.5 to 8.5.
Embodiment 7
[0048] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution is a Ringer's deuterium oxide solution containing 0.85 g sodium chloride, 0.012 g potassium chloride and 0.024 g calcium chloride (CaCl.sub.2.2H.sub.2O) per 100 ml pharmaceutical solution, which is prepared with deuterium oxide, sodium chloride, potassium chloride and calcium chloride and has a pH of 4.5 to 7.5.
Embodiment 8
[0049] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises sodium hyaluronate and deuterium oxide, being a sodium hyaluronate deuterium oxide solution containing 0.04 to 3 g, preferably 0.08 to 1.4 g sodium hyaluronate, most preferably being a sodium hyaluronate deuterium oxide solution containing 0.08 or 1.0 g sodium hyaluronate, 0.9 g sodium chloride, 0.142 g disodium hydrogen phosphate and 0.027 g sodium dihydrogen phosphate per 100 ml pharmaceutical solution and having a pH adjusted to 6.5 to 7.5.
Embodiment 9
[0050] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises hydroxyethyl starch and deuterium oxide, being a hydroxyethyl starch deuterium oxide solution containing 3 to 8 g hydroxyethyl starch, most preferably being a solution of hydroxyethyl starch and sodium chloride in deuterium oxide containing 6 g hydroxyethyl starch and 0.9 g sodium chloride per 100 ml pharmaceutical solution and having a pH adjusted to 6.0 to 7.0.
Embodiment 10
[0051] The pharmaceutical solution according to Embodiment 1, characterized in that, the pharmaceutical solution comprises hydroxypropyl-.beta.-cyclodextrin (HP-.beta.-CD) and deuterium oxide, being a HP-.beta.-CD deuterium oxide solution containing 0.4 to 10 g HP-.beta.-CD, most preferably being a HP-.beta.-CD sodium chloride deuterium oxide solution containing 10 g HP-.beta.-CD and 0.9 g sodium chloride per 100 ml pharmaceutical solution and having a pH adjusted to 5.0 to 7.0.
Embodiment 11
[0052] The pharmaceutical solution according to Embodiments 1 to 10, characterized in that, the pharmaceutical solution is a solution for lavage and perfusion, a solution for injection, a suspension, an emulsion, or a solution for embolization.
Embodiment 12
[0053] An anti-tumor medicament for combinational administration, characterized in that the medicament comprises the pharmaceutical solution according to any one of Embodiments 1 to 10, at least one anti-tumor drug, and optionally one or more pharmaceutically acceptable excipients.
Embodiment 13
[0054] The medicament for combinational administration according to Embodiment 12, wherein the anti-tumor drug is selected from anti-tumor cytotoxic drugs and monoclonal antibody anti-tumor drugs.
Embodiment 14
[0055] The medicament for combinational administration according to Embodiment 13, wherein the anti-tumor cytotoxic drugs include antimetabolites, phytogenic anti-tumor drugs, tumor antibiotics, alkylating agents, monoclonal antibodies, and platinum preparations.
Embodiment 15
[0056] The medicament for combinational administration according to Embodiment 14, wherein the antimetabolites include 5-fluorouracil, gemcitabine, floxuridine, pemetrexed, raltitrexed, fludarabine, cytarabine; the tumor antibiotics include mitomycin, epirubicin, peplomycin, daunorubicin, adriamycin, pirarubicin, aclarubicin; the platinum preparations include cisplatin, oxaliplatin, carboplatin, nedaplatin; the phytogenic anti-tumor drugs include paclitaxel, paclitaxel liposomes, paclitaxel albumin, docetaxel, etoposide, hydroxycamptothecin; the alkylating agents include thiotepa, carmustine, nimustine, fotemustine, estramustine, cyclophosphamide, myleran.
Embodiment 16
[0057] The medicament for combinational administration according to Embodiment 14, wherein the monoclonal antibody anti-tumor drugs include bevacizumab, cetuximab, trastuzumab, panitumumab, nimotuzumab, recombinant human endostatin.
Embodiment 17
[0058] An anti-tumor pharmaceutical composition, characterized in that the pharmaceutical composition comprises the pharmaceutical solution according to any one of Embodiments 1 to 11, at least one anti-tumor drug, and optionally one or more pharmaceutically acceptable excipients.
Embodiment 18
[0059] The pharmaceutical composition according to Embodiment 17, wherein the anti-tumor drug is selected from anti-tumor cytotoxic drugs and monoclonal antibody anti-tumor drugs.
Embodiment 19
[0060] The pharmaceutical composition according to Embodiment 18, wherein the anti-tumor cytotoxic drugs include antimetabolites, phytogenic anti-tumor drugs, tumor antibiotics, alkylating agents, monoclonal antibodies, and platinum preparations.
Embodiment 20
[0061] The pharmaceutical composition according to Embodiment 19, wherein the antimetabolites include 5-fluorouracil, gemcitabine, floxuridine, pemetrexed, raltitrexed, fludarabine, cytarabine; the tumor antibiotics include mitomycin, epirubicin, peplomycin, daunorubicin, adriamycin, pirarubicin, aclarubicin; the platinum preparations include cisplatin, oxaliplatin, carboplatin, nedaplatin; the phytogenic anti-tumor drugs include paclitaxel, paclitaxel liposomes, paclitaxel albumin, docetaxel, etoposide, hydroxycamptothecin; the alkylating agents include thiotepa, carmustine, nimustine, fotemustine, estramustine, cyclophosphamide, myleran.
Embodiment 21
[0062] The pharmaceutical composition according to Embodiment 20, wherein the monoclonal antibody anti-tumor drugs include bevacizumab, cetuximab, trastuzumab, panitumumab, nimotuzumab, recombinant human endostatin.
Embodiment 22
[0063] Use of the pharmaceutical solution according to any one of Embodiments 1 to 11, the medicament for combinational administration according to any one of Embodiments 12 to 16, or the pharmaceutical composition according to any one of Embodiments 17 to 21, in the manufacture of an anti-tumor agent.
Embodiment 23
[0064] The pharmaceutical solution according to any one of Embodiments 1 to 11, the medicament for combinational administration according to any one of Embodiments 12 to 16, or the pharmaceutical composition according to any one of Embodiments 17 to 21, for use in treatment or prevention of tumors.
Embodiment 24
[0065] A method for treating or preventing tumors, comprising administering a therapeutically effective amount of the pharmaceutical solution, the pharmaceutical composition, or the medicament for combinational administration according to the present disclosure to a mammal having a tumor by various administration routes, excluding oral administration, including intravenous injection or instillation, intra-arterial injection, topical perfusion and lavage, topical hyperthermic perfusion and lavage, transcatheter embolization, topical intrathecal injection, and intratumoral and peritumoral injection.
Embodiment 25
[0066] In Embodiment 23 or 24, in the administration by hyperthermic perfusion and lavage, the temperature of the solution for lavage and perfusion is 40.degree. C. to 48.+-.1.degree. C., preferably 42.degree. C. to 44.5.+-.1.degree. C., and more preferably 42.+-.1.degree. C.
Embodiment 26
[0067] In Embodiment 25, the administration by topical perfusion and lavage or by topical hyperthermic perfusion and lavage is performed by perfusing and lavaging topical sites, such as thoracic cavity, peritoneal cavity, pelvic cavity, bladder cavity, buccal cavity, nasal cavity, enteric cavity, uterine cavity, articular cavity, and skin, of the mammal with a solution for lavage and perfusion in a dose of 5 to 6,000 ml/administration.
Embodiment 27
[0068] In Embodiment 24, in the administration by intravenous injection, intra-arterial injection, intrathecal injection, or intratumoral and peritumoral injection, the dose of the solution for injection is 1 ml/kg to 20 ml/kg.
Embodiment 28
[0069] A method for treating or preventing tumors, comprising administering a therapeutically effective amount of the pharmaceutical solution according to any one of Embodiments 1 to 12, the medicament for combinational administration according to any one of Embodiments 13 to 17, or the pharmaceutical composition according to any one of Embodiments 18 to 22 to a mammal having a tumor.
Embodiment 29
[0070] Use of deuterium oxide in the manufacture of the pharmaceutical solution according to any one of Embodiments 1 to 11.
Embodiment 30
[0071] The use according to Embodiment 29, wherein the pharmaceutical solution serves as a broad-spectrum anti-tumor efficacy-enhancing agent, and synergistically enhances the efficacy of anti-tumor drugs.
[0072] In the pharmaceutical solution or pharmaceutical composition according to the present disclosure, every 100 ml of the pharmaceutical solution or pharmaceutical composition contains 1 to 99.9 g deuterium oxide, preferably 9 to 99.9 g deuterium oxide, more preferably 20 to 99.9 g deuterium oxide, even more preferably 30 to 99.9 g deuterium oxide, even more preferably 40 to 99.9 g deuterium oxide, even more preferably 50 to 99.9 g deuterium oxide, even more preferably 60 to 99.9 g deuterium oxide, even more preferably 70 to 99.9 g deuterium oxide, even more preferably 80 to 99.9 g deuterium oxide, even more preferably 90 to 99.9 g deuterium oxide, and most preferably 95 to 99.9 g deuterium oxide.
[0073] Deuterium oxide according to the present disclosure is produced by deuterium oxide plants, and is commercially available. In the deuterium oxide the isotope abundance of deuterium is 1 to 99.9%, preferably 30 to 99.9%, more preferably 50 to 99.9%, more preferably 70 to 99.9%, and most preferably 90 to 99.9%. For example the deuterium oxide provided by Cambridge Isotope Laboratories, Inc. USA, may be used, which has an isotope abundance of deuterium of 90 to 99.9% (D 90-99.9%).
[0074] The pharmaceutically excipients may be water, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, sodium lactate, sodium acetate, sodium citrate, sodium bicarbonate, potassium dihydrogen phosphate, disodium hydrogenphosphate, sodium bicarbonate, glucose, fructose, albumin, liposomes, hyaluronic acid, and/or polyethylene glycol.
[0075] The anti-tumor drug is selected from anti-tumor cytotoxic drugs and monoclonal antibody anti-tumor drugs.
[0076] The anti-tumor cytotoxic drugs include antimetabolites, phytogenic anti-tumor drugs, tumor antibiotics, alkylating agents, monoclonal antibodies, and platinum preparations. The antimetabolites include 5-fluorouracil, gemcitabine, floxuridine, pemetrexed, raltitrexed, fludarabine, cytarabine; preferably 5-fluorouracil, gemcitabine, and pemetrexed. The tumor antibiotics include mitomycin, epirubicin, peplomycin, daunorubicin, adriamycin, pirarubicin, aclarubicin; preferably mitomycin and epirubicin. The platinum preparations include cisplatin, oxaliplatin, carboplatin, nedaplatin; preferably cisplatin and oxaliplatin. The phytogenic anti-tumor drugs include paclitaxel, paclitaxel liposomes, paclitaxel albumin, docetaxel, etoposide, hydroxycamptothecin; preferably paclitaxel, paclitaxel liposomes, paclitaxel albumin, and docetaxel. The alkylating agents include thiotepa, carmustine, nimustine, fotemustine, estramustine, cyclophosphamide, myleran; preferably thiotepa and carmustine.
[0077] The monoclonal antibody anti-tumor drugs include bevacizumab, cetuximab, trastuzumab, panitumumab, nimotuzumab, recombinant human endostatin; preferably bevacizumab and recombinant human endostatin.
[0078] The pharmaceutical solution according to the present disclosure is prepared by standard methods for preparing solution in the field of pharmaceutics, and satisfies relevant standards in Chinese Pharmacopoeia, United States Pharmacopoeia, and European Pharmacopoeia.
[0079] When the pharmaceutical solution according to the present disclosure is combined with anti-tumor drugs, the dissolution or dilution ratios of anti-tumor drugs in the pharmaceutical solution prescribed in Chinese Pharmacopoeia, United States Pharmacopoeia, and European Pharmacopoeia are used.
[0080] The medicament for combinational administration according to the present disclosure refers to combined administration of one pharmaceutical solution according to the present disclosure and one or more anti-tumor drugs.
[0081] The pharmaceutical composition according to the present disclosure is a composite formulation prepared from a pharmaceutical solution and one or more anti-tumor drugs.
[0082] The components in the pharmaceutical solution, the pharmaceutical composition, or the medicament for combinational administration according to the present disclosure may be administered separately or concomitantly.
[0083] The pharmaceutical solution, the pharmaceutical composition, or the medicament for combinational administration according to the present disclosure is various preparations such as a solution for perfusion and lavage, a solution for injection, a suspension, an emulsion, or a solution for embolization.
[0084] The tumor according to the present disclosure is selected from lung cancer, colorectal cancer, primary liver cancer, esophageal cancer, gastric cancer and cardiac cancer, pancreatic cancer, renal cell carcinoma, bladder cancer, prostate cancer, head and neck cancer, nasopharyngeal cancer, cervical cancer, ovarian cancer, breast cancer, brain tumor, bone and joint sarcoma, thyroid cancer, skin cancer, malignant melanoma, malignant lymphoma, leukemia, and complications and recurrence of various malignant tumors, such as thoracic, peritoneal and/or pelvic cavity metastasis and implantation of tumor cells, and malignant effusion in thoracic, peritoneal and/or pelvic cavity.
[0085] Lung cancer includes small cell lung cancer (SCLC), non-small cell lung cancer and the like; colorectal cancer includes early stage colorectal cancer, advanced stage colorectal cancer, and the like; primary liver cancer include the hepatic cell type, hepatic duct cell type, a mixed type, and the like; esophageal cancer includes adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, small cell carcinoma, and the like; gastric cancer and cardiac cancer include adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, small cell carcinoma, malignant gastrointestinal stromal tumor, and the like; pancreatic cancer includes ductal cell carcinoma, osteoclast-like giant cell carcinoma, and the like; renal cell carcinoma includes clear cell carcinoma and papillary renal cell carcinoma; bladder cancer includes urothelial carcinoma, squamous cell carcinoma, adenocarcinoma, and the like; prostate cancer includes adenocarcinoma, ductal adenocarcinoma, urothelial carcinoma, and squamous cell carcinoma; head and neck cancer includes squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma; nasopharyngeal carcinoma includes squamous cell carcinoma and adenocarcinoma; cervical cancer includes squamous cell carcinoma, adenocarcinoma, and sarcoma; ovarian cancer includes ovarian epithelial cancer, germ cell tumors, and ovarian sex cord-stromal tumors; breast cancer includes epithelial tumors, mesenchymal tumors, and myoepitheliomas; brain tumors include primary brain tumors and brain tumor metastases; bone and joint sarcomas includes chondrosarcoma, osteosarcoma, Ewing's sarcoma, and soft tissue sarcoma; thyroid cancer includes papillary carcinoma, follicular carcinoma, and medullary carcinoma; skin cancer includes basal cell carcinoma and squamous cell carcinoma; malignant melanoma includes superficial diffusive melanoma, nodular melanoma, and acral-lentiginous melanoma; malignant lymphomas include Hodgkin's lymphoma and non-Hodgkin's lymphoma; leukemia includes acute leukemia and chronic leukemia.
[0086] In the method according to the present disclosure, the mammal is selected from a rodent and a human.
[0087] The word "comprise" or "comprising" used herein encompasses both the case where the substance(s) or component(s) referred to is exclusively included, and the case where other substance(s) or component(s) that does not interfere with the accomplishment of the intended objective is also included in addition to the substance or component referred to.
[0088] Unless otherwise specified, all values used herein to indicate the amount of a substance should be construed as being modified by the term "approximately".
[0089] The present disclosure will be further described in the below examples. It is to be understood that the scope of the present disclosure is not limited to the examples.
[0090] All cell lines used in the examples of the present disclosure are commercially available.
Example 1
[0091] 0.9 g sodium chloride was weighed out, to which 99 g deuterium oxide (D, 99.8%, from Cambridge Isotope Laboratories, Inc., USA, the same below) was added to dissolve the sodium chloride under stirring. Drops of a sodium hydroxide solution were added to adjust the solution pH to 7.0, and then deuterium oxide was added to a final volume of 100 ml. The solution was sterilized by filtration through a 0.2 .mu.m millipore filter and sealed in a container, to obtain a sodium chloride deuterium oxide solution containing 0.9 g sodium chloride per 100 ml pharmaceutical solution according to the present disclosure.
Example 2
[0092] 5 g glucose was weighed out, to which 99 g deuterium oxide was added to dissolve the glucose under stirring. Drops of a hydrochloric acid solution were added to adjust the pH to 5.5, and then deuterium oxide was added to a final volume of 100 ml. The solution was sterilized by filtration through a 0.2 .mu.m millipore filter and sealed in a container, to obtain a 5% glucose deuterium oxide solution containing 5 g glucose per 100 ml pharmaceutical solution according to the present disclosure.
Example 3
[0093] 10 g glucose was weighed out, to which 99 g deuterium oxide was added to dissolve the glucose under stirring. Drops of a hydrochloric acid solution were added to adjust the pH to 5.5, and then deuterium oxide was added to a final volume of 100 ml. The solution was sterilized by filtration through a 0.2 .mu.m millipore filter and sealed in a container, to obtain a 10% glucose deuterium oxide solution containing 10 g glucose per 100 ml pharmaceutical solution according to the present disclosure.
Example 4
[0094] 0.9 g sodium chloride and 5 g glucose were separately weighed out, to which 99 g deuterium oxide was added to dissolve them under stirring. Drops of a hydrochloric acid solution were added to adjust the pH to 5.0, and then deuterium oxide was added to a final volume of 100 ml. The solution was sterilized by filtration through a 0.2 .mu.m millipore filter, sealed in a container, to obtain a glucose sodium chloride deuterium oxide solution containing 0.9 g sodium chloride and 5 g glucose per 100 ml pharmaceutical solution according to the present disclosure.
Example 5
[0095] 5 g sodium bicarbonate was separately weighed out, to which 99 g deuterium oxide was added to dissolve it under stirring. Drops of a hydrochloric acid solution were added to adjust the pH to 8.0, and then deuterium oxide was added to a final volume of 100 ml. The solution was sterilized by filtration through a 0.2 .mu.m millipore filter and sealed in a container, to obtain a 5% sodium bicarbonate deuterium oxide solution containing 5 g sodium bicarbonate per 100 ml pharmaceutical solution according to the present disclosure.
Example 6
[0096] 0.85 g sodium chloride, 0.03 g potassium chloride and 0.033 g calcium chloride (CaCl.sub.2.2H.sub.2O) were separately weighed out, to which 99 g deuterium oxide was added to dissolve them under stirring. Drops of a sodium hydroxide solution were added to adjust the pH to 7.0, and then deuterium oxide was added to a final volume of 100 ml. The solution was sterilized by filtration through a 0.2 .mu.m millipore filter, and sealed in a container, to obtain a Ringer's deuterium oxide solution according to the present disclosure.
Example 7
[0097] 0.8 g sodium chloride, 0.04 g potassium chloride, 0.1 g glucose, 0.006 g potassium dihydrogen phosphate, 0.00475 g disodium hydrogen phosphate, and 0.22 g sodium bicarbonate were separately weighed out, to which 99 g deuterium oxide was added to dissolve them under stirring. Drops of a sodium hydroxide solution were added to adjust the pH to 7.0, and then deuterium oxide was added to a final volume of 100 ml. The solution was sterilized by filtration through a 0.2 .mu.m millipore filter, and sealed in a container, to obtain an Earle's balanced salt deuterium oxide solution according to the present disclosure.
Example 8
[0098] A method for preparing a warm (40.degree. C. to 48.degree. C.) 0.08% sodium hyaluronate deuterium oxide solution. Sodium hyaluronate, (HA, CAS: 9067-32-7, Formula: (C.sub.14H.sub.2O.sub.11N).sub.n, Mw: 10.sup.5 to 10.sup.7). The solution comprising 0.08 g sodium hyaluronate, 0.8 g sodium chloride, 0.142 g disodium hydrogen phosphate (Na.sub.2HPO.sub.4.12H.sub.2O) and 0.027 g sodium dihydrogen phosphate (NaH.sub.2PO.sub.4) per 100 ml solution, and having a pH of 6.5 to 7.5. Detailed steps of the method: 0.8 g sodium chloride, 0.142 g disodium hydrogen phosphate (Na.sub.2HPO.sub.4.12H.sub.2O) and 0.027 g sodium dihydrogen phosphate (NaH.sub.2PO.sub.4) was separately weighed out, dissolved in 80 g deuterium oxide to make a solution, which was boiled and sterilized, then 0.08 g sterile sodium hyaluronate was added and dissolved, the volume was metered to 100 ml with deuterium oxide, the pH was adjusted to 6.0 to 7.0, and the solution was sterilized by 0.2 .mu.m millipore filtration to obtain 0.08% sterile sodium hyaluronate deuterium oxide solution, which was filled into a container. For topical perfusion and lavage, the solution was warmed to a temperature of 40.degree. C. to 48.degree. C. in a hyperthermic perfusion and lavage apparatus, mixed with various anti-tumor drugs, and administered by topical perfusion and lavage.
Example 9
[0099] A method for preparing a 1.0% sodium hyaluronate deuterium oxide solution comprising 10 g sodium hyaluronate, 8 g sodium chloride, 1.42 g disodium hydrogen phosphate (Na.sub.2HPO.sub.4.12H.sub.2O) and 0.27 g sodium dihydrogen phosphate (NaH.sub.2PO.sub.4) per 1000 ml solution, and having a pH of 6.5 to 7.5. Detailed steps of the method: 8 g sodium chloride, 1.42 g disodium hydrogen phosphate (Na.sub.2HPO.sub.4.12H.sub.2O) and 0.27 g sodium dihydrogen phosphate (NaH.sub.2PO.sub.4) was separately weighed out, dissolved in 800 g deuterium oxide to make a solution, which was boiled and sterilized by filtration, then 10 g sterile sodium hyaluronate was added and dissolved, the volume was metered to 1000 ml with deuterium oxide, the pH was adjusted to 6.0 to 7.5, and the solution was sterilized by 0.2 .mu.m millipore filtration to obtain a 1.0% sterile sodium hyaluronate deuterium oxide solution, which was filled into a container and was ready for use.
Example 10
[0100] A method for preparing a perfusion and lavage solution of 6% hydroxyethyl starch (200/0.5) deuterium oxide solution. Hydroxyethyl Starch (CAS: 9005-27-0, Mw: 580.5. Formula: C.sub.22H.sub.44O.sub.17). There are 4 types of hydroxyethyl starches having different molecular weights: hydroxyethyl starch (20), hydroxyethyl starch (40), hydroxyethyl starch (130/0.4), and hydroxyethyl starch (200/0.5), all of which can be made into a perfusion and lavage solution of hydroxyethyl starch, and among which hydroxyethyl starch (200/0.5) is preferred. Detailed steps of the method: 60 g hydroxyethyl starch (200/0.5) was weighed out, dissolved in 500 g deuterium oxide to make a solution; 9 g sodium chloride was dissolved in another 500 g deuterium oxide, then the solution of hydroxyethyl starch (200/0.5) deuterium oxide and the solution of sodium chloride deuterium oxide were mixed in a ratio of 1:1, the volume was metered to 1000 ml with deuterium oxide, the pH was adjusted to 6.0 to 7.0, and the solution was sterilized by 0.2 .mu.m millipore filtration and sealed in a container and was ready for use.
Example 11
[0101] A method for preparing a hydroxypropyl-.beta.-cyclodextrin deuterium oxide solution. Hydroxypropyl-.beta.-cyclodextrin (HP-.beta.-CD, CAS: 128446-35-5. Mw: 1431-1806. Formula: (C.sub.42H.sub.70O.sub.35)-Hn+(C.sub.3H.sub.7O.sub.2).sub.n) comprising HP-.beta.-CD as a solute and deuterium oxide as a solvent, wherein the concentration of HP-.beta.-CD in the solution is 0.4 to 10 wt %, preferably 10 wt %. Detailed steps of the method: 10 g HP-.beta.-CD was weighed out and dissolved in 100 g deuterium oxide under stirring. The solution contained 10 g HP-.beta.-CD per 100 ml pharmaceutical solution, the pH was adjusted to 5.0 to 7.0, and the solution was sterilized by 0.2 .mu.m millipore filtration and sealed in a container and was ready for use.
Example 12
[0102] A method for preparing a sulfobutylether-.beta.-cyclodextrindeuterium oxide solution. Sulfobutylether-.beta.-cyclodextrin (SBE-.beta.-CD. CAS: 25167-62-8) comprising SBE-.beta.-CD as a solute and deuterium oxide as a solvent, wherein the concentration of SBE-.beta.-CD in the solution is 3 to 30 wt %, preferably 10 wt %. Most preferably, the solution contained 10 g SBE-.beta.-CD per 100 ml pharmaceutical solution. Detailed steps of the method: 10 g SBE-.beta.-CD was weighed out and dissolved in 100 g deuterium oxide under stirring, the pH was adjusted to 5.0 to 7.0, and the solution was sterilized by 0.2 pin millipore filtration and sealed in a container and was ready for use.
Example 13
[0103] The method for warming the solution to a temperature of 40.degree. C. to 48.degree. C.: for a solution of or less than 50 ml, the container containing the solution to be used was placed on a small heater or in a water bath at the corresponding temperature for 10 to 20 min; for a solution more than 50 ml, the solution was heated to a temperature of 40.degree. C. to 48.degree. C. in a hyperthermic perfusion and lavage apparatus (BR-TRG-1 hyperthermic perfusion intraperitoneal treatment system, manufactured by Baorui Medical Technology Co., Ltd, Guangzhou, China) and was ready for use.
Example 14
[0104] Studies of in vitro inhibition of growth of human tumor cells by deuterium oxide, by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
[0105] Cell line: all cell lines of various types shown in Table 1 were provided by NANJING KEYGEN BIOTECH. CO., LTD and from ATCC, and were commercially available (the same below).
Method:
[0106] 1. A bottle of cells that had been cultured for 3 to 4 days and were in the exponential phase was taken, a suitable amount of 0.25% Trypsin-EDTA solution was added thereto to detach the cells from the wall, and the cells were suspended in 10 ml RPMI 1640 medium containing 10% FBS. 2. The cells were counted on a hemocytometer, and generally viable cells should be not less than 97%. 3. The cell suspension was diluted with a complete medium to prepare a suspension containing 1.times.10.sup.4 cells/ml. 4. To each well of a 12-well culture plate, 300 .mu.l cell suspension was added. The addition of cells should be finished within 4 hours. The plate was placed in an incubator at 37.degree. C. and 5% CO.sub.2 for 24 hours. 5. Powdery RPMI Medium 1640 (Gibco, USA, Cat#: 31800-022) was dissolved in deuterium oxide (provided by Cambridge Isotope Laboratories, Inc. (US)) to prepare a cell culture medium (unless particularly specified, all culture media used in the Examples hereinafter were prepared from powdery RPMI Medium 1640 and deuterium oxide) at a concentration shown in Table 1, which was added into the cell culture plate. 6. The cell culture plate was incubated for 3 days in an incubator at 37.degree. C., 5% CO.sub.2 and 100% humidity. 7. A solution of 1 mg/ml MTT was prepared in a serum-free RPMI1640 medium, added to the plate at 200 .mu.l/well, and incubated at 37.degree. C. for 4 hours, to allow MTT to be reduced to formazan. 8. The supernatant was removed by pipetting, 200 .mu.l DMSO was added to dissolve formazan, and the solution was well mixed on a platform shaker. 9. Absorbance of each well was measured on an ELISA reader with a detection wavelength of 570 nm and a reference wavelength of 450 nm. 10. The assay was repeated one more time.
[0107] Calculation of results: Inhibition of growth of tumor cells by deuterium oxide was calculated using tumor cells treated with sodium chloride physiological solution as a control group. Inhibition of cell growth was plotted against amounts of deuterium oxide to obtain a dose response curve, from which the half maximal inhibitory concentration (IC.sub.50) of deuterium oxide was derived.
Inhibition of tumor cell growth (%)=(1-OD test/OD control).times.100%
TABLE-US-00001 TABLE 1 (a-1) In vitro inhibition of growth of human tumor cells by deuterium oxide Inhibition of growth (%) Deuterium Colon Gastric oxide cancer cell cancer cell conc. Lung cancer cell line Pancreatic cancer cell line line line (v/v) A549 NCI-H460 NCI-1975 CFPAC-1 ASPC-1 PANC-1 HCT-116 BGC-823 80% 92.34 98.06 87.23 91.85 85.83 79.05 94.62 74.57 70% 70.88 82.33 79.65 77.94 70.14 57.29 70.42 61.90 60% 55.42 69.50 51.93 61.42 52.22 50.31 66.31 37.83 50% 42.67 53.09 39.10 45.30 44.14 31.62 48.43 15.99 40% 39.37 42.05 24.03 33.67 23.99 26.73 37.43 7.50 30% 27.71 30.58 6.66 26.16 9.70 17.81 30.21 12.32 20% 16.19 11.60 5.60 14.84 -7.26 9.06 23.32 9.09 10% 18.88 9.47 -4.95 10.75 -4.88 8.43 18.58 -3.60 5% 18.05 11.27 -7.44 13.42 -0.20 14.41 21.64 -4.23 2.5% 17.64 9.40 -8.29 14.05 6.35 13.88 31.55 2.15 1.2% 7.71 1.15 0.84 6.82 0.64 7.37 11.81 2.74
TABLE-US-00002 TABLE 1 (a-2) In vitro inhibition of growth of human tumor cells by deuterium oxide Inhibition of growth (%) Deuterium Cervical Breast Bladder Ovarian Prostate Brain oxide cancer cancer cancer cell cancer cell cancer cancer cell Osteosarcoma Leukemia conc. cell line cell line line line cell line line cell line cell line (v/v) HeLa MCF-7 5637 SKOV3 PC-3 SF767 U2-OS K562 80% 90.11 98.08 88.43 90.42 87.08 71.22 45.84 93.24 70% 85.82 89.06 74.86 74.07 73.42 49.65 29.64 88.59 60% 73.32 86.41 59.76 59.84 60.84 4053 23.43 70.13 50% 65.63 63.22 52.11 41.80 59.42 21.23 18.21 55.51 40% 58.26 52.45 44.32 32.76 43.19 16.50 10.23 39.51 30% 45.41 40.38 36.69 16.69 26.67 8.42 11.99 22.72 20% 36.12 31.60 25.56 12.64 -16.46 7.66 -3.12 9.10 10% 28.87 22.47 16.15 9.65 8.81 -8.43 -8.55 -7.60 5% 16.25 15.71 10.44 8.33 -2.50 -4.41 -1.64 4.23 2.5% 14.44 11.04 -3.09 7.35 4.33 3.94 4.20 6.51 1.2% 12.11 9.78 0.84 1.82 0.46 4.76 -2.81 -2.47
[0108] Table 1 (b) shows IC.sub.50 and IC.sub.10 (expressed in v/v %) of deuterium oxide against different types tumor cells.
TABLE-US-00003 TABLE 1 (b-1) IC.sub.50 and IC.sub.10 for in vitro inhibition of growth of human tumor cells by deuterium oxide Colon Gastric cancer cancer cell Lung cancer cell line Pancreatic cancer cell line cell line line A549 NCI-H460 NCI-1975 CFPAC-1 ASPC-1 PANC-1 HCT-116 BGC-823 IC.sub.50 55.32% 48.75% 62.61% 59.43% 57.00% 65.38% 51.13% 96.68% IC.sub.10 1.52% 5.60% 27.00% 3.20% 11.80% 3.03% 0.25% 26.92%
TABLE-US-00004 TABLE 1 (b-2) IC.sub.50 and IC.sub.10 for in vitro inhibition of growth of human tumor cells by deuterium oxide Cervical Breast Bladder Ovarian Prostate Brain cancer cancer cancer cancer cell cancer cancer cell Osteosarcoma Leukemia cell line cell line cell line line cell line line cell line cell line HeLa MCF-7 5637 Caov-3 PC-3 SF767 U2-OS K562 IC.sub.50 68.33% 61.29% 59.08% 46.83% 61.34% 91.12% 86.25% 51.72% IC.sub.10 1.8% 3.28% 4.46% 11.51% 9.35% 31.85% 24.99% 14.83%
[0109] It can be seen from Table 1 that deuterium oxide shows different IC.sub.50 and IC.sub.10 against different types of tumor cells.
[0110] The results demonstrate that deuterium oxide shows a certain inhibitory effect on the growth of various cancer cell lines, and higher contents of deuterium oxide show more significant inhibition of growth of tumor cells.
Example 15
[0111] In vitro inhibition of growth of human colon cancer HCT-116 cells (a moderately drug resistant strain) by deuterium oxide in combination with 5-fluorouracil (5-FU)
[0112] Cell lines: human colon cancer cells HCT-116 were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0113] Test drugs: 5-fluorouracil (5-FU), and the sodium chloride deuterium oxide solution, prepared in Example 1.
[0114] Method: 5-fluorouracil was diluted with the sodium chloride deuterium oxide solution according to the concentrations shown in Table 2 and added to a cell culture plate; the in vitro inhibition of tumor cell growth was measured by the MTT assay in the same manner as Example 14.
[0115] The assay was repeated one more time.
[0116] Result: it was found that 5-FU showed a certain inhibitory effect on growth of human colon cancer HCT-116 cells (a moderately drug resistant strain), and can significantly enhance the anti-tumor efficacy when combined with deuterium oxide, as shown in Table 2.
TABLE-US-00005 TABLE 2 Inhibition of growth of human colon cancer HCT-116 cells (a moderately drug resistant strain) by deuterium oxide in combination with 5-FU Inhibition Inhibition of cell growth (%) 5-FUFinal conc. of cell growth (%) [5-FU + 10% Deuterium (.mu.g/ml) (5-FU alone) oxide (v/v)] 12.5 35.32 80.51 6.25 26.52 67.15 3.13 18.58 62.24 1.56 11.04 51.15 0.78 9.82 45.36 0.39 6.98 32.94 0.20 3.24 28.82 0.10 2.30 16.45 0.05 2.55 11.14 0.02 2.18 10.76
[0117] The results demonstrate that the combination of deuterium oxide and 5-FU enhances the inhibitory effect on growth of drug-resistant tumor cells (a moderately drug resistant strain); and especially at a low concentration of 5-FU, deuterium oxide shows a more significant efficacy-enhancing effect. Therefore, deuterium oxide has a sensitizing effect against drug resistance.
Example 16
[0118] Inhibition of invasion of human colon cancer HCT-116 cells and human lung cancer A549 cells by deuterium oxide in combination with 5-fluorouracil or gemcitabine (by the Transwell method)
[0119] Cell lines: human colon cancer HCT-116 cells and human lung cancer A549 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0120] Test drugs: gemcitabine (GEM), 5-fluorouracil (5-FU), and the sodium chloride deuterium oxide solution, prepared in Example 1.
Method:
Cell Culturing
1. Cell Resuscitation
[0121] 1.1. Warm water at 37.degree. C. to 40.degree. C. was prepared, a cryogenic vial was taken out of liquid nitrogen and immediately put into the 37.degree. C.-40.degree. C. warm water, followed by vigorous shaking until the cryogenic liquid was completely thawed; 1.2. The suspension of cryogenic cells was transferred to a centrifugal tube, 5 ml culture medium was added thereto, and the cells were well mixed in the medium by gentle pipetting; 1.3. The cell suspension was centrifuged at 800 to 1,000 rpm for 5 min, and the supernatant was discarded; 1.4. A complete culture medium was added to the cell pellet which was well mixed by gentle pipetting, the cell suspension was transferred to a culture flask, and the cells were cultured by supplementing culture medium.
Cell Invasion Assayed by Transwell
[0122] 1. Test drugs were added according to different cell treatment groups: gemcitabine or 5-FU diluted in sodium chloride physiological solution only, or gemcitabine or 5-FU diluted in the sodium chloride deuterium oxide solution according to the concentrations shown in Table 3 (i.e. gemcitabine+deuterium oxide, or 5-FU+deuterium oxide), was separately used to treat A549 and HCT116 cells, and the cells were incubated for 24 hours in an incubator at 37.degree. C., 5% CO.sub.2; 2. After incubation of cells with the test drugs for 24 hours, serum was removed, and the cells were incubated under starvation with an incomplete culture medium for 24 hours; 3. Matrigel was placed at 4.degree. C. in advance to thaw overnight; 4. Thawed Matrigel was 1:1 diluted with the incomplete culture medium, 30 .mu.l diluted Matrigel was added to the upper compartment of Transwell and incubated at 37.degree. C. for 120 min to gel the Matrigel; 5. Cells from different groups were digested, counted, and then adjusted to a density of 1.times.10.sup.5 cells/ml with the incomplete culture medium, 100 .mu.l cell suspension was added to the upper compartment (small compartment) of Transwell, and 500 .mu.l medium containing 20% FBS was added to the lower compartment of Transwell; 6. A 24-well cell culture plate was placed in an incubator at 37.degree. C. and 5% CO.sub.2 to culture the cells for 24 hours; 7. Cell counting: cells in the Matrigel and the upper compartment were wiped off with a cotton swab, the Transwell was removed, followed by inversion and air drying, 500 .mu.l medium containing 0.1% crystal violet was added to the 24-well plate, the small compartment was placed in the medium to allow the membrane to be immersed in the dye, removed after holding at 37.degree. C. for 30 min, washed with PBS, and photographed in 3 fields along the diameter (200.times. magnification) for cell counting. 8. The assay was repeated one more time.
Results
TABLE-US-00006
[0123] TABLE 3 Inhibition of invasion of A549 and HCT-116 cells by deuterium oxide Number of cells Number of cells Group (HCT-116) (A549) Control (Sodium chloride 65.0 .+-. 12.0 183.0 .+-. 12.3 physiological solution) Deuterium oxide (1.8%) 29.3 .+-. 5.1 74.3 .+-. 11.0 Deuterium oxide (3.7%) 16.3 .+-. 3.2 54.0 .+-. 2.0 Deuterium oxide (7.5%) 3.7 .+-. 0.6 43.0 .+-. 4.4 Deuterium oxide (15%) 3.0 .+-. 1.0 29.3 .+-. 1.5 Deuterium oxide (30%) 1.70 .+-. 0.6 22.0 .+-. 1.7
[0124] In Table 3, for example, Deuterium oxide (1.8%) means that the concentration of deuterium oxide in the cell culture medium is 1.8% (v/v), and the same applies hereinafter.
[0125] The results demonstrate that deuterium oxide alone shows inhibition of invasion of A549 and HCT-116 cells, and the inhibition is proportional to its content.
TABLE-US-00007 TABLE 4 Inhibition of invasion of HCT-116 cells by 5-fluorouracil (5-FU) Group Number of cells Control 76.0 .+-. 4.6 5-FU (0.195 ug/ml) 66.0 .+-. 2.6 5-FU (0.39 ug/ml) 47.3 .+-. 4.0 5-FU (0.78 ug/ml) 31.3 .+-. 1.5 5-FU (1.56 ug/ml) 23.0 .+-. 4.6 5-FU (3.125 ug/ml) 12.0 .+-. 2.0 5-FU (6.25 ug/ml) 10.7 .+-. 1.5 5-FU (12.5 ug/ml) 6.3 .+-. 0.6
[0126] In Table 4, for example, 5-FU (0.195 .mu.g/ml) means that the concentration of 5-FU is 0.195 .mu.g/ml in sodium chloride physiological solution as the medium.
TABLE-US-00008 TABLE 5 Inhibition of invasion of HCT-116 cells by 5-FU in combination with deuterium oxide Number of Cell line Group cells HCT-116 Control 98.7 .+-. 3.5 Deuterium oxide(30%) + 5-FU (0.195 ug/ml) 8.7 .+-. 1.5 Deuterium oxide(30%) + 5-FU (0.39 ug/ml) 8.3 .+-. 1.2 Deuterium oxide(30%) + 5-FU (0.78 ug/ml) 6.3 .+-. 0.6 Deuterium oxide(30%) + 5-FU (1.56 ug/ml) 5.0 .+-. 1.0 Deuterium oxide(30%) + 5-FU (3.125 ug/ml) 5.7 .+-. 0.6 Deuterium oxide(30%) + 5-FU (6.25 ug/ml) 4.3 .+-. 0.6 Deuterium oxide(30%) + 5-FU (12.5 ug/ml) 3.3 .+-. 0.6
[0127] In Table 5, for example, Deuterium oxide (30%)+5-FU (0.195 .mu.g/ml) means that in the cell culture medium the concentration of deuterium oxide is 30% (v/v), and the concentration of 5-FU is 0.195 .mu.g/ml.
[0128] The results demonstrate that the combination of 30% deuterium oxide and 5-FU significantly inhibit invasion of HCT-116 cells; in particular, when the concentration of 5-FU is only 0.195 .mu.g/ml, the combination can significantly inhibit invasion of HCT-116 cells.
TABLE-US-00009 TABLE 6 Inhibition of invasion of A549 cells by gemcitabine (GEM) Group Number of cells Control 178.3 .+-. 13.8 GEM (1.95 nM) 160.0 .+-. 6.6 GEM (3.9 nM) 112.7 .+-. 3.5 GEM (7.8 nM) 59.0 .+-. 2.0 GEM (15.6 nM) 51.7 .+-. 3.1 GEM (31.2 nM) 37.3 .+-. 2.3 GEM (62.5 nM) 22.3 .+-. 0.6 GEM (125 nM) 16.0 .+-. 1.0 GEM (250 nM) 9.7 .+-. 2.1
TABLE-US-00010 TABLE 7 Inhibition of invasion of A549 cells by GEM in combination with deuterium oxide Cell line Group Number of cells A549 Control 161.3 .+-. 3.8 Deuterium oxide(30%) + GEM (1.95 nM) 31.0 .+-. 1.0 Deuterium oxide(30%) + GEM (3.9 nM) 23.3 .+-. 2.5 Deuterium oxide(30%) + GEM (7.8 nM) 21.0 .+-. 1.0 Deuterium oxide(30%) + GEM (15.6 nM) 13.3 .+-. 0.6 Deuterium oxide(30%) + GEM (31.2 nM) 10.3 .+-. 0.6 Deuterium oxide(30%) + GEM (62.5 nM) 11.0 .+-. 1.0 Deuterium oxide(30%) + GEM (125 nM) 8.0 .+-. 1.0 Deuterium oxide(30%) + GEM (250 nM) 6.7 .+-. 0.6
[0129] The results demonstrate that the combination of deuterium oxide and gemcitabine significantly inhibit invasion of A549 cells; in particular, when the concentration of gemcitabine is only 1.95 nM, the combination can significantly inhibit invasion of A549 cells.
[0130] The results demonstrate that deuterium oxide shows an inhibitory effect on metastasis of various tumor cells, proportional to its dose, and can be combined with other drugs to inhibit metastasis of tumor cells.
Example 17
[0131] Effect of deuterium oxide on the cell cycle of human lung cancer A549 cells and human colon cancer HCT-116 cells
Materials
[0132] Human lung cancer A549 cells and human colon cancer HCT-116 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD., and cultured in an incubator at 37.degree. C., 5% CO.sub.2 and saturated humidity.
[0133] A KGA511 Cell cycle assay kit was provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0134] The flow cytometer was FACS Calibur provided by Becton-Dickinson, US.
[0135] Test drugs: gemcitabine (GEM), 5-fluorouracil (5-FU), and the sodium chloride deuterium oxide solution, prepared in Example 1.
Method:
Cell Culturing
1. Cell Resuscitation
[0136] 1.1. Warm water at 37.degree. C. to 40.degree. C. was prepared, a cryogenic vial of cells was taken out of liquid nitrogen and immediately put into the 37.degree. C.-40.degree. C. warm water, followed by vigorous shaking until the cryogenic liquid was completely thawed; 1.2. The suspension of cryogenic cells was transferred to a centrifugal tube, 5 ml culture medium was added thereto, and the cells were well mixed in the medium by gentle pipetting; 1.3. The cell suspension was centrifuged at 800 to 1,000 rpm for 5 min, and the supernatant was discarded; 1.4. A complete culture medium was added to the cell pellet which was well mixed by gentle pipetting, the cell suspension was transferred to a culture flask, and the cells were cultured by supplementing culture medium.
2. Cell Subculturing
[0137] 2.1. The original culture medium was removed by pipetting when the cell coverage reached 80% to 90% in the culture bottle; 2.2. A suitable amount of trypsin (0.25%) was added to digest for 1 to 2 min; 2.3. When all the cells became round, an equal volume of culture medium containing serum was added to cease the digestion; 2.4. The cells were blown by pipetting to suspend, then drawn into a 15 ml centrifugal tube, and centrifuged at 1000 rpm for 5 min; 2.5. The supernatant was discarded, and the cells were re-suspended in 1 to 2 ml culture medium and transferred to a culture bottle for further culturing.
Detection of Cell Cycle by PI Single Staining
[0138] 1. Cells growing in the exponential phase were digested and inoculated to a 6-well plate; on the next day, after the cells attached to the wall, cell culture media containing corresponding test drugs were added according to different groups, and a negative control group was also set; 2. After treatment with test drugs for 72 hours, cells were digested with 0.25% pancreatin (EDTA-free) and collected; 3. The cells were washed with PBS once (centrifuged at 2,000 rpm for 5 min), and 5.times.10.sup.5 cells were collected; 4. The prepared suspension of individual cells was fixed with 70% (v/v) ethanol for 2 hours (or overnight), and stored at 4.degree. C.; before staining, the fixing solution was washed away with PBS (if necessary, the cell suspension was filtered once through a 200-mesh screen); 5. 100 .mu.l RNase A was added, and the cells were water-bathed at 37.degree. C. for 30 min; 6. 400 .mu.l PI was added and well mixed for staining, and the cells were kept at 4.degree. C. in darkness for 30 min; 7. In a detection apparatus, red fluorescence with an excitation wavelength of 488 nm was recorded.
Results
TABLE-US-00011
[0139] TABLE 8 Effect of deuterium oxide on the cell cycle of human colon cancer HCT-116 cells(%, x .+-. SD, n = 6) G1 Phase S Phase G2 Phase Control(sodium chloride 82.53 .+-. 1.76 9.40 .+-. 1.83 8.06 .+-. 1.89 physiological solution) Deuterium oxide(30%, v/v) 89.19 .+-. 3.81 6.61 .+-. 1.41 4.21 .+-. 0.95 5-FU(12.5 ug/mlin sodium 32.69 .+-. 8.66 26.07 .+-. 11.32 41.24 .+-. 2.97 chloride physiological solution) Deuterium oxide(30%, 49.16 .+-. 4.47 0.30 .+-. 0.26 50.53 .+-. 4.26 v/v) + 5-FU(12.5 ug/ml)
TABLE-US-00012 TABLE 9 Effect of deuterium oxide on the cell cycle of human lung cancer A549 cells(%, x .+-. SD, n = 6) G1 Phase S Phase G2 Phase Control (sodium chloride 80.29 .+-. 5.02 13.50 .+-. 1.33 6.21 .+-. 4.52 physiological solution) Deuterium oxide(30%, v/v) 86.23 .+-. 3.69 0.31 .+-. 0.28 13.47 .+-. 3.94 GM (250 nM in sodium 73.73 .+-. 3.73 15.55 .+-. 5.51 10.71 .+-. 2.39 chloride physiological solution) Deuterium oxide(30%, v/v) + 87.32 .+-. 1.60 0.08 .+-. 0.01 12.6 .+-. 1.46 GM(250 nM)
[0140] 5-FU and gemcitabine are cytotoxic metabolite anti-tumor drugs, and mainly act on tumor cells in the DNA synthesis phase, i.e. the S phase. It can be seen in the results of Tables 8 and 9 and FIGS. 1-4 that deuterium oxide impeded tumor cells entering the G2 phase from the S phase; when deuterium oxide was combined with 5-FU and gemcitabine, 5-FU and gemcitabine kill the S-phase tumor cells more effectively, resulting in significant reduction in the S-phase tumor cells, and exerting a synergistic effect.
Example 18
[0141] Studying 1: Efficacy of intraperitoneal perfusion (lavage) with deuterium oxide in combination with 5-fluorouracil on Ehrlich ascites tumor (EAC)-bearing mice.
[0142] Studying 2: Evaluate the effectiveness of different preparations and administration routes of deuterium oxide on nude mice beard human colon cancer HCT-116 cell.
[0143] Laboratory animal: 4 to 5 week-aged male ICR mice, provided by Shanghai SIPPR-BK Laboratory Animal Co. Ltd.
[0144] Cell lines: Ehrlich ascites tumor cells, human colon cancer HCT-116 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0145] Test drugs: 5-fluorouracil (5-FU), and the sodium chloride deuterium oxide solution, prepared in Example 1.
Studying 1 Experimental Method:
[0146] 1. Modeling: EAC ascitic fluid was taken and adjusted to 1.times.10.sup.7 cells/ml, which was inoculated into the peritoneal cavity of mice at 0.1 ml/mouse. 2. Grouping and dosing: the animals were randomized 3 days after inoculation, and meanwhile drugs were given to mice in each group, following the dosing scheme shown in Table 10.
TABLE-US-00013 TABLE 10 Grouping and dosing scheme, NS means sodium chloride physiological solution (same below). Drugs, I.P. Groups administration Inoculation Dosing Data Group 1 NS. 0.4 ml/animal Giving NS for 3 days first, Then giving 0.4 ml NS on then giving drug on day 3, each day until death of the followed by inoculation animal Group 2 5-FU20 mg/kg Giving 5-FU for 3 days Then giving5-FU20 mg/kg on Days that the alone (in NS) first, then giving drug on each day for 7 successive animal had day 3, followed by days (10 days in total), then survived were inoculation discontinuing the drug recorded Group 3 5-FU 30 mg/kg Giving 5-FU for 3 days Then giving 5-FU 30 mg/kg Days that the alone (in NS) first, then giving drug on on each day for 7 successive animal had day 3, followed by days (10 days in total), then survived were inoculation discontinuing the drug recorded Group 4 Sodium chloride Giving Sodium chloride Then giving0.4 ml Sodium Days that the deuterium oxide deuterium oxide solution chloride deuterium oxide animal had solution, alone, for 3 days first, then giving solution on each day until survived were 0.4 ml/animal drug on day 3, followed by death of animal, without drug recorded inoculation discontinuance The above 4 groups and Group 5, Group 6, Group 7below, seven groups in total: Group 5 Sodium chloride Giving drug for 3 days first, Then giving drug on each day Days that the deuterium oxide then giving drug on day 3, for 10 days in total, then animal had solution0.1 followed by inoculation discontinuing the drug survived were ml/animal + recorded 5-FU20 mg/kg Group 6 Sodium chloride Giving drug for 3 days first, Then giving drug on each day Days that the deuterium oxide then giving drug on day 3, for 10 days in total, then animal had solution0.2 followed by inoculation discontinuing the drug survived were ml/animal + recorded 5-FU20 mg/kg Group 7 Sodium chloride Giving drug for 3 days first, Then giving drug on each day Days that the deuterium oxide then giving drug on day 3, for 10 days in total, then animal had solution 0.4 followed by inoculation discontinuing the drug survived were ml/animal + recorded 5-FU20 mg/kg For the 4 groups below, Sodium chloride deuterium oxide solution (0.6 ml/animal)was given 3 times by intraperitoneal lavage followed by intraperitoneal administration of drugs to corresponding groups Group 8 NS 0.4 ml/animal Giving NS for 3 days first, Then giving 0.4 ml NS on Days that the then giving drug on day 3, each day until death of the animal had followed by inoculation animal survived were recorded Group 9 Sodium chloride Giving drug for 3 days first, Then giving drug on each day Days that the deuterium oxide then giving drug on day 3, for 10 days in total, then animal had solution 0.1 followed by inoculation discontinuing the drug survived were ml/animal + 5-FU, recorded 20 mg/kg Group 10 Sodium chloride Giving drug for 3 days first, Then giving drug on each day Days that the deuterium oxide then giving drug on day 3, for 10 days in total, then animal had solution 0.2 followed by inoculation discontinuing the drug survived were ml/animal + 5-FU, recorded 20 mg/kg Group 11 Sodium chloride Giving drug for 3 days first, Then giving drug on each day Days that the deuterium oxide then giving drug on day 3, for 10 days in total, then animal had solution 0.4 followed by inoculation discontinuing the drug survived were ml/animal + 5-FU, recorded 20 mg/kg
[0147] In Table 10, "Sodium chloride deuterium oxide solution 0.1 ml/animal+5-FU 20 mg/kg" means that each mouse was given 0.1 ml sodium chloride deuterium oxide solution, in which 5-FU had been dissolved to make a dose of 20 mg/kg.
3. Observation of Indicators
[0148] After the dosing, ascitic fluid was taken from 3 mice from each group, the volume of the ascitic fluid was measured, the number of tumor cells in the ascitic fluid was counted, and the rest of animals were continuously fed. Median survival time (MST) was recorded for animals in each group to evaluate the survival time for each group.
[0149] The comparison between the treatment group and the control group was expressed by T/C (%) which is calculated by the following equation:
T / C % = TMST CMST .times. 100 % ##EQU00001##
[0150] T MST: MST of the treatment group; C MST: MST of the negative control group.
[0151] The evaluation criterion uses 125% as a cut-off threshold. When T/C %.gtoreq.125%, effective, otherwise ineffective.
4. Statistics
[0152] The mean value was expressed in X.+-.SD. Inter-group analysis was statistically performed with t test. The results were statistically analyzed using SPSS (Statistical Package for the Social Science) 17.0.
5. Results
5.1. Survival Time: Effect of Intraperitoneal Administration of Drugs on Survival Time (in Days) of Animals
TABLE-US-00014
[0153] TABLE 11 Effect of intraperitoneal administration of drugs on survival time (in days) of animals(x .+-. SD, n = 8) Group MST(days) T/C(%) control group: NS (0.4 ml/animal) 14.1 .+-. 1.9 5-FU (20 mg/kg)(in NS) 21.8 .+-. 2.9* 154.6% 5-FU (30 mg/kg)(in NS) 22.3 .+-. 4.9* 158.1% Sodium chloride deuterium oxide 17.2 .+-. 3.2** 121.9% solution (0.4 ml/animal) Sodium chloride deuterium oxide solution 21.4 .+-. 3.1* 151.7% (0.1 ml/animal) + 5-FU(20 mg/kg) Sodium chloride deuterium oxide solution 24.1 .+-. 2.7* 170.9% (0.2 ml/animal) + 5-FU(20 mg/kg) Sodium chloride deuterium oxide solution 25.7 .+-. 2.6* 182.2% (0.4 ml/animal) + 5-FU(20 mg/kg) Compared to the control group: *P < 0.001, **P < 0.05.
TABLE-US-00015 TABLE 12 Effect of intraperitoneal lavage with sodium chloride deuterium oxide solution, followed by intraperitoneal administration of drugs, on survival time (in days) of animals(x .+-. SD, n = 8) Group MST(days) T/C(%) control group: NS (0.4 ml/animal) 15.3 .+-. 1.9 Sodium chloride deuterium oxide solution 26.1 .+-. 3.7* 170.5% (0.1 ml/animal) + 5-FU (20 mg/kg) Sodium chloride deuterium oxide solution 29.1 .+-. 3.1* 190.1% (0.2 ml/animal) + 5-FU (20 mg/kg) Sodium chloride deuterium oxide solution 34.5 .+-. 3.3* 225.4% (0.4 ml/animal) + 5-FU (20 mg/kg) Compared to the control group: *P < 0.001.
[0154] The results demonstrate that, as compared to the NS control group, the group receiving combined sodium chloride deuterium oxide solution, and 5-FU showed an extended MST, with a P value<0.01 and T/C (%).gtoreq.150%, which was also better than the group receiving 5-FU alone.
[0155] Intraperitoneal lavage with the sodium chloride deuterium oxide solution first, followed by intraperitoneal administration of drugs, can achieve a significantly extended MST in mice, longer than the MST resulting from only intraperitoneal administration of drugs in the sodium chloride deuterium oxide solution, namely 29.1.+-.3.1 days vs. 24.1.+-.2.7 days; and 34.5.+-.3.3 days vs. 25.7.+-.2.6 days, both having a P value<0.05, demonstrating an advantage of intraperitoneal lavage with deuterium oxide followed by administration of drugs.
Studying 2: Experimental Method:
[0156] 1. Modeling: human colon cancer HCT-116 cells was taken and adjusted to 1.times.107 cells/ml, which was inoculated into the peritoneal cavity of mice at 0.1 ml/mouse. 2. Grouping and dosing: the animals were randomized 3 days after inoculation, and meanwhile agents were given to mice in each group, following the dosing scheme and results shown in Table 13.
TABLE-US-00016 TABLE 13 Effects of the oral drinking or i.v. dosing of deuterium oxide on survival time(in days) in mice bearing human colon cancer HCT- 116 cells(x .+-. SD, n = 6), grouping, dosing scheme and results. administration Groups agent route and dosing MST (days) T/C (%) Group 1 drinking water drinking freely 36 .+-. 3.1 Group 2 30% deuterium drinking freely 39 .+-. 2.2 8.3 oxide, Group 3 sodium chloride 20 ml/kg, i.v. x 7 46 .+-. 2.2* 27.7 deuterium oxide solution Group 4 30% deuterium 1. deuterium oxide 53 .+-. 4.3** 47.2 oxide + 5-FU drinking freely 2. 5-FU 10 mg/kg dissolving in saline solution, i.v. x 7 Group 5 sodium chloride 5-FU 10 mg/kg 65 .+-. 6.2** 80.5 deuterium oxide dissolving in sodium solution + 5-FU chloride deuterium oxide solution, 20 ml/kg, i.v. x 7 Compared to group 1: *P < 0.01, **P < 0.001; i.v. means intravenous route.
[0157] The intravenous route of deuterium oxide show unexpected effect of extension the survival time of mice bearing human colon cancer HCT-116 cells. The drinking of 30% deuterium oxide only extended 3 days for survival time, P>0.05. But the intravenous administration of deuterium oxide increased 10 days for survival time. The drinking of 30% deuterium oxide in combination with 5-FU could extend 17 days for survival time, but the intravenous route of deuterium oxide in combination with 5-FU would increase 30 days for survival time ultimately, in a T/C 80.5%, P<0.001. Both intravenous route of deuterium oxide were significantly superior to the oral drinking of administration. Conclusion: the intravenous route changes the pharmacodynamics of deuterium oxide and enhances the efficacy of it, also improves the survival time. Finally, it will be beneficial to patients with cancer therapy.
Example 19
[0158] Inhibition of liver cancer H22 ascites tumor in tumor-bearing mice by 42.+-.1.degree. C. hyperthermic intraperitoneal retention lavage using deuterium oxide in combination with cisplatin, and using deuterium oxide (42.degree. C.) in combination with cisplatin.
Experimental Method:
[0159] Animal: 4 to 5 week-aged male Kunming mice, provided by Shanghai SIPPR-BK Laboratory Animal Co. Ltd.
[0160] Cell lines: Liver cancer H22 ascites tumor cell line was provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0161] Modeling: 0.2 ml suspension of H22 tumor cells containing 1.times.10.sup.7 cells/ml (2.times.10.sup.6 H22 cells in total) was inoculated into the peritoneal cavity of mice.
[0162] Test drugs: cisplatin, and the Ringer's deuterium oxide solution as prepared in Example 6.
[0163] Grouping and dosing: the mouse model was established 7 to 8 days after inoculation, and the mice were randomized with 18 animals/group.
[0164] Cisplatin were dissolved in a 42.+-.1.degree. C. Ringer's deuterium oxide solution, and used to lavage the peritoneal cavity of the tumor-bearing mice.
1). Blank control group (normal Ringer's solution, 10 ml/kg, room temperature); 2). Normal chemo group (cisplatin 0.6 mg/kg+normal Ringer's solution, 10 ml/kg, room temperature); 3). Room temperature low-dose deuterium oxide+cisplatin group (Ringer's deuterium oxide solution, 5 ml/kg+cisplatin 0.6 mg/kg); 4). Room temperature mid-dose deuterium oxide+cisplatin group (Ringer's deuterium oxide solution, 20 ml/kg+cisplatin 0.6 mg/kg); 5). Room temperature high-dose deuterium oxide+cisplatin group (Ringer's deuterium oxide solution, 40 ml/kg+cisplatin 0.6 mg/kg); 6). Hyperthermic low-dose deuterium oxide+cisplatin group (42.+-.1.degree. C. Ringer's deuterium oxide solution, 5 ml/kg+cisplatin 0.6 mg/kg); 7). Hyperthermic mid-dose deuterium oxide+cisplatin group (42.+-.1.degree. C. Ringer's deuterium oxide solution, 20 ml/kg+cisplatin 0.6 mg/kg); 8). Hyperthermic high-dose deuterium oxide+cisplatin group (42.+-.1.degree. C. Ringer's deuterium oxide solution, 40 ml/kg+cisplatin 0.6 mg/kg);
[0165] The peritoneal cavity of mice was lavaged with the above compositions which were retained for 10 min in the peritoneal cavity after being introduced, which was repeated for 3 times. The lavage was performed once another day for 5 successive times. The body weight and abdomen circumference of mice were measured on each day before administration of the drugs, and the daily living status of mice was observed. 24 hours after the administration of drugs was completed (on day 11), 8 mice from each group were sacrificed, the volume of ascitic fluid was measured, and bodies of the mice were dissected to observe the peritoneal organs and metastasis to lung. The rest of mice were observed for their survival time, and the extension of life was calculated.
4. Observation of indicators: the same as Example 18. 5. Statistics: the same as Example 18.
6. Results
TABLE-US-00017
[0166] TABLE 14 Effect of 42 .+-. 1.degree. C. intraperitoneal lavage with deuterium oxide in combination with cisplatin on survival time (in days) of animals(x .+-. SD, n = 10) Group MST(days) T/C(%) Blank control group (Ringer's solution for 13.2 .+-. 1.9 injection, 5 ml/kg) Normal chemo group (cisplatin 0.6 mg/kg + 18.1 .+-. 3.2* 137.1 Ringer's solution5 ml/kg) RT low-dose deuterium oxide + cisplatin group 20.3 .+-. 2.2* 153.7 (Ringer's deuterium oxide solution5 ml/kg + cisplatin 0.6 mg/kg) RT mid-dose deuterium oxide + cisplatin 24.7 .+-. 1.8* 187.1 group (Ringer's deuterium oxide solution20 ml/kg + cisplatin 0.6 mg/kg) RT high-dose deuterium oxide + cisplatin group 26.1 .+-. 5.9* 197.7 (Ringer's deuterium oxide solution 40ml/kg + cisplatin 0.6 mg/kg) Hyperthermic low-dose deuterium oxide + 24.3 .+-. 3.3* 184.0 cisplatin group(Ringer's deuterium oxide solution 5 ml/kg + cisplatin0.6 mg/kg) Hyperthermic mid-dose deuterium oxide + 28.6 .+-. 6.4* 216.6 cisplatin group(Ringer's deuterium oxide solution 20 ml/kg + cisplatin0.6 mg/kg) Hyperthermic high-dose deuterium oxide + 32.1 .+-. 3.1* 243.1 cisplatin group(Ringer's deuterium oxide solution40 ml/kg + cisplatin0.6 mg/kg) Compared to the negative control group: *P < 0.001. RT means room temperature.
TABLE-US-00018 TABLE 15 Effect of 42 .+-. 1.degree. C. intraperitoneal lavage with deuterium oxide in combination with cisplatinon the volume of ascitic fluid(x .+-. SD, n = 8) Ascitic Groups fluid (ml) Blank control group (Ringer's solution for 14.85 .+-. 2.56 injection, 5 ml/kg) Normal chemo group (cisplatin 0.6 mg/kg + 7.30 .+-. 2.32* Ringer's solution for injection 5 ml/kg) RT low-dose deuterium oxide + cisplatin group 6.55 .+-. 3.95* (Ringer's deuterium oxide solution5 ml/kg + cisplatin 0.6 mg/kg) RT mid-dose deuterium oxide + cisplatin group 4.93 .+-. 3.05* (Ringer's deuterium oxide solution20 ml/kg + cisplatin 0.6 mg/kg) RT high-dose deuterium oxide + cisplatin group 4.08 .+-. 1.22* (Ringer's deuterium oxide solution40 ml/kg + cisplatin 0.6 mg/kg) Hyperthermic low-dose deuterium oxide + cisplatin 3.11 .+-. 0.56** group (Ringer's deuterium oxide solution 5 ml/kg + cisplatin0.6 mg/kg); Hyperthermic mid-dose deuterium oxide + cisplatin 2.49 .+-. 0.62** group (Ringer's deuterium oxide solution20 ml/kg + cisplatin0.6 mg/kg); Hyperthermic high-dose deuterium oxide + cisplatin 0.44 .+-. 0.46** group (Ringer's deuterium oxide solution40 ml/kg + cisplatin0.6 mg/kg). Compared to the negative control group: *P < 0.001, **P < 0.0001. RT means room temperature.
[0167] Cisplatin is a representative anti-tumor platinum preparation, and deuterium oxide has a broad-spectrum effect of enhancing anti-tumor efficacy. The results demonstrate that the 42.degree. C. hyperthermic intraperitoneal lavage with deuterium oxide in combination with cisplatin could significantly extend the survival time, inhibit production of ascitic fluid than deuterium oxide under room temperature in mice bearing H22 ascites tumors. The application of hyperthermic deuterium oxide exhibits an unexpected anti-tumor effect of enhancing, thereby improves quality of life of the tumor-bearing mice.
Example 20
[0168] Inhibition of sarcoma S180 (ascites type) in tumor-bearing mice by 42.+-.1.degree. C. hyperthermic intraperitoneal retention lavage using deuterium oxide in combination with oxaliplatin and using deuterium oxide in combination with oxaliplatin and mitomycin.
Experimental Method:
[0169] Animal: 4 to 5 week-aged male Kunming mice, provided by Shanghai SIPPR-BK Laboratory Animal Co. Ltd.
[0170] Cell lines: sarcoma S180 (ascites type) cell line was provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0171] Modeling: 0.2 ml suspension of sarcoma S180 cells containing 1.times.10.sup.7 cells/ml was inoculated into the peritoneal cavity of mice.
[0172] Test drugs: oxaliplatin, mitomycin, and the glucose deuterium oxide solution as prepared in Example 2.
[0173] Grouping and dosing: the mouse model was established 7 to 8 days after inoculation, and the mice were randomized with 12 animals/group.
[0174] Oxaliplatin and mitomycin were dissolved in a 42.+-.1.degree. C. glucose deuterium oxide solution, and used to lavage the peritoneal cavity of the tumor-bearing mice.
1). Blank control group (5% glucose injection, 5 ml/kg); 2). Chemo group (oxaliplatin 0.8 mg/kg+5% glucose injection, 5 ml/kg); 3). Low-dose deuterium oxide+oxaliplatin group (5% glucose deuterium oxide solution, 5 ml/kg+oxaliplatin 0.8 mg/kg); 4). Mid-dose deuterium oxide+oxaliplatin group (5% glucose deuterium oxide solution, 20 ml/kg+oxaliplatin 0.8 mg/kg); 5). High-dose deuterium oxide+oxaliplatin group (5% glucose deuterium oxide solution, 40 ml/kg+oxaliplatin 0.8 mg/kg); 6). Low-dose deuterium oxide+oxaliplatin+mitomycin group (42.+-.1.degree. C. 5% glucose deuterium oxide solution, 5 ml/kg+oxaliplatin 0.8 mg/kg+mitomycin 0.4 mg/kg); 7). Mid-dose deuterium oxide+oxaliplatin+mitomycin group (42.+-.1.degree. C. 5% glucose deuterium oxide solution, 20 ml/kg+oxaliplatin 0.8 mg/kg+mitomycin 0.4 mg/kg); 8). High-dose deuterium oxide+oxaliplatin+mitomycin group (42.+-.1.degree. C. 5% glucose deuterium oxide solution, 40 ml/kg+oxaliplatin 0.8 mg/kg+mitomycin 0.4 mg/kg).
[0175] The peritoneal cavity was lavaged with the 42.+-.1.degree. C. glucose deuterium oxide solution in combination with the above composition, which were retained for 10 min in the peritoneal cavity after being introduced, which was repeated for 3 times. The lavage was performed once another day for 5 successive times. The body weight and abdomen circumference of mice were measured on each day before administration of the drugs, and the daily living status of mice was observed. 24 hours after the administration of drugs was completed (on day 11), 4 mice from each group were sacrificed, the volume of ascitic fluid was measured, and bodies of the mice were dissected to observe the peritoneal organs and metastasis to lung. The rest of mice were observed for their survival time, and the extension of life was calculated.
4. Observation of indicators: the same as Example 18. 5. Statistics: the same as Example 18.
6. Results
TABLE-US-00019
[0176] TABLE 16 Effect of 42 .+-. 1.degree. C. intraperitoneal lavage with deuterium oxide in combination with oxaliplatin and mitomycin on survival time (in days) of animals(x .+-. SD, n = 8) Group MST(days) T/C(%) Blank control group (glucose injection, 5 ml/kg) 12.2 .+-. 3.0 Chemo group (oxaliplatin 0.8 mg/kg + glucose 21.7 .+-. 2.8* 177.6 injection 5 ml/kg) Low-dose deuterium oxide + oxaliplatin(glucose 23.4 .+-. 3.2* 191.8 deuterium oxide solution5 ml/kg + oxaliplatin 0.8 mg/kg) Mid-dose deuterium oxide + oxaliplatin(glucose 27.7 .+-. 4.9* 227.0 deuterium oxide solution 20 ml/kg + oxaliplatin 0.8 mg/kg) High-dose deuterium oxide + oxaliplatin(glucose 31.1 .+-. 6.1* 254.9 deuterium oxide solution 40 ml/kg + oxaliplatin 0.8 mg/kg) Low-dose deuterium oxide + oxaliplatin + 26.4 216.3 mitomycin(glucose deuterium oxide solution 5 ml/kg + oxaliplatin 0.8 mg/kg + mitomycin 0.4 mg/kg) Mid-dose deuterium oxide + oxaliplatin + 30.1 246.7 mitomycin(glucose deuterium oxide solution 20 ml/kg + oxaliplatin 0.8 mg/kg + mitomycin 0.4 mg/kg) High-dose deuterium oxide + oxaliplatin + 32.8 268.8 mitomycin(glucose deuterium oxide solution 40 ml/kg + oxaliplatin 0.8 mg/kg + mitomycin 0.4 mg/kg) Compared to the negative control group: *P < 0.0001.
TABLE-US-00020 TABLE 17 Effect of 42 .+-. 1.degree. C. intraperitoneal lavage with deuterium oxide in combination with oxaliplatin and mitomycin on the volume of ascitic fluid(x .+-. SD, n = 8) Ascitic Group fluid (ml) Blank control group (glucose injection, 5 ml/kg) 16.5 .+-. 4.3 Chemo group (oxaliplatin0.8 mg/kg + glucose 8.7 .+-. 1.9* injection 5 ml/kg) Low-dose deuterium oxide + oxaliplatin(oxaliplatin 8.1 .+-. 2.8* 0.8 mg/kg + glucose deuterium oxide solution 5 ml/kg) Mid-dose deuterium oxide + oxaliplatin(oxaliplatin 7.3 .+-. 2.9* 0.8 mg/kg + glucose deuterium oxide solution 20 ml/kg) High-dose deuterium oxide + oxaliplatin(oxaliplatin 2.1 .+-. 1.3** 0.8 mg/kg + glucose deuterium oxide solution 40 ml/kg) Low-dose deuterium oxide + oxaliplatin + 7.5 .+-. 3.1* mitomycin(oxaliplatin 0.8 mg/kg + mitomycin 0.4 mg/kg + glucosedeuterium oxide solution 5 ml/kg) Mid-dose deuterium oxide + oxaliplatin + 3.7 .+-. 1.6** mitomycin(oxaliplatin 0.8 mg/kg + mitomycin 0.4 mg/kg + glucose deuterium oxide solution 20 ml/kg) High-dose deuterium oxide + oxaliplatin + 1.1 .+-. 0.5** mitomycin(oxaliplatin 0.8 mg/kg + mitomycin 0.4 mg/kg + glucose deuterium oxide solution 40 ml/kg) Compared to the negative control group: *P < 0.0001, **P < 0.00001.
[0177] Oxaliplatin is a third-generation anti-tumor platinum preparation, mitomycin is an anti-tumor antibiotic to which tumor cells in G0 to S phases are sensitive, and deuterium oxide has a broad-spectrum effect of enhancing anti-tumor efficacy, to which tumor cells in the S phase are also sensitive. Their combination can further enhance anti-tumor efficacy. The results demonstrate that the hyperthermic deuterium oxide in combination with oxaliplatin and mitomycin can also significantly extend the survival time of mice bearing sarcoma S180, and inhibits production of ascitic fluid in mice bearing sarcoma S180. This indicates that combination of hyperthermic deuterium oxide with platinum preparation and anti-tumor antibiotics has general applicability in inhibition of growth of various tumors.
Example 21
[0178] Inhibition of growth of xenograft tumors from human colon cancer HCT-166 cells in nude mice by intravenous injection of deuterium oxide in combination with 5-fluorouracil
[0179] Animal: 6 to 7 week-aged female BALB/c nude mice, each weighing 18 to 20 g, provided by Shanghai SLAC Laboratory Animal Co. Ltd.
[0180] Cell lines: human colon cancer cells HCT-116 were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0181] Test drugs: 5-fluorouracil (5-FU), and the glucose (5%) deuterium oxide solution (containing 5 g glucose per 100 ml) prepared in Example 2.
Experimental Method:
1. Modeling
[0182] Tumor cells were resuscitated and cultured according to a routine procedure, a suspension of cultured HCT-116 cells was collected, sterile sodium chloride physiological solution was added thereto to make a suspension at a concentration of 1.times.10.sup.7 cells/ml, and 0.1 ml of the suspension was subcutaneously inoculated into the right axillary fossa of each nude mouse.
2. Grouping and Dosing Scheme
[0183] The diameter of xenograft tumors in the nude mice was measured with a vernier caliper, and 10 days after inoculation, when tumors grew to 100 to 110 cm.sup.3, the animals were randomized with 8 mice per group. Meanwhile drugs were administered by injection into tail vein, once every three days, for 21 successive days. By measuring the tumor diameter, the anti-tumor efficacy of test samples was dynamically observed, and the body weight of animals was also recorded to observe the toxicity of the test samples. 21 days after administration, the nude mice were sacrificed, and tumors were harvested by surgery, weighed and photographed. Bone marrow nucleated cells were isolated by a routine method, DNA was extracted from the cells, the OD value was measured in a UV-260 spectrometer as a representative of the DNA content, and inhibition of DNA synthesis by the test samples was observed.
[0184] The test used 7 groups in total, with 8 animals per group.
1) Negative control group: 5% glucose injection, 10 ml/kg; 2) Paclitaxel positive control group: paclitaxel (8 mg/kg) diluted in sodium chloride physiological solution; 3) High-dose 5-FU positive control group: 5-FU (30 mg/kg) diluted in 5% glucose injection; 4) Low-dose 5-FU positive control group: 5-FU (12 mg/kg) diluted in 5% glucose injection. 5) Test group 1: 5-FU (12 mg/kg) diluted in the glucose deuterium oxide solution (5 ml/kg). 6) Test group 2: 5-FU (12 mg/kg) diluted in the glucose deuterium oxide solution (10 ml/kg). 7) Test group 3: 5-FU (12 mg/kg) diluted in the glucose deuterium oxide solution (20 ml/kg).
3. Observation of Indicators
1) Anti-Tumor Activity Evaluation by Relative Tumor Volume
[0185] Tumor volume (TV) was calculated by the following equation:
TV=1/2.times.a.times.b.sup.2,
wherein a and b refer to length and width, respectively.
[0186] From the measurements, relative tumor volume (RTV) was calculated by the following equation:
RTV=V.sub.t/V.sub.0
wherein V.sub.0 is the tumor volume measured on initial administration (i.e. do), and V.sub.t is the tumor volume measured each time afterwards.
[0187] Relative tumor growth ratio T/C (%) was calculated by the following equation:
TC ( % ) = T RTV C RTV .times. 100 ##EQU00002##
[0188] T.sub.RTV: RTV of the treatment group; C.sub.RTV: RTV of the negative control group.
[0189] Criteria of efficacy evaluation: T/C %>40%, not efficacious; T/C %.ltoreq.40% and statistically P<0.05, efficacious.
2) Anti-Tumor Activity Evaluation by Tumor Weight
[0190] Inhibition of tumor growth (%) was calculated by the following equation:
Inhibition of tumor growth = ATW NCG - ATW DG ATW NCG .times. 100 % ##EQU00003##
[0191] ATW.sub.NCG: Average tumor weight in negative control group
[0192] ATW.sub.DG: Average tumor weight in drug-dosed group
[0193] Criteria of efficacy evaluation: Inhibition of tumor growth<40%, not efficacious; Inhibition of tumor growth.gtoreq.40% and statistically P<0.05, efficacious.
3) Observation of Drug's Inhibition of Bone Marrow and Monitoring of Drug's Toxicity Based on Bone Marrow DNA Content.
4. Statistics
[0194] The mean value was expressed in X.+-.SD. Inter-group analysis was statistically performed with t test. The results were statistically analyzed using SPSS (Statistical Package for the Social Science) 17.0.
5. Results
[0195] Anti-tumor activity evaluation by xenograft tumor volume: the results demonstrate that deuterium oxide in combination with low-dose 5-FU showed a good inhibitory effect on the xenograft tumors; 21 days after administration, the tumor volumes were 1.22.+-.0.15 cm.sup.3 in the group receiving the deuterium oxide solution (5 ml/kg) in combination with 5-FU, 1.45.+-.0.25 cm.sup.3 in the group receiving the deuterium oxide solution (10 ml/kg) in combination with 5-FU, and 1.27.+-.0.24 cm.sup.3 in the group receiving the deuterium oxide solution (20 ml/kg) in combination with 5-FU, all with a T/C<40% as compared to the negative control group, and satisfied the criteria for pharmacological efficacy. But the group receiving low-dose 5-FU alone showed a reduced tumor volume to 2.27.+-.0.30 cm.sup.3 only, with a T/C of 80.91%, which is >40% and did not satisfy the criteria for pharmacological efficacy. Therefore, when combined with 5-FU, deuterium oxide significantly enhance the anti-tumor efficacy of 5-FU, as shown in FIG. 5.
[0196] Anti-tumor activity evaluation by xenograft tumor weight: the results demonstrate that deuterium oxide in combination with low-dose 5-FU showed a good inhibitory effect on the xenograft tumors; 21 days after administration, the inhibitions were 56.32% in the group receiving the deuterium oxide solution (5 ml/kg) in combination with 5-FU, 53.70% in the group receiving the deuterium oxide solution (10 ml/kg) in combination with 5-FU, and 54.22% in the group receiving the deuterium oxide solution (20 ml/kg) in combination with 5-FU, all with a T/C>40% and P<0.001 as compared to the tumor weight in the negative control group, and all satisfied the criteria for pharmacological efficacy. The group receiving low-dose 5-FU alone showed an inhibition of 23.64% and did not satisfy the criteria for pharmacological efficacy. Therefore, when combined with 5-FU, deuterium oxide significantly enhance the anti-tumor efficacy of 5-FU, as shown in Table 18 and FIG. 6.
TABLE-US-00021 TABLE 18 Effect of deuterium oxide in combination with 5-FU on xenograft tumors from human colon cancer HCT-116 cells in nude mice(x .+-. SD, n = 8) Bone marrow Body weight (g) Tumor weight Inhibition DNA content Group Dose .times. Times (Initial/End) (g) (%) (OD) Negative Control 10 ml/kg 18.5/+0.4 2.92 .+-. 0.27 -- 0.950 .+-. 0.2711 Paclitaxel positive 8 mg/kg .times. 11 18.4/-1.2 0.94 .+-. 0.25 67.80%* 0.841 .+-. 0.3014** control 5-FU positive control 30 mg/kg .times. 11 18.4/-1.0 1.04 .+-. 0.13 64.38%* 0.836 .+-. 0.5023** 5-FU(H) 5-FU low-dose 12 mg/kg .times. 11 18.0/-0.6 2.23 .+-. 0.2 23.63% 0.897 .+-. 0.3783** 5-FU(L) Test group 1 Glucose deuterium 18.3/-0.4 1.34 .+-. 0.38 54.10%* 0.913 .+-. 0.5122 5-FU(L) + HW(L) oxide solution (5 ml/kg) + 5-FU(12 mg/kg) .times. 11 Test group 2 Glucose deuterium 18.5/+0.2 1.35 .+-. 0.34 53.76%* 0.901 .+-. 0.4655 5-FU(L) + HW(M) oxide solution(10 ml/kg) + 5-FU(12 mg/kg) .times. 11 Test group 3 Glucose deuterium 18.4/+0.2 1.28 .+-. 0.46 56.16%* 0.921 .+-. 0.2910 5-FU(L) + HW(H) oxide solution(20 ml/kg) + 5-FU(12 mg/kg) .times. 11 Compared to the negative control group: *P < 0.001, **P < 0.05. Inhibition of tumor growth (%) >40% indicates pharmacological efficacy.
[0197] Toxicity reduction: importantly, in the high-dose paclitaxel and 5-FU positive control groups, despite apparent inhibition of tumor growth, the body weight of tumor-bearing mice dropped greatly from 18.4.+-.0.5 g to 16.8.+-.0.9 g and to 17.4.+-.0.5 g, respectively, and the bone marrow DNA content also decreased, indicating significant toxicity of paclitaxel and 5-FU to animals. The deuterium oxide solution in combination with 5-FU (10 mg/kg, one third of that of control) can achieve the same efficacy as high-dose paclitaxel and 5-FU (30 mg/kg) (equivalence), while the body weight of the tumor-bearing mice during dosing did not drop, and the bone marrow DNA content did not decrease, without the toxicity caused by high-dose paclitaxel and 5-FU. Because toxicity of anti-tumor drugs is very harmful to patients and even unacceptable to some weak or aged patients, chemotherapeutic drugs having low toxic side effects and a strong killing effect are urgently needed in tumor treatment. Hence, deuterium oxide in combination with low-dose 5-FU has a great advantage in clinical application, as shown in Table 18 and FIG. 7.
Example 22
[0198] Inhibition of growth of xenograft tumors from human breast cancer MCF-7 cells in nude mice by intravenous injection of deuterium oxide in combination with gemcitabine
[0199] Animal: 6 to 7 week-aged female BALB/c nude mice, each weighing 18 to 20 g, provided by Shanghai SLAC Laboratory Animal Co. Ltd.
[0200] Cell lines: human breast cancer MCF-7 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0201] Test drugs: gemcitabine (GEM) and the sodium chloride deuterium oxide solution prepared in Example 1.
Experimental Method:
1. Modeling
[0202] Tumor cells were resuscitated and cultured according to a routine procedure, suspension of cultured MCF-7 cells was collected, sterile sodium chloride physiological solution was added thereto to make a suspension at a concentration of 1.times.10.sup.7 cells/ml, and 0.1 ml of the suspension was subcutaneously inoculated into the right axillary fossa of each nude mouse.
2. Grouping and Dosing Scheme
[0203] The diameter of xenograft tumors in the nude mice was measured with a vernier caliper, and 10 days after inoculation, when tumors grew to 100 to 110 cm.sup.3, the animals were randomized with 8 mice per group. Meanwhile drugs were administered by injection into tail vein, once every three days, for 21 successive days. By measuring the tumor diameter, the anti-tumor efficacy of test samples was dynamically observed. Meanwhile the body weight of animals was also recorded to observe the toxicity of the test samples. 21 days after administration, the nude mice were sacrificed, and tumors were harvested by surgery, weighed and photographed. Bone marrow nucleated cells were isolated by a routine method, DNA was extracted from the cells, the OD value was measured in a UV-260 spectrometer as a representative of the DNA content, and inhibition of DNA synthesis by the test samples was observed.
[0204] The test used 7 groups in total, with 8 animals per group.
[0205] The test used 7 groups:
1) Negative control group: sodium chloride physiological solution, 10 ml/kg; 2) Paclitaxel positive control group: paclitaxel diluted in sodium chloride physiological solution (8 mg/kg); 3) Gemcitabine positive control group: gemcitabine diluted in sodium chloride physiological solution (20 mg/kg); 4) Low-dose gemcitabine control group: gemcitabine diluted in sodium chloride physiological solution (10 mg/kg); 5) Test group 1: gemcitabine (10 mg/kg) diluted in the sodium chloride deuterium oxide solution (5 ml/kg). 6) Test group 2: gemcitabine (10 mg/kg) diluted in the sodium chloride deuterium oxide solution (10 ml/kg). 7) Test group 3: gemcitabine (10 ml/kg) diluted in the sodium chloride deuterium oxide solution (20 ml/kg). 3. Observation of indicators: the same as Example 21. 4. Statistics: the same as Example 21.
5. Results
[0206] Anti-tumor activity evaluation by xenograft tumor volume: the sodium chloride deuterium oxide solution in combination with gemcitabine 10 mg/kg showed a good inhibitory effect on the xenograft tumors; 21 days after administration, the tumor volumes were 1.949.+-.0.491 cm.sup.3 in the group receiving the deuterium oxide solution 5 ml/kg in combination with gemcitabine, 1.743.+-.0.503 cm.sup.3 in the group receiving the deuterium oxide solution 10 ml/kg in combination with gemcitabine, and 1.671.+-.0.386 cm.sup.3 in the group receiving the deuterium oxide solution 20 ml/kg in combination with gemcitabine, all with a T/C<40% as compared to the negative control group, and satisfied the criteria for pharmacological efficacy. The group receiving gemcitabine 10 mg/kg alone showed only a slightly reduced tumor volume of 2.757.+-.0.342 cm.sup.3 with a T/C of 60.39%, which was >40% and did not satisfy the criteria for pharmacological efficacy. When combined with gemcitabine, deuterium oxide significantly enhances the anti-tumor efficacy of gemcitabine, as shown in FIG. 8.
[0207] Anti-tumor activity evaluation by xenograft tumor weight: the deuterium oxide solution in combination with gemcitabine showed a good inhibitory effect on the xenograft tumors; 21 days after administration, the inhibitions were 48.82% in the group receiving the deuterium oxide solution 5 ml/kg in combination with gemcitabine, 57.78% in the group receiving the deuterium oxide solution 10 ml/kg in combination with gemcitabine, and 60.70% in the group receiving the deuterium oxide solution 20 ml/kg in combination with gemcitabine, all with a T/C>40% and P<0.001 as compared to the negative control group, and all satisfied the criteria for pharmacological efficacy. The group receiving gemcitabine 10 mg/kg alone showed only a slightly reduced tumor weight and an inhibition of 39.18%, which was less than 40% and did not satisfy the criteria for pharmacological efficacy. Therefore, deuterium oxide significantly enhances the anti-tumor efficacy of gemcitabine, as shown in Table 19 and FIG. 9.
TABLE-US-00022 TABLE 19 Effect of deuterium oxide in combination with gemcitabine on xenograft tumors from human breast cancer MCF-7 cells in nude mice (x .+-. SD, n = 8) Body Bone marrow weight Tumor weight Inhibition DNA content Group Dose .times. Times (g) (Initial/End) (g) (%) (OD) Negative Control 10 ml/kg .times. 11 18.3/+3.3 4.07 .+-. 0.76 -- 0.931 .+-. 0.2011 Paclitaxel positive 8 mg/kg .times. 11 18.3/+1.0 1.59 .+-. 0.40 60.93%* 0.881 .+-. 0.2502 control Gemcitabine positive 20 mg/kg .times. 11 18.1/+0.9 1.57 .+-. 0.21 61.42%* 0.823 .+-. 0.1416** control GEM(H) Gemcitabine low-dose 10 mg/kg .times. 11 18.0/+2.9 2.48 .+-. 0.41 39.06% 0.908 .+-. 0.2543 GEM(L) Test group 1 Sodium chloride 18.4/+3.1 2.08 .+-. 0.28 48.89%* 0.923 .+-. 0.3101 GEM(L) + HW(L) deuterium oxide solution (5 ml/kg) + GEM (10 mg/kg) .times. 11 Test group 2 Sodium chloride 18.0/+3.3 1.72 .+-. 0.51 57.73%* 0.942 .+-. 0.2896 GEM(L) + HW(M) deuterium oxide solution (10 ml/kg) + GEM (10 mg/kg) .times. 11 Test group 3 Sodium chloride 18.4/+2.4 1.6 .+-. 0.55 60.68%* 0.920 .+-. 0.4167 GEM(L) + HW(H) deuterium oxide solution (20 ml/kg) + GEM (10 mg/kg) .times. 11 Compared to the negative control group: *P < 0.001, **P < 0.05. Inhibition of tumor growth (%) >40% indicates pharmacological efficacy.
[0208] Toxicity reduction: importantly, during the experiment, the average body weight of tumor-bearing mice in the negative control group increased by 3.3 g; in the paclitaxel and gemcitabine positive control groups, despite apparent reduction in tumor weight, the body weight of tumor-bearing mice only slightly increased, and the bone marrow DNA content also only slightly increased, indicating certain toxicity of paclitaxel and gemcitabine to animals. The sodium chloride deuterium oxide solution in combination with low-dose gemcitabine (10 mg/kg, half of that of control) can achieve the same efficacy as high-dose paclitaxel (8 mg/kg) and high-dose gemcitabine (20 mg/kg) (equivalence), while the body weight of the tumor-bearing mice increased similarly to the negative control group, and the bone marrow DNA content also increased, without the toxicity caused by high-dose paclitaxel and gemcitabine. Toxicity of anti-tumor drugs is very harmful to patients and even unacceptable to some weak or aged patients, and thus causes a serious problem to clinical anti-tumor chemotherapy. Hence, deuterium oxide in combination with low-dose gemcitabine has a great advantage in clinical applications, as shown in Table 19 and FIG. 10.
[0209] 5-FU and gemcitabine are antimetabolites among cytotoxic anti-tumor drugs. It can be also seen in Examples 19 and 20 that deuterium oxide in combination with antimetabolite anti-tumor drugs has general applicability for inhibition of growth of various tumors.
Example 23
[0210] Inhibition of growth of xenograft tumors on nude mice-bearing human lung cancer A549 cell by intravenous injection of deuterium oxide in combination with gemcitabine
[0211] Animal: 6 to 7 week-aged female BALB/c nude mice, each weighing 18 to 20 g, provided by Shanghai SLAC Laboratory Animal Co. Ltd.
[0212] Cell lines: human lung cancer A549 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0213] Test drugs: gemcitabine (GEM) and the sodium chloride deuterium oxide solution prepared in Example 1.
1. Tumor cells were resuscitated and inoculated, and sterile sodium chloride physiological solution was added thereto to make a cell suspension which was subcutaneously inoculated into the upper right axillary fossa of animals. 5.times.10.sup.6 tumor cells were inoculated into each animal.
2. Grouping and Dosing Scheme
[0214] The diameter of xenograft tumors in the nude mice was measured with a vernier caliper, and 10 days after inoculation, when tumors grew to 100 to 110 cm.sup.3, the animals were randomized with 8 mice per group. Meanwhile drugs were administered by injection into tail vein, once every three days, for 21 successive days. By measuring the tumor diameter, the anti-tumor efficacy of test samples was dynamically observed. Meanwhile the body weight of animals was also recorded to observe the toxicity of the test samples. 21 days after administration, the nude mice were sacrificed, and tumors were harvested by surgery, weighed and photographed.
[0215] The test used 7 groups:
1) Negative control group: sodium chloride physiological solution, 10 ml/kg; 2) Paclitaxel positive control group: paclitaxel diluted in sodium chloride physiological solution (8 mg/kg); 3) Gemcitabine positive control group: gemcitabine diluted in sodium chloride physiological solution (20 mg/kg); 4) Low-dose gemcitabine control group: gemcitabine diluted in sodium chloride physiological solution (10 mg/kg); 5) Test group 1: gemcitabine (10 mg/kg) diluted in the sodium chloride deuterium oxide solution (5 ml/kg). 6) Test group 2: gemcitabine (10 mg/kg) diluted in the sodium chloride deuterium oxide solution (10 ml/kg). 7) Test group 3: gemcitabine (10 ml/kg) diluted in the sodium chloride deuterium oxide solution (20 ml/kg). 3. Observation of indicators: the same as Example 21. 4. Statistics: the same as Example 21.
5. Results
[0216] Anti-tumor activity evaluations by xenograft tumor volume and weight: the results demonstrate that, 21 days after administration, as compared to the sodium chloride physiological solution control group, the group receiving low-dose gemcitabine alone showed a reduced tumor weight to 2.23.+-.0.2 g and an inhibition of 23.6%, which however did not satisfy the criteria for pharmacological efficacy. As compared to the control group, the deuterium oxide solution in combination with gemcitabine all showed a good inhibitory effect on the xenograft tumors; the group receiving the deuterium oxide solution 5 ml/kg in combination with gemcitabine showed a tumor weight of 1.34.+-.0.38 g and a tumor proliferation ratio of 54.22%, the group receiving the deuterium oxide solution 10 ml/kg in combination with gemcitabine showed a tumor weight of 1.35.+-.0.34 g and a tumor proliferation ratio of 53.70%, and the group receiving the deuterium oxide solution 20 ml/kg in combination with gemcitabine showed a tumor weight of 1.28.+-.0.46 g and a tumor proliferation ratio of 56.32%, all having P<0.001 and satisfying the criteria for pharmacological efficacy, as shown in FIGS. 11 and 12.
TABLE-US-00023 TABLE 20 Effect of sodium chloride deuterium oxide solution on tumor weight of human lung cancer xenograft tumors in nude mice (x .+-. SD, n = 8) Body weight Tumor weight Inhibition Group Dose .times. Times (g) (Initial/End) (g) (%) Negative control 10 ml/kg 18.5/+1.4 2.92 .+-. 0.27 -- Paclitaxel positive 8 mg/kg .times. 11 18.4/+0.9 0.94 .+-. 0.25 67.97* control Gemcitabine positive 20 mg/kg .times. 11 18.4/+0.5 1.04 .+-. 0.13 64.54* control GEM(H) Gemcitabine low-dose 10 mg/kg .times. 11 18.0/+0.4 2.23 .+-. 0.2 23.64 GEM(L) Test group 1 Sodium chloride 18.3/+1.5 1.34 .+-. 0.38 54.22* GEM(L) + HW(L) deuterium oxide solution (5 ml/kg) + GEM (10 mg/kg) .times. 11 Test group 2 Sodium chloride 18.5/+1.1 1.35 .+-. 0.34 53.70* GEM(L) + HW(M) deuterium oxide solution (10 ml/kg) + GEM (10 mg/kg) .times. 11 Test group 3 Sodium chloride 18.4/+0.7 1.28 .+-. 0.46 56.32* GEM(L) + HW(H) deuterium oxide solution (20 ml/kg) + GEM (10 mg/kg) .times. 11 As compared to the control group, *P < 0.001, and inhibition of tumor weight >40% indicates pharmacological efficacy.
[0217] Importantly, in the paclitaxel and gemcitabine positive control groups, despite an apparent reduction in tumor weight to 0.94.+-.0.25 g and 1.04.+-.0.13 g, respectively, and a tumor proliferation ratio of 67.97% and 64.54%, respectively, the body weight of tumor-bearing mice barely increased, indicating significant toxicity of paclitaxel and gemcitabine to animals. Deuterium oxide in combination with low-dose gemcitabine can achieve the same efficacy as high-dose paclitaxel and gemcitabine, does not cause the corresponding toxicity, and results in an increased body weight of tumor-bearing mice during administration, as shown in Table 20 and FIG. 13.
Example 24
[0218] Inhibition of growth of xenograft tumors from human ovarian cancer SKOV3 cells in nude mice by intravenous injection of deuterium oxide in combination with paclitaxel
[0219] Animal: 6 to 7 week-aged female BALB/c nude mice, each weighing 18 to 20 g, provided by Shanghai SLAC Laboratory Animal Co. Ltd.
[0220] Cell lines: human ovarian cancer SKOV3 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0221] Test drugs: paclitaxel and the sodium chloride deuterium oxide solution, prepared in Example 1.
Experimental Method:
1. Tumor Cell Resuscitation and Inoculation
[0222] Tumor cells were resuscitated and inoculated, and sterile sodium chloride physiological solution was added thereto to make a cell suspension which was subcutaneously inoculated into the upper right axillary fossa of animals. 5.times.10.sup.6 tumor cells were inoculated into each animal.
2. Grouping and Dosing Scheme
[0223] The diameter of xenograft tumors in the nude mice was measured with a vernier caliper, and 10 days after inoculation, when tumors grew to 100 to 110 cm.sup.3, the animals were randomized. Meanwhile drugs were administered by injection into tail vein, once every three days, for 21 successive days. The body weight of animals was also recorded to observe the toxicity of the test samples. 21 days after administration, the nude mice were sacrificed, and tumors were harvested by surgery, and weighed.
[0224] The test used 6 groups in total, with 5 animals per group.
1) Control group: sodium chloride physiological solution, 10 ml/kg; 2) Paclitaxel positive control group: paclitaxel (8 mg/kg) diluted in sodium chloride physiological solution; 3) Sodium chloride deuterium oxide solution (10 ml/kg); 4) Test group 1: paclitaxel (8 mg/kg) diluted in the sodium chloride deuterium oxide solution (5 ml/kg); 5) Test group 2: paclitaxel (8 mg/kg) diluted in the sodium chloride deuterium oxide solution (10 ml/kg); 6) Test group 3: paclitaxel (8 mg/kg) diluted in the sodium chloride deuterium oxide solution (20 ml/kg). 3. Observation of indicators: the same as Example 21. 4. Statistics: the same as Example 21.
[0225] The results are shown in Table 21.
TABLE-US-00024 TABLE 21 Effect of sodium chloride deuterium oxide solution in combination with paclitaxel on tumor weight of xenograft tumors from human ovarian cancer SKOV3 cells in nude mice (x .+-. SD, n = 5) Body weight Tumor weight Inhibition Group Dose .times. Times (g) (Initial/End) (g) (%) Control 10 ml/kg 20.8/+4.0 3.22 .+-. 1.59 -- Paclitaxel 8 mg/kg .times. 11 20.1/+2.8 2.07 .+-. 1.06 35.7 Sodium chloride 10 ml/kg .times. 11 21.1/+3.9 2.44 .+-. 1.51 24.2 deuterium oxide solution Test group 1 Sodium chloride 21.0/+3.8 1.95 .+-. 0.57 39.4 Sodium chloride deuterium oxide deuterium oxide solution (5 ml/kg) + solution + Paclitaxel Paclitaxel (8 mg/kg) .times. 11 Test group 2 Sodium chloride 20.4/+3.7 1.71 .+-. 0.87 46.8* Sodium chloride deuterium oxide deuterium oxide solution (10 ml/kg) + solution + Paclitaxel Paclitaxel (8 mg/kg) .times. 11 Test group 3 Sodium chloride 20.9/+4.1 1.45 .+-. 0.42 54.9* Sodium chloride deuterium oxide deuterium oxide solution (20 ml/kg) + solution + Paclitaxel Paclitaxel (8 mg/kg) .times. 11 Compared to the control group: *P < 0.01.
[0226] Paclitaxel is an important phytogenic anti-tumor drug, having various types of a similar mechanism of action, including paclitaxel, paclitaxel liposomes, paclitaxel albumin, and docetaxel. The results demonstrate that, 21 days after administration, as compared to the control group, the groups receiving the deuterium oxide solution (10 ml/kg and 20 ml/kg) in combination with paclitaxel (8 mg/kg) showed a good inhibitory effect on xenograft tumors, with a significant reduction in tumor weight, while the tumor inhibition T/C was 54.9% on day 21 with P<0.001. On the other hand, as compared to the control group, the group receiving paclitaxel (8 mg/kg) alone showed a reduction, but did not satisfy the criteria of >40% for pharmacological efficacy. As compared to the control group, the group receiving the deuterium oxide solution (5 ml/kg) in combination with paclitaxel (12 mg/kg) showed a reduction, but did not satisfy the criteria of >40% for pharmacological efficacy. Deuterium oxide as a broad-spectrum anti-tumor efficacy-enhancing agent can also enhance the efficacy of phytogenic anti-tumor drugs.
Example 25
[0227] Inhibition of growth of xenograft tumors from human bladder cancer 5637 cells in nude mice by intravenous injection of deuterium oxide in combination with thiotepa
[0228] Animal: 6 to 7 week-aged female BALB/c nude mice, each weighing 18 to 20 g, provided by Shanghai SLAC Laboratory Animal Co. Ltd.
[0229] Cell lines: human bladder cancer 5637 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0230] Test drugs: thiotepa and the sodium chloride deuterium oxide solution, prepared in Example 1.
Experimental Method:
1. Tumor Cell Resuscitation and Inoculation
[0231] Tumor cells were resuscitated and inoculated, and sterile sodium chloride physiological solution was added thereto to make a cell suspension which was subcutaneously inoculated into the upper right axillary fossa of animals. 5.times.10.sup.6 tumor cells were inoculated into each animal.
2. Grouping and Dosing Scheme
[0232] The diameter of xenograft tumors in the nude mice was measured with a vernier caliper, and 10 days after inoculation, when tumors grew to 100 to 110 cm.sup.3, the animals were randomized. Meanwhile drugs were administered by injection into tail vein, once every three days, for 21 successive days. The body weight of animals was also recorded to observe the toxicity of the test samples. 21 days after administration, the nude mice were sacrificed, and tumors were harvested by surgery, and weighed.
[0233] The test used 5 groups in total, with 5 animals per group.
1) Control group: sodium chloride physiological solution, 10 ml/kg; 2) Thiotepa positive control group: thiotepa (0.2 mg/kg) diluted in sodium chloride physiological solution; 3) Test group 1: thiotepa (0.2 mg/kg) diluted in the sodium chloride deuterium oxide solution (1 ml/kg); 4) Test group 2: thiotepa (0.2 mg/kg) diluted in the sodium chloride deuterium oxide solution (10 ml/kg); 5) Test group 3: thiotepa (0.2 mg/kg) diluted in the sodium chloride deuterium oxide solution (25 ml/kg). 3. Observation of indicators: the same as Example 21. 4. Statistics: the same as Example 21.
[0234] The results demonstrate that, 21 days after administration, as compared to the control group, the groups receiving the two dosages of deuterium oxide solution (10 ml/kg and 20 ml/kg) in combination with thiotepa (0.2 mg/kg) showed a good inhibitory effect on xenograft tumors, with a significant reduction in tumor weight, while the tumor inhibitions T/C were 47.0% and 63.4% on day 21, with P<0.01 and P<0.001, respectively. On the other hand, as compared to the control group, the group receiving thiotepa (0.2 mg/kg) alone did not show a considerable difference. As compared to the control group, the group receiving the deuterium oxide solution (1 ml/kg) in combination with thiotepa (0.2 mg/kg) did not show a considerable difference. The results are shown in Table 22.
TABLE-US-00025 TABLE 22 Effect of sodium chloride deuterium oxide solution in combination with thiotepa on tumor weight of xenograft tumors from human bladder cancer 56373 cells in nude mice (x .+-. SD, n = 5) Body weight Tumor weight Inhibition Groups Dose .times. Times (g) (Initial/End) (g) (%) Control 10 ml/kg 19.8/+3.1 2.87 .+-. 1.11 -- Thiotepa 0.2 mg/kg .times. 11 20.6/+2.6 1.75 .+-. 1.06 39.0 Test group 1 Sodium chloride 20.4/+3.3 1.72 .+-. 0.73 40.1 Sodium chloride deuterium deuterium oxide oxide solution + thiotepa solution (1 ml/kg) + thiotepa (0.2 mg/kg) .times. 11 Test group 2 Sodium chloride 20.1/+3.0 1.32 .+-. 0.55 54.0* Sodium chloride deuterium deuterium oxide oxide solution + thiotepa solution (10 ml/kg) + thiotepa (0.2 mg/kg) .times. 11 Test group 3 Sodium chloride 19.9/+4.1 1.05 .+-. 0.38 63.4** Sodium chloride deuterium deuterium oxide oxide solution + thiotepa solution (25 ml/kg) + thiotepa (0.2 mg/kg) .times. 11 Compared to the control group: *P < 0.01, **P < 0.001.
[0235] Thiotepa is an ethyleneimine alkylating agent involved in guanine binding and affecting DNA synthesis, and is effective on various types of tumor cells, especially bladder cancer cells. Deuterium oxide in combination with thiotepa can synergistically enhance the effect of thiotepa against bladder cancer cells, increased the inhibition of cancer cell growth from 39% (when thiotepa is used alone) to 54%-63.4%, which verifies the function of deuterium oxide as a broad-spectrum anti-tumor efficacy-enhancing agent. However, when the dose of deuterium oxide is very low (1 ml/kg), the effect is not significant.
Example 26
[0236] Inhibition of microvessel density of xenograft tumors from human pancreatic cancer PANC-1 cells in nude mice by intravenous injection of deuterium oxide in combination with bevacizumab
[0237] Animal: 6 to 7 week-aged female BALB/c nude mice, each weighing 18 to 20 g, provided by Shanghai SLAC Laboratory Animal Co. Ltd.
[0238] Cell lines: human pancreatic cancer PANC-1 cells were provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0239] Test drugs: bevacizumab (Avastin, Roche) and the sodium chloride deuterium oxide solution, prepared in Example 1.
Experimental Method:
1. Modeling
[0240] Tumor cells were resuscitated and cultured according to a routine procedure, suspension of cultured PANC-1 cells was collected, sterile sodium chloride physiological solution was added thereto to make a suspension at a concentration of 1.times.10.sup.7 cells/ml, and 0.1 ml of the suspension was subcutaneously inoculated into the right axillary fossa of each nude mouse.
2. Grouping and Dosing Scheme
[0241] The diameter of xenograft tumors in the nude mice was measured with a vernier caliper, and 10 days after inoculation, when tumors grew to 100 to 110 cm.sup.3, the animals were randomized. Meanwhile drugs were administered by injection into tail vein, once every three days, for 14 successive days. The body weight of animals was also recorded to observe the toxicity of the test samples. 14 days after administration, the dosing was discontinued for 1 week, then the nude mice were sacrificed, tumors were harvested by surgery and weighed, and the microvessel density (MVD) was examined. Tumors were stained by a standard EnVision.TM. method, the number of microvessels was counted by the Weidner microvessel method, the entire field was observed under 100.times. magnification, the areas having the highest microvessel density of the tumor were selected for counting, the microvessel density in three magnified fields was counted under 400.times. magnification, and the obtained values of microvessel density were averaged as the MVD.
[0242] The test used 4 groups in total, with 5 animals per group.
1) Negative control group: 0.9% sodium chloride injection, 10 ml/kg; 2) Bevacizumab positive control group: bevacizumab (5 mg/kg) diluted in 0.9% sodium chloride injection; 3) Test group 1: bevacizumab (5 mg/kg) diluted in the sodium chloride deuterium oxide solution (10 ml/kg); 4) Test group 2: bevacizumab (5 mg/kg) diluted in the sodium chloride deuterium oxide solution (20 ml/kg). 3. Observation of indicators: the same as Example 21. 4. Statistics: the same as Example 21. 5. Results: The results are shown in Tables 23 and 24.
TABLE-US-00026 TABLE 23 Effect of deuterium oxide in combination with bevacizumab on tumor weight of xenograft tumors (x .+-. SD, n = 5) Inhibition of Groups Tumor weight (g) growth (%) Negative control 1.97 .+-. 0.29 Bevacizumab positive control 1.12 .+-. 0.17* 43.1 Bevacizumab (5 mg/kg) diluted in 1.04 .+-. 0.13* 47.2 sodium chloride deuterium oxide solution. (10 ml/kg) Bevacizumab (5 mg/kg) diluted in 0.93 .+-. 0.2* 53.7 sodium chloride deuterium oxide solution. (20 ml/kg) Compared to the negative control group: *P < 0.01.
TABLE-US-00027 TABLE 24 Effect of deuterium oxide in combination with bevacizumab on microvessel density (MVD) (x .+-. SD, n = 5) Groups MVD Negative control 31.10 .+-. 4.47 Bevacizumab positive control 22.40 .+-. 3.22* Bevacizumab (5 mg/kg) diluted in 18.80 .+-. 1.58* sodium chloride deuterium oxide solution. (10 ml/kg) Bevacizumab (5 mg/kg) diluted in 16.60 .+-. 1.92* sodium chloride deuterium oxide solution. (20 ml/kg) Compared to the negative control group: *P < 0.05.
[0243] Growth of tumor tissue requires a large amount of blood supply, and bevacizumab is an angiogenesis inhibitor. When the growth of microvessels in tumor tissue is inhibited, microvessels and blood supply are reduced. The results demonstrate that deuterium oxide as a broad-spectrum anti-tumor efficacy-enhancing agent can also enhance the effect of bevacizumab in reducing the microvessel density (MVD) in tumor tissue, reducing the blood supply to pancreatic cancer PANC-1 cells, and in turn inhibiting tumor growth.
Example 27
[0244] Comparison of survival time of nude mice bearing human colon cancer HCT-116 cells upon 42.degree. C. hyperthermic intraperitoneal retention lavage using a hydroxyethyl starch deuterium oxide solution in combination with 5-FU and using a sodium chloride deuterium oxide solution in combination with 5-FU.
Experimental Method:
[0245] Animal: 4 to 5 week-aged male nude mice, provided by Shanghai SIPPR-BK Laboratory Animal Co. Ltd.
[0246] Cell lines: human colon cancer cell line HCT-116 was provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0247] Modeling: 0.2 ml suspension of HCT-116 cancer cells containing 1.times.10.sup.7 cells/ml (2.times.10.sup.6 cells in total) was inoculated into the peritoneal cavity of the nude mice.
[0248] Test drugs: 5-FU, the 6% hydroxyethyl starch (200/0.5) deuterium oxide solution prepared in Example 10, and the 0.9% sodium chloride deuterium oxide solution, prepared in Example 1.
[0249] Grouping and dosing: the mouse model was established 7 to 8 days after inoculation into the nude mice, and the mice were randomized with 18 animals/group.
[0250] 5-FU was separately dissolved in the hydroxyethyl starch deuterium oxide solution at 42.degree. C., and in the sodium chloride deuterium oxide solution at 42.degree. C., for intraperitoneal lavage of tumor-bearing mice.
1). Blank control group (42.degree. C. sodium chloride physiological solution, 10 ml/kg); 2). Normal chemo group (42.degree. C. sodium chloride physiological solution, 10 ml/kg+ 5-FU 20 mg/kg); 3). Low-dose sodium chloride deuterium oxide solution+5-FU group (42.degree. C. sodium chloride deuterium oxide solution 5 ml/kg+5-FU 20 mg/kg); 4). Mid-dose sodium chloride deuterium oxide solution+5-FU group (42.degree. C. sodium chloride deuterium oxide solution 20 ml/kg+5-FU 20 mg/kg); 5). High-dose sodium chloride deuterium oxide solution.+5-FU group (42.degree. C. sodium chloride deuterium oxide solution 40 ml/kg+5-FU 20 mg/kg); 6). Low-dose hydroxyethyl starch deuterium oxide solution+5-FU group (42.degree. C. hydroxyethyl starch deuterium oxide solution, 5 ml/kg+5-FU 20 mg/kg); 7). Mid-dose hydroxyethyl starch deuterium oxide solution+5-FU group (42.degree. C. hydroxyethyl starch deuterium oxide solution, 20 ml/kg+5-FU 20 mg/kg); 8). High-dose hydroxyethyl starch deuterium oxide solution+5-FU group (42.degree. C. hydroxyethyl starch deuterium oxide solution, 40 ml/kg+5-FU 20 mg/kg).
[0251] The peritoneal cavity of nude mice was lavaged with the above compositions which were retained for 20 min in the peritoneal cavity after being introduced, which was performed once a day for 5 successive times. The body weight and abdomen circumference of the nude mice were measured on each day before administration of the drugs, and the daily living status of mice was observed. After administration of drugs was completed, 4 nude mice from each group were sacrificed, and bodies of the nude mice were dissected to observe the cancer cells and peritoneal organs. The rest of nude mice were observed for their survival time, and the extension of life was calculated.
4. Observation of indicators: the same as Example 18. 5. Statistics: the same as Example 18.
6. Results:
TABLE-US-00028
[0252] TABLE 25 Effect of 42.degree. C. intraperitoneal lavage with hydroxyethyl starch deuterium oxide solution in combination with 5-FU on survival time (in days) of animals (x .+-. SD, n = 10) Groups MST (days) T/C (%) Blank control group 12.4 .+-. 2.9 Normal chemo group 17.2 .+-. 2.2* 138.7 Low-dose sodium chloride deuterium 21.6 .+-. 2.5* 174.1 oxide solution + 5-FU Mid-dose sodium chloride deuterium 23.2 .+-. 1.0* 187.0 oxide solution + 5-FU High-dose sodium chloride deuterium 27.4 .+-. 4.4* 220.9 oxide solution + 5-FU Low-dose hydroxyethyl starch 30.7 .+-. 4.3** 247.5 deuterium oxide solution + 5-FU Mid-dose hydroxyethyl starch 38.6 .+-. 4.3** 311.2 deuterium oxide solution + 5-FU High-dose hydroxyethyl starch 42.1 .+-. 4.7** 339.5 deuterium oxide solution + 5-FU Compared to the blank control group: *P < 0.001, **P < 0.0001.
[0253] In patients with mid-stage and late stage colon cancer, intraperitoneal metastasis often occurs, and the patients can survive only several months, and intraperitoneal lavage with anti-tumors drugs may have a certain effect thereon. The sodium chloride deuterium oxide solution, as an anti-tumors drug carrier, in which 5-FU is dissolved showed an anti-tumor efficacy-enhancing effect. However, deuterium oxide is a medium of small molecules, resides in the peritoneal cavity for only a short time, and is rapidly metabolized, thereby lowering the anti-tumors effect of 5-FU. Hydroxyethyl starch is a polymerexcipients for drugs, shows a long retention time in blood, and now has been a regular plasma replacement in clinical settings. The results demonstrate that the 42.degree. C. hyperthermic intraperitoneal lavage using a hydroxyethyl starch deuterium oxide solution in combination with 5-FU can significantly extend the survival time of nude mice bearing colon cancer cell and enhance the anti-tumor efficacy, apparently superior to the intraperitoneal lavage using a sodium chloride deuterium oxide solution in combination with 5-FU.
Example 28
Effect of Sodium Bicarbonate Deuterium Oxide Solution on Model of Transplanted Liver Cancer
[0254] 12 rabbits as a model of VX2 transplanted liver cancer were randomized into 3 groups (4 animals per group). Group 1: Embolization treatment by hepatic arterial infusion with sodium chloride physiological solution 5 ml+5-FU (20 mg/kg)+ultra fluid lipiodol 0.5 ml; Group 2: Embolization treatment by hepatic arterial infusion with 5% sodium bicarbonate solution 5 ml+5-FU (20 mg/kg)+ultra fluid lipiodol 0.5 ml; Group 3: Embolization treatment by hepatic arterial infusion with 5% sodium bicarbonate deuterium oxide solution 5 ml+5-FU (20 mg/kg)+ultra fluid lipiodol 0.5 ml. 2 weeks after the operation, the laboratory rabbits were sacrificed and tumors were taken. The tumor growth rate was calculated by macropathological examination, pathological examinations were performed byoptical and electron microscopy, and comparative studies were made between the groups, the results shown in Table 26.
TABLE-US-00029 TABLE 26 Effect of sodium bicarbonate deuterium oxide solution on model of transplanted liver cancer Partial central Size Tumor Formation of necrosis in tumor Groups (cm) nodule pseudocapsule nodule Embolization with sodium chloride 3 .times. 4 Intact No Present, apparent physiological solution + 5-FU (20 mg/kg) + lipiodol Embolization with sodium bicarbonate solution + 2.0 .times. 0.9 Non-intact Yes Absent 5-FU (20 mg/kg) + lipiodol Embolization with sodium bicarbonate 1.2 .times. 1 Non-intact No Absent deuterium oxide solution + 5-FU (20 mg/kg) + lipiodol
[0255] Analysis of various observed indices demonstrates that the group receiving embolization with sodium bicarbonate deuterium oxide solution+lipiodol is apparently better than the group receiving embolization with sodium chloride physiological solution or sodium bicarbonate solution+lipiodol, in terms of the degree of necrosis of tumors and the anti-tumors effect.
Example 29
[0256] Effect of 42.degree. C. hyperthermicintraperitoneal retention lavage with hydroxypropyl-.beta.-cyclodextrin (HP-.beta.-CD) deuterium oxide solution in combination with gemcitabine (GEM) on the survival of nude mice bearing human pancreatic cancer PANC-1 cell.
Experimental Method:
[0257] Animal: 4 to 5 week-aged male nude mice, provided by Shanghai SIPPR-BK Laboratory Animal Co. Ltd.
[0258] Cell lines: human pancreatic cancer PANC-1 cell line was provided by NANJING KEYGEN BIOTECH. CO., LTD.
[0259] Modeling: 0.2 ml suspension of pancreatic cancer PANC-1 cells containing 1.times.10.sup.7 cells/ml (2.times.10.sup.6 cells in total) was inoculated into the peritoneal cavity of the nude mice.
[0260] Test drugs: gemcitabine, the HP-.beta.-CD deuterium oxide solution prepared in Example 12, and the 0.9% sodium chloride deuterium oxide solution prepared in Example 2.
[0261] Grouping and dosing: the mouse model was established 7 to 8 days after inoculation into the nude mice, and the mice were randomized with 18 animals/group.
[0262] Gemcitabine was separately dissolved in the HP-.beta.-CD deuterium oxide solution at 42.degree. C., and in the sodium chloride deuterium oxide solution at 42.degree. C., for intraperitoneal lavage of tumor-bearing mice.
1). Blank control group (42.degree. C. sodium chloride physiological solution, 10 ml/kg); 2). Normal chemo group (42.degree. C. sodium chloride physiological solution, 10 ml/kg+gemcitabine 20 mg/kg); 3). Low-dose sodium chloride deuterium oxide solution+gemcitabine group (42.degree. C. sodium chloride deuterium oxide solution, 5 ml/kg+gemcitabine 20 mg/kg); 4). Mid-dose sodium chloride deuterium oxide solution+gemcitabine group (42.degree. C. sodium chloride deuterium oxide solution, 20 ml/kg+gemcitabine 20 mg/kg); 5). High-dose sodium chloride deuterium oxide solution+gemcitabine group (42.degree. C. sodium chloride deuterium oxide solution, 40 ml/kg+gemcitabine 20 mg/kg); 6). Low-dose HP-.beta.-CD deuterium oxide solution+gemcitabine group (42.degree. C. HP-.beta.-CD deuterium oxide solution 2.5 ml/kg+gemcitabine 20 mg/kg); 7). Mid-dose HP-.beta.-CD deuterium oxide solution+gemcitabine group (42.degree. C. HP-.beta.-CD deuterium oxide solution 5 ml/kg+gemcitabine 20 mg/kg); 8). High-dose HP-.beta.-CD deuterium oxide solution+gemcitabine group (42.degree. C. HP-.beta.-CD deuterium oxide solution 10 ml/kg+gemcitabine 20 mg/kg).
[0263] The peritoneal cavity of nude mice was lavaged with the above compositions which were retained for 20 min in the peritoneal cavity after being introduced, which was performed once a day for 5 successive times. The body weight and abdomen circumference of the nude mice were measured on each day before administration of the drugs, and the daily living status of mice was observed. After administration of drugs was completed, 4 nude mice from each group were sacrificed, and bodies of the nude mice were dissected to observe the cancer cells and peritoneal organs. The rest of nude mice were observed for their survival time, and the extension of life was calculated.
4. Observation of indicators: the same as Example 18. 5. Statistics: the same as Example 18.
6. Results
TABLE-US-00030
[0264] TABLE 27 Effect of 42.degree. C. intraperitoneal lavage with HP-.beta.-CD deuterium oxide solutionin combination with gemcitabine on survival time (in days) of animals (x .+-. SD, n = 10) Groups MST (days) T/C (%) Blank control group 11.8 .+-. 2.2 Normal chemo group 17.8 .+-. 1.8* 150.8 Low-dose sodium chloride deuterium 20.6 .+-. 2.2* 174.5 oxide solution + GEM Mid-dose sodium chloride deuterium 22.2 .+-. 1.4* 188.1 oxide solution + GEM High-dose sodium chloride deuterium 26.8 .+-. 4.4* 227.1 oxide solution + GEM Low-dose HP-.beta.-CD deuterium 29.7 .+-. 3.3** 251.6 oxide solution + GEM Mid-dose HP-.beta.-CD deuterium 41.6 .+-. 3.4** 352.5 oxide solution + GEM High-dose HP-.beta.-CD deuterium 46.2 .+-. 3.7** 391.5 oxide solution + GEM Compared to the blank control group: *P < 0.001, **P < 0.0001.
[0265] .beta.-cyclodextrin is a cyclic oligosaccharide and useful as a drug stabilizer. HP-.beta.-CD and sulfobutylether-.beta.-cyclodextrin (SBE-.beta.-CD) have similar properties, are both readily soluble in water, can also solubilize insoluble drugs, can control drug release, have good safety, and are suitable for preparation of lavage solutions and mucosa administrating systems.
[0266] In this experiment, the sodium chloride deuterium oxide solution as an anti-tumors drug carrier in which gemcitabine was dissolved showed an anti-tumor efficacy-enhancing effect. However, deuterium oxide is a medium of small molecules, resides in the peritoneal cavity for only a short time, and is rapidly metabolized, lowering the anti-tumors effect of gemcitabine. HP-.beta.-CD shows a long retention time in blood, and slowly releases the gemcitabine dissolved therein. The results demonstrate that the 42.degree. C. hyperthermicintraperitoneal lavage using a HP-.beta.-CD deuterium oxide solution in combination with gemcitabine can significantly extend the survival time of nude mice bearing pancreatic cancer cell and enhance the anti-tumor efficacy, superior to the intraperitoneal lavage using a sodium chloride deuterium oxide solution in combination with gemcitabine, and only requires a small dose which is readily acceptable to patients.
Example 30
[0267] Intrabladder perfusion and lavage experiment with the sodium hyaluronate deuterium oxide solution.
[0268] Four bladder cancer patients, diagnosed with superficial bladder cancer (SBC) by cystoscopy and pathological examination and having undergone transurethral resection of bladder tumor (TURBt), were subjected to intrabladder perfusion and lavage for 60 min with a 50 ml 0.08% sodium hyaluronate deuterium oxide solution which had been warmed to 43.5.+-.1.degree. C. in a hyperthermic perfusion and lavage apparatus (BR-TRG-1 hyperthermic perfusion intraperitoneal treatment system, manufactured by Baorui Medical Technology Co., Ltd, Guangzhou, China) and mixed with mitomycin (MMC, 40 mg/50 ml). The above procedure was carried out once a week for 6 successive weeks, and the following indices were observed: (1) adverse reaction of bladder to chemotherapeutic drugs after perfusion: VAS score (pain), and hematuria; (2) 12 months later, recurrence of bladder cancer was examined by cystoscopy, and the results are shown in Table 28.
TABLE-US-00031 TABLE 28 Effect of intrabladder perfusion and lavage with the sodium hyaluronate deuterium oxide solution. Age Tumor No. Gender (years) location Tumor size/cm VASscore Hematuria Recurrence 1 Male 61 Trigone 0.2 .times. 0.2 (3 tumors) 0.12 No No 2 Male 57 Posterior wall 0.5 .times. 0.3 (1 tumor) 0.11 Occasional, in very No small amounts 3 Female 49 Lateral walls 0.2 .times. 0.2 (6 tumors) 0.16 No No 4 Male 38 Lateral walls 0.1 .times. 0.1 (8 tumors) 0.22 No No
[0269] When the anti-tumors drug mitomycin dissolved in only a sodium chloride solution is used for intrabladder perfusion and lavage to treat SBC, urine stimulates the inner cavity of bladder and often causes great pain to patients because the anti-tumors drug damages the glucosamine protection layer lining the epithelium of the bladder cavity, which results in hematuria and cease of the treatment. Furthermore, the anti-tumors drug mitomycin has a short life in a sodium chloride solution, which reduces its effect and leads to a tumor recurrence rate as high as 78% and eventually to a failure of treatment. The sodium hyaluronate is a linear macromolecule and acid mucopolysaccharide, has excellent anti-inflammatory and anti-viscous performance, and can slow down drug release. When the sodium hyaluronate deuterium oxide solution is used for intrabladder perfusion, the sodium hyaluronate can temporarily play the role of the glucosamine protection layer lining the epithelium of the bladder and prolong the retention time of the anti-tumors drug mitomycin in bladder, while deuterium oxide also enhances the effect of mitomycin. Their combination can not only enhance the efficacy of the anti-tumors drug, but also reduce the side effects of the anti-tumors drug. The use of a 0.08% sodium hyaluronate deuterium oxide solution in combination with mitomycin for intrabladder hyperthermic perfusion and lavage in a preliminary treatment experiment has shown efficacy, in that the pain in patients was significantly reduced and no recurrence was seen within 12 months.
Example 31
[0270] Studies on drug safety: toxicity test for intravenous injection of sodium chloride deuterium oxide solution.
Experimental Method:
[0271] 6 to 7 week-aged SD rats, half male and half female, with males each weighing 280 to 338 g and females each weighing 212 to 268 g, were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd.
[0272] Test sample: the sodium chloride deuterium oxide solution (abundance 99.9%), prepared in Example 1.
Experiment 1
Observation of Toxicity after Single Dosing
[0273] Dosing scheme: A maximum dosing method was used, in which the sodium chloride deuterium oxide solution prepared in Example 1 was intravenously injected at a dose of 20 ml/kg into 20 rats, half male and half female, at an injection speed of 2 ml/min. An equal volume of a 0.9% sodium chloride rejection was given to the control group containing 10 rats, half male and half female.
TABLE-US-00032 TABLE 29 Dosing and grouping. Dose Drug volume Number Serial No. Serial No. Groups (mg/kg) (ml/100 g) of animals of Male of Female sodium -- 2 20 1101-1110 2101-2110 chloride deuterium oxide solution Control 0* 2 10 1001-1005 2001-2005 *indicates that an equal volume of a 0.9% sodium chloride rejection was given to the control group.
Experimental Results:
1. Observation of General Symptoms
[0274] In the toxicity experiment in which a single dose of sodium chloride deuterium oxide solution was intravenously administered to rats, a maximum dose of 20 ml/kg was given to the subjects. Immediately after administration, animals receiving the test sample all showed symptoms such as reduced activity, shortness of breath, and increased urination, which were recovered to normal within 1 hour after the administration, and no other apparent abnormalities were found.
[0275] Observations were made at least once a day for 14 successive days, and no abnormalities in appearance and body sign, behavior and activity, and excrement characteristics or death was seen.
2. Body Weight
[0276] During the experiment, the animals showed a good general status and a normal increase in body weight.
TABLE-US-00033 TABLE 30 Body weight change of rats (male) in the toxicity experiment with single intravenous administration of sodium chloride deuterium oxide solution (g, x .+-. SD) 0 day(s) post 7 day(s) post 14 day(s) post Groups administration administration administration sodium chloride 304.40 .+-. 21.10 333.00 .+-. 21.47 360.50 .+-. 16.30 deuterium oxide solution Control 309.40 .+-. 10.09 334.80 .+-. 9.58 364.40 .+-. 11.19 Note: the group receiving sodium chloride deuterium oxide solution: n = 10; the control group: n = 5.
TABLE-US-00034 TABLE 31 Body weight change of rats (female) in the toxicity experiment with single intravenous administration of sodium chloride deuterium oxide solution (g, x .+-. SD) 0 day(s) post 7 day(s) post 14 day(s) post Groups administration administration administration sodium chloride 248.80 .+-. 17.08 264.20 .+-. 19.58 275.80 .+-. 19.45 deuterium oxide solution Control 250.20 .+-. 6.22 267.60 .+-. 10.97 278.40 .+-. 11.72 Note: the group receiving sodium chloride deuterium oxide solution: n = 10; the control group: n = 5.
[0277] Discussion: in the toxicity experiment in which a single dose of sodium chloride deuterium oxide solution, as the test sample was intravenously administered to rats, a maximum dose of 20 ml/kg of the sodium chloride deuterium oxide solution was given to the subjects. Immediately after administration, symptoms such as reduced activity and shortness of breath were observed, and no other toxicities associated with the test sample were found. Observations were continued for two weeks, the animals showed a normal increase in body weight, and no death was seen. According to the results of this experiment, for single-dose intravenous administration to rats, the minimum lethal dose (MLD) of the sodium chloride deuterium oxide solution, as the test sample is greater than 20 ml/kg.
Experiment 2
Observation of Toxicity During Multiple Dosing
[0278] Dosing scheme: A method of repeatedly administrating the maximum dose was used, in which the sodium chloride deuterium oxide solution, prepared in Example 1 was intravenously injected once a day at a dose of 20 ml/kg and an injection speed of 2 ml/min to 20 rats, half male and half female, for 5 successive days. An equal volume of a 0.9% sodium chloride rejection was given to the control group containing 10 rats, half male and half female.
TABLE-US-00035 TABLE 32 Dosing and grouping. Serial Dose Drug volume Number of No. of Serial No. of Groups (mg/kg) (ml/100 g) animals Male Female sodium -- 2 .times. 5 times 20 1201-1210 2201-2210 chloride deuterium oxide solution Control 0* 2 .times. 5 times 10 1101-1105 2101-2105 *indicates that an equal volume of a 0.9% sodium chloride rejection was given to the control group.
Experimental Results:
1. Observation of General Symptoms
[0279] In the toxicity experiment in which multiple doses of sodium chloride deuterium oxide solution were intravenously administered to rats, a maximum dose of 20 ml/kg was given to the subjects once a day, for 5 successive days. Immediately after each administration, animals receiving the test sample all showed symptoms such as reduced activity, shortness of breath, and increased urination, which were recovered to normal within 1 hour after the administration, and no other apparent abnormalities were found.
[0280] Observations were made at least once a day for 3 successive months, and no abnormalities in appearance and body sign, behavior and activity, and excrement characteristics or death was seen.
2. Body Weight
[0281] During the experiment, the animals showed a good general status and a normal increase in body weight.
TABLE-US-00036 TABLE 33 Body weight change of rats (male) in the toxicity experiment with multiple repeating intravenous administration of sodium chloride deuterium oxide solution (g, x .+-. SD) 0 day(s) post 30 day(s) post 90 day(s) post Groups administration administration administration sodium chloride 305.20 .+-. 20.90 352.00 .+-. 31.72 452.40 .+-. 30.14 deuterium oxide solution Control 303.20 .+-. 11.10 361.01 .+-. 29.65 461.91 .+-. 31.19 Note: the group receiving sodium chloride deuterium oxide solution: n = 10; the control group: n = 5.
TABLE-US-00037 TABLE 34 Body weight change of rats (female) in the toxicity experiment with multiple repeating intravenous administration of sodium chloride deuterium oxide solution (g, x .+-. SD) 0 day(s) post 30 day(s) post 90 day(s) post Groups administration administration administration sodium chloride 243.52 .+-. 14.88 293.20 .+-. 18.83 395.63 .+-. 29.54 deuterium oxide solution Control 251.28 .+-. 11.22 298.12 .+-. 14.67 405.42 .+-. 26.12 Note: the group receiving sodium chloride deuterium oxide solution: n = 10; the control group: n = 5.
[0282] In the toxicity experiment in which multiple doses of sodium chloride deuterium oxide solution, as the test sample were repeatedly intravenously administered to rats, a maximum dose of 20 ml/kg of the sodium chloride deuterium oxide solution was given to the subjects. Immediately after administration, symptoms such as reduced activity and shortness of breath were observed, and no other toxicities associated with the test sample were found. Observations were continued for 3 months, the animals showed a normal increase in body weight, and no death was seen. According to the results of this experiment, for multiple-dose intravenous administration to rats, the minimum lethal dose (MLD) of the sodium chloride deuterium oxide solution, as the test sample is greater than 20 ml/kg.
Statistic Analysis
[0283] A comparison of the data was carried out between the treatment groups and control group. The scale variable (expressed as mean.+-.standard deviation) was proceeded by a Mann-Whitney test, and unpaired t-test). A two-side alpha of 0.05 was used for all tests.
[0284] Although some aspects of the present disclosure have been set forth and discussed herein, it would be appreciated by a person skilled in the art that modifications can be made to the above aspects without departing from the principle and spirit of the present disclosure. Therefore, the scope of the present disclosure is defined by the claims and equivalents therefore.
User Contributions:
Comment about this patent or add new information about this topic: