Patent application title: LINER-BASED SHIPPING AND DISPENSING CONTAINERS FOR THE SUBSTANTIALLY STERILE STORAGE, SHIPMENT, AND DISPENSE OF MATERIALS
Inventors:
Alfredo Daniel Botet (Avon, CT, US)
IPC8 Class: AB65D2514FI
USPC Class:
220 6221
Class name: Receptacles receptacle side wall made of two or more layers of material permanently attached together bag liner
Publication date: 2014-08-21
Patent application number: 20140231427
Abstract:
A liner-based assembly including an overpack, a liner disposed within the
overpack, and a filling connector securable to at least one of the
overpack and the liner and ineloding a membrane for substantially aseptic
filling. The membrane may be an elastomeric stopper, which may be
configured, for re-sealing with or without the assistance of heat The
liner-based assembly may further include a dispense connector that is
operably connected with the overpack and/or the liner. The dispense
connector may comprise or be operably connected to a one-way valve, the
one-way valve having --an elastomeric portion defining an opening and
generally operably coupled with a plug member positioned within
the--opening. In a closed position, the elastomeric portion may be sealed
against the plug member, and when a pressure is applied against the
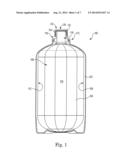
elastomeric portion, the elastomeric portion may flex in a manner
configured --to permit only one--way flow through the opening.Claims:
1. A liner-based assembly for use with pressure dispense comprising: an
overpack; a liner disposed within the overpack; and a filling connector
that is securable to at least one of the overpack and the liner, the
connector including a membrane for substantially aseptic filling.
2. The liner-based assembly of claim 1, wherein the membrane comprises a re-sealable elastomeric stopper.
3. The liner-based assembly of claim 2, wherein the re-sealable elastomeric stopper is configured for re-sealing with the assistance of heat.
4. The liner-based assembly of claim 2, wherein the liner and overpack are irradiated.
5. The liner-based assembly of claim 1, further comprising a dispense connector operably connected with at least one of the overpack and the liner, over the filling connector, the dispense connector at least one of comprising or operably connected to a one-way valve.
6. The liner-based assembly of claim 5, wherein the liner is collapsible away from the overpack.
7. The liner-based assembly of claim 6, further comprising a portable pressure source for pressurizing an annular space between the overpack and liner for collapsing the liner and dispensing the contents thereof.
8. The liner-based assembly of claim 5, wherein the one-way valve comprises an elastomeric portion defining an opening and generally operably coupled with a plug member positioned within the opening, the plug member being relatively stiff as compared to the elastomeric portion, wherein in a closed position, the elastomeric portion is sealed against the plug member, and wherein when a pressure is applied against the elastomeric portion, the elastomeric portion is flexes in a manner configured to permit only one-way flow through the opening.
9. A method for providing a sterile transporting and pressure dispense system, the method comprising: providing an overpack and a liner disposed within the overpack; irradiating the overpack and liner for sterility; and securing a connector to at least one of the overpack and the liner, the connector including a membrane for substantially aseptic filling.
10. The method of claim 9, wherein the membrane comprises a re-sealable elastomeric stopper.
11. The method of claim 10, further comprising filling the liner by piercing the elastomeric stopper with a needle and passing a material through the needle into the liner.
12. The method of claim 11, further comprising removing the needle upon completion of filling and applying heat to assist in re-sealing the elastomeric stopper.
13. The method of claim 11, further comprising removing headspace gas from the interior of the liner upon completion of filling the liner.
14. The method of claim 13, wherein the connector comprises a one-way valve for permitting headspace gas to pass in one direction out of the liner.
15. The method of claim 9, further comprising providing a dispense connector that is operably connected with at least one of the overpack and the liner, the dispense connector at least one of comprising or operably connected to a one-way valve.
16. The method of claim 15, wherein providing a dispense connector comprises providing a portable pressure source for pressurizing an annular space between the overpack and liner.
17. The method of claim 16, wherein the portable pressure source comprises a disposable compressed gas cartridge.
18. A liner-based assembly for use with pressure dispense comprising: an overpack; a liner disposed within the overpack, the liner configured for collapsing away from the overpack upon the introduction of a gas or fluid into an annular space between the overpack and liner; and a dispense connector that is securable to at least one of the overpack and the liner, the dispense connector comprising a passage in fluid communication with the annular space and at least one of comprising or operably connected to a one-way dispense valve in fluid communication with an interior of the liner.
19. The liner-based assembly of claim 18, wherein the one-way valve comprises an elastomeric portion defining an opening and generally operably coupled with a plug member positioned within the opening, the plug member being relatively stiff as compared to the elastomeric portion, wherein in as closed position, the elastomeric portion is sealed against the plug member, and wherein when a pressure is applied against the elastomeric portion, the elastomeric portion is flexes in a manner configured to permit only one-way flow through the opening.
20. The liner-based assembly of claim 8, wherein the liner and overpack irradiated.
Description:
FIELD OF THE INVENTION
[0001] The present disclosure relates to novel and advantageous shipping and dispensing systems. Particularly, the present disclosure relates to novel and advantageous liner-based systems for the substantially sterile storage, shipment, and dispense of materials used in the biotechnology and pharmaceutical industries, and which, in some cases, may be completely or substantially disposable.
BACKGROUND OF THE INVENTION
[0002] Numerous materials, such as culture media, buffers, reagents and other biological materials, for example, are used extensively by biotech companies, for example, in research and development, vaccine creation and usage, protein production and purification, and the development of other biologics. To be safe and effective for their intended use, as well as to he in compliance with various rules and regulations, these materials must be pure and sterile,
[0003] Container systems may be used in the biopharmaceutical, and other Industries, for storing, shipping, mixing, reacting, processing, and/or dispensing materials such as those described above. Such materials are often fragile and/or expensive, and/or must be maintained in a sterile environment, Accordingly, any container system used with such materials must be substantially air-tight to prevent contamination and to prevent escape of the material into the outside environment. Further a container system must be safe, sterile, reliable and leak proof, such that it may withstand the stresses of shipping and dispense.
[0004] Container systems that are used to store and dispense the types of materials described, above, as well as other liquid-based contents, typically include a container of some kind, and/or a Liner, a cap that may be used to seal and protect the contents of the storage system when the contents are not being dispensed, and a connector that may be used to dispense the contents from the container. However, traditional storage and dispense container systems are typically not configured to permit for the safe and secure shipment of the types of materials described, particularly not in a disposable container system. Specifically, such traditional dispensers may not adequately maintain or ensure the purity of the contents of the dispenser and, for example, are unable to keep gas or other contaminants from getting into the contents stored in the liner. Similarly, in cases where the material being transferred is noxious or harmful, with traditional storage and dispense container systems not configured to permit for the safe and secure shipment, the user may be undesirably exposed to the material during transfer,
[0005] Accordingly, there is a need for a dispenser that limits or substantially eliminates contamination and/or degradation of the contents of the dispenser. Further yet, a need exists for isolating the contents of a dispenser from the environment from the point of filling to final dispense of the contents.
BRIEF SUMMARY OF THE INVENTION
[0006] The present disclosure, in one embodiment, relates to a liner-based assembly for use with pressure dispense. The liner-based assembly may include an overpack, a liner disposed within the overpack, and a filling connector that is securable to at least one of the overpack and the liner, the connector including a membrane for substantially aseptic filling. The membrane, in some eases, may be a re-sealable elastomeric stopper which may be configured for re-scaling with or without the assistance of heat. The liner and overpack could be irradiated for sterility. The liner-based assembly may further include a dispense connector that is operably connected with the overpack and/or the liner, over the filling connector. The dispense connector may comprise or be operably connected to a one-way valve. The one-way valve may have an elastomeric portion defining an opening and generally operably coupled with it plug member positioned within the opening. The plug member may he relatively stiff as compared to the elastomeric portion. In a closed position, the etastomeric portion may be sealed against the plug member, and when a pressure is applied against the elastomeric portion, the elastomeric portion may flex in a manner configured to permit only one-way flow through the opening. The liner may be configured to collapsible away from the overpack during pressure dispense. Furthermore, the liner-based assembly may include a portable pressure source for pressurizing an annular space between the overpack overpack and liner for collapsing the liner and dispensing the contents thereof.
[0007] The present disclosure, in another embodiment, relates to a method for providing a sterile transporting and pressure dispense system. The method includes providing an overpack and a liner disposed within the overpack; irradiating the overpack and liner for sterility; and securing as connector at least one of the overpack and the liner, the connector including a membrane, which in some cases may be an elastomeric stopper, for substantially aseptic filling. Again, the membrane may be a re-sealable elastomeric stopper. The method may further include filling the liner by piercing the elastomeric stopper with a needle and passing a material through the needle into the liner. Upon completion of filling, the needle may be removed, and in some cases heat may be applied to assist in re-sealing the elastomeric stopper. Also upon completion of filling the liner, headspace gas may be removed from the interior of the liner. A one-way valve may be provided on the connector for permitting headspace gas to pass in one direction out of the liner. The method may also include providing dispense connector that is operably connected with the overpack and/or the liner, the dispense connector comprising or operably connected to a one-way valve. A portable pressure source may be provided for pressurizing an annular space between the overpack and liner. The portable pressure source may be, for example, a disposable compressed gas cartridge.
[0008] In another embodiment, the present disclosure relates to a liner-based assembly for use with pressure dispense. The liner-based assembly may include an overpack, a liner disposed within the overpack, the liner configured for collapsing away from the overpack upon the introduction of a gas or fluid into an annular space between the overpack and liner, and a dispense connector that is securable to at least one of the overpack and the liner, the dispense connector comprising a passage in fluid communication with the annular space and comprising or operably connected to a one-way dispense valve in fluid communication with an interior of the liner. The one-way valve may include an elastomeric portion defining an opening and generally operably couple with a plug member positioned within the opening, the plug member being relatively stiff as to the elastomeric portion. In a closed position, the elastomeric portion may be sealed against the plug member, and when a pressure is applied against the elastomeric portion, the elastomeric portion may flex in a manner configured to permit only one-way flow through the opening. In some embodiments, the liner and overpack may be irradiated.
[0009] While multiple embodiments are disclosed, still other embodiments of the present disclosure will become apparent to those skilled in the art from the following detailed description, which shows and describes illustrative embodiments of the disclosure. As will be realized, the various embodiments of the present disclosure are capable of modifications in various obvious aspects, all without departing from the spirit and scope of the present disclosure. Accordingly, the drawings and detailed description are to be regarded as illustrative in nature and not restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] While the specification concludes with claims particularly pointing out and distinctly claiming the subject matter that is regarded as forming the various embodiments of the present disclosure, it is believed that the disclosure will be better understood from the following description taken in conjunction with the accompanying Figures, in which:
[0011] FIG. 1 is a cross-sectional view of a shipping and dispensing system according to one embodiment of the present disclosure.
[0012] FIG. 2 is a cross-sectional view of a outlet valve of a dispense connector according to one embodiment of the present disclosure.
[0013] FIG. 3 is a dispense system schematic according to one embodiment of the present disclosure.
[0014] FIG. 4 is a dispense system schematic according to another embodiment of the present disclosure.
[0015] FIG. 5 shows a shipping and storage system for use with indirect pressure dispense according to one embodiment of the present disclosure.
[0016] FIG. 6 shows statistics related to the indirect pressure dispense method shown in FIG. 5 provided in graphical form in accordance with one embodiment of the present disclosure.
[0017] FIG. 7 is a cross-sectional view of a shipping and dispensing system including a packaging element according to one embodiment of the present disclosure that.
DETAILED DESCRIPTION
[0018] The present disclosure relates to novel and advantageous shipping and dispensing systems. More particularly, the present disclosure relates to novel and advantageous liner-based systems for use, in some embodiments, with materials that must maintain their purity, or some high level of purity during shipping and/or dispense, wherein, in some cases, the liner-based system may be completely or substantially disposable. For example, the shipping and dispense system of the present disclosure, in one aspect, may be configured for a single use and/or remain substantially air-tight for use in industries that use materials that must remain substantially pure, uncontaminated, and/or sterile, such as many materials used in, for example, the biopharmaceutical manufacturing and analytical processes industries. Examples of some of the types of materials that may be used with embodiments of the present disclosure include, but are not limited to, reagents, buffers, cell culture media, or other sterile media. Applications may include, but are not limited to, sterile media transfer, vaccine manufacture, filling and formulation, bioreactors feed and harvest, pharmaceutical process fluid transfer, high containment operations, in-process pooling, and transferring buffers. It is, however, recognized that embodiments of the present disclosure may also be used with as variety of materials to a variety of different industries. For example, dispensers of the present disclosure may contain, but are not limited in use to: ultrapure liquids, such as acids, solvents, bases, photoresists, slurries, detergents, cleaning formulations, dopants, inorganic, organic, metalorganics, TEOS, and biological solutions, DNA and RNA solvents and reagents, pharmaceuticals, nanomaterials (including for example, fullerenes, inorganic nanoparticles, sol-gels, and other ceramics), and radioactive chemicals; pesticides/fertilizers; paints/glosses/solvents/coating-materials etc.; power washing fluids; lubricants for use in the automobile or aviation industry, for example; food products, such as but not limited to, condiments, cooking oils, and soft drinks, for example; reagents or other materials for use in the biomedical or research industry; hazardous materials used by the military, for example; polyurethanes; agrochemicals; industrial chemicals; cosmetic chemicals; petroleum and lubricants; adhesives; sealants; health and oral hygiene products and toiletry products; or any other material that may be dispensed by pressure dispense, for example. Materials that may be used with embodiments of the present disclosure may have any viscosity, including high viscosity and low viscosity fluids. Those skilled in the art will recognize the benefits of the disclosed embodiment and therefore will recognize the suitability of the disclosed embodiments to various industries and for the transportation and dispense of various products.
[0019] The use creation, and/or storage of some materials that may he used with embodiments of the present disclosure may be subject to various rules, regulations, and/or standards. Accordingly, in some embodiments of the present disclosure, the liner-based system and/or the use of the liner-based system may meet guidelines set by the United States Pharmacopeia ("USP"). Specifically, some embodiments of the present disclosure may be suitable to meet Class VI USP guidelines to ensure biocompatibility with plastics. USP's official Reference Standards are highly characterized specimens of drug substances, excipients, impurities, degradation products, dietary supplements, compendial reagents, and performance calibrators. They are specified for use in conducting official USP-NF tests and assays. USP also provides Reference Standards specified in the Food Chemicals Codex as well as authentic substances, high-quality chemical samples, as a service to analytical, clinical, pharmaceutical, and research laboratories. USP's Reference Standards are used in more than 130 countries around the world. USP Reference Standards that are based directly on official monographs in the USP-NF, whose standards and procedures are enforceable by the U.S. Food and Drug Administration (FDA), are recognized as official standards in the U.S., and their use is effective in demonstrating compliance with statutory requirements.
[0020] In other embodiments, various components or all components of the shipping and dispensing systems of the present disclosure the liner may be, or may also be, animal derived component free ("ADCF"). Using ADCF materials may be important, for example, because bovine spongiform encephalopathy ("BSE"), and its potential to affect humans has emerged as a serious concern. Accordingly, suppliers of many essential animal-sourced components used in cell culture and fermentation processes, for example, became concerned about the potential for material contamination with prions. Viruses also can be present in raw materials derived from animal origins. Many important drug and vaccine products are made by mammalian cell culture or bacterial fermentation, so their biological safety is paramount. However, it is very difficult to ensure that any material from an animal source carries no infection. Even the rigorous cleaning methods designed to minimize carry-over of biohazards from one batch to the next is no guarantee of safety. Thus the use of ACDF materials for storing, shipping and dispensing biological and/or biopharmaceutical media may be advantageous.
[0021] The liner-based systems of the present disclosure may hold up to approximately 200 liters, in some embodiments. Alternatively, the liner-based systems may hold up to approximately 20 liters, Alternatively, the liner-based systems may hold. approximately 1 to 5 liters, or less. It will be appreciated that the referenced container sizes are examples only and that the liner-based systems of the present disclosure may be readily adapted for use with a wide variety of sized and shaped shipping, and dispensing containers. The entire liner-based system of the present disclosure may be used a single-time and then disposed of, in some embodiments. In other embodiments, the overpack, for example, may be reused while the liner and/or any connectors may be used only a single time.
[0022] FIG. 1 illustrates one embodiment of a liner-based shipping and dispense system 100 of the present disclosure. In some embodiments, the shipping and dispense system 100 may include an overpack 102, a liner 104, and one or more connectors or connector assemblies, which may be, for example, a filling connector and/or a dispensing connector or connector assembly, as will be described in further detail below.
[0023] The overpack 102 may include an overpack wall 106, an interior cavity 108, and a mouth 110. The overpack 102 may be comprised of any suitable material or combination of materials, for example but not limited to one or more polymers, including plastics, nylons, EVOH, polyolefins, or other natural or synthetic polymers. In further embodiments, the overpack 102 may be manufactured using polyethylene terephthalate (PET), polyethylene naphthalate (PEN), poly(butylene 2,6-naphthalate) (PBN), polyethylene (PE), linear low-density polyethylene (LLDPE), low-density polyethylene (LDPE), medium-density polyethylene (MDPE), high-density polyethylene (HDPE), polypropylene (PP), and/or fluoropolymer, such as but not limited to, Polychlorotrifluoroethylene (PCTFE), polytetrafluoroethylene (PTFE), fluorinated ethylene propylene (FEP), and perfluoroalkoxy (PFA). The overpack 102 may be of any suitable shape or configuration, such as, but not limited to, a bottle, a can, a drum, etc.
[0024] As described above, the shipping and dispense system 100 may include a liner 104, which may be disposed within the overpack 102. The liner 104 may include a liner wall 112, an interior cavity 114, and a mouth 116. The mouth 116 of the liner 104 may include a fitment portion 118. The fitment portion 118 may be made of the same or different material than the rest of the liner 104 and may be harder, more resilient, and/or less flexible than the rest of the liner. The fitment portion 118 may include threads 120 that may couple with complementary threads on a connector or connector assembly (discussed more fully below). It is appreciated, however, that the fitment portion 118 may alternatively or additionally include any other means for coupling with a connector, such as but not limited to, snap-fit or friction-fit means, bayonet means, or any other suitable mechanism or combination of mechanisms for coupling, as will be appreciated by those skilled in the art. In some embodiments, a connector or connector assembly may couple to, or may also couple to, the mouth 110 of the overpack 102.
[0025] In some embodiments, the liner 104 may be a collapsible liner that is substantially flexible, while in other embodiments the liner may be somewhat rigid but still collapsible, e.g., a rigid collapsible liner. The liner 104 may be manufactured using any suitable material or combination of materials, such as but not limited to, any of the materials or combination of materials listed above with respect to the overpack 102, However, the overpack 102 and liner 104 need not be manufactured from the same materials. In some embodiments, the material or materials selected and the thickness of that material or those materials may determine the rigidity of the liner 104. The liner 104 may have one or more layers and may have any desirable thickness. A liner 104 may have a thickness of, for example, from about 0.05 mm to about 3 mm, or any other suitable thickness.
[0026] The liner 104 may be configured to comprise any desirable shape that is appealing to the user, and/or assists in the collapse of the liner. The liner 104, in some embodiments, may be dimensioned and shaped to substantially conform to the interior of the overpack 102. As such, the liner 102 may have a relatively simplistic design with a generally smooth outer surface, or the liner may have a relatively complicated design including, for example but not limited to, indentations and/or protrusions, In some embodiments, the liner wall 112 may include a generally textured surface in order to minimize adhesion. For example, in some embodiments, the surface may include a plurality of bumps, scales, or projections, which may each have any appropriate size, for example, but not limited to, from about 0.5-100 μm. Texturizing features may be spaced any suitable distance from one another. In some embodiments, the texturizing may comprise a framework, such as a lattice or scaffold, for example. Examples of some suitable texturizing features are described in greater detail in U.S. Provisional Patent Appln. No. 61/334,006, titled, "Fluid Processing Components with Textured Surface for Decreased Adhesion and Related Methods," filed May 12, 2010, which is hereby incorporated by reference herein in its entirety. The liner 104 may have a relatively thin liner wall 112, as compared to the thickness of the overpack wall 106. In some embodiments, the liner 102 may be flexible such that the liner wall 112 may be readily collapsed, such as by vacuum through the mouth 116 or by pressure between the liner wall 112 and overpack wall 106, referred to herein as the annular space therebetween.
[0027] The liner 104, in a further embodiment, may have a shape, when inflated or filled, that is different from, but complimentary with, the shape of the overpack 102 such that it may be disposed therein. In some embodiments, the liner 104 may be removably attached to the interior of the overpack wall 102. The liner 104 may provide a barrier, such as a gas barrier, against drive gas migration from the annular space between the liner wall 112 and the overpack wall 106. Accordingly, the liner 104 may generally ensure and/or maintain the purity of the contents within the liner.
[0028] In some embodiments, particularly where sterility of the contents of the liner must be substantially maintained, the liner 102 may be comprised of a material that may help ensure or maintain as sterile environment for the contents disposed in the liner. For example, in some embodiments the liner may be comprised of TK8 manufactured by ATMI of Danbury, Conn., or any other suitable material. As noted above, in some embodiments, the liner 104 may comprise multiple layers. The multiple layers may comprise one or more different polymers or other suitable materials. In some embodiments, the thickness, ply, and/or the composition of the liner and/or the layers of the liner may allow for the secure and substantially uncontaminated shipment of the contents of the liner-based system of the present disclosure by limiting or eliminating typical weaknesses or problems associated with traditional liners or packages, such as, for example weld tears, pin holes, gas entrainment and/or any other means of contamination. Similarly, or in addition, the liner 104 may also contribute to the secure and substantially uncontaminated shipment of the contents of the shipping and dispense system 100 of the present disclosure b configuring the liner to substantially conform to the shape of the overpack when the liner is filled, thereby reducing the amount of movement of the contents during shipping. Further, in embodiments where the liner substantially conforms to the shape of the overpack, the amount of movement of the liner during shipment may be reduced or substantially reduced, advantageously reducing or eliminating the occurrence of pin holes.
[0029] The overpack 102 and liner 104 may each be manufactured using any suitable manufacturing process, such as but not limited to, injection blow molding, injection stretch blow molding, extrusion, etc., and may each be manufactured as a single component or may be a combination of multiple components. To some embodiments, the overpack 102 and liner 104 may be blow molded in a nested fashion, also referred to herein as co-blow molded. Examples of liner-based systems and methods utilizing co-blow molding techniques have been described in greater detail in U.S. Prov. Appl. No. 61/506,807, titled "Nested Blow Molded Liner and Overpack and Methods of Making Same," filed Jul. 12, 2011, which is hereby incorporated herein by reference in its entirety. In some embodiments a liner may be blow molded into an already formed overpack, whereby the overpack may function as the mold for the liner, and may be referred to herein as "dual blow molding," which is described in further detail in U.S. Prov. Appl. No. 61/703,996, titled "Liner-based Shipping and Dispensing Systems," filed Sep. 21, 2012, which is hereby incorporated herein by reference in its entirety. In such embodiments, the overpack may be manufactured by any suitable process.
[0030] Examples of the type of liners and overpacks that may be used are disclosed in more detail in: International PCT Appl. No. PCT/US11/55558, titled, "Substantially Rigid Collapsible Liner, Container and/or Liner for Replacing Glass Bottles, and Enhanced Flexible Liners," filed Oct. 10, 2011; International PCT Appl, No PCP/US11/55560, titled, "Nested Blow Molded Liner and Overpack and Methods of Making Same," filed Oct. 10, 2011; International PCT Appl. No. PCT/US11/64141, titled "Generally Cylindrically-Shaped Liner for Use in Pressure Dispense Systems and Methods of Manufacturing the Same," filed Dec. 9, 2011; U.S. Prov. Appl. No. 61/468,832, titled "Liner-Based Dispenser," filed Mar. 29, 2011; U.S. Prov. Appl. No. 61/525,540, titled "Liner-Based Dispensing Systems," filed Aug. 19, 2011; U.S. Prov. Appl. No, 61/703,996, titled "Liner-based Shipping and Dispensing Systems," filed Sep. 21, 2012; U.S. Pat. Appl. No. 11/915,996, titled "Fluid Storage and Dispensing Systems and Processes," filed Jun. 5, 2006; International PCT Appl. No. PCT/US10/51786, titled "Material Storage and Dispensing System and Method With Degassing Assembly," filed Oct. 7, 2010, international PCT Appl, No. PCT/US10/41629, U.S. Pat. No. 7,335,721, U.S. Pat. Appl. No. 11/912,629, U.S. Pat. Appl. No. 12/302,287 and International PCT Appl. No. PCT/US08/85264, each of which is hereby incorporated by reference herein in its entirety. The overpack 102 and liner 104 for use with the shipping and dispense system 100 of the present disclosure may include any of the embodiments, features, and/or enhancements disclosed in any of the above noted applications, including, but not limited to, flexible, rigid collapsible, 2-dimensional, 3-dimensional, welded, molded, gusseted, and/or non-gusseted liners, and/or liners that contain folds and/or liners that comprise methods for limiting or eliminating choke-off and linen sold under the brand name NOWpak® by ATMI, Inc. for example.
[0031] As described above, the liner 104 may include a fitment portion 118, which may include coupling means, such as but not limited to, threads 120, for coupling with complementary threads on a connector or connector assembly. Various types of connectors or connector assemblies may be utilized with the shipping and dispensing system 100 of the present disclosure. In one embodiment, the shipping and dispensing system 100 may include one or both of a shipping and filling connector and a dispense connector assembly, each of which is described more fully below.
[0032] As illustrated in FIG. 1, a shipping and filling connector 122 of the present disclosure, in some embodiments, may include complementary threads 124 that may couple with threads 120 on the fitment portion 118 of the liner 104. As with fitment portion 118, it is appreciated, however, that the connector 122 may alternatively or additionally include any other means for coupling with one or more portions of the liner based system, such as but not limited to, snap-fit or friction-fit means, bayonet means, or any other suitable mechanism or combination of mechanisms for coupling, as will be appreciated by those skilled, in the art.
[0033] In some embodiments, the connector 122 may be configured for aseptic filling of the interior cavity 114 of the liner 104. In some embodiments, connector 122 may include a membrane 126 through which aseptic filling may be accomplished. The membrane 126 may be, for example, a re-sealable stopper made from an elastomeric material, such that a filling tube or needle, such as but not limited to a non-coring needle, may be passed through the membrane for filling the liner 104. Upon removal of the filling tube or needle, the elastomeric material of the membrane 126 may reseal, thereby eliminating or substantially eliminating germ ingress to the liner 104. In some embodiments, the membrane 126 resealing may be assisted with the use of heat, such as by laser. Such a connector 122 permitting aseptic filling can eliminate the need for costly and complex isolators. Rather, the shipping and dispense system 100, or more particularly, the overpack 102 and/or liner 104 may be sterile and irradiated, thereby themselves becoming the isolators. In general, with some such embodiments of connector 122, at no time during filling and shipping of the filled liner are the contents within the interior cavity 114 of the liner 104 exposed to the external environment, completely or substantially preventing contamination of the contents. In one embodiment, the shipping and filling connector 122 may be, or may be similar to, filling connectors sold under the brand name Intact® Sterile Filling System by Medical Instill Technologies, Inc, of New Milford, Conn. While a connector having a membrane of an elastomeric substance is described, it will be understood that any suitable Class VI plastic, or combinations thereof, for example, may be used to provide a sterile connector within the spirit and scope of the present disclosure.
[0034] A dispense connector or assembly may, or may also be used with embodiments of the present disclosure. In some cases, the dispense assembly may comprise a dispense connector, features for dispensing by pressure dispense, and/or a one-way dispense valve. In some embodiments, a dispense connector may include dispensing features, as well as features for filling such as those described above, thereby allowing the same connector to be used for both filling and dispense, while in other cases, the dispense connector may be a separate connector from the connector used to fill the liner based system. Accordingly, the dispense connector may be directly coupled to or over the connector 122 used to fill and/or ship the container in some embodiments. In other embodiments where the dispense connector is separate from the connector 122 used to fill and/or ship, some or all of the fill and/or shipping connector may be removed from the liner and overpack system before coupling the dispense connector thereto. The dispense connector may be comprised of any suitable material, such as metal, plastic, or any other material or combination of materials. In some embodiments, the dispense connector may be comprised of a material that may be suitable for use in a sterile environment, such as those listed above, or any other suitable material, or combination of materials.
[0035] The dispense assembly may also include features used to dispense the contents of the liner based system. In some embodiments, the dispense assembly may be configured to work with existing static systems, for instance existing pressure-dispense systems that may not be portable. In other embodiments, the dispense system may be configured to be substantially completely portable. Generally, dispensing features of the dispense assembly may include a pressurizing gas inlet that generally permits a gas pressure in-line to be inserted through or coupled with the dispense connector and be in fluid communication with the annular space between the liner 104 and the overpack 102. In such a system, a fluid, gas, or other suitable substance may be introduced into the annular space, thereby pushing the contents of the filled liner 104 out of the liner 104. The dispense assembly may also include one or more ports that may include dip tubes that may extend any suitable distance into the interior of the liner 104 that may be used, for example, to puncture the membrane 126 on the fill connector 122 in embodiments where the dispense connector may be coupled to or over the fill connector 122.
[0036] The dispense assembly may also include a one-way dispense valve 200 as shown in FIG. 2. In some embodiments the dispense valve 200 may act as a non-contamination barrier, protecting the contents of the liner during dispense from air, moisture, bacteria and/or any other harmful contaminants. The one-way valve 200 may generally only allow material to flow in one direction, i.e. out of the valve and not back into the liner, during dispense. In this way, the one-way valve 200 functions similarly to the arterial valve in the cardiovascular system. The one-way valve 200 may include an elastomeric portion 202 that may be coupled to and abut up against a plug 204 of the valve. The plug 204 may be relatively stiff as compared to the elastomeric portion 202. As may be seen in FIG. 2, in some embodiments the elastomeric portion 202 may generally surround an outlet tube 206, for example. The elastomeric portion 202 may generally taper to provide a relatively smaller dispense opening 210, in which the plug 204 may be positioned. The plug 204 may generally seal the dispense end of the elastomeric portion 202 when contents are not being dispensed. In use, the one-way valve 200 may be used to dispense materials in a substantially aseptic manner. During pressure dispense, the contents of the liner may be directed toward the one-way valve 200, thereby creating pressure on the valve 200. The pressure created by the force of the material on the valve 200 may cause the elastomeric portion 202 to open. The material may then pass through the valve 200 as a result of the pressure applied to the material, ensuring that there is direct flow in only the dispense direction 220. As the desired amount of material is dispensed, the pressure source may be lessened, and eventually may be turned off, causing the elastomeric portion 202 to close in on itself beginning with the thicker portion at the base 208, thereby allowing no ingress, and squeezing out the remaining material in the valve 200. In one embodiment, the dispense connector may be, or may be similar to, dispense connectors or dispense valves sold under the brand name Pure-Dose® One-Way Visco-Elastic Valve by Medical Instill Technologies, Inc. of New Milford, Conn. In other embodiments, the valve may be comprised of any other suitable material, including any other plastic or combination of plastics or other materials.
[0037] Filling connectors and dispense connectors and/or valves, such as those like the connectors sold under the brand names Intact® Sterile Filling System and Pure-Dose® One-Way Visco-Elastic Valve by Medical Instill Technologies, Inc., are further described in detail in: U.S. Pat. No. 7,997,447; U.S. Pat. No. 7992,597; U.S. Pat. No. 7,980,276; U.S. Pat. No. 7,975,453; U.S. Pat. No. 7,967034; U.S. Pat. No. 7,966,746; U.S. Pat. No. 7,954,521; U.S. Pat. No. 7,886,937; U.S. Pat. No. 7,874,129; U.S. Pat. No. 7,861,750; U.S. Pat. No. 7,850,051; U.S. Pat. No. 7,845,517; U.S. Pat. No. 7,810,677; U.S. Pat. No. 7,798,185; U.S. Pat. No. 7,780,023; U.S. Pat. No. 7,779,609; U.S. Pat. No. 7,726,357; U.S. Pat. No. 7,678,089; U.S. Pat. No. 7,669,390; U.S. Pat. No. 7,665,923; U.S. Pat. No. 7,651,291; U.S. Pat. No. 7,637,401; U.S. Pat. No. 7,637,400; U.S. Pat. No. 7,628,184; U.S. Pat. No. 7,568,509; U.S. Pat. No. 7,556,066; U.S. Pat. No. 7,410,050; U.S. Pat. No. 7,331,944; U.S. Pat. No. 7,322,491; U.S. Pat. No. 7,270,158; and U.S. Pat. No. 7,077,176; U.S. Pat. No. 7,000,806, each of which is hereby incorporated by reference herein in its entirety.
[0038] The liner-based system may also include features for helping prevent or limit choke-off. Generally speaking, choke-off may be described as what occurs when a liner ultimately collapses on itself, or a structure internal to the liner, to form a choke point disposed above a substantial amount of liquid. When choke-off occurs, it may preclude complete utilization of the liquid disposed within the liner, which can be a significant problem, as many materials used in the biotechnology and/or pharmaceutical industry, for example, can be very expensive. A variety of ways of preventing or handling choke-off are described in PCT Application Number PCT/US08/52506, entitled, "Prevention Of Liner Choke-off In Liner-based Pressure Dispensation System," with an international filing date of Jan. 30, 2008, which is hereby incorporated herein by reference in its entirety. Additional ways of preventing or handling choke-off are described in International PCT Appl. No. PCT/US11/55558, titled, "Substantially Rigid Collapsible Liner, Container and/or Liner for Replacing Glass Bottles, and Enhanced Flexible Liners," filed Oct. 10, 2011, which was previously incorporated herein by reference in its entirety.
[0039] In use, in some embodiments, as liner-based system may arrive at a first filling site, for example, with the system fully assembled including an overpack 102, liner 104, and filling connector 122. In some cases, the overpack 102, liner 104, and/or the connector may be irradiated and sterilized at a manufacturing site, such that that the sterilization process may not need to be performed at the filling site. As part of the sterilization process, the liner may be evacuated and therefore may be in a collapsed state and may include substantially no gases and be ready for filling upon arrival, for example, at the fill site. In other embodiments, the filling connector may be provided at the till site and/or the overpack 102, liner 104, and/or filling connector may be irradiated and/or sterilized at the fill site prior to filling.
[0040] At the fill site, the filling connector 122 may be used to aseptically fill the contents of the liner. As explained above, in some embodiments the filling connector may be configured to comprise a closed needle that may puncture a membrane that may seal the liner. Once the needle has passed through the membrane, the needle may open to allow material to be introduced into the liner in a sterile manner. After the fill is complete, in some embodiments, any headspace may be removed from the liner. Headspace generally refers to any gas space in a liner, for example, that may exist above the material stored in the liner. Headspace may be undesirable because it may allow for some of the headspace gas to enter the material, thereby contaminating the material. Limiting or eliminating headspace may be particularly important for systems that may be transported. The movement of the material in the liner that may occur when headspace is present may cause foaming, bubbling, stress, protein damage, and/or gas contamination of the material, for example, which can be highly undesirable when maintaining the purity of the contents of the system is crucial. The headspace may be removed by introducing a suitable gas or fluid into the annular space between the liner and the overpack. The increased pressure in the annular space may push the liner in upon itself, thereby forcing out any excess gas in the liner. This may be accomplished in some embodiments by including a sterile outlet valve, for example, as part of the connector 122 that may allow any headspace to be removed and captured in an isolated part of the fill connector 122, for example. In some embodiments, the outlet valve may be a one-way valve that may only allow headspace gas to move out of the liner and may not allow it back into the liner. In other embodiments headspace may be removed at the dispense location prior to dispense.
[0041] Once the liner has been filled, and in some embodiments, the headspace has been substantially removed, the filling connector may reseal, as described above, thereby aseptically sealing the liner 104. In some cases, the system 100 may then be shipped to a second site, while in other cases, the filled system may be stored at the first site prior to dispense at a subsequent time at the first site.
[0042] Prior to dispense, dispense connector or dispense connector assembly may be coupled to the system 100. In some embodiments, the headspace gas may be removed at the dispense site just prior to dispense, for example, substantially in the same manner as explained above. Depending on the embodiment being used, in some cases the fill connector may also include features used for dispense, and therefore another connector may not need to be added to the system for dispense. In other embodiments, however, some portion, or all of the fill connector may be removed from the liner and overpack and the dispense connector and/or assembly may be coupled thereto for dispense. In still other embodiments, a dispense connector and/or dispense assembly may be coupled to or over the fill connector. In embodiments where the dispense connector may be coupled to or over the fill connector, a portion of the dispense connector may puncture the fill connector membrane 126 so as to allow access to the material of the liner for dispense. Once the dispense connector and/or dispense assembly have been operably coupled to the liner 104 and/or overpack 102, dispense may occur. Dispense may be by pressure dispense, as discussed in more detail below.
[0043] In some embodiments, after dispense, the dispense connector and/or assembly, the liner, and/or the overpack may be disposed of. However, in other embodiments, one or more of the dispense connector/assembly, liner, or overpack may be cleaned, sterilized, and reused in another cycle.
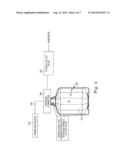
[0044] FIG. 3 generally shows how the system of the present disclosure may operate during liquid dispense, specifically pressure dispense, according to one embodiment. One end of a gas pressure in-line 306 may be connected to the pressurizing gas inlet fitting of a dispense connector 360, while the other end may be connected to a pressurized gas or fluid source 340. In one embodiment, the gas or fluid source 340 may be regulated to push pressurized gas or fluid into the area in the annular space between the inside wall of the container 320 and the outside wall of the liner 310. As can be seen, as the amount of gas or fluid increases in the space between the wall of the container 320 and the wall of the liner 310, the collapsible liner 312 will begin to collapse in upon itself, which will force the contents M of the liner 312 up through the dispense connector 360. In some embodiments, the dispense connector 360 may include a one-way dispense valve, while in other embodiments, a one-way outlet valve 362 may be distant from, but operably coupled to, the dispense connector 360. Pressurized gas or fluid may continue to be added until substantially all of the contents M of the liner 312 have been dispensed. Once liquid dispense has been completed and/or the liner 312 has been substantially emptied, the gas pressure in-line 306 may be removed from the pressurizing gas inlet of the dispense connector 360, which in some embodiments may also release the pressure gas in the annular space between the inside wall of the container 320 and the inside wall of the liner 310. As discussed above, in some embodiments, the liner and/or the connector may be removed and disposed of while the overpack may be cleaned, sterilized, and reused. In other embodiments the overpack may also be disposed of after a single use.
[0045] In some embodiments, the controlled and varied introduction of pressurized gas or liquid into the annular space between the inside of the container wall 320 and the outside of the liner wall 310 may be used to mix the contents of the liner. In some such embodiments, headspace may not be removed prior to mixing so as to allow room for mixing. A controlled cycle of pressurization and depressurization resulting in compression and relaxation of the liner may cause the contents of the liner to mix, for example. In use, this embodiment would allow for the sterile mixing of the contents of the liner without the need for impellers or paddles. Because introducing objects into the interior of the liner may increase the risk of contamination, not needing to introduce impellers or paddles into the liner may advantageously help minimize the risk of contamination. In other embodiments two containers may be used for mixing two or more materials.
[0046] The use of pressure dispense may he advantageous over methods currently used in relevant industries, such as dispense by peristaltic pumps. The use of pumps to dispense the contents of a liner may cause bubbling and stress on the material and the system, which may be undesirable because the purity of the contents of the liner may be crucial. The use of pressure dispense may help avoid or eliminate these problems. Further, in some cases a higher rate of dispense may be achieved by pressure dispense as opposed to pump dispense.
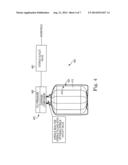
[0047] As explained above, in some embodiments, the entire storage and dispense system may be configured to be portable, as shown in FIG. 4. In some embodiments of a portable storage and dispense system 400 a pressure source 460 may be included as part of the dispense assembly 420. The pressure source 460 may be used to pressure dispense the contents of the liner 412 by forcing a gas, for example, into the annular space between the inner wall of the overpack 420 and the outer wall 410 of the liner 412. The pressure source 460, in some embodiments, may be connected directly to, or be integral with the dispense connector as shown in FIG. 4 by any suitable manner, while in other embodiments, the pressure source may be remotely connected to the dispense connector via any suitable means, for example tubing or hosing. In some embodiments, the pressure source 460 may comprise a carbon dioxide (CO2), nitrogen (N2), or other disposable compressed gas cartridge, for example. In still other embodiments, the pressure source may be generally directly detachably connected or integrally connected to the dispense assembly. While particular embodiments have been described herein, it will be understood that the pressure source may be positioned at any suitable place on and/or near the dispenser by any suitable means. While the pressure source has been discussed as a disposable cartridge for use with a disposable system, in other embodiments the pressure source may be any suitable or known pressure source to which the dispenser may be operably connected. In some embodiments, a user may activate the dispensing system by activating the pressure source. The pressure source may be activated, or turned "on," in a variety of suitable ways, for example but not limited to, via as button, flip-switch, sliding switch, or any other suitable actuator or combination of actuators. In some embodiments, such as in a fully disposable and/or recyclable embodiment, wherein the pressure source may comprise, for example, a CO2, N2, or other disposable compressed gas cartridge, the pressure source may be activated by activating the compressed gas cartridge as would be understood by those skilled in the art. In some embodiments, activating the pressure source may cause the contents of the liner or dispensing system to be dispensed. In still further embodiments, the contents of the liner or dispensing system may be dispensed continuously until the pressure source is deactivated or in the case of a compressed gas cartridge, for example, until the contents of the pressure source are entirely or substantially depleted, and/or until the contents of the liner are substantially depleted or are otherwise down to a desired level. In some embodiments the one-way dispense valve 480, as described above, may be coupled to the dispense connector 420, while in other embodiments the one-way dispense valve 480 may be remote from the dispense connector 420 but may be operably coupled thereto by any suitable means, such as tubing, for example,
[0048] In one embodiment, the portable pressure source 460 may be, or may be similar to, the portable pressure source and dispense systems sold under the brand name Tap-A-Draft by Sturman B G, LLC of Woodland Park, Colo., which have traditionally been used to maintain and dispense carbonated beverages. Generally, as the liquid stored in the container to which the Tap-A-Draft system is attached is poured through the Tap-A-Draft dispense connector, gas, such as CO2, Nitrous, or Argon, is allowed to enter the container to maintain the pressure within the container. Such portable pressure source and dispense systems, like the pressure dispense assemblies sold under the brand name Tap-A-Draft by Sturman B G, LLC, are further described in detail in U.S. Pat. No. 5,395,012; U.S. Pat. No. 5,443,186; U.S. Pat. No. 5,979,713; U.S. Pat. No. 6,036,054; U.S. Pat. No. 7845,522; U.S. Pat. No. D582,722; U.S. Publ. No. 2009/0242044; and U.S. Publ. No. 2011/0147406, each of which is hereby incorporated by reference herein in its entirety.
[0049] In another embodiment, the liner may first be filled with a solid material, for example, but not limited to, a peptide, API, etc. The solid may take up relatively little space within the liner. The liner-based system may then be stored, or in other cases, shipped to another site, whereupon the liner may be filled with a sterile liquid. In order to dissolve the solid in the liquid, the liner may be shaken or otherwise moved. The contents of the liner may then be dispensed or shipped to another location for dispense. In use, such an embodiment may be substantially similar to the embodiments described above. Using such an embodiment would allow a user to avoid having to transfer the solid material to a new container for sterile mixing, thereby minimizing the risk of contamination and saving time, labor, act any associated costs, for example. Alternatively, two or more containers may be used to mix the contents of one or more liners.
[0050] In some embodiments, a dispenser as disclosed herein may include more than one liner that my contain different materials. The dispense assembly may include a connector/cover that may connect to or align with the fitments of each of the liners. Alternatively, one liter may comprise two or more compartments that may contain different materials. The dispense assembly may draw the material from some or all of the liners and may mix the material prior to or at the time the material is dispensed from the dispenser, for example, such that the resulting material that is dispensed out of the dispenser may be a mixture of the contents of all or some of the liners. In some such embodiments, the one or more liners may not be completely filled, and/or headspace may not be removed, and/or headspace may be introduced into the liners to allow for mixing room in one or more liners.
[0051] This embodiment may also be advantageously used with substances that may be unstable and require a catalyst to cure. Accordingly, one liner may contain the substance and another liner may contain the catalyst, thereby allowing a mixture of both to be applied. In multiple liner embodiments, the ratio of the material of each liner that is included in the mixture may be controlled by a variety of means, for example, by varying the pressure applied, by varying the size of a terminal apparatus configured as a nozzle, by varying the size of the channels through the connector/cover, by varying the size of any diptube(s) used, or any other suitable method or combination of methods.
[0052] In some dispenser embodiments, the liner, overpack, and/or connectors may be configured for high flow dispense or dispense of contents of relatively higher viscosity. In one embodiment, such high flow or high viscous dispense can be achieved by providing larger orifice sizes in the liner, overpack, and/or dispensing assembly, which would allow for higher flow rates or the larger flow paths for materials with relatively higher viscosity.
[0053] Embodiments of liners of the present disclosure, in some cases, may be dispensed at pressures less than about 100 psi, or more preferably at pressures less than about 50 psi, and still more preferably at pressures less than about 20 psi, In some cases, the contents of the liners of some embodiments, however, may be dispensed at significantly lower pressures, as may be desirable, depending on the intended use or application involved.
[0054] In additional embodiments, a dispense assembly, including the connector, may also include control components to control the incoming gas and outgoing liquid. For example, a controller can be operably coupled to control components to control the dispense of the liquid from the liner. One or more transducers may also be included in some embodiments to sense the inlet and/or outlet pressure. In this regard, such control components may be utilized to detect when the liner is near empty. Means for controlling such dispense of fluid from the liner and determining when a liner nears empty are described for example in U.S. Pat. No. 7,172,096, entitled "Liquid Dispensing System," issued Feb. 6, 2007 and PCT Application Number PCT/US07/70911, entitled "Liquid Dispensing Systems Encompassing Gas Removal," with an international filing date of Jun. 11, 2007, each of which is hereby incorporated herein by reference in its entirety, and International Patent Application No. PCT/US2011/055558, previously incorporated by reference in its entirety.
[0055] In an additional or alternative embodiment, shown in FIG. 5, an empty detect mechanism may include a liner and overpack system 502 that may be operably connected to an indirect pressure dispensing assembly 504. The dispense assembly 504 may include a pressure transducer or sensor 506, as pressure solenoid or other control valve 508, and a vent solenoid or other control valve 510. A microcontroller may he used to control the pressure solenoid 508 and/or the vent solenoid 510. The outlet liquid pressure may be read and measured by the pressure transducer 506. If the pressure is too low, i.e. lower than a set value, the pressure solenoid 508 may be turned on for a period a time (Pon), thereby causing more pressurizing gas or other substance to be introduced into the annular space between the overpack and liner and raising the outlet liquid pressure. If the pressure is too high, i.e. higher than a predetermined value, the vent solenoid 510 may be turned on for a period of time (Pvent), somewhat relieving the pressure in the annular space between the overpack and liner, and thus the outlet liquid pressure. As may be seen in FIG. 6, as the contents of the liner near empty, the liquid pressure drops 610. The drop in liquid pressure triggers the pressure solenoid to turn on for a longer period of time. The increase in the time that the pressure solenoid is turned on (Pon) rises rapidly as the liner nears empty 612. Accordingly, the amount of time that the pressure valve is on (Pon) may be used to determine when the endpoint of the dispense has been reached.
[0056] Alternatively or additionally, the frequency of the on/off switching of the inlet pressure solenoid may be monitored. As indicated above, as the liner approaches empty, the inlet pressure will need to increase in order to maintain the constant liquid outlet pressure. The inlet pressure solenoid may thus switch on/off at a higher frequency as the liner nears empty to permit the required amount of pressurized gas into the annular space between the liner and the container. This frequency of the on/off switching can be a useful empty detect indicator. Empty detect mechanisms such as those disclosed herein, may help save time and energy, and consequently money.
[0057] After dispense is completed or substantially completed and the liner is empty or substantially empty, the end-user may dispose of the liner-based system, and/or recycle or reuse some or all of the liner-based system, including some or all of the closure/connector assembly. In order to assist in making the dispensers described herein more sustainable, the dispensers or one or more components thereof, including any overpack, liner(s), handles, etc., may be manufactured from biodegradable materials or biodegradable polymers, including but not limited to: polyhydroxyalkanoates (PHAs), like poly-3-hydroxybutyrate (PHB), polyhydroxyvalerate (PHV), and polyhydroxyhexanoate (PHH); polylactic acid (PLA); polybutylene succinate (PBS); polycaprolactone (PCL); polyanhydrides; polyvinyl alcohol; starch derivatives; cellulose esters, like cellulose acetate and nitrocellulose and their derivatives (celluloid); etc. Similarly, in some embodiments, and if suitable for the industry application, the dispensers or one or more components thereof may be manufactured from materials that can be recycled or recovered, and in some embodiments, used in another process by the same or a different end user, thereby allowing such end user(s) to lessen their impact on the environment or lower their overall emissions. For example, in one embodiment, the dispensers or one or more components thereof may be manufactured from materials that may be incinerated, such that the heat generated therefrom may be captured and incorporated or used in another process by the same or different end user. In general the dispensers or one or more components thereof may be manufactured from materials that can be recycled, or that may be converted into raw materials that may be used again.
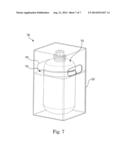
[0058] In one embodiment of the present disclosure a storage and dispense system 700 may include an additional optional packaging element 720, in which the liner and overpack 702 may be positioned. The packaging element 720 may be used to store, transport, and/or carry the liner and overpack 702, in some cases relatively easily. The packaging element 720 may generally be a box configured from a corrugated material, such as but not limited to cardboard. However, in other embodiments, the packaging element 720 may be comprised of any suitable material or combination of materials including paper, wood, metal, glass, or plastic, for example. The packaging element 720 may include one or more reinforcing elements 730 that may provide support and/or stability for the liner and overpack 702 disposed therein. A reinforcing element 730 may be positioned at any appropriate or desired height in the packaging element 720. For example, as may be seen in FIG. 7, one reinforcing element 730 may be provided near the top of the body of the overpack and liner 702. However, in other embodiments, one or more reinforcing elements may be positioned at other areas of the overpack, for example at the bottom of the overpack, or the middle of the overpack. In still another embodiment, the reinforcing element may generally fill substantially all of, or some portion of the space not taken up by the liner and overpack. The reinforcing element(s) 730 may be comprised of any suitable material or combination of materials, such as but not limited to the materials listed above for the packaging element. In some embodiments, the reinforcing element(s) 730 may be comprised of the same material as the remainder of the packaging element 720, although use of the same materials is not necessary. The packaging element 720 may also have one or more handles or handle slots/openings 740 that may make the packaging element 720 relatively easy to move and/or carry. The packaging element 720 may be any desired shape, and in some cases may be a generally rectangular box, as shown. A plurality of systems, such as those shown in FIG. 7, may be easily and conveniently packed for storage and/or shipping due to the rectangular box shape of the packaging element. Additionally, the packaging element may further protect the liner and overpack disposed therein from exposure, such as exposure to potentially harmful UV rays.
[0059] In some embodiments including a packaging element 720, the liner and overpack system may not include a handle or chime because the storage unit 720 may provide handle slots/openings and the support otherwise provided by the chime. Accordingly, a cost associated with the liner and overpack related to the handle and/or chime may be reduced or eliminated in such embodiments. Nonetheless, in other embodiments, the liner and overpack may still include a handle and/or chime in embodiments including a packaging element.
[0060] In still further embodiments, co-blow molded or nested preforms and liners, such as those described in International PCT Appl. No. PCT/US11/55560, titled, "Nested Blow Molded Liner and Overpack and Methods of Making Same," filed Oct. 10, 2011, which was previously incorporated herein, may be used to manufacture a dispenser having greater than two layers. Two or more separate materials may be filled into the spaces between the layers. The dispenser may be configured to mix the separate materials upon dispense.
[0061] In other embodiments, the dispensers of the present disclosure may include baffles, baffling features, or other discontinuities in the interior surface(s) thereof to retard settling of the suspended solids contained therein during storage and/or transportation.
[0062] The dispensers described herein may be configured as any suitable shape, including but not limited to square, rectangular, triangular or pyramidal, cylindrical, or any other suitable polygon or other shape. Differently shaped dispensers can improve packing density during storage and/or transportation, and may reduce overall transportation costs. Additionally, differently shaped dispensers can be used to differentiate dispensers from one another, such as to provide an indicator of the contents provided within the dispensers or to identify for which application or applications the contents are to be used, etc. In still further embodiments, the dispensers described herein may be configured as any suitable shape in order to "retrofit" the dispensers with existing dispense assemblies or dispense systems.
[0063] In some embodiments, the dispensers described herein may include symbols and/or writing that is molded into the dispensers or one or more components thereof. Such symbols and/or writing may include, but is not limited to names, logos, instructions, warnings, etc. Such molding may be done during or after the manufacturing process of the dispensers or one or more components thereof. In one embodiment, such molding may be readily accomplished during the fabrication process by, for example, embossing the mold for the dispensers or one or more components thereof. The molded symbols and/or writing may be used, for example, to differentiate products.
[0064] In some embodiments, one or more colors and/or absorbant materials may be added to the materials of the dispensers or one or more components thereof during or after the manufacturing process to help protect the contents of the dispensers from the external environment, to decorate the dispensers, or to use as an indicator or identifier of the contents within the dispensers or otherwise to differentiate multiple dispensers, etc. Colors may be added using, for example, dyes, pigments, nanoparticles, or any other suitable mechanism. Absorbant materials may include materials that absorb ultraviolet light, infrared light, and/or radio frequency signals, etc.
[0065] Similarly, in some embodiments, the dispensers or one or more components thereof may be provided with different textures or finishes. As with color and molded symbols and/or writing, the different textures or finishes may he used to differentiate products, to provide an indicator of the contents provided within the dispensers, or to identify for which application or applications the contents are to be used etc. In one embodiment, the texture or finish may be designed to be a substantially non-slip texture or finish or the like, and including or adding such a texture or finish, to the dispensers or one or more components thereof may help improve graspability or handling of the packaging system, and thereby reduce or minimize the risk of dropping of the dispensers. The texture or finish may be readily accomplished during the fabrication process by, for example, providing a mold for the dispensers or one or more components thereof with the appropriate surface features. In other embodiments, the molded dispensers may be coated with the texture or finish. In some embodiments, the texture or finish may be provided on substantially the entire dispenser or substantially the entirety of one or more components thereof. However, in other embodiments, the texture or finish may be provided on only a portion of the dispenser or a portion of one or more components thereof.
[0066] Similarly, in some embodiments, the exterior and/or interior walls of the dispensers or one or more components thereof may have any suitable coating provided thereon. The coating may increase material compatibility, decrease permeability, increase strength, increase pinhole resistance, increase stability, provide anti-static capabilities or otherwise reduce static, etc. Such coatings can include coatings of polymers or plastic, metal, glass, adhesives, etc. and may be applied during the manufacturing process by, for example coating a preform used in blow-molding, or may be applied post manufacturing, such as by spraying, dipping, filling, etc.
[0067] In some embodiments, the dispensers may include one or more handles. The one or more handles can be of any shape or size, and may be located at any suitable position of the dispensers. Types of handles can include, but are not limited to, handles that are located at the top and/or sides; are ergonomic; are removable or detachable; are molded into the dispensers or are provided after fabrication of the dispensers (such as by, for example, snap fit, adhesive, riveting, screwed on, bayonet-fit, etc.); etc. Different handles and/or handling options can be provided and may depend on, for example but not limited to, the anticipated contents of the dispensers, the application for the dispensers, the size and shape of the dispensers, the anticipated dispensing system for the dispensers, etc.
[0068] In some embodiments, the dispensers may include two or more layers, such as an overpack and a liner, multiple overpacks, or multiple liners. In further embodiments, a dispenser may include at least three layers, which may help ensure enhanced containment of the contents therein, increase structural strength, and/or decrease permeability, etc. Any of the layers may be made from the same or different materials, such as but not limited to, the materials previously discussed herein.
[0069] In some embodiments, and if suitable for the industry application, the dispensers or one or more components thereof may be manufactured from materials that can be recycled or recovered, and in some embodiments, used in another process by the same or a different end user, thereby allowing such end user(s) to lessen their impact on the environment or lower their overall emissions. For example, in one embodiment, the dispensers or one or more components thereof may be manufactured from materials that may be incinerated, such that the heat generated therefrom may be captured and incorporated or used in another process by the same or different end user. In general the dispensers or one or more components thereof may be manufactured from materials that can be recycled, or that may be converted into raw materials that may be used again.
[0070] In some embodiments, structural features may be designed into the dispensers that add strength and integrity to the dispensers or one or more components thereof. For example, the base (or chime in some embodiments), top, and sides of the dispensers may all be areas that experiences increased shake and external forces during filling, transportation, installation, and use (e.g., dispensing). Accordingly, in one embodiment, added thickness or structural edifices e.g., bridge tressel design) may be added to support stressed regions of the dispensers, which can add strength and integrity to the dispensers. Furthermore, any connection region in the dispensers may also experience increased stress during use. Accordingly, any of these such regions may include structural features that add strength through, for example, increased thickness and/or specifically tailored designs. In further embodiments, the use of triangular shapes could be used to add increased strength to any of the above described structures; however, other designs or mechanical support features may be used.
[0071] In some embodiments, the dispensers or one or more components thereof, including any overpack or liner(s), may include reinforcement features, such as but not limited to, a mesh, fiber(s), epoxy, or resin, etc. that may be integrated or added to the dispensers or one or more components thereof, or portions thereof, in order to add reinforcement or strength. Such reinforcement may assist in high pressure dispense applications, or in applications for dispensing high viscosity contents or corrosive contents.
[0072] In some embodiments, the dispensers may include level sensing features or sensors. Such level sensing features or sensors may use visual, electronic, ultrasonic, or other suitable mechanisms for identifying, indicating, or determining the level of the contents stored in the dispensers. For example, in one embodiment, the dispensers or a portion thereof may be made from a substantially translucent or transparent material that may be used to view the level of the contents stored therein.
[0073] In further embodiments, flow metering technology may be integrated into or operably coupled with the connectors for a direct measurement of material being delivered from the packaging system to a down stream process. A direct measurement of the material being delivered could provide the end user with data which may help ensure process repeatability or reproducibility. In one embodiment, the flow meter may provide an analog or digital readout of the material flow. The flow meter, or other component of the system, can take the characteristics of the material (including but not limited to viscosity and concentration) and other flow parameters into consideration to provide an accurate flow measurement, Additionally, or alternatively, the flow meter can be configured to work with, and accurately measure, a specific material stored and dispensed from the dispenser. In one embodiment, the inlet pressure can be cycled, or adjusted, to maintain a substantially constant outlet pressure or flow rate.
[0074] The various shipping and dispensing system embodiments of the present disclosure have several advantages over traditional packaging systems. Many of the advantages have been noted throughout the application and include, but are not limited to: sterility of the contents from the time of fill to complete dispense; substantially reduced or eliminated germ ingress during filling; longer shelf life of the contents of the liner; portable dispense; enables sterile dispense automation; easier portable dispense, which can eliminate hand-pump dispense. Other advantages will be recognized by those skilled in the art and may vary from industry application to industry application.
[0075] In the foregoing description various embodiments of the invention have been presented for the purpose of illustration and description. They are not intended to be exhaustive or to limit the invention to the precise form disclosed. Obvious modifications or variations are possible in light of the above teachings. The embodiments were chosen and described to provide the best illustration of the principals of the invention and its practical application, and to enable one of ordinary skill in the art to utilize the invention in various embodiments and with various modifications as are suited to the particular use contemplated. All such modifications and variations are within the scope of the invention as determined by the appended claims when interpreted in accordance with the breadth they are fairly, legally, and equitably entitled.
User Contributions:
Comment about this patent or add new information about this topic: