Patent application title: NOVEL PEPTIDES THAT ENHANCE TIGHT JUNCTION PERMEABILITY
Inventors:
Amir Tamiz (Silver Spring, MD, US)
Min Li (Forest Park, IL, US)
Assignees:
Alba Therapeutics, Inc.
IPC8 Class: AA61K3828FI
USPC Class:
514 59
Class name: Designated organic active ingredient containing (doai) peptide (e.g., protein, etc.) containing doai insulin or derivative utilizing
Publication date: 2010-11-11
Patent application number: 20100286036
Claims:
1. A peptide tight junction agonist comprising the amino acid sequence of
SEQ ID NO:1, with at least one amino acid substitution, deletion, or
insertion, or at least one chemical modification, with respect to SEQ ID
NO: 1.
2. The tight junction agonist of claim 1, wherein the agonist has from one to five amino acid substitutions with respect to SEQ ID NO: 1.
3. The tight junction agonist of claim 1, wherein the agonist has 2 or 3 amino acid substitutions with respect to SEQ ID NO: 1.
4. The tight junction agonist of claim 1, wherein the position corresponding to position 1 of SEQ ID NO: 1 is Phe.
5. The tight junction agonist of claim 1, wherein the position corresponding to position 2 of SEQ ID NO: 1 is AllylGly.
6-9. (canceled)
10. The tight junction agonist of claim 1, wherein the tight junction agonist has a terminal basic group after the position corresponding to position 5 or 6 of SEQ ID NO: 1.
11. The tight junction agonist of claim 10, wherein the basic functional group is an amine.
12. The tight junction agonist of claim 1, comprising a substitution of at least one non-genetically encoded amino acid.
13-14. (canceled)
15. The tight junction agonist of claim 1, wherein the agonist is from 3 to 10 amino acids in length.
16-17. (canceled)
18. The peptide tight junction agonist of claim 1, wherein the peptide comprises an amino acid sequence selected from the group of SEQ ID NOs: 2-90.
19-27. (canceled)
28. A peptide tight junction agonist that comprises the amino acid sequence of SEQ ID NO: 2.
29-34. (canceled)
35. A pharmaceutical composition comprising the peptide tight junction agonist of claim 1 and at least one pharmaceutically acceptable excipient and/or active ingredient.
36-42. (canceled)
43. A method of treating a disease in a subject in need thereof, comprising:administering to the subject an effective dose of the pharmaceutical composition of claim 35.
44-48. (canceled)
Description:
PRIORITY
[0001]This application claims priority to US Provisional Application No 60,952,144 filed Jul. 26, 2007, U.S. Provisional Application No. 60/953,398 filed Aug. 1, 2007, U.S. Provisional Application No. 60/953,403 filed Aug. 1, 2007, and U.S. Provisional Application No. 60/953,405 filed Aug. 1, 2007, each of which is hereby incorporated by reference in its entirety.
FIELD OF THE INVENTION
[0002]The present invention provides novel peptides that enhance tight junction permeability and their use as therapeutic agents, and their use in materials and methods to facilitate the delivery of therapeutic agents. In some embodiments, novel peptides that enhance tight junction permeability (i.e., peptide tight junction agonists) are used in compositions to facilitate the uptake of therapeutic agents across biological barriers comprising tight junctions. In some embodiments, such peptide tight junction agonists are used in compositions to modulate an immune response in a subject. In some embodiments, such peptide tight junction agonists are used in compositions to raise an immune response against an antigen.
BACKGROUND
[0003]The tight junctions (tj) or zonula occludens (ZO) are one of the hallmarks of absorptive and secretory epithelia (Madara, J. Clin. Invest., 83:1089-1094 (1989); and Madara, Textbook of Secretory Diarrhea Eds. Lebenthal et al, Chapter 11, pages 125-138 (1990)). Tight junctions act as a barrier between apical and basolateral compartments, selectively regulating the passive diffusion of ions and water-soluble solutes through the paracellular (between cells) pathway (Gumbiner, Am. J. Physiol., 253 (Cell Physiol. 22):C749-C758 (1987)). This barrier maintains any gradient generated by the activity of pathways associated with the transcellular route (Diamond, Physiologist, 20:10-18 (1977)).
[0004]Variations in transepithelial conductance can usually be attributed to changes in the permeability of the paracellular pathway, since the resistances of enterocyte plasma membranes are relatively high (Madara, supra). The ZO represents the major barrier in this paracellular pathway, and the electrical resistance of epithelial tissues seems to depend on the number of transmembrane protein strands, and their complexity in the ZO, as observed by freeze-fracture electron microscopy (Madara et al, J. Cell Biol., 101:2124-2133 (1985)).
[0005]Zonula occludens toxin (ZOT), which is produced by Vibrio cholerae, has been characterized by Fasano et al., (Proc. Natl. Acad. Sci., USA, 8:5242-5246 (1991)) and the sequence has been determined (GenBank accession no. A43864). ZOT is a tight junction agonist and increases the intestinal permeability of rabbit ileal mucosa by modulating the structure of intercellular tight junctions. U.S. Pat. Nos. 5,827,534, 5,665,389, 5,908,825 disclose the use of ZOT to facilitate the uptake of therapeutic agents. U.S. patent publication nos. US-2006-0276403-A1 and US 2006-0165722 A1, and application Ser. No. 11/673,192 disclose peptide tight junction agonists that can be used to facilitate the uptake of therapeutic agents.
Drug Delivery
[0006]The low bioavailability (BA) of efficacious pharmacotherapeutic drugs continues to be a major obstacle in drug development and in many instances may be the deciding factor on whether or not a potent agent is developed. Many therapeutic agents experience low BA after oral administration due to poor absorption or susceptibility to first pass metabolism. A means of enhancing the gastrointestinal absorption of such drugs would significantly extend their therapeutic usefulness while decreasing the dose required to produce efficacy.
[0007]Absorption enhancers, including surfactants, fatty acids, and chitosan derivatives, have been used to modify bioavailability by either disruption of the cell membrane or modulation of the tight junctions (TJ). In general, the optimal absorption enhancer should possess the following qualities: its effect should be reversible, it should provide a rapid permeation enhancing effect on the intestinal cellular membrane, it should be non-cytotoxic at the effective concentration level without deleterious and/or irreversible effects on the cellular membrane or cytoskeleton of the TJ. Zonula Occludens Toxin (Zot), a 44.8 kDa protein (399 amino acids; AA) located in the cell envelope of the bacterial strain Vibrio cholerae, is capable of reversibly opening the TJ between cells and increasing the paracellular transport of many drugs in a non-toxic manner. Intensive investigation of the biological activity of Zot as an absorption enhancer was triggered by reports of effective oral administration of insulin with Zot in diabetic rats. Recently, a smaller 12 kDa fragment (AA 265-399) of Zot, referred to as delta G (AG), was introduced as the biologically active fragment of Zot. Amino acid comparison between Zot active fragment and Zonulin, combined with site-directed mutagenesis experiments, confirmed the presence of an octapeptide receptor-binding domain toward the amino terminus of the processed Zot.
[0008]Applicants disclose novel peptides that enhance tight junction permeability, and methods of increasing bioavailability of pharmacotherapeutic drugs. The novel peptides facilitate transport of pharmacotherapeutic drugs across biological barriers whose permeability is regulated by tight junctions and thereby allows for increased bioavailability of such drugs. The novel peptides of the present invention are advantageous in that they are non-toxic, their effects are reversible, they are devoid of endotoxin contamination, readily synthesized and inexpensive to produce and purify.
Vaccines
[0009]Vaccines have proven to be successful, highly acceptable methods for the prevention of infectious diseases. They are cost effective, and do not induce antibiotic resistance to the target pathogen or affect normal flora present in the host. In many cases, such as when inducing anti-viral immunity, vaccines can prevent a disease for which there are no viable curative or ameliorative treatments available.
[0010]As is well known in the art, vaccines function by triggering the immune system to mount a response to an immunogenic agent, or antigen (antigenic agent), typically an infectious organism or a portion thereof that is introduced into the body in a non-infectious or non-pathogenic form. Once the immune system has been "primed" or sensitized to the organism, later exposure of the immune system to this organism as an infectious pathogen results in a rapid and robust immune response that destroys the pathogen before it can multiply and infect enough cells in the host organism to cause disease symptoms. The agent or antigen used to induce the immune system can be the entire organism in a less infectious state, known as an attenuated organism, or in some cases, components of the organism such as carbohydrates, proteins or peptides representing various structural components of the organism.
[0011]In many cases, it is necessary to enhance the immune response to the antigens present in a vaccine in order to stimulate the immune system to a sufficient extent to make a vaccine effective, i.e., to confer immunity. Many protein and most peptide and carbohydrate antigens, administered alone, do not elicit a sufficient antibody response to confer immunity. Such antigens need to be presented to the immune system in such a way that they will be recognized as foreign and will elicit an immune response. To this end, adjuvants have been devised which stimulate the immune response.
[0012]The best known adjuvant, Freund's complete adjuvant, consists of a mixture of mycobacteria in an oil/water emulsion. Freund's adjuvant works in two ways: first, by enhancing cell and humoral-mediated immunity, and second, by blocking rapid dispersal of the antigen challenge (the "depot effect"). However, due to frequent toxic physiological and immunological reactions to this material, Freund's adjuvant cannot be used in humans. Another molecule that has been shown to have immunostimulatory or adjuvant activity is endotoxin, also known as lipopolysaccharide (LPS). LPS stimulates the immune system by triggering an "innate" immune response--a response that has evolved to enable an organism to recognize endotoxin (and the invading bacteria of which it is a component) without the need for the organism to have been previously exposed. While LPS is too toxic to be a viable adjuvant, molecules that are structurally related to endotoxin, such as monophosphoryl lipid A ("MPL") are being tested as adjuvants in clinical trials. Currently, however, the only FDA-approved adjuvant for use in humans is aluminum salts (Alum) which are used to "depot" antigens by precipitation of the antigens. Alum also stimulates the immune response to antigens.
[0013]Thus, there is a recognized need in the art for compounds which can be co-administered with antigens in order to stimulate the immune system to generate a more robust antibody response to the antigen than would be seen if the antigen were injected alone or with Alum. Further, because development of mucosal vaccines requires the use of specific adjuvants, adjuvants that work for systemic immunization such as Alum are generally not effective for mucosal immunization. Despite intensive research on adjuvants for mucosal vaccines in the last decade, no adjuvants have been registered for human use so far. The main issues in adjuvant research are efficacy and toxicity, and candidate mucosal adjuvants do not completely satisfy the criteria of high efficacy and absence of toxicity. Furthermore, most of the proposed mucosal adjuvants are complex molecules whose mechanism of action is poorly understood. Applicants provide herein non-toxic alternative peptide tight junction agonist adjuvants for inducing immune responses to an antigen.
[0014]Zonula Occludens Toxin (ZOT) from Vibrio cholerae was identified as an adjuvant for mucosal vaccination (Infect. Immun. 1999, 67:1287; Infect. Immun. 2003, 71:1897). Intranasal administration of ZOT with a soluble antigen in mice stimulated systemic humoral and cell-mediated responses as well as mucosal responses specific for the antigen Ovalbumin (Infect. Immun. 2003, 71:1897). ZOT is a protein of 44.8 kDa that binds a receptor on epithelial cells and modulates tight junctions, inducing the increase of mucosal barrier permeability. The effect of ZOT on tight junctions is reversible and does not cause tissue damage (J. Clin. Invest. 1995, 96:710). The receptor for ZOT on epithelial cells has been partially characterized and recently a mammalian protein with homology to ZOT has been identified and named Zonulin. Interestingly, this protein has been shown to be an endogenous regulator of tight junctions that is released by epithelial cells and binds to the same receptor used by ZOT (Ann. NY. Acad Sci. 2000, 915:214). The mechanism of ZOT as an adjuvant may involve binding to its receptor on the nasal mucosa, modulation of tight junctions and antigen passage in the submucosa, with subsequent exposure to cells of the immune system.
[0015]The development of mucosal vaccines for the prevention of infectious diseases is highly desirable. Mucosal vaccination has several advantages over parenteral vaccination. Mucosal immunization induces an immune response at the site of infection (locally). Furthermore, because of the intrinsic properties of the mucosal immune system, the immunization at one mucosal site can induce specific responses at distant sites (regionally). Such flexibility is important to address cultural and religious barriers to vaccination because protective immunity (for instance against sexually-transmitted diseases) may then be induced in segregated mucosal sites in a practical way. In addition to local responses against mucosally-acquired pathogens, mucosal vaccines induce systemic immunity, including humoral and cell-mediated responses. Thus, mucosal vaccination could be exploited for combating infections acquired through other routes (i.e., blood or skin). Finally, the administration of mucosal vaccines does not require the use of needles, which could increase vaccine compliance and negate concerns with blood transmissible infections. For all the above reasons mucosal vaccines may be used also to combat cancer, either with preventive or therapeutic vaccination. These vaccines may be both against cancers caused by infectious agents (such as Helicobacter pylori, Papilloma Virus, Herpes Virus) and cancers of different etiology (such as melanoma, colon cancer and others).
[0016]Interestingly, most human pathogens are acquired through the mucosal route, however, few mucosal vaccines are presently used. Of those currently used, the vaccine is based on a living attenuated microorganism. Further, purified antigens are not able to stimulate/induce an immune response per se when delivered at mucosal surfaces. Therefore, such vaccines require the use of specific adjuvants. Unfortunately, development of mucosal vaccines has been so far hampered by the lack of safe and effective adjuvants as described above. An effective mucosal adjuvant allows antigen (Ag) passage through a mucosal barrier and facilitates the induction of an Ag-specific immune response.
[0017]Applicants disclose novel peptides that enhance tight junction permeability, and methods of mucosal delivery of an antigen together with such peptides to induce systemic and/or mucosal responses specific for the antigen. The novel peptides facilitate delivery of the antigen through the mucosa and induce systemic and mucosal responses to the antigen. The novel peptides of the present invention are advantageous in that they are non-toxic, their effects are reversible, they are devoid of endotoxin contamination, readily synthesized and inexpensive to produce and purify.
[0018]There remains a need in the art for materials and methods to modulate immune responses and to facilitate the delivery of therapeutic agents. This need and others are met by the present invention.
SUMMARY OF THE INVENTION
[0019]The present invention provides novel peptide tight junction agonists (inducers). Generally, the peptide tight junction agonists comprise the amino acid sequence of SEQ ID NO:1, with at least one amino acid deletion, insertion, or substitution, or at least one chemical modification, with respect to SEQ ID NO: 1. For example, the peptide tight junction agonist may have from one to five amino acid substitutions with respect to SEQ ID NO: 1, while maintaining the activity as an agonist (inducer) of tight junction permeability. In certain embodiments, the tight junction agonist has an AllylGly at the position corresponding to position 2 of SEQ ID NO: 1.
[0020]Such peptide tight junction agonists of the invention may vary in length. In some embodiments, peptide tight junction agonists according to the invention may be from about three to about ten amino acids in length. In some embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs: 2-90. In some embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of the amino acid sequence of SEQ ID NO:2. In other embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:5-21. In other embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:22-38. In other embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:39-54. In other embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:55-72. In other embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:73-90. In some embodiments, peptide tight junction agonists of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:15, 23, 25, 26, 27, 29, 30 and 42. Additional tight junction agonists of the invention are as shown in the sequence listing filed concurrently herewith.
[0021]The present invention also provides compositions, e.g., pharmaceutical compositions, comprising one or more peptide tight junction agonists of the invention. Suitable peptide tight junction agonists for use in the compositions of the invention include, but are not limited to, peptide tight junction agonists that comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:2-90. In some embodiments, peptide tight junction agonists for use in the compositions of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of the amino acid sequence of SEQ ID NO:2. In other embodiments, peptide tight junction agonists for use in the compositions of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:5-21. In other embodiments, peptide tight junction agonists for use in the compositions of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:22-38. In other embodiments, peptide tight junction agonists for use in the compositions of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists an amino acid sequence selected from the group consisting of SEQ ID NOs:39-54. In other embodiments, peptide tight junction agonists for use in the compositions of the invention may comprise, consist essentially of or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:55-72. In other embodiments, peptide tight junction agonists for use in the compositions of the invention may comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:73-90. In some embodiments, peptide tight junction agonists for use in the compositions of the invention include, but are not limited to, peptide tight junction agonists that comprise, consist essentially of, or consist of a peptide that comprises, consists essentially of, or consists of an amino acid sequence selected from the group consisting of SEQ ID NOs:15, 23, 25, 26, 27, 29, 30 and 42. Compositions of the invention may further comprise one or more additional active agents. Typically additional active agents may be therapeutic agents, imaging agents, and/or immunogenic agents. Examples of suitable additional therapeutic agents include, but are not limited to, glucose metabolism agents (e.g., insulin), antibiotics, antineoplastics, antihypertensives, antiepileptics, central nervous system agents, and immune system suppressants. Examples of suitable additional immunogenic agents include, but are not limited to, antigens. Suitable additional imaging agents include, but are not limited to, agents comprising one or more radioactive atoms. A pharmaceutical composition of the invention may comprise one or more pharmaceutically acceptable excipients.
[0022]Compositions of the invention, for example, pharmaceutical compositions, may be formulated for any type of delivery. For example, compositions of the invention may be formulated for intestinal delivery, e.g., may be delayed release compositions. Compositions of the invention may also be formulated for pulmonary delivery, oral delivery and/or transcutaneous delivery.
[0023]In one embodiment, the present invention provides a method of treating a disease in a subject in need thereof. Methods of the invention may comprise administering to the subject a pharmaceutical composition comprising one or more peptide tight junction agonists and one or more additional therapeutic agents. In one embodiment, the present invention provides a method of treating diabetes in a subject in need thereof. In another embodiment, the present invention provides a method of treating an excessive or undesirable immune response in a subject in need thereof. In another embodiment, the present invention provides a method of treating inflammation in a subject in need thereof. In specific embodiments, the present invention provides methods of treating inflammatory bowel disease in a subject in need thereof. Inflammatory bowel disease that can be treated using methods of the present invention may be Crohn's disease or ulcerative colitis. In another embodiment, the present invention provides methods of treating cancer in a subject in need thereof.
[0024]In certain embodiments, pharmaceutical compositions of the present invention may comprise one or more insulins and/or derivatives thereof. In other embodiments, pharmaceutical compositions of the present invention may comprise one or more anti-inflammatory agents. In other embodiments, pharmaceutical compositions of the present invention may comprise one or more immune-suppressive drugs, for example, cyclosporin A. In another embodiment, pharmaceutical compositions of the present invention may comprise one or more anticancer agents.
BRIEF DESCRIPTION OF THE DRAWINGS
[0025]FIG. 1 is a schematic showing the steps involved in solid phase synthesis of an exemplary tight junction agonist of the invention.
[0026]FIG. 2 is a bar graph showing the results of a Real-Time Cell Electronic Sensing assay comparing the activity of various compounds of the invention to known tight junction agonist peptide FCIGRL (SEQ ID NO: 1).
[0027]FIG. 3 is a fluorescence microscopy analysis of the effects of tight junction agonist FCIGRL (SEQ ID NO:1) on IEC6 cells grown in monolayer and stained for F-actin.
[0028]FIG. 4 is a fluorescence microscopy analysis of the effects of PT-gliadin, tight junction agonist FCIGRL (SEQ ID NO:1), and various doses of tight junction agonist FCIGR on IEC6 cells grown in monolayer and stained for F-actin.
[0029]FIG. 5 is a fluorescence microscopy analysis of the effects of tight junction agonist FCIGRL (SEQ ID NO:1) on CaCo-2 cells grown in monolayer and stained for tight junction protein ZO-1. Cells were grown 21 days in a 6 well filter and treated with the junction agonist (apical and basolateral) for 3 hours.
[0030]FIG. 6 is a fluorescence microscopy analysis of the effects of tight junction agonist FCIGR on CaCo-2 cells grown in monolayer and stained for tight junction protein ZO-1.
[0031]FIG. 7 is a dose response curve for the tight junction agonist of SEQ ID NO:2 in a Lucifer Yellow (LY) permeability assay as described in Example 1.
[0032]FIG. 8 is a fluorescence microscopy analysis comparing the effects of tight junction agonist FCIGRL (SEQ ID NO:1) and F-(Allyl)GIGRL (SEQ ID NO:2) on CaCo-2 cells grown in monolayer and stained for tight junction protein ZO-1. Cells were grown 21 days in a 6 well filter and treated with the junction agonist (apical and basolateral) for 3 hours.
[0033]FIG. 9 is a fluorescence microscopy analysis showing a dose response of F-(Allyl)GIGRL (SEQ ID NO:2) on redistribution of ZO-1 in CaCo-2 BBE (Brush Border Expressing) cells grown in monolayer.
[0034]FIG. 10 is a fluorescence microscopy analysis showing the effects of FCIGRL (SEQ ID NO:1) and F-(Allyl)GIGRL (SEQ ID NO:2) on actin rearrangement in HeLa cells after 15 minutes and 60 minutes.
[0035]FIG. 11 is a magnified version of the 60 minute panels of FIG. 10, showing actin cytoskeletal rearrangement to the borderline of cells with the tight junction agonists FCIGRL (SEQ ID NO:1) and F-(Allyl)GIGRL (SEQ ID NO:2).
DETAILED DESCRIPTION OF THE INVENTION
Tight Junction Agonists
[0036]As used herein, a "tight junction agonist" is a compound that mediates or induces or facilitates or augments the physiological, transient opening of tight junctions, for example, the tight junctions between adjacent epithelial cells. An example of a tight junction agonist is zonula occludens toxin (ZOT), which is produced by Vibrio cholerae. A ZOT receptor agonist is a compound which is believed to mediate tight junction opening through the same receptor utilized by ZOT. In some embodiments, a tight junction agonist may comprise a peptide.
[0037]As used herein a subject is any animal, e.g., mammal, upon which methods of the invention may be practiced and/or to which materials of the present invention may be administered. Subjects include, but are not limited to, humans.
[0038]Tight junction agonists of the invention may comprise peptide tight junction agonists. An exemplary peptide tight junction agonist is a peptide that comprises the amino acid sequence Phe Cys Ile Gly Arg Leu (SEQ ID NO:1). Additional examples of peptide tight junction agonists of the invention include, but are not limited to, peptides wherein one or more amino acids of SEQ ID NO:1 have been substituted with a different amino acid.
[0039]In certain embodiments, position 2 of SEQ ID NO:1 will be substituted. In preferred embodiments the Cysteine (Cys or C) residue at position 2 of SEQ ID NO:1 is replaced by an AllylGly residue. In such preferred embodiments the peptide tight junction agonist of the invention comprises the amino acids sequence Phe-AllylGly-Ile-Gly-Arg-Leu of SEQ ID NO:2. In some embodiments, more than one position of SEQ ID NO:2 will be substituted. Substitutions may be made at any of position(s) 1, 3, 4, 5 or 6 of SEQ ID NO:2. In some embodiments, substitutions are made at position 1 of SEQ ID NO:2. In some embodiments, substitutions are made at position 3 of SEQ ID NO:2. In some embodiments, substitutions are made at position 4 of SEQ ID NO:2. In some embodiments, substitutions are made at position 5 of SEQ ID NO:2. In some embodiments, substitutions are made at position 6 of SEQ ID NO:2. In some embodiments, additional substitutions may be made at positions 1, 3, 4, 5 and 6 of SEQ ID NO:2 In some embodiments, position 1 of SEQ ID NO:2 will be substituted with another naturally occurring amino acid. In some embodiments, position 1 of SEQ ID NO:2 will be substituted with a non-naturally occurring amino acid. In some embodiments, position 3 of SEQ ID NO:2 will be substituted with another naturally occurring amino acid. In some embodiments, position 3 of SEQ ID NO:2 will be substituted with a non-naturally occurring amino acid. In some embodiments, position 4 of SEQ ID NO:2 will be substituted with another naturally occurring amino acid. In some embodiments, position 4 of SEQ ID NO:2 will be substituted with a non-naturally occurring amino acid (e.g., non-genetically encoded). In some embodiments, position 5 of SEQ ID NO:2 will be substituted with another naturally occurring amino acid. In some embodiments, position 5 of SEQ ID NO:2 will be substituted with a non-naturally occurring amino acid. In some embodiments, position 6 of SEQ ID NO:2 will be substituted with another naturally occurring amino acid. In some embodiments, position 6 of SEQ ID NO:2 will be substituted with a non-naturally occurring amino acid. Non-naturally occurring amino acids of the present invention are listed in Table 7. In some embodiments, a peptide tight junction agonist may comprise one or more D-amino acids.
[0040]When the tight junction agonist is a peptide, any length of peptide may be used. Generally, the size of the peptide tight junction agonist will range from about 3 to about 100, from about 3 to about 90, from about 3 to about 80, from about 3 to about 70, from about 3 to about 60, from about 3 to about 50, from about 3 to about 40, from about 3 to about 30, from about 3 to about 25, from about 3 to about 20, from about 3 to about 15, from about 3 to about 10, from about 3 to about 9, from about 3 to about 8, from about 3 to about 7, from about 3 to about 6, from about 3 to about 5, or from about 3 to about 4 amino acids in length. As used herein, "about" used to modify a numerical value means within about 10% of the value. Peptide tight junction agonists of the invention may be about 3, 4, 5, 6, 7, 8, 9, 10 or more amino acids in length.
[0041]The peptide tight junction agonists can be chemically synthesized and purified using well-known techniques, such as described in High Performance Liquid Chromatography of Peptides and Proteins: Separation Analysis and Conformation, Eds. Mant et al., C.R.C. Press (1991), and a peptide synthesizer, such as Symphony (Protein Technologies, Inc); or by using recombinant DNA techniques, i.e., where the nucleotide sequence encoding the peptide is inserted in an appropriate expression vector, e.g., an E. coli or yeast expression vector, expressed in the respective host cell, and purified therefrom using well-known techniques. A schematic representation of a solid phase synthesis of an exemplary tight junction agonist of the invention is shown in FIG. 1.
[0042]As used herein, the term "peptide" includes molecules with conventional peptide backbones as well as peptidomimetics having modified backbones. Such modifications include, but are not limited to, cyclization, N-terminus modification and C-terminus modification (including addition or modification with a basic group such as an amine), peptide bond modification, including, but not limited to backbones containing CH2--NH, CH2--S, CH2--S--O, O═C--NH, CH2--O, CH2--CH2, S═C--NH, CH═CH or CF═CH. Methods for preparing peptidomimetic compounds are well known in the art and are specified in Quantitative Drug Design, C. A. Ramsden Gd., Chapter 17.2, F. Choplin Pergamon Press (1992), which is hereby incorporated by reference.
[0043]Compositions
[0044]Typically, compositions, such as pharmaceutical compositions, comprise a peptide tight junction agonist and optionally one or more additional active agents. Peptide tight junction agonists may be present in an amount sufficient to facilitate the opening of tight junctions, for example, the tight junctions between adjacent epithelial cells; or in amount sufficient to modulate an immune response to an antigen; or in an amount sufficient to reduce inflammation, in a subject in need thereof. The amount of agonist (e.g., peptide tight junction agonist) employed in any given composition may vary according to factors such as the disease state, age, sex, and weight of the subject. Dosage regimens may be adjusted to provide the optimum therapeutic response. For example, a single bolus may be administered, several divided doses may be administered over time or the dose may be proportionally reduced or increased as indicated by the exigencies of the therapeutic situation.
[0045]Generally, a pharmaceutical composition of the invention will comprise an amount of peptide tight junction agonist in the range of about 1 μg to about 1 g, preferably about 1 mg to about 1000 mg, from about 10 mg to about 100 mg, from about 10 mg to about 50 mg, or from about 10 mg to about 25 mg of peptide tight junction agonist. As used herein, "about" used to modify a numerical value means within 10% of the value.
[0046]Compositions of the invention may comprise one or more peptide tight junction agonists at a level of from about 0.1 wt % to about 20 wt %, from about 0.1 wt % to about 18 wt %, from about 0.1 wt % to about 16 wt %, from about 0.1 wt % to about 14 wt %, from about 0.1 wt % to about 12 wt %, from about 0.1 wt % to about 10 wt %, from about 0.1 wt % to about 8 wt %, from about 0.1 wt % to about 6 wt %, from about 0.1 wt % to about 4 wt %, from about 0.1 wt % to about 2 wt %, from about 0.1 wt % to about 1 wt %, from about 0.1 wt % to about 0.9 wt %, from about 0.1 wt % to about 0.8 wt %, from about 0.1 wt % to about 0.7 wt %, from about 0.1 wt % to about 0.6 wt %, from about 0.1 wt % to about 0.5 wt %, from about 0.1 wt % to about 0.4 wt %, from about 0.1 wt % to about 0.3 wt %, or from about 0.1 wt % to about 0.2 wt % of the total weight of the composition. As used herein, "about" used to modify a numerical value means within 10% of the value. Compositions of the invention may comprise one or more peptide tight junction agonists at a level of about 0.1 wt %, about 0.2 wt %, about 0.3 wt %, about 0.4 wt %, about 0.5 wt %, about 0.6 wt %, about 0.7 wt %, about 0.8 wt %, or about 0.9 wt % based on the total weight of the composition.
[0047]Compositions of the invention may comprise one or more peptide tight junction agonists at a level of from about 1 wt % to about 20 wt %, from about 1 wt % to about 18 wt %, from about 1 wt % to about 16 wt %, from about 1 wt % to about 14 wt %, from about 1 wt % to about 12 wt %, from about 1 wt % to about 10 wt %, from about 1 wt % to about 9 wt %, from about 1 wt % to about 8 wt %, from about 1 wt % to about 7 wt %, from about 1 wt % to about 6 wt %, from about 1 wt % to about 5 wt %, from about 1 wt % to about 4 wt %, from about 1 wt % to about 3 wt %, or from about 1 wt % to about 2 wt % of the total weight of the composition. As used herein, "about" used to modify a numerical value means within 10% of the value. Compositions of the invention may comprise one or more peptide tight junction agonists at a level of about 1 wt %, about 2 wt %, about 3 wt %, about 4 wt %, about 5 wt %, about 6 wt %, about 7 wt %, about 8 wt %, or about 9 wt % based on the total weight of the composition.
[0048]Compositions of the invention, for example, pharmaceutical compositions comprising one or more peptide tight junction agonists and one or more additional active agents, may be formulated for pulmonary delivery (e.g., may be pulmonary dosage forms). Typically such compositions may be provided as pharmaceutical aerosols, e.g., solution aerosols or powder aerosols. Those of skill in the art are aware of many different methods and devices for the formation of pharmaceutical aerosols, for example, those disclosed by Sciarra and Sciarra, Aerosols, in Remington: The Science and Practice of Pharmacy, 20th Ed., Chapter 50, Gennaro et al. Eds., Lippincott, Williams and Wilkins Publishing Co., (2000).
[0049]In one embodiment, the dosage forms are in the form of a powder aerosol (i.e, comprise particles). These are particularly suitable for use in inhalation delivery systems. Powders may comprise particles of any size suitable for administration to the lung.
[0050]Powder formulations may optionally contain at least one particulate pharmaceutically acceptable carrier known to those of skill in the art. Examples of suitable pharmaceutical carriers include, but are not limited to, saccharides, including monosaccharides, disaccharides, polysaccharides and sugar alcohols such as arabinose, glucose, fructose, ribose, mannose, sucrose, trehalose, lactose, maltose, starches, dextran, mannitol or sorbitol. In one embodiment, a powder formulation may comprise lactose as a carrier.
[0051]Powder formulations may be contained in any container known to those in the art. Containers may be capsules of, for example, gelatin or plastic, or in blisters (e.g. of aluminum or plastic), for use in a dry powder inhalation device. In some embodiments, the total weight of the formulation in the container may be from about 5 mg to about 50 mg. In other embodiments, powder formulations may be contained in a reservoir in a multi-dose dry powder inhalation device adapted to deliver a suitable amount per actuation.
[0052]Powder formulations typically comprise small particles. Suitable particles can be prepared using any means known in the art, for example, by grinding in an airjet mill, ball mill or vibrator mill, sieving, microprecipitation, spray-drying, lyophilisation or controlled crystallisation. Typically, particles will be about 10 microns or less in diameter. Particles for use in the compositions of the invention may have a diameter of from about 0.1 microns to about 10 microns, from about 0.1 microns to about 9 microns, from about 0.1 microns to about 8 microns, from about 0.1 microns to about 7 microns, from about 0.1 microns to about 6 microns, from about 0.1 microns to about 5 microns, from about 0.1 microns to about 4 microns, from about 0.1 microns to about 3 microns, from about 0.1 microns to about 2 microns, from about 0.1 microns to about 1 micron, from about 0.1 microns to about 0.5 microns, from about 1 micron to about 10 microns, from about 1 micron to about 9 microns, from about 1 micron to about 8 microns, from about 1 micron to about 7 microns, from about 1 micron to about 6 microns, from about 1 micron to about 5 microns, from about 1 micron to about 4 microns, from about 1 micron to about 3 microns, from about 1 micron to about 2 microns, from about 2 microns to about 10 microns, from about 2 microns to about 9 microns, from about 2 microns to about 8 microns, from about 2 microns to about 7 microns, from about 2 microns to about 6 microns, from about 2 microns to about 5 microns, from about 2 microns to about 4 microns, or from about 2 microns to about 3 microns. As used herein, "about" used to modify a numerical value means within 10% of the value. In some embodiments, particles for use in the invention may be about 1 micron, about 2 microns, about 3 microns, about 4 microns, about 5 microns, about 6 microns, about 7 microns, about 8 microns, about 9 microns, or about 10 microns in diameter.
[0053]In one embodiment, the dosage forms are in the form of a solution aerosol (i.e., comprise droplets). Typically, droplets will be about 10 microns or less in diameter. Droplets for use in the compositions of the invention may have a diameter of from about 0.1 microns to about 10 microns, from about 0.1 microns to about 9 microns, from about 0.1 microns to about 8 microns, from about 0.1 microns to about 7 microns, from about 0.1 microns to about 6 microns, from about 0.1 microns to about 5 microns, from about 0.1 microns to about 4 microns, from about 0.1 microns to about 3 microns, from about 0.1 microns to about 2 microns, from about 0.1 microns to about 1 micron, from about 0.1 microns to about 0.5 microns, from about 1 micron to about 10 microns, from about 1 micron to about 9 microns, from about 1 micron to about 8 microns, from about 1 micron to about 7 microns, from about 1 micron to about 6 microns, from about 1 micron to about 5 microns, from about 1 micron to about 4 microns, from about 1 micron to about 3 microns, from about 1 micron to about 2 microns, from about 2 microns to about 10 microns, from about 2 microns to about 9 microns, from about 2 microns to about 8 microns, from about 2 microns to about 7 microns, from about 2 microns to about 6 microns, from about 2 microns to about 5 microns, from about 2 microns to about 4 microns, or from about 2 microns to about 3 microns. As used herein, "about" used to modify a numerical value means within 10% of the value. In some embodiments, particles and/or droplets for use in the invention may be about 1 micron, about 2 microns, about 3 microns, about 4 microns, about 5 microns, about 6 microns, about 7 microns, about 8 microns, about 9 microns, or about 10 microns in diameter.
[0054]The compositions of the invention may be formulated for enteric delivery, for example, may comprise one or more coatings including, for example, a delayed release coating containing one or more enteric agents. A delayed release coating is typically substantially stable in gastric fluid and substantially unstable (e.g., dissolves rapidly or is physically unstable) in intestinal fluid, thus providing for substantial release of the peptide tight junction agonist and/or active agent from the composition in the duodenum or the jejunum.
[0055]The term "stable in gastric fluid" refers to a composition that releases 30% or less by weight of the total peptide tight junction agonist and/or active agent in the composition in gastric fluid with a pH of 5 or less, or simulated gastric fluid with a pH of 5 or less, in approximately sixty minutes. Examples of simulated gastric fluid and simulated intestinal fluid include, but are not limited to, those disclosed in the 2005 Pharmacopeia 23NF/28USP in Test Solutions at page 2858 and/or other simulated gastric fluids and simulated intestinal fluids known to those of skill in the art, for example, simulated gastric fluid and/or intestinal fluid prepared without enzymes.
[0056]Compositions of the of the invention may release from about 0% to about 30%, from about 0% to about 25%, from about 0% to about 20%, from about 0% to about 15%, from about 0% to about 10%, from about 5% to about 30%, from about 5% to about 25%, from about 5% to about 20%, from about 5% to about 15%, from about 5% to about 10% by weight of the total peptide tight junction agonist and/or active agent in the composition in gastric fluid with a pH of 5 or less, or simulated gastric fluid with a pH of 5 or less, in approximately sixty minutes. As used herein, "about" used to modify a numerical value means within 10% of the value. Compositions of the invention may release about 1%, about 2%, about 3%, about 4%, about 5%, about 6%, about 7%, about 8%, about 9%, or about 10% by weight of the total peptide tight junction agonist in the composition in gastric fluid with a pH of 5 or less, or simulated gastric fluid with a pH of 5 or less, in approximately sixty minutes.
[0057]The term "unstable in intestinal fluid" refers to a composition that releases 70% or more by weight of the total peptide tight junction agonist and/or active agent in the composition in intestinal fluid or simulated intestinal fluid in approximately sixty minutes. The term "unstable in near neutral to alkaline environments" refers to a composition that releases 70% or more by weight of the total amount of tight junction agonist and/or active agent in the composition in intestinal fluid with a pH of 5 or greater, or simulated intestinal fluid with a pH of 5 or greater, in approximately ninety minutes. For example, a composition that is unstable in near neutral or alkaline environments may release 70% or more by weight of a tight junction agonist and/or active agent in a fluid having a pH greater than about 5 (e.g., a fluid having a pH of from about 5 to about 14, from about 6 to about 14, from about 7 to about 14, from about 8 to about 14, from about 9 to about 14, from about 10 to about 14, or from about 11 to about 14) in from about 5 minutes to about 90 minutes, from about 10 minutes to about 90 minutes, from about 15 minutes to about 90 minutes, from about 20 minutes to about 90 minutes, from about 25 minutes to about 90 minutes, from about 30 minutes to about 90 minutes, from about 5 minutes to about 60 minutes, from about 10 minutes to about 60 minutes, from about 15 minutes to about 60 minutes, from about 20 minutes to about 60 minutes, from about 25 minutes to about 60 minutes, or from about 30 minutes to about 60 minutes. As used herein, "about" used to modify a numerical value means within 10% of the value.
[0058]Compositions of the invention may be formulated for transcutaneous delivery (e.g., may be transcutaneous dosage forms). Typically such compositions may be provided as topical solutions and/or gels. Those of skill in the art are aware of many different methods and devices for the formation of topical medications, for example, those disclosed by Block, Medicated Topicals, in Remington: The Science and Practice of Pharmacy, 20th Ed., Chapter 44, Gennaro et al. Eds., Lippincott, Williams and Wilkins Publishing Co., (2000).
[0059]Additional Active Agents
[0060]In addition to one or more peptide tight junction agonists, compositions of the invention may further comprise one or more additional active agents, e.g., therapeutic agents, immunogenic agents and/or imaging agents.
[0061]Additional therapeutic agents that can be used in the compositions of the invention include agents that act on any organ of the body, such as heart, brain, intestine, or kidneys. Suitable additional therapeutic agents include, but are not limited to, glucose metabolism agents (e.g., insulin), antibiotics, antineoplastics, antihypertensives, antiepileptics, central nervous system agents, anti-inflammatory agents and immune system suppressants.
[0062]Additional therapeutic agents that can be used in the compositions of the invention include immunosuppressive agents. Such immunosuppressants used in the method and composition of the invention can be any agent which tends to attenuate the activity of the humoral or cellular immune systems. In particular, in one aspect the invention comprises compositions wherein the immunosuppressant is selected from the group consisting of cyclosporin A, FK506, prednisone, methylprednisolone, cyclophosphamide, thalidomide, azathioprine, and daclizumab, physalin B, physalin F, physalin G, seco-steroids purified from Physalis angulata L., 15-deoxyspergualin (DSG, 15-dos), MMF, rapamycin and its derivatives, CCI-779, FR 900520, FR 900523, NK86-1086, depsidomycin, kanglemycin-C, spergualin, prodigiosin25-c, cammunomicin, demethomycin, tetranactin, tranilast, stevastelins, myriocin, gliooxin, FR 651814, SDZ214-104, bredinin, WS9482, mycophenolic acid, mimoribine, misoprostol, OKT3, anti-IL-2 receptor antibodies, azasporine, leflunomide, mizoribine, azaspirane (SKF 105685), paclitaxel, altretamine, busulfan, chlorambucil, ifosfamide, mechlorethamine, melphalan, thiotepa, cladribine, fluorouracil, floxuridine, gemcitabine, thioguanine, pentostatin, methotrexate, 6-mercaptopurine, cytarabine, carmustine, lomustine, streptozotocin, carboplatin, cisplatin, oxaliplatin, iproplatin, tetraplatin, lobaplatin, JM216, JM335, fludarabine, aminoglutethimide, flutamide, goserelin, leuprolide, megestrol acetate, cyproterone acetate, tamoxifen, anastrozole, bicalutamide, dexamethasone, diethylstilbestrol, bleomycin, dactinomycin, daunorubicin, doxirubicin, idarubicin, mitoxantrone, losoxantrone, mitomycin-c, plicamycin, paclitaxel, docetaxel, topotecan, irinotecan, 9-amino camptothecan, 9-nitro camptothecan, GS-211, etoposide, teniposide, vinblastine, vincristine, vinorelbine, procarbazine, asparaginase, pegaspargase, octreotide, estramustine, and hydroxyurea, and combinations thereof. In one more particular aspect, the immunosuppressant is cyclosporin A.
[0063]Furthermore, the additional therapeutic agent can be selected from the group consisting of a chemotherapeutic, a gene therapy vector, a growth factor, a contrast agent, an angiogenesis factor, a radionuclide, an anti-infection agent, an anti-tumor compound, a receptor-bound agent, a hormone, a steroid, a protein, a complexing agent, a polymer, a thrombin inhibitor, an antithrombogenic agent, a tissue plasminogen activator, a thrombolytic agent, a fibrinolytic agent, a vasospasm inhibitor, a calcium channel blocker, a nitrate, a nitric oxide promoter, a vasodilator, an antihypertensive agent, an antimicrobial agent, an antibiotic, a glycoprotein IIb/IIIa inhibitor, an inhibitor of surface glycoprotein receptors, an antiplatelet agent, an antimitotic, a microtubule inhibitor, a retinoid, an antisecretory agent, an actin inhibitor, a remodeling inhibitor, an antisense nucleotide, an agent for molecular genetic intervention, an antimetabolite, an antiproliferative agent, an anti-cancer agent, a dexamethasone derivative, an anti-inflammatory steroid, a non-steroidal anti-inflammatory agent, an immunosuppressive agent, a PDGF antagonist, a growth hormone antagonist, a growth factor antibody, an anti-growth factor antibody, a growth factor antagonist, a dopamine agonist, a radiotherapeutic agent, an iodine-containing compound, a barium-containing compound, a heavy metal functioning as a radiopaque agent, a peptide, a protein, an enzyme, an extracellular matrix component, a cellular component, an angiotensin converting enzyme inhibitor, a 21-aminosteroid, a free radical scavenger, an iron chelator, an antioxidant, a sex hormone, an antipolymerase, an antiviral agent, an IgG2 Kappa antibody against Pseudomonas aeruginosa exotoxin A and reactive with A431 epidermoid carcinoma cells, monoclonal antibody against the noradrenergic enzyme dopamine beta-hydroxylase conjugated to saporin or other antibody targeted therapy agents, gene therapy agents, a prodrug, a photodynamic therapy agent, and an agent for treating benign prostatic hyperplasia (BHP), a 14C-, 3H-, 131I-, 32P- or 36S-radiolabelled form or other radiolabelled form of any of the foregoing, and combinations thereof.
[0064]More particularly, the additional therapeutic agent can be selected from the group consisting of parathyroid hormone, heparin, human growth hormone, covalent heparin, hirudin, hirulog, argatroban, D-phenylalanyl-L-poly-L-arginyl chloromethyl ketone, urokinase, streptokinase, nitric oxide, triclopidine, aspirin, colchicine, dimethyl sulfoxide, cytochalasin, deoxyribonucleic acid, methotrexate, tamoxifen citrate, dexamethasone, dexamethasone sodium phosphate, dexamethasone acetate, cyclosporin, trapidal, angiopeptin, angiogenin, dopamine, 60Co, 192Ir, 32P, 111In, 90Y, 99mTc, pergolide mesylate, bromocriptine mesylate, gold, tantalum, platinum, tungsten, captopril, enalapril, ascorbic acid, α-tocopherol, superoxide dismutase, deferoxamine, estrogen, azidothymidine (AZT), acyclovir, famciclovir, rimantadine hydrochloride, ganciclovir sodium, 5-aminolevulinic acid, meta-tetrahydroxyphenylchlorin, hexadecafluoro zinc phthalocyanine, tetramethyl hematoporphyrin, and rhodamine 123, and combinations thereof.
[0065]Compositions of the invention may comprise one or more immunogenic agents, for example, antigens. Examples of antigens that can be used in the compositions of the invention (e.g., immunogenic and/or vaccine compositions) include peptides, proteins, microorganisms (e.g., attenuated and/or recombinant microorganisms), cells (e.g., cancer cells and/or recombinant cells) and viruses (e.g., attenuated and/or recombinant viruses). Examples of peptide antigens include the B subunit of the heat-labile enterotoxin of enterotoxigenic E. coli, the B subunit of cholera toxin, capsular antigens of enteric pathogens, fimbriae or pili of enteric pathogens, HIV surface antigens, cancer antigens (e.g., cancer cells comprising antigens, isolated antigens, etc.), dust allergens, and acari allergens. Other immunogenic compounds as are known in the art can also be used.
[0066]Examples of attenuated microorganisms and viruses that can be used in the compositions of the invention (e.g., vaccine compositions) include those of enterotoxigenic Escherichia coli, enteropathogenic Escherichia coli, Vibrio cholerae, Shigella flexneri, Salmonella typhi and rotavirus (Fasano et al, In: Le Vaccinazioni in Pediatria, Eds. Vierucci et al, CSH, Milan, pages 109-121 (1991); Guandalini et al, In: Management of Digestive and Liver Disorders in Infants and Children, Elsevior, Eds. Butz et al, Amsterdam, Chapter 25 (1993); Levine et al, Sem. Ped. Infect. Dis., 5.243-250 (1994); and Kaper et al, Clin. Micrbiol. Rev., 8:48-86 (1995), each of which is incorporated by reference herein in its entirety).
[0067]Any antigen capable of inducing a protective immune response may be used in the vaccine compositions of the invention. Examples of suitable antigens include, but are not limited to, measles virus antigens, mumps virus antigens, rubella virus antigens, Corynebacterium diphtheriae antigens, Bordetella pertussis antigens, Clostridium tetani antigens, Bacillus anthracis antigens, Haemophilus influenzae antigens, smallpox virus antigens, and influenza virus antigens.
[0068]Compositions of the invention may further comprise one or more protease inhibitors. Any protease inhibitor can be used, including, but not limited to, a proteinase, peptidase, endopeptidase, or exopeptidase inhibitor. A cocktail of inhibitors can also be used. Alternatively, the protease inhibitors can be selected from the group consisting of bestatin, L-trans-3-carboxyoxiran-2-carbonyl-L-leucylagmatine, ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonylfluoride (PMSF), aprotinin, amyloid protein precursor (APP), amyloid beta precursor protein, α1-proteinase inhibitor, collagen VI, bovine pancreatic trypsin inhibitor (BPTI), 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), antipain, benzamidine, chymostatin, ε-aminocaproate, N-ethylmaleimide, leupeptin, pepstatin A, phosphoramidon, and combinations thereof. Novel protease inhibitors can also be used. Indeed, protease inhibitors can be specifically designed or selected to decrease the proteolysis of the tight junction agonist and/or the therapeutic agent.
[0069]Compositions of the invention may also comprise one or more pharmaceutically acceptable excipients. Suitable excipients include, but are not limited to, buffers, buffer salts, bulking agents, salts, surface active agents, acids, bases, sugars, binders, and the like.
[0070]Methods of Treatment
[0071]Peptide tight junction agonists and pharmaceutical compositions of the invention can be used for treating, ameliorating, and/or preventing a disease. Any disease may be treated using the compositions of the invention by selection of an appropriate active agent, e.g., therapeutic and/or immunogenic agent. In one embodiment, the present invention provides a method of treating diabetes response in a subject (e.g., a mammal such as a human) by administering a composition comprising one or more peptide tight junction agonists together with one or more insulins and/or derivatives thereof. In another embodiment, the invention provides a method of suppressing an excessive or undesirable immune response in a subject (e.g., a mammal such as a human) by administering a composition comprising one or more peptide tight junction agonists together with one or more immune-suppressive drugs that may include, for example, cyclosporin A.
[0072]Examples of diseases that can be treated using the compositions of the invention include, but are not limited to, cancer, autoimmune diseases, vascular disease, bacterial infections, gastritis, gastric cancer, collagenous colitis, inflammatory bowel disease, osteoporosis, systemic lupus erythematosus, food allergy, asthma, and irritable bowel syndrome. For example, to treat inflammatory bowel disease, a composition comprising one or more peptide tight junction agonists may be administered to the subject (e.g., a mammal such as a human) in need thereof.
[0073]In another example, to treat cancer of the colon or rectal area, a composition comprising a therapeutically effective amount of Erbitux® (Cetuximab) together with an absorption enhancing amount of one or more peptide tight junction agonists may be administered to the subject (e.g., a mammal such as a human) in need thereof. In another example, to treat breast cancer, a composition comprising a therapeutically effective amount of Herceptin® (Trastuzumab) together with an absorption enhancing amount of one or more peptide tight junction agonists may be administered to the subject (e.g., a mammal such as a human) in need thereof. In another example, to treat various types of cancer, a composition comprising a therapeutically effective amount of Avastin® (Bevacizumab) together with an absorption enhancing amount of one or more peptide tight junction agonists may be administered to the subject (e.g., a mammal such as a human) in need thereof. Another example involves treatment of osteoporosis by administration of a composition comprising one or more peptide tight junction agonists together with a therapeutically effective amount of Fosamax® (Alendronate) to the subject in need thereof. Another example involves treatment of transplant rejection by administration of a composition comprising one or more peptide tight junction agonists together with a therapeutically effective amount of Cyclosporin A to the subject in need thereof. Another example involves treatment of anemia by administration of a composition comprising one or more peptide tight junction agonists together with a therapeutically effective amount of erythropoietin to the subject in need thereof. Another example involves treatment of hemophilia by administration of a composition comprising one or more peptide tight junction agonists together with a therapeutically effective amount of Factor VIII to the subject in need thereof.
[0074]In some embodiments, compositions of the invention (e.g., pharmaceutical compositions) may be given repeatedly over a protracted period, i.e., may be chronically administered. Typically, compositions may be administered one or more times each day in an amount suitable to prevent, reduce the likelihood of an attack of, or reduce the severity of an attack of the underlying disease condition (e.g., diabetes, cancer, transplant rejection, etc). Such compositions may be administered chronically, for example, one or more times daily over a plurality of days.
[0075]In some embodiments, compositions of the invention (e.g., pharmaceutical compositions) may be used to treat acute attacks of the underlying disease (e.g., diabetes, cancer, transplant rejection, etc). Typically, embodiments of this type will require administration of the compositions of the invention to a subject undergoing an attack in an amount suitable to reduce the severity of the attack. One or more administrations may be used.
[0076]In some embodiments, peptide tight junction agonists of the invention may be used in the manufacture of compositions and pharmaceutical compositions for use in the methods described above.
[0077]The following examples are provided for illustrative purposes only, and are in no way intended to limit the scope of the present invention.
EXAMPLES
Example 1
Measurement of Trans Epithelial Electric Resistance (TEER) and Epithelial Flux of a Fluorescent Marker Lucifer Yellow
[0078]CaCo2 cells form monolayers that exhibit tight junctions between adjacent cells. Agonists of tight junctions can be identified by their ability to enhance the flux of compounds (e.g. ions, Lucifer Yellow) through a cell monolayer that comprises tight junctions; or by their ability to reduce TEER across a cell monolayer that comprises tight junctions. Treatment of CaCo2 monolayers with peptide FCIGRL (SEQ ID NO: 1) led to a 51-fold enhancement of Lucifer Yellow permeability through CaCo2 monolayers compared to vehicle alone. Further, FIG. 7 shows a dose response curve for the tight junction agonist of SEQ ID NO:2 in the Lucifer Yellow (LY) permeability assay.
[0079]Treatment of CaCo2 monolayers with peptide FCIGRL led to a 16-fold decrease in TEER across CaCo2 monolayers compared to vehicle alone.
[0080]Tight junction agonists can be identified and tested using the following method:
[0081]Determination of TEER and Lucifer Yellow flux
[0082]Prepare Modified Hank's Balanced Salt Solution (MHBSS) by obtaining 1 L bottle of HBSS removing 10 ml of HBSS and replacing it with 10 ml HEPES buffer pH 7.0. Adjust pH to 7.4±0.1 using concentrated NaOH (10N).
[0083]Remove CaCo-2 cells from incubator, grown on 12-well, 3.0 μM, polycarbonate Transwell® filters (Corning) and record passage#, date cells seeded and age in days.
[0084]Aspirate cell culture medium from both the apical (AP) and basolateral (BL) compartments, replacing with 0.5 ml and 1.5 ml of MHBSS, respectively. Incubate cells at 37° C. for 30 minutes.
[0085]Using the MilliCell®-ERS instrument (Millipore), measure and record the transepithelial electrical resistance (TEER) across each filter and record.
[0086]Aspirate solution from the apical compartment of each filter (n=3 per condition) and replace with 0.5 ml of control and test solutions containing Lucifer Yellow and test compound If appropriate.
[0087]Place all plates into incubator set at 37° C. (±0.2), 50 RPM (±5) for a total of 180 minutes.
[0088]At t=30, 60, 120 and 180 minutes, measure and record the transepithelial electrical resistance (TEER) across each filter using the MilliCell-ERS instrument.
[0089]At t=60, 120 and 180 minutes remove 1000 from each basolateral compartment and place it in a 96-well plate for Lucifer Yellow analysis, replace with 100 μl of MHBSS.
[0090]Make a Lucifer Yellow standard curve with the following dilutions (7500 μM, 3750 μM, 750 μM, 375 μM, 75 μM, 37.5 μM, 7.5 μM, 3.75 μM, 0.75 μM) and pipette 100 μL of each into a 96-well plate except for the first three standards mentioned above which require a 1:10 dilutions prior to transferring to the 96-well plate.
[0091]Harvest the remaining start solutions and what is left in each apical compartment into 1.5 ml vials. Freeze at -20° C. for future analysis.
[0092]Analyze each 96-well plate in a Tecan Spectra Fluor Plus using Magellan at 485 and 535 nm.
[0093]Materials:
[0094]Cells: CaCo-2 cells passage 40-60 grown on Transwell plates for 21-28 days
[0095]Culture Medium: DMEM supplemented with 10% fetal bovine serum, 1% NEAA, 1% Penn/Strep
[0096]Buffers: Hank's Balanced Salt Solution (HBSS) without calcium and magnesium
[0097]Flasks: 100×20 mm Tissue culture dish Falcon.
[0098]Plates: 12 well polycarbonate Transwell filters; 0.3 uM pore size
Example 2
Identification of Tight Junction Agonists Using Real-Time Cell Electronic Sensing (RT-CES)
[0099]IEC6 cells form monolayers that exhibit tight junctions between adjacent cells. Agonists of tight junctions can be identified by their ability to induce changes in morphology of cells in a monolayer of cells that comprise tight junctions. Such changes in the morphology of IEC6 cells may be measured using a Real-Time Cell Electronic Sensing protocol as described below.
[0100]Tight junction agonists can be identified using the following method:
[0101]Materials: cells: IEC6 passage 30-50, medium: DMEM 10% no calcium no magnesium, foetal bovine serum 0.1 unit per ml bovine insulin, buffers: phosphate buffered saline (PBS) no calcium no magnesium, trypsin: 0.25% porcine trypsin in HBSS no calcium no magnesium, flasks: 100×20 mm Tissue culture dish Falcon, plates: 16× E-Plate, machine: RT-CES® 16× system (ACEA Biosciences, Inc., San Diego, Calif.)
[0102]Wash a 75 cm2 flask of confluent IEC6 cells twice with 25 ml of PBS.
[0103]Add 2.5 ml of trypsin to the flask and place back in the incubator at 37° C.
[0104]Wash cells from the surface of the flask with 10 ml of serum containing media to quench the trypsin.
[0105]Pellet the cells by centrifugation at 1500 rpm for 5 minutes aspirate the media.
[0106]Resuspend the cell pellet in 10 ml of serum free media and centrifuge at 1500 rpm for 5 minutes aspirate the media and repeat for a total of 5 washes.
[0107]Take 100 μl of cells and mix with 100 μl of trypan blue.
[0108]Count the cells four times and use the average cell concentration.
[0109]Dilute the cells to 1×106 per ml in serum free media.
[0110]Add 100 μl of serum free media to each well of the ACEA plates to be used.
[0111]Insert the ACEA plate and press scan.
[0112]Run step 1 of the program to measure background.
[0113]Add 50 μl of cells to each well of the ACEA plate tap each slide of the plate 10 times and place the cells on the bench for 15 minutes to allow the cells to settle.
[0114]Insert the ACEA plate and run the scan step to check connections and run step 2-1 and 2-2 overnight.
[0115]Step 2-1 sample every 2 minutes 30 times.
[0116]Step 2-2 sample every 15 minutes 100 times.
[0117]The cell indices should be between 6-10 after the overnight run and should have reached a plateau.
[0118]Remove 100 μl of media carefully from each well.
[0119]Make up the compounds to be tested so that 50 μl contains 2× the desired final concentration.
[0120]Add 50 μl of compounds to the designated wells.
[0121]Scan the plate to check connections.
[0122]Run steps 3-1 and 3-2.
[0123]Step 3-1 sample every 2 minutes 30 times.
[0124]Step 3-2 sample every 15 minutes 100 times.
[0125]FIG. 2 shows a comparison of ACEA data for the indicated tight junction agonists.
Example 3
Assay of Cytoskeletal Rearrangement Induced by Tight Junction Agonists
[0126]Gliadin treated with the peptidases pepsin and trypsin (termed PT-gliadin or PTG) induces a cytoskeletal arrangement in CaCo2 cells grown in monolayers.
[0127]The rearrangement can be visualized using a Nikon-TE2000 epifluorescence microscope and a 40× objective and Alexa Fluor 555 conjugated phalloidin (Invitrogen, Carlsbad, Calif.), which binds specifically to F-actin. Exposure times were identical for control and agonist treated samples. The figures were generated using Adobe Photoshop CS2 v 9.0.2. The concentration of agonist was as indicated. Tight junction agonists can be identified by their ability to induce the cytoskeletal rearrangement as shown by the effects of peptide FCIGRL, a known tight junction agonist. FIGS. 3-6 and 8-11 show the cytoskeletal rearrangements induced by exemplary tight junction agonists of the invention, including with respect to the distribution of F-actin and the tight junction protein ZO-1.
[0128]Additional results are provided in the following tables. The first column of the table provides SEQ ID NO: of the peptide, the second column provides the sequence of the peptides tested, the third column provides the results of the indicated assay (i.e., ACEA, TEER reduction, or Lucifer Yellow flux).
[0129]In the following tables + indicates an enhancement of the permeability of the tight junctions were observed and - indicates no enhancement of permeability was observed.
TABLE-US-00001 TABLE 1 Amino acid substitutions at position 2 of SEQ ID NO: 1. SEQ ID Enhanced LY Reduced NO: Sequence permeability TEER 1 Phe-Cys-Ile-Gly-Arg-Leu + + 2 Phe-AllylGly-Ile-Gly-Arg-Leu-OH + + 3 Phe-AllylGly-Ile-Gly-Arg-NH2 - - 4 Phe-(d)AllylGly-Ile-Gly-Arg-NH2 - -
TABLE-US-00002 TABLE 2 Amino acid substitutions at position 1 of SEQ ID NO: 2. SEQ ID Enhanced LY Reduced NO: Sequence permeability TEER 1 Phe-Cys-Ile-Gly-Arg-Leu + + 5 Met-AllylGly-Ile-Gly-Arg-Leu-NH2 + - 6 Gln-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 7 Leu-AllylGly-Ile-Gly-Arg-Leu-NH2 + - 8 Ser-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 9 Thr-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 10 Glu-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 11 Val-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 12 Tyr-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 13 Gly-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 14 Asp-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 15 Trp-AllylGly-Ile-Gly-Arg-Leu-NH2 + - 16 Lys-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 17 Ala-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 18 His-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 19 Pro-AllylGly-Ile-Gly-Arg-Leu-NH2 - - 20 Arg-AllylGly-Ile-Gly-Arg-Leu-NH2 + - 21 Ile-AllylGly-Ile-Gly-Arg-Leu-NH2 - -
TABLE-US-00003 TABLE 3 Amino acid substitutions at position 3 of SEQ ID NO: 2. SEQ ID Enhanced LY Reduced NO: Sequence permeability TEER 1 Phe-Cys-Ile-Gly-Arg-Leu + + 22 Phe-AllylGly-Pro-Gly-Arg-Leu-NH2 - - 23 Phe-AllylGly-Phe-Gly-Arg-Leu-NH2 + + 24 Phe-AllylGly-Thr-Gly-Arg-Leu-NH2 - - 25 Phe-AllylGly-Leu-Gly-Arg-Leu-NH2 + + 26 Phe-AllylGly-Ser-Gly-Arg-Leu-NH2 + + 27 Phe-AllylGly-Phe-Gly-Arg-Leu-NH2 + + 28 Phe-AllylGly-Val-Gly-Arg-Leu-NH2 - - 29 Phe-AllylGly-Gly-Gly-Arg-Leu-NH2 + + 30 Phe-AllylGly-Ala-Gly-Arg-Leu-NH2 + + 31 Phe-AllylGly-His-Gly-Arg-Leu-NH2 + + 32 Phe-AllylGly-Asp-Gly-Arg-Leu-NH2 + + 33 Phe-AllylGly-Glu-Gly-Arg-Leu-NH2 + + 34 Phe-AllylGly-Gln-Gly-Arg-Leu-NH2 + + 35 Phe-AllylGly-Arg-Gly-Arg-Leu-NH2 + + 36 Phe-AllylGly-Lys-Gly-Arg-Leu-NH2 + + 37 Phe-AllylGly-Asn-Gly-Arg-Leu-NH2 + + 38 Phe-AllylGly-Tyr-Gly-Arg-Leu-NH2 + +
TABLE-US-00004 TABLE 4 Amino acid substitutions at position 4 of SEQ ID NO: 2. SEQ ID Enhanced LY Reduced NO: Sequence permeability TEER 1 Phe-Cys-Ile-Gly-Arg-Leu + + 39 Phe-AllylGly-Ile-Thr-Arg-Leu-NH2 + + 40 Phe-AllylGly-Ile-Leu-Arg-Leu-NH2 41 Phe-AllylGly-Ile-Ile-Arg-Leu-NH2 42 Phe-AllylGly-Ile-Ala-Arg-Leu-NH2 + + 43 Phe-AllylGly-Ile-Pro-Arg-Leu-NH2 - - 44 Phe-AllylGly-Ile-Gly-Arg-Leu-NH2 45 Phe-AllylGly-Ile-His-Arg-Leu-NH2 + + 46 Phe-AllylGly-Ile-Asp-Arg-Leu-NH2 + + 47 Phe-AllylGly-Ile-Glu-Arg-Leu-NH2 + + 48 Phe-AllylGly-Ile-Gln-Arg-Leu-NH2 + + 49 Phe-AllylGly-Ile-Phe-Arg-Leu-NH2 + + 50 Phe-AllylGly-Ile-Arg-Arg-Leu-NH2 51 Phe-AllylGly-Ile-Lys-Arg-Leu-NH2 - - 52 Phe-AllylGly-Ile-Asn-Arg-Leu-NH2 + + 53 Phe-AllylGly-Ile-Ser-Arg-Leu-NH2 + + 54 Phe-AllylGly-Ile-Val-Arg-Leu-NH2 - +
TABLE-US-00005 TABLE 5 Amino acid substitutions at position 5 of SEQ ID NO: 2. SEQ ID Enhanced LY Reduced NO: Sequence permeability TEER 1 Phe-Cys-Ile-Gly-Arg-Leu + + 55 Phe-AllylGly-Ile-Gly-His-Leu-NH2 - - 56 Phe-AllylGly-Ile-Gly-Asp-Leu-NH2 + + 57 Phe-AllylGly-Ile-Gly-Glu-Leu-NH2 - - 58 Phe-AllylGly-Ile-Gly-Gln-Leu-NH2 - - 59 Phe-AllylGly-Ile-Gly-Gly-Leu-NH2 + + 60 Phe-AllylGly-Ile-Gly-Ala-Leu-NH2 + 61 Phe-AllylGly-Ile-Gly-Phe-Leu-NH2 62 Phe-AllylGly-Ile-Gly-Lys-Leu-NH2 + + 63 Phe-AllylGly-Ile-Gly-Leu-Leu-NH2 64 Phe-AllylGly-Ile-Gly-Met-Leu-NH2 65 Phe-AllylGly-Ile-Gly-Asn-Leu-NH2 66 Phe-AllylGly-Ile-Gly-Ser-Leu-NH2 67 Phe-AllylGly-Ile-Gly-Tyr-Leu-NH2 68 Phe-AllylGly-Ile-Gly-Thr-Leu-NH2 + 69 Phe-AllylGly-Ile-Gly-Ile-Leu-NH2 - 70 Phe-AllylGly-Ile-Gly-Trp-Leu-NH2 + + 71 Phe-AllylGly-Ile-Gly-Pro-Leu-NH2 + - 72 Phe-AllylGly-Ile-Gly-Val-Leu-NH2 - +
TABLE-US-00006 TABLE 6 Amino acid substitutions at position 6 of SEQ ID NO: 2. SEQ ID Enhanced LY Reduced NO: Sequence permeability TEER 1 Phe-Cys-Ile-Gly-Arg-Leu + + 73 Phe-AllylGly-Ile-Gly-Arg-His-NH2 + + 74 Phe-AllylGly-Ile-Gly-Arg-Asp-NH2 + + 75 Phe-AllylGly-Ile-Gly-Arg-Arg-NH2 + - 76 Phe-AllylGly-Ile-Gly-Arg-Phe-NH2 + + 77 Phe-AllylGly-Ile-Gly-Arg-Ala-NH2 + - 78 Phe-AllylGly-Ile-Gly-Arg-Gly-NH2 + - 79 Phe-AllylGly-Ile-Gly-Arg-Gln-NH2 - - 80 Phe-AllylGly-Ile-Gly-Arg-Glu-NH2 + + 81 Phe-AllylGly-Ile-Gly-Arg-Thr-NH2 - + 82 Phe-AllylGly-Ile-Gly-Arg-Tyr-NH2 + + 83 Phe-AllylGly-Ile-Gly-Arg-Ser-NH2 - - 84 Phe-AllylGly-Ile-Gly-Arg-Asn-NH2 + + 85 Phe-AllylGly-Ile-Gly-Arg-Met-NH2 + + 86 Phe-AllylGly-Ile-Gly-Arg-Lys-NH2 + - 87 Phe-AllylGly-Ile-Gly-Arg-Ile-NH2 + + 88 Phe-AllylGly-Ile-Gly-Arg-Trp-NH2 + + 89 Phe-AllylGly-Ile-Gly-Arg-Pro-NH2 - - 90 Phe-AllylGly-Ile-Gly-Arg-Val-NH2 + +
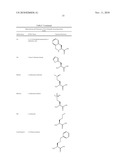
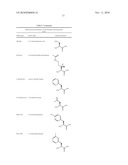
TABLE-US-00007 TABLE 7 Abbreviations and Structures of Non-Naturally Occurring Amino Acids. Abbreviation IUPAC Name Chemical Structure Sar Sarcosine ##STR00001## Aib alpha-aminoisobutyric acid ##STR00002## Tle L-tert-Leucine ##STR00003## MeAla L-N-methyl Alanine ##STR00004## Abu L-alpha-aminobutyric acid ##STR00005## Phe(4-NO2) L-4-nitro-phenylalanine ##STR00006## Phe(4-Cl) L-4-chlorophenylalanine ##STR00007## Tic L-1,2,3,4-tetrahydroisoquinoline-3- carboxylic acid ##STR00008## Thi L-beta-(2-thienyl)-alanine ##STR00009## Met(O) L-methionine-sulfoxide ##STR00010## Met(O)2 L-methionine-sulfone ##STR00011## Nle L-norleucine ##STR00012## Cys(S-benzyl) L-S-benzyl-cysteine ##STR00013## Cys(t-buthiol) L-S-tert-butylthio-cysteine ##STR00014## Phg L-phenylglycine ##STR00015## Cha L-beta-cyclohexyl-alanine ##STR00016## (Allyl)Gly L-allylglycine ##STR00017## (t-Bu)Gly L-tert-butylglycine ##STR00018## Dab L-1,4-diaminobutyric acid ##STR00019## (cyclopropane)Pro L-(R,S)-3,4-cis-methanoproline ##STR00020## Dap/Dpr L-1,3-diaminopropionic acid ##STR00021## Pen(Acm) L-S-acetamidomethyl-penicillamine ##STR00022## (2-pyridyl)Ala L-beta-(2-pyridyl)-alanine ##STR00023## (4,5-dehydro)Leu L-4,5-dehydro-leucine ##STR00024## Phe(4-CN) L-4-cyano-phenylalanine ##STR00025## Phe(3-Me) L-3-methyl-phenylalanine ##STR00026## (cyclopropyl)Ala L-beta-cyclopropyl-alanine ##STR00027## Pra L-propargylglycine ##STR00028## (2-furyl)Ala L-beta-(2-furyl)-alanine ##STR00029## Thh 1,2,3,4-tetrahydroharmane-3- carboxylic acid ##STR00030## (styryl)Gly L-beta-styryl-alanine ##STR00031## HOCit Homocitrulline ##STR00032##
[0130]All publications, patents and patent applications mentioned in this specification are indicative of the level of skill of those skilled in the art to which this invention pertains, and are herein incorporated by reference to the same extent as if each individual publication, patent or patent application was specifically and individually indicated to be incorporated by reference.
Sequence CWU
1
27416PRTUnknownPeptide tight junction agonist 1Phe Cys Ile Gly Arg Leu1
526PRTUnknownPeptide tight junction agonist 2Phe Xaa Ile Gly
Arg Leu1 535PRTUnknownPeptide tight junction agonist 3Phe
Xaa Ile Gly Arg1 545PRTUnknownPeptide tight junction
agonist 4Phe Xaa Ile Gly Arg1 556PRTUnknownPeptide tight
junction agonist 5Met Xaa Ile Gly Arg Leu1
566PRTUnknownPeptide tight junction agonist 6Gln Xaa Ile Gly Arg Leu1
576PRTUnknownPeptide tight junction agonist 7Leu Xaa Ile Gly
Arg Leu1 586PRTUnknownPeptide tight junction agonist 8Ser
Xaa Ile Gly Arg Leu1 596PRTUnknownPeptide tight junction
agonist 9Thr Xaa Ile Gly Arg Leu1 5106PRTUnknownPeptide
tight junction agonist 10Glu Xaa Ile Gly Arg Leu1
5116PRTUnknownPeptide tight junction agonist 11Val Xaa Ile Gly Arg Leu1
5126PRTUnknownPeptide tight junction agonist 12Tyr Xaa Ile
Gly Arg Leu1 5136PRTUnknownPeptide tight junction agonist
13Gly Xaa Ile Gly Arg Leu1 5146PRTUnknownPeptide tight
junction agonist 14Asp Xaa Ile Gly Arg Leu1
5156PRTUnknownPeptide tight junction agonist 15Trp Xaa Ile Gly Arg Leu1
5166PRTUnknownPeptide tight junction agonist 16Lys Xaa Ile
Gly Arg Leu1 5176PRTUnknownPeptide tight junction agonist
17Ala Xaa Ile Gly Arg Leu1 5186PRTUnknownPeptide tight
junction agonist 18His Xaa Ile Gly Arg Leu1
5196PRTUnknownPeptide tight junction agonist 19Pro Xaa Ile Gly Arg Leu1
5206PRTUnknownPeptide tight junction agonist 20Arg Xaa Ile
Gly Arg Leu1 5216PRTUnknownPeptide tight junction agonist
21Ile Xaa Ile Gly Arg Leu1 5226PRTUnknownPeptide tight
junction agonist 22Phe Xaa Pro Gly Arg Leu1
5236PRTUnknownPeptide tight junction agonist 23Phe Xaa Phe Gly Arg Leu1
5246PRTUnknownPeptide tight junction agonist 24Phe Xaa Thr
Gly Arg Leu1 5256PRTUnknownPeptide tight junction agonist
25Phe Xaa Leu Gly Arg Leu1 5266PRTUnknownPeptide tight
junction agonist 26Phe Xaa Ser Gly Arg Leu1
5276PRTUnknownPeptide tight junction agonist 27Phe Xaa Phe Gly Arg Leu1
5286PRTUnknownPeptide tight junction agonist 28Phe Xaa Val
Gly Arg Leu1 5296PRTUnknownPeptide tight junction agonist
29Phe Xaa Gly Gly Arg Leu1 5306PRTUnknownPeptide tight
junction agonist 30Phe Xaa Ala Gly Arg Leu1
5316PRTUnknownPeptide tight junction agonist 31Phe Xaa His Gly Arg Leu1
5326PRTUnknownPeptide tight junction agonist 32Phe Xaa Asp
Gly Arg Leu1 5336PRTUnknownPeptide tight junction agonist
33Phe Xaa Glu Gly Arg Leu1 5346PRTUnknownPeptide tight
junction agonist 34Phe Xaa Gln Gly Arg Leu1
5356PRTUnknownPeptide tight junction agonist 35Phe Xaa Arg Gly Arg Leu1
5366PRTUnknownPeptide tight junction agonist 36Phe Xaa Lys
Gly Arg Leu1 5376PRTUnknownPeptide tight junction agonist
37Phe Xaa Asn Gly Arg Leu1 5386PRTUnknownPeptide tight
junction agonist 38Phe Xaa Tyr Gly Arg Leu1
5396PRTUnknownPeptide tight junction agonist 39Phe Xaa Ile Thr Arg Leu1
5406PRTUnknownPeptide tight junction agonist 40Phe Xaa Ile
Leu Arg Leu1 5416PRTUnknownPeptide tight junction agonist
41Phe Xaa Ile Ile Arg Leu1 5426PRTUnknownPeptide tight
junction agonist 42Phe Xaa Ile Ala Arg Leu1
5436PRTUnknownPeptide tight junction agonist 43Phe Xaa Ile Pro Arg Leu1
5446PRTUnknownPeptide tight junction agonist 44Phe Xaa Ile
Gly Arg Leu1 5456PRTUnknownPeptide tight junction agonist
45Phe Xaa Ile His Arg Leu1 5466PRTUnknownPeptide tight
junction agonist 46Phe Xaa Ile Asp Arg Leu1
5476PRTUnknownPeptide tight junction agonist 47Phe Xaa Ile Glu Arg Leu1
5486PRTUnknownPeptide tight junction agonist 48Phe Xaa Ile
Gln Arg Leu1 5496PRTUnknownPeptide tight junction agonist
49Phe Xaa Ile Phe Arg Leu1 5506PRTUnknownPeptide tight
junction agonist 50Phe Xaa Ile Arg Arg Leu1
5516PRTUnknownPeptide tight junction agonist 51Phe Xaa Ile Lys Arg Leu1
5526PRTUnknownPeptide tight junction agonist 52Phe Xaa Ile
Asn Arg Leu1 5536PRTUnknownPeptide tight junction agonist
53Phe Xaa Ile Ser Arg Leu1 5546PRTUnknownPeptide tight
junction agonist 54Phe Xaa Ile Val Arg Leu1
5556PRTUnknownPeptide tight junction agonist 55Phe Xaa Ile Gly His Leu1
5566PRTUnknownPeptide tight junction agonist 56Phe Xaa Ile
Gly Asp Leu1 5576PRTUnknownPeptide tight junction agonist
57Phe Xaa Ile Gly Glu Leu1 5586PRTUnknownPeptide tight
junction agonist 58Phe Xaa Ile Gly Gln Leu1
5596PRTUnknownPeptide tight junction agonist 59Phe Xaa Ile Gly Gly Leu1
5606PRTUnknownPeptide tight junction agonist 60Phe Xaa Ile
Gly Ala Leu1 5616PRTUnknownPeptide tight junction agonist
61Phe Xaa Ile Gly Phe Leu1 5626PRTUnknownPeptide tight
junction agonist 62Phe Xaa Ile Gly Lys Leu1
5636PRTUnknownPeptide tight junction agonist 63Phe Xaa Ile Gly Leu Leu1
5646PRTUnknownPeptide tight junction agonist 64Phe Xaa Ile
Gly Met Leu1 5656PRTUnknownPeptide tight junction agonist
65Phe Xaa Ile Gly Asn Leu1 5666PRTUnknownPeptide tight
junction agonist 66Phe Xaa Ile Gly Ser Leu1
5676PRTUnknownPeptide tight junction agonist 67Phe Xaa Ile Gly Tyr Leu1
5686PRTUnknownPeptide tight junction agonist 68Phe Xaa Ile
Gly Thr Leu1 5696PRTUnknownPeptide tight junction agonist
69Phe Xaa Ile Gly Ile Leu1 5706PRTUnknownPeptide tight
junction agonist 70Phe Xaa Ile Gly Trp Leu1
5716PRTUnknownPeptide tight junction agonist 71Phe Xaa Ile Gly Pro Leu1
5726PRTUnknownPeptide tight junction agonist 72Phe Xaa Ile
Gly Val Leu1 5736PRTUnknownPeptide tight junction agonist
73Phe Xaa Ile Gly Arg His1 5746PRTUnknownPeptide tight
junction agonist 74Phe Xaa Ile Gly Arg Asp1
5756PRTUnknownPeptide tight junction agonist 75Phe Xaa Ile Gly Arg Arg1
5766PRTUnknownPeptide tight junction agonist 76Phe Xaa Ile
Gly Arg Phe1 5776PRTUnknownPeptide tight junction agonist
77Phe Xaa Ile Gly Arg Ala1 5786PRTUnknownPeptide tight
junction agonist 78Phe Xaa Ile Gly Arg Gly1
5796PRTUnknownPeptide tight junction agonist 79Phe Xaa Ile Gly Arg Gln1
5806PRTUnknownPeptide tight junction agonist 80Phe Xaa Ile
Gly Arg Glu1 5816PRTUnknownPeptide tight junction agonist
81Phe Xaa Ile Gly Arg Thr1 5826PRTUnknownPeptide tight
junction agonist 82Phe Xaa Ile Gly Arg Tyr1
5836PRTUnknownPeptide tight junction agonist 83Phe Xaa Ile Gly Arg Ser1
5846PRTUnknownPeptide tight junction agonist 84Phe Xaa Ile
Gly Arg Asn1 5856PRTUnknownPeptide tight junction agonist
85Phe Xaa Ile Gly Arg Met1 5866PRTUnknownPeptide tight
junction agonist 86Phe Xaa Ile Gly Arg Lys1
5876PRTUnknownPeptide tight junction agonist 87Phe Xaa Ile Gly Arg Ile1
5886PRTUnknownPeptide tight junction agonist 88Phe Xaa Ile
Gly Arg Trp1 5896PRTUnknownPeptide tight junction agonist
89Phe Xaa Ile Gly Arg Pro1 5906PRTUnknownPeptide tight
junction agonist 90Phe Xaa Ile Gly Arg Val1
5916PRTUnknownPeptide tight junction agonist 91Phe Cys Ile Gly Arg Leu1
5926PRTUnknownPeptide tight junction agonist 92Phe Thr Ile
Gly Arg Leu1 5936PRTUnknownPeptide tight junction agonist
93Phe Ser Ile Gly Arg Leu1 5946PRTUnknownPeptide tight
junction agonist 94Phe Met Ile Gly Arg Leu1
5956PRTUnknownmisc_feature(6)..(6)Leu is modified with a C terminal -NH2
moiety 95Phe Leu Ile Gly Arg Leu1 5967PRTUnknownPeptide
tight junction agonist 96Phe Phe Leu Ile Gly Arg Leu1
5976PRTUnknownPeptide tight junction agonist 97Phe Phe Ile Gly Arg Leu1
5986PRTUnknownPeptide tight junction agonist 98Phe Pro Ile
Gly Arg Leu1 5996PRTUnknownPeptide tight junction agonist
99Phe Trp Ile Gly Arg Leu1 51006PRTUnknownPeptide tight
junction agonist 100Phe His Ile Gly Arg Leu1
51016PRTUnknownPeptide tight junction agonist 101Phe Pro Ile Gly Arg Leu1
51026PRTUnknownPeptide tight junction agonist 102Phe Asp
Ile Gly Arg Leu1 51036PRTUnknownPeptide tight junction
agonist 103Phe Leu Ile Gly Arg Leu1 51046PRTUnknownPeptide
tight junction agonist 104Phe Phe Ile Gly Arg Leu1
51056PRTUnknownPeptide tight junction agonist 105Phe Arg Ile Gly Arg Leu1
51066PRTUnknownPeptide tight junction agonist 106Phe Gly
Ile Gly Arg Leu1 51076PRTUnknownPeptide tight junction
agonist 107Phe Gln Ile Gly Arg Leu1 51086PRTUnknownPeptide
tight junction agonist 108Phe Glu Ile Gly Arg Leu1
51096PRTUnknownPeptide tight junction agonist 109Phe Lys Ile Gly Arg Leu1
51106PRTUnknownPeptide tight junction agonist 110Phe Asn
Ile Gly Arg Leu1 51116PRTUnknownPeptide tight junction
agonist 111Phe Tyr Ile Gly Arg Leu1 51126PRTUnknownPeptide
tight junction agonist 112Phe Leu Ile Gly Arg Leu1
51136PRTUnknownPeptide tight junction agonist 113Phe Val Ile Gly Arg Leu1
51146PRTUnknownPeptide tight junction agonist 114Phe Ile
Ile Gly Arg Leu1 51156PRTUnknownPeptide tight junction
agonist 115Ser Leu Ile Gly Arg Leu1 51166PRTUnknownPeptide
tight junction agonist 116Leu Arg Gly Ile Leu Phe1
51175PRTUnknownPeptide tight junction agonist 117Phe Leu Ile Gly Arg1
51186PRTUnknownPeptide tight junction agonist 118Phe Xaa Ile
Gly Arg Leu1 51196PRTUnknownPeptide tight junction agonist
119Phe Xaa Ile Gly Arg Leu1 51206PRTUnknownPeptide tight
junction agonist 120Phe Xaa Ile Gly Arg Leu1
51216PRTUnknownPeptide tight junction agonist 121Phe Xaa Ile Gly Arg Leu1
51226PRTUnknownPeptide tight junction agonist 122Phe Xaa
Ile Gly Arg Leu1 51236PRTUnknownPeptide tight junction
agonist 123Phe Cys Ile Gly Arg Leu1 51246PRTUnknownPeptide
tight junction agonist 124Phe Xaa Ile Gly Arg Leu1
51256PRTUnknownPeptide tight junction agonist 125Phe Xaa Ile Gly Arg Leu1
51266PRTUnknownPeptide tight junction agonist 126Phe Xaa
Ile Gly Arg Ala1 51276PRTUnknownPeptide tight junction
agonist 127Phe Xaa Ile Gly Arg Ser1 51286PRTUnknownPeptide
tight junction agonist 128Phe Xaa Ile Gly Arg Phe1
51296PRTUnknownPeptide tight junction agonist 129Phe Xaa Ile Gly Arg Phe1
51306PRTUnknownPeptide tight junction agonist 130Phe Xaa
Ile Gly Arg Leu1 51316PRTUnknownPeptide tight junction
agonist 131Phe Xaa Ile Gly Arg Leu1 51326PRTUnknownPeptide
tight junction agonist 132Phe Xaa Ile Gly Arg Leu1
51336PRTUnknownPeptide tight junction agonist 133Phe Val Ile Gly Arg Leu1
51346PRTUnknownPeptide tight junction agonist 134Phe Xaa
Ile Gly Arg Leu1 51356PRTUnknownPeptide tight junction
agonist 135Phe Xaa Ile Gly Arg Leu1 51366PRTUnknownPeptide
tight junction agonist 136Phe Xaa Ile Gly Arg Leu1
51376PRTUnknownPeptide tight junction agonist 137Phe Xaa Ile Gly Arg Leu1
51386PRTUnknownPeptide tight junction agonist 138Phe Xaa
Ile Gly Arg Leu1 51396PRTUnknownPeptide tight junction
agonist 139Phe Xaa Ile Gly Arg Leu1 51406PRTUnknownPeptide
tight junction agonist 140Phe Xaa Ile Gly Arg Leu1
51416PRTUnknownPeptide tight junction agonist 141Phe Xaa Ile Gly Arg Leu1
51426PRTUnknownPeptide tight junction agonist 142Phe Leu
Ile Gly Arg Leu1 51436PRTUnknownPeptide tight junction
agonist 143Phe Leu Ile Gly Arg Leu1 51446PRTUnknownPeptide
tight junction agonist 144Leu Arg Gly Ile Leu Phe1
51456PRTUnknownPeptide tight junction agonist 145Phe Xaa Ile Gly Arg Leu1
51466PRTUnknownPeptide tight junction agonist 146Phe Xaa
Ile Gly Arg Leu1 51475PRTUnknownPeptide tight junction
agonist 147Phe Xaa Ile Gly Arg1 51485PRTUnknownPeptide
tight junction agonist 148Phe Xaa Ile Gly Arg1
51496PRTUnknownPeptide tight junction agonist 149Phe Xaa Ile Gly Arg Leu1
51506PRTUnknownPeptide tight junction agonist 150Phe Xaa
Ile Gly Arg Leu1 51516PRTUnknownPeptide tight junction
agonist 151Xaa Phe Ile Gly Arg Leu1 51526PRTUnknownPeptide
tight junction agonist 152Xaa Phe Ile Gly Arg Leu1
51536PRTUnknownPeptide tight junction agonist 153Phe Xaa Ile Gly Arg Leu1
51545PRTUnknownPeptide tight junction agonist 154Phe Xaa
Ile Gly Arg1 51555PRTUnknownPeptide tight junction agonist
155Phe Xaa Ile Gly Arg1 51565PRTUnknownPeptide tight
junction agonist 156Phe Xaa Ile Gly Arg1
51576PRTUnknownPeptide tight junction agonist 157Phe Xaa Ile Gly Arg Leu1
51586PRTUnknownPeptide tight junction agonist 158Phe Xaa
Ile Gly Arg Leu1 51596PRTUnknownPeptide tight junction
agonist 159Phe Xaa Ile Gly Arg Leu1 51606PRTUnknownPeptide
tight junction agonist 160Phe Xaa Ile Gly Arg Leu1
51616PRTUnknownPeptide tight junction agonist 161Phe Cys Ile Gly Arg Leu1
51626PRTUnknownPeptide tight junction agonist 162Phe Cys
Ala Gly Met Ser1 51636PRTUnknownPeptide tight junction
agonist 163Phe Cys Val Gly Met Ser1 51646PRTUnknownPeptide
tight junction agonist 164Pro Cys Ile Gly Arg Leu1
51656PRTUnknownPeptide tight junction agonist 165Gln Cys Ile Gly Arg Leu1
51666PRTUnknownPeptide tight junction agonist 166Gly Cys
Ile Gly Arg Leu1 51676PRTUnknownPeptide tight junction
agonist 167Thr Cys Ile Gly Arg Leu1 51686PRTUnknownPeptide
tight junction agonist 168Ser Cys Ile Gly Arg Leu1
51696PRTUnknownPeptide tight junction agonist 169Asn Cys Ile Gly Arg Leu1
51706PRTUnknownPeptide tight junction agonist 170Arg Cys
Ile Gly Arg Leu1 51716PRTUnknownPeptide tight junction
agonist 171Val Cys Ile Gly Arg Leu1 51726PRTUnknownPeptide
tight junction agonist 172Phe Cys Ile Gly Arg Gly1
51736PRTUnknownPeptide tight junction agonist 173Ala Cys Ile Gly Arg Gly1
51746PRTUnknownPeptide tight junction agonist 174Ala Cys
Ile Gly Arg Gly1 51756PRTUnknownPeptide tight junction
agonist 175Phe Cys Ile Gly Arg Gly1 51766PRTUnknownPeptide
tight junction agonist 176Phe Cys Ile Gly Arg Gly1
51776PRTUnknownPeptide tight junction agonist 177Phe Cys Ile Gly Arg Ser1
51786PRTUnknownPeptide tight junction agonist 178Phe Cys
Ile Gly Arg Gln1 51796PRTUnknownPeptide tight junction
agonist 179Phe Cys Ile Gly Arg Lys1 51806PRTUnknownPeptide
tight junction agonist 180Phe Cys Ile Gly Arg Ala1
51816PRTUnknownPeptide tight junction agonist 181Phe Cys Ile Gly Arg Ile1
51826PRTUnknownPeptide tight junction agonist 182Phe Cys
Ile Gly Arg Gly1 51836PRTUnknownPeptide tight junction
agonist 183Phe Cys Ile Gly Arg Asp1 51846PRTUnknownPeptide
tight junction agonist 184Phe Cys Ile Gly Arg Glu1
51856PRTUnknownPeptide tight junction agonist 185Phe Cys Ile Gly Arg Phe1
51866PRTUnknownPeptide tight junction agonist 186Phe Cys
Ile Gly Arg Asn1 51876PRTUnknownPeptide tight junction
agonist 187Phe Cys Ile Gly Arg Pro1 51886PRTUnknownPeptide
tight junction agonist 188Glu Cys Ile Gly Arg Leu1
51896PRTUnknownPeptide tight junction agonist 189Asp Cys Ile Gly Arg Leu1
51906PRTUnknownPeptide tight junction agonist 190Lys Cys
Ile Gly Arg Leu1 51917PRTUnknownPeptide tight junction
agonist 191Phe Cys Ile Gly Arg Leu Cys1
51926PRTUnknownPeptide tight junction agonist 192Pro Gly Pro Gly Arg Leu1
51936PRTUnknownPeptide tight junction agonist 193Phe Cys
Ile Pro Gly Pro1 51946PRTUnknownPeptide tight junction
agonist 194Phe Cys Leu Gly Arg Leu1 51956PRTUnknownPeptide
tight junction agonist 195Gly Cys Ile Gly Arg Gly1
51966PRTUnknownPeptide tight junction agonist 196Tyr Cys Ile Gly Arg Leu1
51976PRTUnknownPeptide tight junction agonist 197Ala Cys
Ile Gly Arg Leu1 51986PRTUnknownPeptide tight junction
agonist 198Trp Cys Ile Gly Arg Leu1 51996PRTUnknownPeptide
tight junction agonist 199Ala Cys Ile Gly Arg Ser1
52006PRTUnknownPeptide tight junction agonist 200Ala Cys Ile Gly Arg Ala1
52016PRTUnknownPeptide tight junction agonist 201Phe Cys
Ile Gly Arg Phe1 52026PRTUnknownPeptide tight junction
agonist 202Ser Leu Ile Gly Arg Leu1 52034PRTUnknownPeptide
tight junction agonist 203Phe Cys Ala Gly12044PRTUnknownPeptide tight
junction agonist 204Phe Cys Gly Gly12057PRTUnknownPeptide tight junction
agonist 205Gly Phe Cys Ile Gly Arg Leu1
52066PRTUnknownPeptide tight junction agonist 206Leu Arg Gly Gly Arg Leu1
52076PRTUnknownPeptide tight junction agonist 207Phe Cys
Ala Gly Met Ser1 52086PRTUnknownPeptide tight junction
agonist 208Phe Cys Val Gly Met Ser1 52096PRTUnknownPeptide
tight junction agonist 209Phe Leu Ile Gly Arg Leu1
52105PRTUnknownPeptide tight junction agonist 210Tyr Ile Gly Ser Arg1
52116PRTUnknownPeptide tight junction agonist 211Xaa Cys Ile
Gly Arg Leu1 52126PRTUnknownPeptide tight junction agonist
212Xaa Cys Ile Gly Arg Leu1 52136PRTUnknownPeptide tight
junction agonist 213Xaa Cys Ile Gly Arg Leu1
52146PRTUnknownPeptide tight junction agonist 214Xaa Cys Ile Gly Arg Leu1
52156PRTUnknownPeptide tight junction agonist 215Xaa Cys
Ile Gly Arg Leu1 52166PRTUnknownPeptide tight junction
agonist 216Xaa Cys Ile Gly Arg Leu1 52176PRTUnknownPeptide
tight junction agonist 217Phe Cys Ile Gly Arg Xaa1
52186PRTUnknownPeptide tight junction agonist 218Phe Cys Ile Gly Arg Xaa1
52196PRTUnknownPeptide tight junction agonist 219Phe Cys
Ile Gly Arg Xaa1 52206PRTUnknownPeptide tight junction
agonist 220Phe Cys Ile Gly Arg Xaa1 52216PRTUnknownPeptide
tight junction agonist 221Phe Cys Ile Gly Arg Xaa1
52226PRTUnknownPeptide tight junction agonist 222Phe Cys Ile Gly Arg Xaa1
52236PRTUnknownPeptide tight junction agonist 223Phe Cys
Ile Gly Arg Xaa1 52246PRTUnknownPeptide tight junction
agonist 224Xaa Cys Ile Gly Arg Leu1 52256PRTUnknownPeptide
tight junction agonist 225Xaa Cys Ile Gly Arg Leu1
52266PRTUnknownPeptide tight junction agonist 226Phe Cys Ile Gly Xaa Leu1
52276PRTUnknownPeptide tight junction agonist 227Xaa Cys
Ile Gly Arg Leu1 52286PRTUnknownPeptide tight junction
agonist 228Xaa Cys Ile Gly Arg Leu1 52296PRTUnknownPeptide
tight junction agonist 229Phe Cys Ile Gly Arg Xaa1
52306PRTUnknownPeptide tight junction agonist 230Phe Cys Ile Gly Arg Xaa1
52316PRTUnknownPeptide tight junction agonist 231Xaa Cys
Ile Gly Arg Leu1 52326PRTUnknownPeptide tight junction
agonist 232Phe Cys Xaa Gly Arg Leu1 52336PRTUnknownPeptide
tight junction agonist 233Phe Cys Ile Gly Arg Xaa1
52346PRTUnknownPeptide tight junction agonist 234Xaa Cys Ile Gly Arg Leu1
52356PRTUnknownPeptide tight junction agonist 235Phe Cys
Ile Gly Arg Xaa1 52366PRTUnknownPeptide tight junction
agonist 236Phe Cys Ile Gly Arg Leu1 52376PRTUnknownPeptide
tight junction agonist 237Ala Cys Ile Gly Arg Leu1
52386PRTUnknownPeptide tight junction agonist 238Phe Ala Ile Gly Arg Leu1
52396PRTUnknownPeptide tight junction agonist 239Phe Cys
Ala Gly Arg Leu1 52406PRTUnknownPeptide tight junction
agonist 240Phe Cys Ile Ala Arg Leu1 52416PRTUnknownPeptide
tight junction agonist 241Phe Cys Ile Gly Ala Leu1
52426PRTUnknownPeptide tight junction agonist 242Phe Cys Ile Gly Arg Ala1
52435PRTUnknownPeptide tight junction agonist 243Cys Ile
Gly Arg Leu1 52444PRTUnknownPeptide tight junction agonist
244Ile Gly Arg Leu12455PRTUnknownPeptide tight junction agonist 245Phe
Cys Ile Gly Arg1 52464PRTUnknownPeptide tight junction
agonist 246Phe Cys Ile Gly12476PRTUnknownPeptide tight junction agonist
247Phe Cys Ile Gly Arg Leu1 52486PRTUnknownPeptide tight
junction agonist 248Phe Cys Ile Gly Arg Leu1
52496PRTUnknownPeptide tight junction agonist 249Phe Cys Ile Gly Arg Leu1
52506PRTUnknownPeptide tight junction agonist 250Phe Cys
Ile Gly Arg Leu1 52516PRTUnknownPeptide tight junction
agonist 251Phe Cys Ile Gly Arg Leu1 52526PRTUnknownPeptide
tight junction agonist 252Leu Arg Gly Ile Cys Phe1
52536PRTUnknownPeptide tight junction agonist 253Leu Arg Gly Ile Cys Phe1
52546PRTUnknownPeptide tight junction agonist 254Leu Arg
Gly Ile Cys Phe1 52556PRTUnknownPeptide tight junction
agonist 255Leu Arg Gly Ile Cys Phe1 52566PRTUnknownPeptide
tight junction agonist 256Leu Arg Gly Ile Cys Phe1
52576PRTUnknownPeptide tight junction agonist 257Leu Arg Gly Ile Cys Phe1
52586PRTUnknownPeptide tight junction agonist 258Leu Arg
Gly Ile Cys Phe1 52596PRTUnknownPeptide tight junction
agonist 259Leu Arg Gly Ile Cys Phe1 52606PRTUnknownPeptide
tight junction agonist 260Leu Arg Gly Ile Cys Phe1
52616PRTUnknownPeptide tight junction agonist 261Leu Arg Gly Ile Cys Phe1
52626PRTUnknownPeptide tight junction agonist 262Leu Arg
Gly Ile Cys Phe1 52636PRTUnknownPeptide tight junction
agonist 263Leu Arg Gly Ile Cys Phe1 52646PRTUnknownPeptide
tight junction agonist 264Phe Cys Ile Gly Arg Leu1
52656PRTUnknownPeptide tight junction agonist 265Phe Cys Ile Gly Arg Leu1
52666PRTUnknownPeptide tight junction agonist 266Phe Cys
Ile Gly Arg Leu1 52676PRTUnknownPeptide tight junction
agonist 267Phe Cys Ile Gly Arg Leu1 52686PRTUnknownPeptide
tight junction agonist 268Phe Cys Ile Gly Arg Leu1
52696PRTUnknownPeptide tight junction agonist 269Phe Cys Ile Gly Arg Leu1
52706PRTUnknownPeptide tight junction agonist 270Phe Cys
Ile Gly Arg Leu1 52716PRTUnknownPeptide tight junction
agonist 271Leu Arg Gly Ile Cys Phe1 52726PRTUnknownPeptide
tight junction agonist 272Leu Arg Gly Ile Cys Phe1
52736PRTUnknownPeptide tight junction agonist 273Leu Arg Gly Ile Cys Phe1
52746PRTUnknownPeptide tight junction agonist 274Leu Arg
Gly Ile Cys Phe1 5
User Contributions:
Comment about this patent or add new information about this topic:
| People who visited this patent also read: | |
| Patent application number | Title |
|---|---|
| 20120248179 | CARTON AND BLANK THEREFOR |
| 20120248178 | Carton Having Novel Opening Features |
| 20120248177 | PADDED PAPER ENVELOPE |
| 20120248176 | SOLDER PASTES FOR PROVIDING IMPACT RESISTANT, MECHANICALLY STABLE SOLDER JOINTS |
| 20120248175 | COATED ROTARY TOOL |