Patent application title: RNA-DEPENDENT DNA POLYMERASE FROM GEOBACILLUS STEAROTHERMOPHILUS
Inventors:
Bert C. Lampson (Johnson City, TN, US)
Jashree Velore (Kingsprot, TN, US)
IPC8 Class: AC12N912FI
USPC Class:
435194
Class name: Enzyme (e.g., ligases (6. ), etc.), proenzyme; compositions thereof; process for preparing, activating, inhibiting, separating, or purifying enzymes transferase other than ribonuclease (2.) transferring phosphorus containing group (e.g., kineases, etc.(2.7))
Publication date: 2011-01-27
Patent application number: 20110020897
Claims:
1. A purified or isolated polynucleotide comprising a nucleic acid
selected from the group consisting of:a) SEQ ID NO:1;b) a nucleic acid
encoding the amino acid sequence of SEQ ID NO: 2; andc) a nucleic acid
which hybridizes with the nucleic acid of b) under stringent conditions
and encodes a polypeptide having a similar reverse transcriptase activity
to that of the polypeptide comprising SEQ ID NO:2.
2. A vector comprising the polynucleotide of claim 1.
3. A host cell comprising the vector of claim 2.
4. A method of producing a reverse transcriptase, the method comprising:a) introducing the vector of claim 2 into an expression system andb) expressing the polypeptide product encoded by the polynucleotide of the vector.
5. The method of claim 4 wherein the expression system is a host cell.
6. The method of claim 4 wherein the expression system is a cell-free expression system.
7. A method of producing a reverse transcriptase, the method comprising:a) culturing a host cell comprising the vector of claim 6;b) expressing a protein encoded by the polynucleotide; andc) isolating the protein from the host cell.
8. The method of claim 7, wherein the host cell is E. coli.
Description:
[0001]This application is a divisional application of and claims the
benefit of priority of earlier-filed U.S. patent application Ser. No.
10/797,262, filed Mar. 10, 2004.
FIELD OF THE INVENTION
[0002]The present invention relates to DNA and protein sequences encoding heat-stable polymerase enzymes, expression vector constructs for recombinant production of such enzymes, and methods of use for heat-stable polymerases. More specifically, the invention relates to a substantially pure thermostable RNA-directed DNA polymerase (i.e., reverse transcriptase) isolated from Geobacillus stearothermophilus. The invention also relates to the cloning and expression of the G. stearothermophilus RNA-directed DNA polymerase in Escherichia coli, to DNA molecules containing the cloned gene, and to hosts which express said genes.
BACKGROUND OF THE INVENTION
[0003]Heat-stable enzymes are essential tools of molecular biology that have proven invaluable in DNA cloning, sequencing, and random mutagenesis. The most often used heat-stable polymerases are the DNA polymerases utilized in polymerase chain reactions (PCR) reactions. These are often coupled with reverse transcriptases in RT-PCR, when an RNA molecule is used as a template to form a complementary DNA (cDNA) molecule, and that cDNA sequence is amplified by polymerase chain reaction (PCR). cDNA synthesis is most often done using reverse transcriptase enzymes of viral origin, with the reactions being performed at temperatures below about 50° C., which is optimal for these enzymes. There are, however, distinct advantages to synthesizing cDNA at temperatures above 50° C., since higher temperatures melt the secondary structures that can form in the RNA template and block further processivity of the transcriptase enzyme. A reverse transcriptase that remains stable and active at higher temperatures is especially useful for cDNA synthesis in combination reverse transcription/polymerase chain reaction (RT-PCR) reactions, as well as in other applications. Since higher temperatures can eliminate the secondary structures that may form in the RNA template, the length of the cDNA product can be extended. Higher temperatures also reduce the amount of non-specific annealing of PCR primers during cDNA synthesis, increasing specificity and amplification of cDNA. Higher temperatures can also melt the 3' end of a mismatched primer, inhibiting further synthesis from the primer and limiting incorporation of mismatched bases in the cDNA product.
[0004]Retroviral reverse transcriptases generally have three potential enzymatic activities associated with them: an RNA-directed DNA polymerase, a DNA-directed DNA polymerase, and an RNAse H activity. Therefore, when retroviral RTs are used to copy RNA or DNA, an RNAse inhibitor must often be included in the reaction to minimize RNAse effects. Unfortunately, this can also inhibit the action of other enzymes. Furthermore, retroviral enzymes are typically most effective at temperatures at or below 50° C.
[0005]To produce cDNA at higher temperatures, a DNA-dependent, DNA polymerase Tth pol, from the thermophilic bacterium Thermus thermophilus, has been used. Although it is not technically classified as a reverse transcriptase, it will reverse transcribe RNA at high temperatures in an RT-PCR reaction. Manganese chloride (MnCl2) must be added to the reaction to boost efficiency, but this also reduces the fidelity of cDNA synthesis so that an added step is necessary to remove it prior to PCR amplification.
[0006]An RNase H-deficient Avian Myeloblastosis Virus reverse transcriptase (AMV-RT) has been used in RT-PCR reactions, the RNase H-deficient enzyme being more thermostable than the native enzyme. This RT does not degrade the RNA template, increasing the amount of full-length cDNA product that can be produced. cDNA synthesis with this enzyme is generally performed at 50° C., but larger amounts of the enzyme and substrate dNTPs are required for cDNA synthesis at higher temperatures, and synthesis from long RNA templates is often truncated, even at the higher temperature. AMV-RT also comprises two polypeptide chains (α and β), making it more difficult and expensive to produce as a recombinant product. When expressed in E. coli, for example, the end product is not appropriately modified to provide a fully active enzyme. An RT from Moloney Murine Leukemia Virus (MMLV) is also commercially available for use in reverse transcription reactions. Invitrogen's (Carlsbad, Calif.) SuperscriptII® RT is a point mutant of M-MLV-RT. According to the product literature, SuperscriptII® RT can be used at temperatures up to 50° C., and native M-MLV-RT can be used at temperatures up to 42° C.
[0007]Although there are currently enzymes which can be used in RT-PCR and other similar types of reactions, there is still a significant need for an improved RT that remains active, accurate, and stable at high temperatures and can be used in a one-step reaction system for RT-PCR.
SUMMARY OF THE INVENTION
[0008]The present invention relates to a novel isolated polynucleotide sequence from the genome of Bacillus stearothermophilus (Geobacillus stearothermophilus) SEQ ID NO 1, and a novel amino acid sequence (SEQ ID NO 2) encoded by the polynucleotide sequence, the corresponding amino acid sequence comprising a heat-stable protein with reverse transcriptase activity. The invention provides a novel enzyme, Tirt (thermostable intron reverse transcriptase), which has reverse transcriptase activity and retains that activity at temperatures of up to about 75° C.
[0009]The invention further relates to a method of using such a reverse transcriptase enzyme to facilitate DNA cloning, and at least one type of kit with prepared reagents to perform the necessary reactions for RT-PCR. The invention also provides a method of performing reverse transcription of an RNA sequence to form a cDNA sequence, and further provides a method for performing RT-PCR with the reverse transcription reaction being performed at temperatures elevated to about 75° C.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010]FIG. 1 is a multiple amino acid sequence alignment comparison of the Tirt sequence with group II intron-encoded ORFs. The open reading frame (ORF) designated Bst 803-2065 encodes the Tirt protein and was cloned from the genome of G. stearothermophilus strain 10. The amino acid sequence of Tirt is compared with the amino acid sequence from three related ORFs encoded by group II introns from bacteria. These sequences include an ORF from Bacillus halodurans (ATCC accession no. NC002570.1), an RT-maturase protein from Clostridium acetobutylicum (ATCC accession no. NC003030), and a group II intron protein from Pseudomonas alcaligenes (ATCC accession no. U77945). Common to RTs, the Tirt sequence contains most of the highly conserved amino acids that fall into seven distinct domains (underlined sequences lableled I-VII). These seven conserved domains correspond to important structural regions proposed to be shared by all RTs. In addition, the Tirt sequence also contains most of the highly conserved amino acids contained in a region of the protein designated "X". This domain is associated with the maturase function of group II intron encoded proteins. The most conserved amino acids found in the "X" region of bacterial group II intron proteins (according to Zimmerly et al. Nucleic Acids Res. (2000) 29: 1238-1250) are shown above the alignment in italics.
[0011]FIG. 2 is the polynucleotide sequence of the tirt ORF from G. stearothermophilus. The location of DNA sequences used to design the primers Bst755, Bst1396, Bst2015, and Bst2198 are shown by the designated arrows. These primers were used to amplify the tirt-ORF for cloning.
[0012]FIG. 3. is a photograph of a polyacrylamide protein gel (stained with Coomassie blue) illustrating over-expression of the Tirt fusion protein in E. coli. E. coli cells (BL21) containing the plasmid pTirt#16 were induced with IPTG to over-express the Tirt fusion protein via the T7 promoter system. Total protein extracts prepared from induced and non-induced cells were analyzed by polyacrylamide gel electrophoresis. A prominent protein band migrating at about 48 kD is apparent from induced cells (lane 4, indicated by arrow) but absent from non-induced cells (lane 3) and also absent from control cells containing just the plasmid vector alone (lane 1, uninduced and lane 2, induced cells). Most of the over-expressed protein band appears in the insoluble cell fraction (lane 8, soluble cell fraction versus lane 9, insoluble cell fraction). No 48 kD protein band appears in control cells containing just the expression plasmid (lane 6, soluble cell fraction and lane 7, insoluble cell fraction).
[0013]FIG. 4. is a photograph of SDS-PAGE analysis of affinity column purification of the Tirt fusion protein. The soluble fraction from a cell extract expressing the Tirt fusion protein was loaded onto a nickel ion affinity column. A single-step elution of the polyhistidine tagged fusion protein yielded a partially purified fraction containing the 48 kD protein band (arrow). Column fractions 3-6 were analyzed by electrophoresis on a polyacrylamide protein gel stained with coomassie blue (lanes 6-9, respectively). The RT activity of each column fraction was stabilized by dialysis into buffer A (lanes 11-14). Lane 3 contains the insoluble cell fraction and lane 4 contains the soluble cell fraction that was loaded onto the column. Lane 5 contains the column flow-through. Lanes 1 and 2 contain a total cell extract from uninduced and induced cells respectively. Lane S contains a protein standard with the size (in kD) of each known protein indicated on the left.
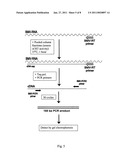
[0014]FIG. 5. is a schematic diagram showing the steps involved in the PERT assay used to detect RT activity from cell fractions. Column purified fractions of the Tirt protein serve as a source of RT activity and are added to a reaction mix containing BMV-RNA as a template. Using a specific primer (BMV-RT primer) the activity of the Tirt protein synthesizes a cDNA copy of the BMV-RNA template. A small region of the cDNA copy is then amplified (using specific primers) by the PCR to produce a final amplified product of 168 bp.
[0015]FIG. 6. is a picture of a polyacrylamide gel illustrating the presence of RT activity in a purified preparation of the Tirt fusion protein. Purified preparations of the Tirt protein were added to the PERT assay as a source of RT. Various control reactions were also run with the PERT assay. The production of a 168 bp amplified DNA indicates RT activity. (A): Lane S, 100 bp molecular weight marker; lane 1, affinity purified column fraction #2; lane 2, column fraction #2 after dialysis in buffer A; lane 3, the same as lane 2 but supplemented with MMLV-RT; lane 4, MMLV-RT in RT buffer (serving as a positive control); lane 5, MMLV-RT but with one of the PCR primers absent from the reaction (a negative control). (B): Lane S, 100 bp molecular weight markers; lanes 1-3 contain column fractions #2-4 respectively after dialysis in buffer A; lane 4, MMLV-RT in RT buffer (positive control); lane 5, MMLV-RT plus RNase A added to the reaction; lane 6, no exogenous (H2O) source of RT added to the reaction; lane 7, column fraction #2 (dialyzed in buffer A) plus RNase A.
[0016]FIG. 7. is a picture of a polyacrylamide gel illustrating the presence of heat stable RT activity associated with purified Tirt protein. The PERT assay was used to detect RT activity of the purified Tirt protein under various temperature conditions. (A) Column purified fractions of Tirt (dialyzed in buffer A) were pooled and incubated at the indicated temperatures for 15 minutes, then added to the PERT assay. Lane S, 100 bp molecular weight marker; lane 1, MMLV-RT with one PCR primer missing (negative control); lane 2, purified Tirt protein with no heat treatment; lane 3, purified Tirt protein heated to 65° C.; lane 4, purified Tirt protein heated to 75° C.; lane 5, commercial preparation of MMLV-RT with no heat treatment; lane 6, MMLV-RT heated to 65° C.; lane 7, MMLV-RT heated to 75° C. (B) The PERT assay itself was run at three different temperatures with either the purified Tirt protein or MMLV-RT added as a source of RT. Lane S, 100 bp molecular weight marker; lane 1, a control reaction containing purified Tirt, but no RNA template was added (negative control); lanes 2-4, are the PERT assay containing the purified Tirt protein incubated at 37° C., 50° C. and 68° C. respectively; lanes 5-7, are the PERT assay containing MMLV-RT incubated at 37° C., 50° C., and 68° C. respectively.
[0017]FIG. 8. is a map of the pTirt#16 plasmid, for the over-expression and purification of the Tirt protein. (A) The restriction endonuclease map of plasmid pTirt#16 indicates the location of the genomic DNA (hatched rectangle) cloned from G. stearothermophilus that contains the Tirt ORF. The Tirt ORF was cloned adjacent to a T7 promoter and in frame with a poly-histidine ([His]6) tag to allow over-expression of the Tirt protein and simplify purification. (B) A partial DNA sequence of the pTirt#16 plasmid shows the junction region where the Tirt ORF is fused in frame with the poly-histidine tag element (underlined sequence) down stream of the T7 promoter, creating a 35 amino acid extension at the N-terminus of the Tirt ORF that includes six consecutive histidine amino acids.
DETAILED DESCRIPTION
[0018]The present invention seeks to overcome the shortcomings of the prior art by providing a thermostable RNA-directed DNA polymerase as found in the thermophilic bacterium Geobacillus stearothermophilus (also known as Bacillus stearothermophilus). Geobacillus stearothermophilus is an organism isolated from the formation waters of Russian oilfields. A DNA-dependent DNA polymerase from this organism has previously been described. The inventors describe here the discovery of a functional RNA-dependent DNA polymerase enzyme encoded within the genome of G. stearothermophilus. While investigating putative coding regions within the sequence data, the inventors discovered a sequence having characteristics similar to those of type II introns, which have reverse transcriptase activity. In methods to be described herein, the inventors isolated DNA encoding a protein they have designated as Tirt (for "thermostable intron reverse transcriptase"), constructed an expression vector for its overexpression, and demonstrated that it has reverse transcriptase activity even after being heated to about 75° C.
[0019]The DNA sequence shown in FIG. 2 is from the incomplete genomic DNA sequence of Bacillus (Geobacillus) stearothermophilus strain 10 obtained from the Genome Sequencing Project, Advanced Center for Genome Technology, University of Oklahoma (Experimental Program to Stimulate Competitive Research Grant #EPS-9550478). The inventors have discovered that the sequence encodes a 420-amino acid ORF containing the Tirt protein.
[0020]Retroelements are genetic elements that code for a reverse transcriptase and employ the process of reverse transcription in some stage of their replication or mobility. A large variety of these retroelements are found in nearly every type of eukaryotic organism. They include some RNA viruses, DNA viruses, transposons, introns, and even mitochondrial plasmids. Bacteria also contain RT-encoding genetic elements (retro-elements) that fall into two basic types. The group II introns, found in a variety of bacteria, contain a reverse transcriptase region as part of the intron-encoded ORF. A retron, on the other hand, produces an unusual satellite DNA called msDNA. The inventors compared the amino acid sequence from ten different retron RTs with the amino acid sequence from five different bacterial group II intron ORFs by multiple sequence alignment (Clustal W alignment). They then used the multiple sequence alignment to generate a consensus amino acid sequence for RTs found in bacteria. The bacterial consensus sequence was used as the query sequence in a BLAST search of both the GenBank database as well as the bacterial genomes database that contains both completed, as well as, unfinished bacterial species. These searches revealed several ORFs with similarity to the consensus sequence that had not been previously described. This included a 420-amino acid ORF from the unfinished genome sequence of G. stearothermophilus.
[0021]The amino acid sequence of this G. stearothermophilus ORF was further analyzed by comparison with other RTs and found to be strongly similar to group II intron ORFs from both bacteria and mitochondria (FIG. 1). The amino acid sequences of RTs are generally highly variable. However, multiple amino acid sequence alignments demonstrate the presence of a few highly conserved amino acids shared among all RTs. These conserved amino acids fall into seven domains (designated I-VII, FIG. 1) that correspond to conserved secondary structures within the folded RT protein. A short distance beyond domain VII is an additional conserved region designated domain "X", that is found only in group II intron encoded proteins. Domain X is associated with the maturase function found in group II intron ORFs. Although domain X is not well conserved among bacterial group II intron proteins, the G. stearothermophilus ORF appears to contain most of the conserved amino acids of domain X shared among bacterial group II introns (FIG. 1). Thus, the G. stearothermophilus ORF appears to have most of the highly conserved amino acids present in both the RT region and the maturase region (or domain X) of group II intron proteins (FIG. 1). For this reason the G. stearothermophilus ORF is clearly not a retron type RT and was thus designated tirt for thermostable intron reverse transcriptase. Some proteins encoded by group II introns also contain a third, zinc finger domain that imparts an endonuclease activity on this multifunctional protein. However, based on sequence comparisons, this endonuclease domain appears to be absent from the tirt ORF.
[0022]An "isolated" or "purified" nucleic acid or an "isolated" or "purified" polypeptide is a nucleic acid or polypeptide that, generally through human intervention, exists apart from its native environment and is therefore not a product of nature. An isolated nucleic acid or polypeptide may exist in a purified form or may exist in a non-native environment such as, for example, a transgenic host cell.
[0023]As used herein, the term "protein" is intended to include mimetics and the term "amino acid" is intended to include L-form, D-form, and modified amino acids. These substitutions may be made by someone of skill in the art, using the known structural similarities between the molecules. The amino acid sequence is also intended to include any peptide or protein sequence that may include additional amino acids either N-terminal or C-terminal to the listed sequence, or both. The term "Tirt protein" is intended to include variants or biologically active fragments of the polypeptide.
[0024]It is well known in the art that a single amino acid may be encoded by more than one nucleotide codon, and that the nucleotide sequence may be modified to produce an alternate nucleotide sequence that encodes the same peptide. Therefore, alternate embodiments of the present invention include alternate DNA sequences encoding peptides containing the amino acid sequences as previously described. DNA sequences encoding peptides containing the claimed amino acid sequence include DNA sequences which encode any combination of the claimed sequence and any other amino acids located N-terminal or C-terminal to the claimed amino acid sequence.
[0025]It is to be understood that amino acid and nucleic acid sequences may include additional residues, particularly N- or C-terminal amino acids or 5' or 3' nucleotide sequences, and still be essentially as set forth in the sequences disclosed herein, as long as the sequence confers heat-stable RNA-dependent DNA polymerase activity upon the polypeptide or protein moiety of the expressed protein.
[0026]The term "nucleic acid" refers to deoxyribonucleotides or ribonucleotides and polymers thereof in either single- or double-stranded form, composed of monomers containing a sugar, phosphate and a base that is either a purine or pyrimidine (i.e., nucleotides). Unless specifically limited, the term includes nucleic acids containing known analogs of natural nucleotides that have similar binding properties as the reference nucleic acid and are metabolized in a manner similar to naturally occurring nucleotides. Unless otherwise indicated, a particular nucleic acid sequence also implicitly encompasses conservatively modified variants thereof (e.g., degenerate codon substitutions) and complementary sequences as well as the indicated reference sequence.
[0027]Additional nucleic acid bases may be added either 5' or 3' to the Tirt ORF, and may be combined with other DNA sequences, such as promoters, polyadenylation signals, additional restriction enzyme sites, multiple cloning sites, other coding segments, and the like. Therefore, overall length of such a polynucleotide may vary considerably. In a method described by the present invention, a nucleotide sequence as shown in FIG. 2 is inserted into a protein expression vector to produce a protein which can be used to synthesize a DNA copy of an RNA molecule. The DNA can then be amplified to form multiple copies, at temperatures elevated to about 68 to about 75 degrees Celsius.
[0028]"Control sequences" are those DNA sequences that are necessary for the expression of a protein from a polynucleotide sequence containing such a sequence, operably linked to the polynucleotide sequence encoding the protein. These sequences include prokaryotic sequences such as, for example, promoters, operators, and ribosome binding sites, and eukaryotic sequences such as, for example, promoters, enhancers, and polyadenylation signals. "Expression systems" are DNA sequences (such as, for example, plasmids) appropriate for expression of a target protein in a particular host cell, these sequences comprising appropriate control sequences for protein expression in the host cell operably linked to the polynucleotide sequence encoding the target protein.
[0029]It is to be understood that a "variant" of a polypeptide is not completely identical to the native protein. A variant Tirt protein, for example, can be obtained by altering the amino acid sequence by insertion, deletion or substitution of one or more amino acids. The amino acid sequence of the protein can be modified, for example, by substitution to create a polypeptide having substantially the same or improved qualities as compared to the native polypeptide. The substitution may be a conserved substitution. A "conserved substitution" is a substitution of an amino acid with another amino acid having a side chain that is similar in polar/nonpolar nature, charge, or size. The 20 essential amino acids can be grouped as those having nonpolar side chains (alanine, valine, leucine, isoleucine, proline, phenylalanine, and tryptophan), uncharged polar side chains (methionine, glycine, serine, threonine, cystine, tyrosine, asparagine and glutamine), acidic side chains (aspartate and glutamate), and basic side chains (lysine, arginine, and histidine). Conserved substitutions might include, for example, Asp to Glu, Asn, or Gln; His to Lys, Arg or Phe; Asn to Gln, Asp or Glu; and Ser to Cys, Thr or Gly. Alanine, for example, is often used to make conserved substitutions.
[0030]To those of skill in the art, variant polypeptides can be obtained by substituting a first amino acid for a second amino acid at one or more positions in the polypeptide structure in order to affect biological activity. Amino acid substitutions may, for example, induce conformational changes in a polypeptide that result in increased biological activity.
[0031]Those of skill in the art may also make substitutions in the amino acid sequence based on the hydrophilicity index or hydropathic index of the amino acids. A variant amino acid molecule of the present invention, therefore, has less than one hundred percent, but at least about fifty percent, and preferably at least about eighty to about ninety percent amino acid sequence homology or identity to the amino acid sequence of a polypeptide comprising SEQ ID NO 2, or a polypeptide encoded by SEQ ID NO 1. Therefore, the amino acid sequence of the variant Tirt protein corresponds essentially to the native Tirt protein amino acid sequence. As used herein, "corresponds essentially to" refers to a polypeptide sequence that will elicit a similar biological and enzymatic activity to that generated by a Tirt protein comprising SEQ ID NO 2, such activity being at least about 70 percent that of the native Tirt protein, and more preferably greater than 100 percent of the activity of the native Tirt protein.
[0032]A variant of the Tirt protein may include amino acid residues not present in a corresponding Tirt protein comprising SEQ ID NO 2, or may include deletions relative to the Tirt protein comprising SEQ ID NO 2. A variant may also be a truncated "fragment," as compared to the corresponding protein comprising SEQ ID NO 2, the fragment being only a portion of the full-length protein.
[0033]In a preferred embodiment of the present invention, a protein expression vector is genetically engineered to incorporate a DNA sequence encoding the Tirt ORF and appropriate control sequences comprising, for example, transcriptional and translational sequences such as promoter and polyadenylation sequences, to produce a functional Tirt protein.
[0034]The isolated polynucleotide and protein of the present invention can be used in a variety of applications, including, but not limited to assays to confirm the presence of viral infection in tissue samples, preparation of cDNA copies of isolated or cellular RNAs, and real-time or standard RT-PCR for genetic analysis. These and other uses known to, or developed by, those of skill in the art are made possible by the discovery of the thermostable intron reverse transcriptase of the present invention.
[0035]Expression vectors may be chosen from among those readily available for prokaryotic or eukaryotic expression systems. Expression system vectors, which incorporate the necessary regulatory elements for protein expression, as well as restriction endonuclease sites that facilitate cloning of the desired sequences into the vector, are known to those of skill in the art. A number of these expression vectors are commercially available. In one preferred embodiment of the present invention, the expression vector is pET28 (Novagen, Madison, Wis.).
[0036]An expression vector host cell system can be chosen from among a number of such systems that are known to those of skill in the art. In one embodiment of the invention, the protein can be expressed in E. coli. In alternate embodiments of the present invention, the enzyme may be expressed and purified using other bacterial expression systems, viral expression systems, eukaryotic expression systems, or cell-free expression systems. Cellular hosts used by those of skill in the art for expression of various proteins include, but are not limited to, Bacillus subtilis, yeast such as Saccharomyces cerevisiae, Saccharomyces carlsbergenesis, Saccharomyces pombe, and Pichia pastoris, as well as mammalian cells such as 3T3, HeLa, and Vero. The expression vector chosen by one of skill in the art will include promoter elements and other regulatory elements appropriate for the host cell or cell-free system in which the recombinant DNA sequence encoding the enzyme will be expressed. In mammalian expression systems, for example, suitable expression vectors can include DNA plasmids, DNA viruses, and RNA viruses. In bacterial expression systems, suitable vectors can include plasmid DNA and bacteriophage vectors.
[0037]One group of vectors that can be used to express and facilitate purification of the protein include those vectors that encode the polyhistidine (6×His) sequence and an epitope tag to allow rapid purification of the fusion protein with a nickel-chelating resin, along with protein detection with specific antibodies to detect the presence of the secreted protein. An example of such a vector for expression in mammalian cells is the pcDNA3.1/V5-His-TOPO eukaryotic expression vector (Invitrogen). In this vector, the fusion protein can be expressed at high levels under the control of a strong cytomegalovirus (CMV) promoter. A C-terminal polyhistidine (6×His) tag enables fusion protein purification using nickel-chelating resin. Secreted protein produced by this vector can be detected using an anti-His (C-term) antibody.
[0038]Since Tirt is a bacterial protein, bacterial expression systems are particularly suited for expression of the Tirt protein as described by the present invention. Such systems include, for example, the pMAL system (New England Biolabs, Beverly, Mass.) which utilizes a maltose binding protein fusion to facilitate purification, and the Impact-CN Protein Fusion and Purification System (New England Biolabs).
[0039]A baculovirus expression system can be used for production of a target protein such as the enzyme of the present invention. A commonly used baculovirus is AcMNPV. Cloning of the target protein DNA can be accomplished by using homologous recombination. The target protein DNA sequence is cloned into a transfer vector containing a baculovirus promoter flanked by baculovirus DNA, particularly DNA from the polyhedrin gene. This DNA is transfected into insect cells, where homologous recombination occurs to insert the target protein into the genome of the parent virus. Recombinants are identified by altered plaque morphology.
[0040]Proteins as described above can also be produced in the method of the present invention by mammalian viral expression systems. The Sindbis viral expression system, for example, can be used to express proteins at high levels. Sindbis vectors have been described, for example, in U.S. Pat. No. 5,091,309 (Schlesinger et al.), incorporated herein by reference. Sindbis expression vectors, such as pSinHis (Invitrogen, Carlsbad, Calif.) can be used to express the Tirt protein under the direction of the subgenomic promoter PSG. In vitro transcribed RNA molecules encoding the fusion protein and the Sindbis proteins required for in vivo RNA amplification can be electroporated into baby hamster kidney (BHK) cells using methods known to those of skill in the art. Alternatively, the RNA encoding the Tirt protein and Sindbis proteins required for in vivo RNA amplification can be cotransfected with helper RNA that permits the production of recombinant viral particles. Viral particles containing genetic material encoding the fusion protein can then be used to infect cells of a wide variety of cell types, including mammalian, avian, reptilian, and Drosophila. Fusion protein expressed from the pSinHis (Invitrogen) vector can be detected with antibody to an Anti-Xpress® epitope encoded by the vector sequence. The pSinHis vector also includes a polyhistidine tag which provides a binding site for metal-chelating resins to facilitate purification of the expressed fusion protein. Furthermore, an enterokinase cleavage site located between the histidine tag and the fusion protein allows the histidine tag to be enzymatically removed following purification.
[0041]An ecdysone-inducible mammalian expression system (Invitrogen, Carlsbad, Calif.) can also be used to express a target protein. Vectors used in the ecdysone-inducible mammalian expression system can be organized to produce the target protein by expressing the target protein from the expression cassette. With the ecdysone-inducible system, higher levels of protein production can be achieved by use of the insect hormone 20-OH ecdysone to activate gene expression via the ecdysone receptor. An inducible expression plasmid provides a multiple cloning site, into which the nucleotide sequence of the Tirt protein can be ligated. The expression vector contains ecdysone response elements upstream of the promoter (a minimal heat shock promoter) and the multiple cloning site. Cotransfection of a second plasmid, pVgRXR (Invitrogen), provides the receptor subunits to make the cell responsive to the steroid hormone ecdysone analog, ponasterone A. A control expression plasmid containing the lacZ gene can be cotransfected with pVgRXR to provide a marker for transfected cells. Upon induction with ponasterone A, the control plasmid expresses β-galactosidase. Cotransfection of the inducible expression construct and pVgRXR into the mammalian cell of choice can be accomplished by any of the standard means known to those of skill in the art. These include, for example, calcium phosphate transfection, lipid-mediated transfection, and electroporation. Levels of expression of the fusion protein in this system can be varied according to the concentration and length of exposure to ponasterone. Stable cell lines that constitutively express the Tirt protein can be established using Zeocin® (Invitrogen), a bleomycin/phleomycin-type antibiotic isolated from Streptomyces, and neomycin or hygromycin.
[0042]Yeast host cells, such as Pichia pastoris, can also be used for the production of the Tirt protein. Expression of heterologous proteins from plasmids transformed into Pichia has previously been described by Sreekrishna, et al. (U.S. Pat. No. 5,002,876, incorporated herein by reference). Vectors for expression in Pichia of a Tirt protein are commercially available as part of a Pichia Expression Kit (Invitrogen, Carlsbad, Calif.). Pichia pastoris is a methylotrophic yeast, which produces large amounts of alcohol oxidase to avoid the toxicity of hydrogen peroxide produced as a result of methanol metabolism. Alcohol oxidase gene expression is tightly regulated by the AOX1 and AOX2 promoters. In Pichia expression vectors, high levels of expression are produced under the control of these promoters. Ohi, et al. (U.S. Pat. No. 5,683,893, incorporated herein by reference) have previously described a mutant AOX2 promoter capable of producing enhanced expression levels.
[0043]PCR primers were designed based on the DNA sequence recovered from a BLAST search of the unfinished genome sequence of G. stearothermophilus from the "BLAST with bacterial genomes" web page at the National Center for Biotech Information (www.ncbi.nlm.nih-gov/blast) (see FIG. 1). The program Primer3 (available at the web site: www-genome.wi.mit.edu/cgi-bin/primer/primer3) was used to aid in design of primers. The DNA sequence of plasmid pTirt#16 and other constructs was determined by BigDye terminator cycle sequencing using an ABI 327 automated DNA sequencer at the sequencing service (Molecular Biology Core facility) provided by the Quillen College of Medicine at East Tennessee State University. Multiple amino acid sequence alignments of the tirt ORF with other known intron ORFs was done using Clustal W available from the Baylor College of Medicine at http://searchlauncher.bc.tmc.edu/).
[0044]Protocols for performing reverse transcription, RT-PCR, real-time RT-PCR, real-time relative RT-PCR, construction of cDNA libraries, and competitive reverse transcriptase PCR analysis of cellular genes are known to those of skill in the art. One such protocol for competitive reverse transcriptase PCR analysis is described by Waha, et al. in Brain Pathology, Vol. 8 (1998), pages 13 to 18. Protocols for detecting the presence of viral infection include, for example, those described by Henrickson, et al., in U.S. Pat. Nos. 5,744,299 (parainfluenza virus type 1) and 6,014,664 (parainfluenza virus, respiratory syncytial virus, and influenza virus). More recently, detection of the Severe Acute Respiratory Syndrome (SARS) Coronavirus by real-time nested PCR following RT-PCR. (Jiang, et al, Clin. Infectious Disease 2004: 38 (15 Jan.) p. 293-296.) The enzyme of the present invention provides a tool for performing these protocols at higher temperatures. It is known, for example, that in the construction of cDNA libraries or in cDNA labeling, that oligo(dT) primers can be used to insure that poly(A) mRNAs are reverse transcribed, and that short random oligonucleotide primers may also be used for reverse transcription. Those of skill in the art, however, also are aware that RT reactions are often performed at approximately 42° C. in order to avoid inactivating the reverse transcriptase needed for the reaction to be catalyzed. The present invention provides an alternate enzyme for reverse transcription reactions, such as those listed above, that can be used at temperatures significantly higher than those used currently in conventional reaction systems.
[0045]The invention also provides at least one kit for performing, for example, reverse transcriptase, RT-PCR, real-time RT-PCR, competitive RT-PCR, or other protocols which rely on the presence of a reverse transcriptase, these protocols being performed at temperatures of up to about 68 to 75 degrees Celsius. Such a kit may comprise, for example, at least one reaction buffer (e.g., 50 mM Tris-pH 8.3, 100 mM KCl, and 10 mM dithiothreitol), an RNase inhibitor (to a final concentration of 10 units), NP-40 (to a final concentration of 0.17%), dNTP mix (dGTP, dCTP, dUTP, and dATP to a final concentration of 0.8 mM each), Tirt protein, Taq polymerase, BMV-RNA (as a positive control to a final concentration of 50 ng), RT primer (as a positive control primer for RT, to a final concentration of 0.02 μM), and BMV-PCR1 primer with BMV-PCR2 primer (as positive control primers for PCR, to a final concentration of 1 μM).
[0046]The present invention provides the isolated polynucleotide encoding the amino acid sequence of SEQ ID NO: 2, which can be used in production of the reverse transcriptase of the invention and the variant thereof. Furthermore, nucleic acids which hybridize with a nucleic acid encoding the amino acid sequence of SEQ ID NO: 2 under stringent conditions and encode a polypeptide having a similar reverse transcriptase activity to that of a polypeptide comprising SEQ ID NO: 2 are also included as embodiments of the present invention.
[0047]The term "stringent conditions", as used herein, means conditions in which non-specific hybridization will not generally occur. Hybridization under such conditions can be performed based on the description provided in Molecular Cloning: A Laboratory Manual 2nd ed., published by cold Spring Harbor Laboratory in 1989, edited by T. Maniatis et al. For example, stringent conditions include incubation with a probe in 6×SSC containing 0.5% SDS, 5×Denhardt's solution and 100 micrograms/ml salmon sperm DNA at 60° C.
[0048]The invention also provides a method of synthesizing a cDNA copy of an mRNA template by hybridizing a primer to an mRNA molecule and incubating the mRNA molecule and hybridized primer in the presence of one or more deoxy- or dideoxyribonucleoside triphosphates along with a reverse transcriptase of the present invention. Additional buffers and other reagents, as well as time and temperature conditions, can be determined by those of skill in the art, and are provided in the examples contained herein.
[0049]The invention will be further described by means of the following non-limiting examples.
Example 1
Cloning and Expression of the Tirt Gene
[0050]Geobacillus stearothermophilus strain 10 was a kind gift from Dr. Bruce Roe, University of Oklahoma, and was used for cloning the tirt gene. Cultures were grown in trypticase soy agar plates at 55° C. The plasmid pUC18 was used for routine subcloning of DNA fragments. The plasmid pET28a and the E. coli strain BL21(DE3) were from Novagen (Madison, Wis.) and were used for heterologous expression of the Tirt protein in E. coli.
[0051]While multiple sets of oligonucleotide primers were synthesized to amplify the tirt ORF, the inventors found that two pairs of primers could be used to successfully amplifying the tirt gene from the chromosome of G. stearothermophilus. The first primer pair, designated Bst755 and Bst2015 was used to specifically amplify most, but not all, of the tirt ORF via a simple PCR protocol (FIG. 2). A second primer pair designated Bst1396 and Bst2198 was used to amplify a region that includes the last 16 amino acids at the C-terminus of the tirt ORF (FIG. 2).
[0052]The primers used to amplify the tirt gene from the genome of G. stearothermophilus strain 10 comprise SEQ ID NO 4: AGACAACATATGCGGCAAGACCTGAATCTCAT-3' (with the underlined sequence indicating an NdeI restriction site for cloning into the pET28a expression vector); SEQ ID NO 5: 5'-AATGGATCCGCTGGCGAACATCCTTCTC-3' (with the underlined sequence indicating a BamHI restriction site): SEQ ID NO 6: 5'-ATTACTGCAGAGCGGTCCAGTAGGTTTTG-3' (with the underlined sequence indicating a PstI restriction site); and SEQ ID NO 7: 5'-ACTCAAGCTTGAGAAGGGCTTGACGTTCATG-3' (with the underlined sequence indicating a HindIII restriction site for cloning into the pET28a expression vector.).
[0053]Amplification of the tirt gene was done in two stages (FIG. 2) using a single colony of G. stearothermophilus as a source of template. A single colony from an overnight plate culture was suspended in 10 μl of water. One μl of this cell suspension was added to a 50 μl PCR reaction mix containing 1.5 mM MgCl2, Taq polymerase buffer (Promega, Madison, Wis.), 0.2 μM each dNTP, 0.5 μM each primer, and 2 units of Taq polymerase. The reaction was amplified using the following conditions: one cycle at 95° C. for 2 minutes, 30 cycles at 95° C. for one minute, 50° C. for 2 minutes, and 72° C. for 2 minutes. Amplified DNAs were purified by gel electrophoresis, digested with the appropriate restriction endonuclease and ligated into either pUC18 or directly into the pET28a expression vector. First, a 1.26 kilobase pair (Kb) amplified DNA product produced by the first primer pair (SEQ ID NO 4 and SEQ ID NO 6) was ligated into the expression plasmid pET28a. This produced an in-frame fusion between the polyhistidine tag element found in the expression plasmid and the tirt ORF. To capture the remaining 16 amino acids at the C-terminus of Tirt, a naturally occurring EcoRI site (within the tirt ORF, FIG. 2) was used to splice the 3 prime end of the PCR product produced by the second primer pair (SEQ ID NO 5 and SEQ ID NO 7) to the first amplified DNA to yield an expression plasmid, pTirt#16, containing the entire predicted ORF of the Tirt protein.
Example 2
Expression of Tirt Protein
[0054]The plasmid pTirt#16 was used to express the Tirt protein in E. coli by induction of the T-7 promoter system with IPTG. Briefly, protein expression in E. coli was achieved using the T7 RNA polymerase system and the pET28a expression vector (Novagen, Madison, Wis.). Since the upstream primer (SEQ ID NO 4) used to amplify the tirt gene contained an NdeI restriction site, the amplified DNA containing the tirt ORF could be ligated into the NdeI restriction site of the expression vector. This produced an in-frame fusion between the polyhistidine tag element in the pET28a vector and the tirt ORF.
[0055]The Tirt fusion protein was expressed in E. coli strain BL21 lysogenic for μDE3. Briefly, cells of E. coli strain BL21(DE3) transformed with the plasmid pTirt#16 (containing the tirt fusion construct) were induced by addition of IPTG (1 mM). After 3 hours of induction, cells from a 100 ml culture were harvested and resuspended in binding buffer (1×) for nickel ion column purification (Novagen). A cell extract containing the Tirt fusion protein was prepared by incubating the cell suspension in fresh lysozyme (1 mg/ml), followed by three cycles of quick freeze-thaw (10 minutes at -80° C., followed by 10 minutes at 37° C.), followed by sonication. Centrifugation (15,000×g) and filtration (0.45μ filter) produced a cleared, crude protein preparation. The cleared extract was then loaded onto a prepared Nickel ion column (His-bind column, Novagen) and purified fractions collected according to manufacturer's instructions (Novagen).
[0056]Soluble and insoluble protein fractions were compared by resuspending cells (from a 50 ml induced culture) in one tenth volume of buffer (50 mM Tris-HCl pH 8.0, 2 mM EDTA) containing lysozyme (100 μg/ml) plus 1% triton X-100. After incubation at 30° C. for 15 minutes and sonication, the cell extract was centrifuged at 12,000×g for 15 minutes. The supernatant was mixed with an equal volume of SDS sample buffer and this served as the soluble protein fraction for protein gels. The pellet of cell debris was mixed with SDS sample buffer and this served as the insoluble protein fraction for protein gels.
[0057]Only cell extracts from IPTG-induced cultures containing pTirt#16 showed a prominent protein band when analyzed by polyacrylamide gel electrophoresis (SDS-PAGE). The expressed protein appeared to be about 48 kilodaltons (kD) in size, according to its migration during electrophoresis in a polyacrylamide protein gel (FIG. 3, lane 4). This was approximately the size expected (52 kD) for the predicted fusion construction of Tirt in plasmid pTirt#16. In addition, Western blot analysis with a specific antibody probe confirmed the presence of a polyhistidine tag in the 48 kD protein band. Most of the expressed fusion protein fractionated into the insoluble cell debris after high speed centrifugation of the cell extract (FIG. 3, lane 9). However, some of the 48 kD fusion protein also appeared in the soluble cell fraction (FIG. 3, lane 8) and the inventors suspected that his fraction might have detectable RT activity. The 48 kD fusion protein was purified from the soluble cell fraction by nickel ion affinity chromatography. A single-step elution of the polyhistidine-tagged fusion protein yielded a partially purified fraction containing predominately a 48 kD protein band (FIG. 4, lanes 6-9, indicated by arrow). Each eluted column fraction was dialyzed and concentrated into a new buffer (buffer A) to stabilize the purified protein (FIG. 4, lanes 11-14). The polyhistidine tag at the N-terminus of the purified Tirt protein was not removed because this short extension of the protein was not expected to affect the RT activity of the fusion protein, since it does not affect the expression of mammalian viral RTs and other recombinant eukaryotic RTs in E. coli when similar technology is used for their expression.
Example 3
Demonstration of Tirt's Reverse Transcriptase Activity
[0058]Column fractions containing the purified Tirt fusion protein were pooled, dialyzed into buffer A (50 mM Tris-pH 7.5, 1 mM EDTA, 1 mM DTT, and 10% glycerol) using a microcon 30 membrane concentrator (Amicon, Beverly, Mass.), and then used to assay for RT activity. A highly sensitive product enhanced reverse transcriptase (PERT) assay was used to detect RT activity. The assay required the reverse transcription of a Brome Mosaic virus (BMV) RNA template to produce a small cDNA that was then further amplified by PCR (FIG. 5). Briefly, the assay was performed by first assembling the PCR amplification reaction mix in the bottom of a 0.2 ml tube containing: MgCl2-free PCR buffer, 1× (Promega, Madison, Wis.), 1 μM each BMV-PCR1 primer (5'-CGTGGTTGACACGCAGACCTCTTAC-3') and BMV-PCR2 primer (5'-TCAACACTGTACGGCACCCGCATTC-3'), 0.8 mM each dNTP, and Taq polymerase (Promega). The RT reaction mix was then assembled on top after sealing the lower PCR reaction mix with a layer of wax using an Ampliwax pellet (PCR-Gem 50, Applied Biosystems, Roche). The RT reaction mix contained: RT buffer (50 mM Tris-pH 8.3, 75 mM KCl, and 10 mM DTT), 2.5 mM MgCl2, 0.17% NP-40, 10 units of RNasin (Promega, Madison, Wis.), 0.8 mM each dNTP, 0.02 μM RT primer (5'-GGTCTCTTTTAGAGATTTACAGTG-3'), 100 ng of Brome Mosaic Virus (BMV) RNA (Promega), and a source of RT. The source of RT added to the reaction was either the purified Tirt fusion protein described above or commercially available Moloney Murine Leukemia Virus RT (MMLV-RT) (2 units). The reaction tube was then placed in a thermocycler under the following conditions: 1 cycle at 37° C. for 1 hour (reverse transcription); 1 cycle at 94° C. for 1 minute; 30 cycles at 94° C. for 15 seconds, 56° C. for 15 seconds, and 72° C. for 15 seconds (amplification); and finally 72° C. for 5 minutes. Amplified DNA was detected by electrophoresis of the reaction mix on a 5% polyacrylamide gel followed by staining with ethidium bromide.
[0059]The presence of a 168 base pair (bp) PCR product in a DNA gel, following electrophoresis, indicated the presence of RT activity. The assay was run on both crude cell extracts, as well as purified column fractions. No RT activity was detected in the crude cell extracts tested. Furthermore, no RT activity was detected in the purified fractions eluted from the nickel ion affinity column used to purify the fusion protein (FIG. 6A, lane 1). However, when the eluted column fractions were dialyzed and concentrated into buffer A, RT activity was detected in some of the column fractions (FIG. 6B, lanes 1-3), indicating the presence of inhibitors of RT activity in the fractions prior to dialysis. As an additional indication that inhibitors of RT activity are present in the column fractions prior to dialysis, when commercially prepared MMLV-RT was added to the PERT assay, as expected, a 168 bp DNA was produced indicating RT activity (FIG. 6A, lane 4). However, when MMLV-RT was mixed with column fraction #7, no RT activity was detected.
[0060]When one of the PCR primers was omitted from the PERT assay, no DNA product was produced (FIG. 6A: lane 4, two primers; lane 5, one primer). This indicated that the 168 bp DNA product was the result of specific amplification of the reverse transcribed cDNA and not some other process. In another control reaction, RNase was added to the fraction to be tested for RT activity. When RNase was mixed with dialyzed column fraction number 2, no DNA product was observed (FIG. 6B lane 1, without RNase; lane 7, RNase added), indicating that the 168 bp DNA was amplified from a cDNA that was reversed transcribed from the BMV RNA template present in the assay and not from contaminating DNA carried over from previous assay reactions. A third control reaction contained only water as the sample extract to be tested for RT activity (FIG. 6B, lane 6). As expected, no DNA product was produced. This confirmed that an exogenous source of RT added to the assay reaction, and not the Taq polymerase present in the assay, was responsible for cDNA formation (and thus the amplification of the 168 bp DNA).
Example 4
Demonstration of Heat Stability of Tirt
[0061]The column purified Tirt fusion protein (as described above) was added (15 μl) to a microfuge tube and heated in a water bath at either 65° C. or 75° C. for 15 minutes. After heat treatment, the tube was centrifuged and 7.5 μl of the supernatant was added directly to the PERT assay reaction. MMLV-RT diluted in 1×RT buffer (5 units) was treated in a similar fashion.
[0062]To run the PERT assay at different temperatures, the reverse transcription reaction was incubated separately at 37° C., 50° C. or 68° C. After 1 hour of incubation the reaction mix was immediately added to the top of the PCR reaction mix, sealed with a layer of wax, and processed as described in Example 3.
[0063]The column-purified Tirt fusion protein was not heated (FIG. 7A, lane 2), heated at 65° C. for 15 minutes (FIG. 7A, lane 3), or heated at 75° C. for 15 minutes (FIG. 7A, lane 4). After heat treatment the purified fraction was tested for RT activity using the PERT assay. As a control, the same procedure was followed using commercially prepared Moloney Murine Leukemia Virus reverse transcriptase (M-MLV-RT). The purified Tirt protein was not reduced in RT activity even after exposure to temperatures of 75° C. (FIG. 7A, lane 4). In contrast, no RT activity was detected after heat treatment (both at 65° C. and 75° C.) of the mesophilic MMLV-RT (FIG. 7A, lanes 6 and 7).
[0064]The purified Tirt protein was also tested for RT activity by running the PERT assay at three different temperatures; 37° C., 50° C., and 68° C. (FIG. 7B). Again, the purified Tirt protein was not affected in its ability to synthesize cDNA by reverse transcription even at the highest temperature tested, 68° C. (FIG. 7B, lanes 2 and 4). By contrast, cDNA production appeared to be greatly reduced, at least at the highest temperature of 68° C., for the mesophilic mammalian RT (compare FIG. 7B, lane 5 versus lane 7).
[0065]Plasmid pTirt#16 (comprising vector pET28a from Escherichia coli with Tirt coding sequence from Geobacillus stearothermophilus inserted) was deposited on Mar. 4, 2004 with the American Type Culture Collection, Manassas, Va., USA, under the terms of the Budapest Treaty and has been given patent deposit designation number PTA-5847. The Tirt DNA and amino acid sequences have been assigned GenBank accession number AY672067.
Sequence CWU
1
1811263DNAGeobacillus stearothermophilusCDS(1)..(1263) 1atg gct ttg ttg
gaa cgc atc tta gcg aga gac aac ctc atc acg gcg 48Met Ala Leu Leu
Glu Arg Ile Leu Ala Arg Asp Asn Leu Ile Thr Ala1 5
10 15ctc aaa cgg gtc gaa gcc aac caa gga gca
ccg gga atc gac gga gta 96Leu Lys Arg Val Glu Ala Asn Gln Gly Ala
Pro Gly Ile Asp Gly Val 20 25
30tca acc gat caa ctc cgt gat tac atc cgc gct cac tgg agc acg atc
144Ser Thr Asp Gln Leu Arg Asp Tyr Ile Arg Ala His Trp Ser Thr Ile
35 40 45cgc gcc caa ctc ttg gcg gga acc
tac cgg ccg gcg cct gtc cgc agg 192Arg Ala Gln Leu Leu Ala Gly Thr
Tyr Arg Pro Ala Pro Val Arg Arg 50 55
60gtc gga atc ccg aaa ccg ggc ggc ggc aca cgg cag cta ggc att ccc
240Val Gly Ile Pro Lys Pro Gly Gly Gly Thr Arg Gln Leu Gly Ile Pro65
70 75 80acc gtg gtg gac cgg
ctg atc caa caa gcc att ctt caa gaa ctc aca 288Thr Val Val Asp Arg
Leu Ile Gln Gln Ala Ile Leu Gln Glu Leu Thr 85
90 95ccc att ttc gat cca gac ttc tcc cct tcc agc
ttc gga ttc cgt ccg 336Pro Ile Phe Asp Pro Asp Phe Ser Pro Ser Ser
Phe Gly Phe Arg Pro 100 105
110ggc cgt aac gcc cac gat gcc gtg cgg caa gcg caa ggc tac atc cag
384Gly Arg Asn Ala His Asp Ala Val Arg Gln Ala Gln Gly Tyr Ile Gln
115 120 125gaa ggg tat cgg tac gtg gtc
gac atg gac ctg gaa aag ttc ttt gat 432Glu Gly Tyr Arg Tyr Val Val
Asp Met Asp Leu Glu Lys Phe Phe Asp 130 135
140cgg gtc aac cat gac atc ttg atg agt cgg gtg gcc cga aaa gtc aag
480Arg Val Asn His Asp Ile Leu Met Ser Arg Val Ala Arg Lys Val Lys145
150 155 160gat aaa cgc gtg
ctg aaa ctg atc cgt gcc tac ctg caa gcc ggc gtt 528Asp Lys Arg Val
Leu Lys Leu Ile Arg Ala Tyr Leu Gln Ala Gly Val 165
170 175atg atc gaa ggg gtg aag gtg cag acg gag
gaa ggg acg ccg caa ggc 576Met Ile Glu Gly Val Lys Val Gln Thr Glu
Glu Gly Thr Pro Gln Gly 180 185
190ggc ccc ctc agc ccc ctg ctg gcg aac atc ctt ctc gac gat tta gac
624Gly Pro Leu Ser Pro Leu Leu Ala Asn Ile Leu Leu Asp Asp Leu Asp
195 200 205aag gaa ttg gag aag cga gga
ttg aaa ttc tgc cgt tac gca gat gac 672Lys Glu Leu Glu Lys Arg Gly
Leu Lys Phe Cys Arg Tyr Ala Asp Asp 210 215
220tgc aac atc tat gtg aaa agt ctg cgg gca gga caa cgg gtg aaa caa
720Cys Asn Ile Tyr Val Lys Ser Leu Arg Ala Gly Gln Arg Val Lys Gln225
230 235 240agc atc caa cgg
ttc ttg gag aaa acg ctc aaa ctc aaa gta aac gag 768Ser Ile Gln Arg
Phe Leu Glu Lys Thr Leu Lys Leu Lys Val Asn Glu 245
250 255gag aaa agt gcg gtg gac cgc ccg tgg aaa
cgg gcc ttt ctg ggg ttt 816Glu Lys Ser Ala Val Asp Arg Pro Trp Lys
Arg Ala Phe Leu Gly Phe 260 265
270agc ttc aca ccg gaa cga aaa gcg cga atc cgg ctc gcc cca agg tcg
864Ser Phe Thr Pro Glu Arg Lys Ala Arg Ile Arg Leu Ala Pro Arg Ser
275 280 285att caa cgt ctg aaa cag cgg
att cga cag ctg acc aac cca aac tgg 912Ile Gln Arg Leu Lys Gln Arg
Ile Arg Gln Leu Thr Asn Pro Asn Trp 290 295
300agc ata tcg atg cca gaa cga att cat cgc gtc aat caa tac gtc atg
960Ser Ile Ser Met Pro Glu Arg Ile His Arg Val Asn Gln Tyr Val Met305
310 315 320gga tgg atc ggg
tat ttt cgg ctc gtc gaa acc ccg tct gtc ctt cag 1008Gly Trp Ile Gly
Tyr Phe Arg Leu Val Glu Thr Pro Ser Val Leu Gln 325
330 335acc atc gaa gga tgg att cgg agg agg ctt
cga ctc tgt caa tgg ctt 1056Thr Ile Glu Gly Trp Ile Arg Arg Arg Leu
Arg Leu Cys Gln Trp Leu 340 345
350caa tgg aaa cgg gtc aga acc aga atc cgt gag tta aga gcg ctg ggg
1104Gln Trp Lys Arg Val Arg Thr Arg Ile Arg Glu Leu Arg Ala Leu Gly
355 360 365ctg aaa gag aca gcg gtg atg
gag atc gcc aat acc cga aaa gga gct 1152Leu Lys Glu Thr Ala Val Met
Glu Ile Ala Asn Thr Arg Lys Gly Ala 370 375
380tgg cga aca acg aaa acg ccg caa ctc cac cag gcc ctg ggc aaa acc
1200Trp Arg Thr Thr Lys Thr Pro Gln Leu His Gln Ala Leu Gly Lys Thr385
390 395 400tac tgg acc gct
caa ggg ctc aag agt ttg acg caa cga tat ttc gaa 1248Tyr Trp Thr Ala
Gln Gly Leu Lys Ser Leu Thr Gln Arg Tyr Phe Glu 405
410 415ctc cgt caa ggt tga
1263Leu Arg Gln Gly
4202420PRTGeobacillus stearothermophilus 2Met Ala Leu Leu Glu Arg Ile Leu
Ala Arg Asp Asn Leu Ile Thr Ala1 5 10
15Leu Lys Arg Val Glu Ala Asn Gln Gly Ala Pro Gly Ile Asp
Gly Val 20 25 30Ser Thr Asp
Gln Leu Arg Asp Tyr Ile Arg Ala His Trp Ser Thr Ile 35
40 45Arg Ala Gln Leu Leu Ala Gly Thr Tyr Arg Pro
Ala Pro Val Arg Arg 50 55 60Val Gly
Ile Pro Lys Pro Gly Gly Gly Thr Arg Gln Leu Gly Ile Pro65
70 75 80Thr Val Val Asp Arg Leu Ile
Gln Gln Ala Ile Leu Gln Glu Leu Thr 85 90
95Pro Ile Phe Asp Pro Asp Phe Ser Pro Ser Ser Phe Gly
Phe Arg Pro 100 105 110Gly Arg
Asn Ala His Asp Ala Val Arg Gln Ala Gln Gly Tyr Ile Gln 115
120 125Glu Gly Tyr Arg Tyr Val Val Asp Met Asp
Leu Glu Lys Phe Phe Asp 130 135 140Arg
Val Asn His Asp Ile Leu Met Ser Arg Val Ala Arg Lys Val Lys145
150 155 160Asp Lys Arg Val Leu Lys
Leu Ile Arg Ala Tyr Leu Gln Ala Gly Val 165
170 175Met Ile Glu Gly Val Lys Val Gln Thr Glu Glu Gly
Thr Pro Gln Gly 180 185 190Gly
Pro Leu Ser Pro Leu Leu Ala Asn Ile Leu Leu Asp Asp Leu Asp 195
200 205Lys Glu Leu Glu Lys Arg Gly Leu Lys
Phe Cys Arg Tyr Ala Asp Asp 210 215
220Cys Asn Ile Tyr Val Lys Ser Leu Arg Ala Gly Gln Arg Val Lys Gln225
230 235 240Ser Ile Gln Arg
Phe Leu Glu Lys Thr Leu Lys Leu Lys Val Asn Glu 245
250 255Glu Lys Ser Ala Val Asp Arg Pro Trp Lys
Arg Ala Phe Leu Gly Phe 260 265
270Ser Phe Thr Pro Glu Arg Lys Ala Arg Ile Arg Leu Ala Pro Arg Ser
275 280 285Ile Gln Arg Leu Lys Gln Arg
Ile Arg Gln Leu Thr Asn Pro Asn Trp 290 295
300Ser Ile Ser Met Pro Glu Arg Ile His Arg Val Asn Gln Tyr Val
Met305 310 315 320Gly Trp
Ile Gly Tyr Phe Arg Leu Val Glu Thr Pro Ser Val Leu Gln
325 330 335Thr Ile Glu Gly Trp Ile Arg
Arg Arg Leu Arg Leu Cys Gln Trp Leu 340 345
350Gln Trp Lys Arg Val Arg Thr Arg Ile Arg Glu Leu Arg Ala
Leu Gly 355 360 365Leu Lys Glu Thr
Ala Val Met Glu Ile Ala Asn Thr Arg Lys Gly Ala 370
375 380Trp Arg Thr Thr Lys Thr Pro Gln Leu His Gln Ala
Leu Gly Lys Thr385 390 395
400Tyr Trp Thr Ala Gln Gly Leu Lys Ser Leu Thr Gln Arg Tyr Phe Glu
405 410 415Leu Arg Gln Gly
4203420PRTGeobacillus stearothermophilusPEPTIDE(1)..(420) 3Met Ala
Leu Leu Glu Arg Ile Leu Ala Arg Asp Asn Leu Ile Thr Ala1 5
10 15Leu Lys Arg Val Glu Ala Asn Gln
Gly Ala Pro Gly Ile Asp Gly Val 20 25
30Ser Thr Asp Gln Leu Arg Asp Tyr Ile Arg Ala His Trp Ser Thr
Ile 35 40 45Arg Ala Gln Leu Leu
Ala Gly Thr Tyr Arg Pro Ala Pro Val Arg Arg 50 55
60Val Gly Ile Pro Lys Pro Gly Gly Gly Thr Arg Gln Leu Gly
Ile Pro65 70 75 80Thr
Val Val Asp Arg Leu Ile Gln Gln Ala Ile Leu Gln Glu Leu Thr
85 90 95Pro Ile Phe Asp Pro Asp Phe
Ser Pro Ser Ser Phe Gly Phe Arg Pro 100 105
110Gly Arg Asn Ala His Asp Ala Val Arg Gln Ala Gln Gly Tyr
Ile Gln 115 120 125Glu Gly Tyr Arg
Tyr Val Val Asp Met Asp Leu Glu Lys Phe Phe Asp 130
135 140Arg Val Asn His Asp Ile Leu Met Ser Arg Val Ala
Arg Lys Val Lys145 150 155
160Asp Lys Arg Val Leu Lys Leu Ile Arg Ala Tyr Leu Gln Ala Gly Val
165 170 175Met Ile Glu Gly Val
Lys Val Gln Thr Glu Glu Gly Thr Pro Gln Gly 180
185 190Gly Pro Leu Ser Pro Leu Leu Ala Asn Ile Leu Leu
Asp Asp Leu Asp 195 200 205Lys Glu
Leu Glu Lys Arg Gly Leu Lys Phe Cys Arg Tyr Ala Asp Asp 210
215 220Cys Asn Ile Tyr Val Lys Ser Leu Arg Ala Gly
Gln Arg Val Lys Gln225 230 235
240Ser Ile Gln Arg Phe Leu Glu Lys Thr Leu Lys Leu Lys Val Asn Glu
245 250 255Glu Lys Ser Ala
Val Asp Arg Pro Trp Lys Arg Ala Phe Leu Gly Phe 260
265 270Ser Phe Thr Pro Glu Arg Lys Ala Arg Ile Arg
Leu Ala Pro Arg Ser 275 280 285Ile
Gln Arg Leu Lys Gln Arg Ile Arg Gln Leu Thr Asn Pro Asn Trp 290
295 300Ser Ile Ser Met Pro Glu Arg Ile His Arg
Val Asn Gln Tyr Val Met305 310 315
320Gly Trp Ile Gly Tyr Phe Arg Leu Val Glu Thr Pro Ser Val Leu
Gln 325 330 335Thr Ile Glu
Gly Trp Ile Arg Arg Arg Leu Arg Leu Cys Gln Trp Leu 340
345 350Gln Trp Lys Arg Val Arg Thr Arg Ile Arg
Glu Leu Arg Ala Leu Gly 355 360
365Leu Lys Glu Thr Ala Val Met Glu Ile Ala Asn Thr Arg Lys Gly Ala 370
375 380Trp Arg Thr Thr Lys Thr Pro Gln
Leu His Gln Ala Leu Gly Lys Thr385 390
395 400Tyr Trp Thr Ala Gln Gly Leu Lys Ser Leu Thr Gln
Arg Tyr Phe Glu 405 410
415Leu Arg Gln Gly 42041370DNAArtificialPlasmid construct
4ccatgggcag cagccatcat catcatcatc acagcagcgg cctggtgccg cgcggcagcc
60atatgcggca agacctgaat ctcatcccgc ggaaggagaa gatcacgatg gctttgttgg
120aacgcatctt agcgagagac aacctcatca cggcgctcaa acgggtcgaa gccaaccaag
180gagcaccggg aatcgacgga gtatcaaccg atcaactccg tgattacatc cgcgctcact
240ggagcacgat ccgcgcccaa ctcttggcgg gaacctaccg gccggcgcct gtccgcaggg
300tcggaatccc gaaaccgggc ggcggcacac ggcagctagg cattcccacc gtggtggacc
360ggctgatcca acaagccatt cttcaagaac tcacacccat tttcgatcca gacttctccc
420cttccagctt cggattccgt ccgggccgta acgcccacga tgccgtgcgg caagcgcaag
480gctacatcca ggaagggtat cggtacgtgg tcgacatgga cctggaaaag ttctttgatc
540gggtcaacca tgacatcttg atgagtcggg tggcccgaaa agtcaaggat aaacgcgtgc
600tgaaactgat ccgtgcctac ctgcaagccg gcgttatgat cgaaggggtg aaggtgcaga
660cggaggaagg gacgccgcaa ggcggccccc tcagccccct gctggcgaac atccttctcg
720acgatttaga caaggaattg gagaagcgag gattgaaatt ctgccgttac gcagatgact
780gcaacatcta tgtgaaaagt ctgcgggcag gacaacgggt gaaacaaagc atccaacggt
840tcttggagaa aacgctcaaa ctcaaagtaa acgaggagaa aagtgcggtg gaccgcccgt
900ggaaacgggc ctttctgggg tttagcttca caccggaacg aaaagcgcga atccggctcg
960ccccaaggtc gattcaacgt ctgaaacagc ggattcgaca gctgaccaac ccaaactgga
1020gcatatcgat gccagaacga attcatcgcg tcaatcaata cgtcatggga tggatcgggt
1080attttcggct cgtcgaaacc ccgtctgtcc ttcagaccat cgaaggatgg attcggagga
1140ggcttcgact ctgtcaatgg cttcaatgga aacgggtcag aaccagaatc cgtgagttaa
1200gagcgctggg gctgaaagag acagcggtga tggagatcgc caatacccga aaaggagctt
1260ggcgaacaac gaaaacgccg caactccacc aggccctggg caaaacctac tggaccgctc
1320aagggctcaa gagtttgacg caacgatatt tcgaactccg tcaaggttga
1370532DNAArtificialNucleotide primer containing NdeI restriction
site 5agacaacata tgcggcaaga cctgaatctc at
32628DNAArtificialNucleotide primer containing BamHI restriction
site 6aatggatccg ctggcgaaca tccttctc
28729DNAArtificialNucleotide primer containing PstI restriction
site 7attactgcag agcggtccag taggttttg
29831DNAArtificialNucleotide primer containing HindIII restriction
site 8actcaagctt gagaagggct tgacgttcat g
319455PRTArtificialAmino acid sequence of fusion protein 9Met Gly Ser
Ser His His His His His His Ser Ser Gly Leu Val Pro1 5
10 15Arg Gly Ser His Met Arg Gln Asp Leu
Asn Leu Ile Pro Arg Lys Glu 20 25
30Lys Ile Thr Met Ala Leu Leu Glu Arg Ile Leu Ala Arg Asp Asn Leu
35 40 45Ile Thr Ala Leu Lys Arg Val
Glu Ala Asn Gln Gly Ala Pro Gly Ile 50 55
60Asp Gly Val Ser Thr Asp Gln Leu Arg Asp Tyr Ile Arg Ala His Trp65
70 75 80Ser Thr Ile Arg
Ala Gln Leu Leu Ala Gly Thr Tyr Arg Pro Ala Pro 85
90 95Val Arg Arg Val Gly Ile Pro Lys Pro Gly
Gly Gly Thr Arg Gln Leu 100 105
110Gly Ile Pro Thr Val Val Asp Arg Leu Ile Gln Gln Ala Ile Leu Gln
115 120 125Glu Leu Thr Pro Ile Phe Asp
Pro Asp Phe Ser Pro Ser Ser Phe Gly 130 135
140Phe Arg Pro Gly Arg Asn Ala His Asp Ala Val Arg Gln Ala Gln
Gly145 150 155 160Tyr Ile
Gln Glu Gly Tyr Arg Tyr Val Val Asp Met Asp Leu Glu Lys
165 170 175Phe Phe Asp Arg Val Asn His
Asp Ile Leu Met Ser Arg Val Ala Arg 180 185
190Lys Val Lys Asp Lys Arg Val Leu Lys Leu Ile Arg Ala Tyr
Leu Gln 195 200 205Ala Gly Val Met
Ile Glu Gly Val Lys Val Gln Thr Glu Glu Gly Thr 210
215 220Pro Gln Gly Gly Pro Leu Ser Pro Leu Leu Ala Asn
Ile Leu Leu Asp225 230 235
240Asp Leu Asp Lys Glu Leu Glu Lys Arg Gly Leu Lys Phe Cys Arg Tyr
245 250 255Ala Asp Asp Cys Asn
Ile Tyr Val Lys Ser Leu Arg Ala Gly Gln Arg 260
265 270Val Lys Gln Ser Ile Gln Arg Phe Leu Glu Lys Thr
Leu Lys Leu Lys 275 280 285Val Asn
Glu Glu Lys Ser Ala Val Asp Arg Pro Trp Lys Arg Ala Phe 290
295 300Leu Gly Phe Ser Phe Thr Pro Glu Arg Lys Ala
Arg Ile Arg Leu Ala305 310 315
320Pro Arg Ser Ile Gln Arg Leu Lys Gln Arg Ile Arg Gln Leu Thr Asn
325 330 335Pro Asn Trp Ser
Ile Ser Met Pro Glu Arg Ile His Arg Val Asn Gln 340
345 350Tyr Val Met Gly Trp Ile Gly Tyr Phe Arg Leu
Val Glu Thr Pro Ser 355 360 365Val
Leu Gln Thr Ile Glu Gly Trp Ile Arg Arg Arg Leu Arg Leu Cys 370
375 380Gln Trp Leu Gln Trp Lys Arg Val Arg Thr
Arg Ile Arg Glu Leu Arg385 390 395
400Ala Leu Gly Leu Lys Glu Thr Ala Val Met Glu Ile Ala Asn Thr
Arg 405 410 415Lys Gly Ala
Trp Arg Thr Thr Lys Thr Pro Gln Leu His Gln Ala Leu 420
425 430Gly Lys Thr Tyr Trp Thr Ala Gln Gly Leu
Lys Ser Leu Thr Gln Arg 435 440
445Tyr Phe Glu Leu Arg Gln Gly 450
4551025DNAArtificialPrimer sequence 10cgtggttgac acgcagacct cttac
251125DNAArtificialPrimer sequence
11tcaacactgt acggcacccg cattc
251224DNAArtificialPrimer sequence 12ggtctctttt agagatttac agtg
2413394PRTBacillus
haloduransPEPTIDE(1)..(394) 13Met Leu Glu Arg Ile Leu Ser Arg Glu Asn Leu
Ile Gln Leu Glu Arg1 5 10
15Val Glu Lys Asn Lys Gly Ser Tyr Gly Val Asp Glu Met Asp Val Lys
20 25 30Ser Leu Arg Leu His Leu His
Glu Asn Trp Thr Ser Ile Arg Asn Glu 35 40
45Ile Ile Glu Gly Ser Tyr Phe Pro Lys Pro Val Arg Arg Val Glu
Ile 50 55 60Pro Lys Pro Asn Gly Gly
Val Arg Lys Leu Gly Ile Pro Thr Val Met65 70
75 80Asp Arg Phe Leu Gln Gln Ala Ile Ala Gln Ile
Leu Thr Gln Leu Tyr 85 90
95Asp Pro Thr Phe Ser Glu Arg Ser Phe Gly Phe Arg Pro His Arg Arg
100 105 110Gly His Asn Ala Val Arg
Gln Ala Lys Gln Trp Met Lys Glu Gly Tyr 115 120
125Arg Trp Val Val Asp Ile Asp Leu Glu Lys Phe Phe Asp Lys
Val Asn 130 135 140His Asp Arg Leu Met
Arg Lys Leu Ser Ser Arg Ile Gln Asp Pro Arg145 150
155 160Val Leu Gly Leu Ile Arg Arg Tyr Leu Gln
Thr Gly Val Met Glu Arg 165 170
175Gly Leu Val Ser Pro Asn Thr Glu Gly Thr Pro Gln Gly Gly Pro Leu
180 185 190Ser Pro Leu Leu Ser
Asn Ile Val Leu Asp Glu Leu Asp Asn Glu Leu 195
200 205Glu Lys Arg Gly Leu Lys Phe Val Arg Tyr Ala Asp
Asp Cys Asn Ile 210 215 220Tyr Val Arg
Ser Lys Arg Ala Gly Leu Arg Ile Met Glu Ser Val Thr225
230 235 240Ser Phe Ile Glu Asn Arg Leu
Lys Leu Lys Val Asn Arg Glu Lys Ser 245
250 255Ala Val Asp Arg Pro Trp Asn Arg Lys Phe Leu Gly
Phe Ser Phe Thr 260 265 270Arg
Gly Lys Asp Pro Lys Met Arg Val Ser Lys Glu Ser Val Lys Arg 275
280 285Leu Lys Gln Arg Ile Arg Glu Leu Thr
Ser Arg Arg His Ser Met Lys 290 295
300Met Ser Asp Arg Leu Arg Arg Leu Asn Arg Tyr Leu Thr Gly Trp Leu305
310 315 320Gly Tyr Tyr Gln
Val Val Asp Thr Pro Ser Ile Leu Ala Gln Ile Asp 325
330 335Ala Trp Ile Arg Arg Arg Leu Arg Met Ile
Arg Trp Lys Glu Trp Lys 340 345
350Thr Thr Ser Ala Arg Gln Lys Asn Leu Val Arg Leu Gly Ile Lys Lys
355 360 365Ala Lys Ala Trp Gln Trp Ala
Asn Ser Arg Lys Gly Tyr Trp Arg Val 370 375
380Ala His Ser Pro Ile Met Asp Tyr Ala Leu385
39014449PRTClostridium acetobutylicumPEPTIDE(1)..(449) 14Met Lys Asn Ser
Lys Glu Met Gln Lys Leu Gln Thr Thr Ser Tyr Lys1 5
10 15Glu Gly Trp Ser Cys Glu Ile Arg Val Glu
Leu Gln Asn Ser Thr Arg 20 25
30Ala His Ser Ile Ser Thr Ala Phe Asp Arg Arg Lys Asp Asp Gly Lys
35 40 45Leu Tyr Glu Thr Asn Leu Leu Glu
Arg Ile Leu Asp Arg Gln Asn Met 50 55
60Asn Leu Ala Tyr Lys Arg Val Lys Ser Asn Lys Gly Ser His Gly Val65
70 75 80Asp Gly Met Lys Val
Asp Glu Leu Leu Gln Tyr Leu Lys Gln Asn Gly 85
90 95Lys Thr Leu Ile Ala Ser Ile Phe Asn Gly Lys
Tyr Cys Pro Lys Ala 100 105
110Val Arg Arg Val Glu Ile Pro Lys Pro Asp Gly Gly Ile Arg Leu Leu
115 120 125Gly Ile Pro Thr Val Val Asp
Arg Thr Ile Gln Gln Ala Ile Ser Gln 130 135
140Val Leu Thr Pro Ile Phe Glu Lys Thr Phe Ser Glu Asn Ser Tyr
Gly145 150 155 160Phe Arg
Pro Lys Arg Ser Ala Lys Gln Ala Ile Lys Lys Ala Lys Glu
165 170 175Tyr Met Glu Glu Gly Tyr Lys
Trp Val Val Asp Ile Asp Leu Ala Lys 180 185
190Tyr Phe Asp Thr Val Asn His Asp Lys Leu Met Ala Leu Val
Ala Arg 195 200 205Lys Ile Lys Asp
Lys Arg Val Leu Lys Leu Ile Arg Leu Tyr Leu Gln 210
215 220Ser Gly Val Met Ile Asn Gly Val Val Ser Glu Thr
Glu Arg Gly Cys225 230 235
240Pro Gln Gly Gly Pro Leu Ser Pro Leu Leu Ser Asn Ile Met Leu Thr
245 250 255Glu Leu Asp Arg Glu
Leu Glu Lys Arg Gly His Lys Phe Cys Arg Tyr 260
265 270Ala Asp Asp Asn Asn Val Tyr Val Arg Ser Lys Lys
Ala Gly Asp Arg 275 280 285Val Met
Arg Ser Ile Thr Arg Phe Ile Glu Asn Lys Leu Lys Leu Lys 290
295 300Val Asn Lys Glu Lys Ser Ala Val Asp Arg Pro
Trp Arg Arg Lys Phe305 310 315
320Leu Gly Phe Thr Phe Tyr Gln Trp Tyr Gly Lys Ile Gly Ile Arg Val
325 330 335His Glu Lys Ser
Val Lys Lys Phe Lys Ala Lys Ile Lys Ala Ile Thr 340
345 350Ala Arg Ser Asn Ala Leu Asn Ile Glu Asn Arg
Ile Ile Lys Leu Arg 355 360 365Gln
Cys Ile Ile Gly Trp Leu Asn Tyr Phe Gly Ile Ala Glu Met Thr 370
375 380Lys Leu Ala Lys Lys Leu Asp Glu Trp Thr
Arg Arg Arg Leu Arg Met385 390 395
400Cys Tyr Trp Lys Gln Trp Lys Lys Val Lys Thr Lys Tyr Asp Asn
Leu 405 410 415Arg Lys Phe
Gly Ile Asn Asn Ser Lys Ala Trp Glu Phe Ala Asn Thr 420
425 430Arg Lys Ser Tyr Trp Arg Ile Ala Asn Ser
Pro Ile Leu Ser Thr Thr 435 440
445Leu 15449PRTPseudomonas alcaligenesPEPTIDE(1)..(449) 15Met Pro Pro Val
Gly Val Ala Val Ser Leu Val Thr Val Met Gln Lys1 5
10 15Phe Pro Thr Ala Glu Thr Val Ile Pro Asn
Pro Gly Gln Lys Pro Arg 20 25
30Val Met Pro Asp Ser Ala Lys Val Pro Ala Ala Ser Ala Thr Trp Thr
35 40 45Asn Ala Glu Pro Asp Thr Leu Met
Glu Arg Val Leu Ala Pro Ala Asn 50 55
60Leu Arg Arg Ala Tyr Gln Arg Val Val Ser Asn Lys Gly Ala Pro Gly65
70 75 80Ala Asp Gly Met Thr
Val Ala Asp Leu Ala Gly Tyr Val Lys Gln Tyr 85
90 95Trp Pro Thr Leu Lys Ala Arg Leu Leu Ala Gly
Glu Tyr His Pro Gln 100 105
110Ala Val Arg Ala Val Glu Ile Pro Lys Pro Gln Gly Gly Thr Arg Gln
115 120 125Leu Gly Ile Pro Ser Val Val
Asp Arg Leu Ile Gln Gln Ala Leu Gln 130 135
140Gln Gln Leu Thr Pro Ile Phe Asp Pro Leu Phe Ser Lys Tyr Ser
Tyr145 150 155 160Gly Phe
Arg Pro Gly Arg Ser Thr His Gln Ala Ile Glu Met Ala Arg
165 170 175Ala His Val Thr Ala Gly His
Arg Trp Cys Val Glu Leu Asp Leu Glu 180 185
190Lys Phe Phe Asp Arg Val Asn His Asp Ile Leu Met Ala Cys
Ile Glu 195 200 205Arg Arg Ile Lys
Asp Lys Cys Val Leu Arg Leu Ile Arg Arg Tyr Leu 210
215 220Glu Ala Gly Ile Met Ser Gly Gly Val Val Ser Pro
Arg Gln Glu Gly225 230 235
240Thr Pro Gln Gly Gly Pro Leu Ser Pro Leu Leu Ser Asn Ile Leu Leu
245 250 255Asp Glu Leu Asp Arg
Glu Leu Glu Arg Arg Gly His Arg Phe Val Arg 260
265 270Tyr Ala Asp Asp Ala Asn Ile Tyr Val Arg Ser Pro
Arg Ala Gly Glu 275 280 285Arg Val
Leu Val Ser Val Glu Arg Phe Leu Arg Glu Arg Leu Lys Leu 290
295 300Thr Val Asn Arg Lys Lys Ser Gln Val Ala Arg
Ala Trp Lys Cys Asp305 310 315
320Tyr Leu Gly Tyr Gly Met Ser Trp His Gln Gln Pro Arg Leu Arg Val
325 330 335Ala Arg Met Ser
Leu Asp Arg Leu Arg Asp Arg Leu Arg Met Leu Leu 340
345 350Arg Ser Val Arg Ala Arg Lys Met Ala Thr Val
Ile Glu Arg Ile Asn 355 360 365Pro
Val Leu Arg Gly Trp Ala Ser Tyr Phe Lys Leu Ser Gln Ser Lys 370
375 380Arg Pro Leu Glu Glu Leu Asp Gly Trp Val
Arg His Lys Leu Arg Cys385 390 395
400Val Ile Trp Arg Gln Trp Lys Gln Pro Pro Thr Arg Leu Arg Asn
Leu 405 410 415Met Arg Leu
Gly Leu Ser Glu Glu Arg Ala Asn Lys Ser Ala Phe Asn 420
425 430Gly Arg Gly Pro Trp Trp Asn Ser Gly Ala
Gln His Met Asn Tyr Ala 435 440
445Leu 161620DNAGeobacillus stearothermophilusgene(1)..(1620)
16gatgttgcgt gtcgaagcag aattcctttc ggaactcatc tgaggaagca agggtgaagc
60ccagagggcc tcagatcgag ggctgagcgc aacccggcaa gacctgaatc tcatcccgcg
120gaaggagaag gagaagatca cgatggcttt gttggaacgc atcttagcga gagacaacct
180catcacggcg ctcaaacggg tcgaagccaa ccaaggagca ccgggaatcg acggagtatc
240aaccgatcaa ctccgtgatt acatccgcgc tcactggagc acgatccgcg cccaactctt
300ggcgggaacc taccggccgg cgcctgtccg cagggtcgga atcccgaaac cgggcggcgg
360cacacggcag ctaggcattc ccaccgtggt ggaccggctg atccaacaag ccattcttca
420agaactcaca cccattttcg atccagactt ctccccttcc agcttcggat tccgtccggg
480ccgtaacgcc cacgatgccg tgcggcaagc gcaaggctac atccaggaag ggtatcggta
540cgtggtcgac atggacctgg aaaagttctt tgatcgggtc aaccatgaca tcttgatgag
600tcgggtggcc cgaaaagtca aggataaacg cgtgctgaaa ctgatccgtg cctacctgca
660agccggcgtt atgatcgaag gggtgaaggt gcagacggag gaagggacgc cgcaaggcgg
720ccccctcagc cccctgctgg cgaacatcct tctcgacgat ttagacaagg aattggagaa
780gcgaggattg aaattctgcc gttacgcaga tgactgcaac atctatgtga aaagtctgcg
840ggcaggacaa cgggtgaaac aaagcatcca acggttcttg gagaaaacgc tcaaactcaa
900agtaaacgag gagaaaagtg cggtggaccg cccgtggaaa cgggcctttc tggggtttag
960cttcacaccg gaacgaaaag cgcgaatccg gctcgcccca aggtcgattc aacgtctgaa
1020acagcggatt cgacagctga ccaacccaaa ctggagcata tcgatgccag aacgaattca
1080tcgcgtcaat caatacgtca tgggatggat cgggtatttt cggctcgtcg aaaccccgtc
1140tgtccttcag accatcgaag gatggattcg gaggaggctt cgactctgtc aatggcttca
1200atggaaacgg gtcagaacca gaatccgtga gttaagagcg ctggggctga aagagacagc
1260ggtgatggag atcgccaata cccgaaaagg agcttggcga acaacgaaaa cgccgcaact
1320ccaccaggcc ctgggcaaaa cctactggac cgctcaaggg ctcaagagtt tgacgcaacg
1380atatttcgaa ctccgtcaag gttgacgaac cgcctagtgc ggacccgcat gctaggtggt
1440gtgaggggac gggggttagc cgccccctcc tactcgattc gtattgtcat tcggcgctat
1500gccacgcgaa acggccatga acgtcaagcc cttctccttg ttagatcgtc tccttcccgc
1560gcacgccgtt gatcgaatag ctcgctgtaa tggcggcatt taacgaatgg gaaacggaac
162017299DNAArtificial SequencePartial DNA sequence of Tirt plasmid #16
17tggnnnnagt ntttaacctt tgnaccgccn taatacnact cactataggg gaattgtgag
60cggataacaa ttcccctcta gaaataattt tntttaactt taagaaggag atataccatg
120ggcagcagcc atcatcatca tcatcacagc agcggcctgg tgccgcgcgg cagccatatg
180cggcaagacc tgaatctcat cccgcggaag gagaagatca cgatggcttt gttggaacgc
240atcttagcga gagacaacct catcacggcg ctcaaacggg tcgaagccaa ccaaggagc
2991861PRTArtificialPartial amino acid sequence generated from Tirt
plasmid #16 (pTirt#16) 18Met Gly Ser Ser His His His His His His Ser Ser
Gly Leu Val Pro1 5 10
15Arg Gly Ser His Met Arg Gln Asp Leu Asn Leu Ile Pro Arg Lys Glu
20 25 30Lys Ile Thr Met Ala Leu Leu
Glu Arg Ile Leu Ala Arg Asp Asn Leu 35 40
45Ile Thr Ala Leu Lys Arg Val Glu Ala Asn Gln Gly Ala 50
55 60
User Contributions:
Comment about this patent or add new information about this topic: