Patent application title: Therapeutic Gene-Switch Constructs and Bioreactors for the Expression of Biotherapeutic Molecules, and Uses Thereof
Inventors:
Bethany Lynn Merenick (Christiansburg, VA, US)
Robert P. Beech (Cincinnati, OH, US)
Robert P. Beech (Cincinnati, OH, US)
Thomas D. Reed (Blacksburg, VA, US)
Anna P. Tretiakova (Royersford, PA, US)
Richard E. Peterson (Blacksburg, VA, US)
Assignees:
Intrexon Corporation
IPC8 Class: AA61K3512FI
USPC Class:
424 9321
Class name: Whole live micro-organism, cell, or virus containing genetically modified micro-organism, cell, or virus (e.g., transformed, fused, hybrid, etc.) eukaryotic cell
Publication date: 2009-05-28
Patent application number: 20090136465
Claims:
1. (canceled)
2. A method for expressing a therapeutic polypeptide or therapeutic polynucleotide in a subject, comprising:(a) introducing into a subject (1) a first polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex through operable association with a therapeutic switch promoter, and (2) a second polynucleotide encoding said therapeutic polypeptide or therapeutic polynucleotide operably associated with a factor-regulated promoter which is activated by said ligand-dependent transcription factor complex, wherein said first and second polynucleotides are introduced so as to permit expression of said ligand-dependent transcription factor complex; and(b) administering ligand to said subject to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
3. The method of claim 2, wherein said therapeutic switch promoter is constitutive.
4. The method of claim 2, wherein said therapeutic switch promoter is activated under conditions associated with a disease, disorder, or condition so as to permit expression of said ligand-dependent transcription factor complex under conditions associated with said disease, disorder, or condition.
5. The method of claim 4, wherein said disease, disorder, or condition is responsive to said therapeutic polypeptide or therapeutic polynucleotide.
6. The method of claim 2, wherein said therapeutic polypeptide or therapeutic polynucleotide is expressed and disseminated at a level sufficient to treat, ameliorate, or prevent said disease, disorder, or condition.
7. The method of claim 2, wherein said gene switch is an ecdysone receptor (EcR)-based gene switch.
8. The method of claim 7, wherein said ligand binds to the EcR ligand binding domain.
9. The method of claim 8, wherein said ligand is a diacylhydrazine.
10. The method of claim 9, wherein said ligand is RG-115819, RG-115932, or RG-115830.
11-16. (canceled)
17. The method of claim 2, wherein said first and second polynucleotides are introduced into said subject in one or more modified cells.
18. The method of claim 17, wherein said modified cells are prepared by introducing said first and second polynucleotides into cells that have been isolated from said subject to produce modified autologous cells, which are then re-introduced into said subject.
19. The method of claim 17, wherein said modified cells are modified non-autologous (MNA) cells into which said first and second polynucleotides have been incorporated.
20-22. (canceled)
23. The method of claim 19, wherein said MNA cells are C2C12 mouse myoblast cells, HEK293 human embryonic kidney cells, ARPE-19 cells, hMSC cells, pancreatic islet cells, MDCK cell, a CHO cell, an astrocyte derived cell, an oligodendrocyte derived cell, or a myoblast derived cell.
24-25. (canceled)
26. The method of claim 17, wherein said modified cells have been treated such that said cells, upon introduction into said subject, are protected from the subject's immune system.
27. The method of claim 26, wherein said modified cells are contained within a barrier system which allows dissemination of said therapeutic protein or therapeutic polynucleotide, but which prevents direct contact of said modified cells with cells of the subject's immune system.
28. The method of claim 27, wherein said barrier system comprises modified cells with a conformal coating.
29. The method of claim 28, wherein said conformal coating comprises a polymer.
30. The method of claim 29, wherein said polymer is selected from the group consisting of polyethylene glycol, and hydroxyethyl methacrylate-methyl methacrylate (HEMA-MMA).
31. The method of claim 27, wherein said barrier system comprises encapsulated modified cells.
32. The method of claim 31, wherein said modified cells are contained in a macroencapsulation device.
33. The method of claim 32, wherein said macroencapsulation device comprises one or more synthetic membranes.
34. The method of claim 33, wherein said macroencapsulation device comprises two or more synthetic membranes, said synthetic membranes comprising different pore sizes so as to regulate mass transit through said macroencapsulation device.
35. The method of claim 32, wherein said macroencapsulation device comprises a semi-permeable polymer outer membrane and an internal scaffold which supports cells.
36-61. (canceled)
62. A method for expressing a therapeutic polypeptide or therapeutic polynucleotide in one or more modified cells, comprising:(a) introducing into a cell (1) a first polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex through operable association with a therapeutic switch promoter, and (2) a second polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide operably associated with a factor-regulated promoter which is activated by said ligand-dependent transcription factor complex, thereby producing a modified cell; and(b) administering ligand to said modified cell to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
63-64. (canceled)
65. The method of claim 62, wherein said method is carried out in vitro.
66. The method of claim 62, wherein said method is carried out ex vivo in a cell that has been isolated from a subject.
67. The method of claim 62, wherein said method is carried out in vivo.
68. A nucleic acid composition comprising a first polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex through operable association to a therapeutic switch promoter.
69. (canceled)
70. The nucleic acid composition of claim 68, wherein said therapeutic switch promoter is activated under conditions associated with a disease, disorder, or condition so as to permit expression of said ligand-dependent transcription factor complex under conditions associated with said disease, disorder, or condition.
71. The nucleic acid composition of claim 68, further comprising a second polynucleotide encoding a polypeptide or polynucleotide associated with a disease, disorder, or condition through operable association with a promoter which is activated by said ligand-dependent transcription factor complex.
72-90. (canceled)
91. A modified cell comprising the nucleic acid composition of claim 71.
92-99. (canceled)
100. A bioreactor device comprising one or more modified cells as recited in claim 91.
101-142. (canceled)
Description:
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001]The present application claims the benefit of the filing date of U.S. Provisional Application No. 61/047,899, files Apr. 25, 2008 and U.S. Provisional Application No. 60/975,986, filed Sep. 28, 2007, both of which are incorporated herein by reference in their entireties.
REFERENCE TO SEQUENCE LISTING SUBMITTED ELECTRONICALLY VIA EFS-WEB
[0002]The content of the electronically submitted sequence listing (Name: Sequence Listing.ST25.txt; Size: 243,000 bytes; and Date of Creation: Sep. 29, 2008) filed herewith the application is incorporated herein by reference in its entirety:
BACKGROUND OF THE INVENTION
[0003]1. Field of the Invention
[0004]The present invention relates to methods and compositions for treating, ameliorating, or preventing a disease, disorder, or condition in a subject by introducing into the subject a therapeutic gene switch construct that controls expression of one or more therapeutic products. In a further embodiment, the present invention relates to methods and compositions for treating, ameliorating, or preventing a disease, disorder, or condition in a subject by introducing into the subject a "bioreactor," a therapeutic implant composed of a cell or cells that secrete a therapeutic protein. A bioreactor may be immuno-isolated by encapsulation or non-immunoisolated. In particular embodiments, the bioreactor comprises a therapeutic gene switch construct.
[0005]2. Background of the Invention
[0006]The concept of treating or preventing a disease in a subject through introduction of a polynucleotide encoding a therapeutic molecule, e.g., a therapeutic polypeptide or therapeutic polynucleotide into cells of the subject, or introducing into the subject modified cells engineered to secrete the therapeutic molecule has been in existence for many years. Several difficulties in the practical aspects of the concept have hindered progress towards successful therapies. Direct introduction of genetic material into a subject to be treated presents difficulties such as: safety of delivery, obtaining sufficient expression levels of the therapeutic product for a sufficient period of time, limiting expression of the therapeutic product to desired cells, and maintaining the ability to modulate or pulse the expression of the therapeutic product, including the ability to turn off expression of the therapeutic product if it is no longer needed. Cell based therapies are subject to rejection via the subject's immune response, therefore immuno-isolation strategies such as cell encapsulation methods have been developed to increase the longevity of implanted cells and allow use of xenogeneic cells, i.e., cells from a different species. Current encapsulated and non-encapsulated cell therapies are engineered to secrete the therapeutic protein constitutively. Once implanted, protein secretion can not be regulated. To improve the safety and clinical application of direct or cell-mediated bioreactor therapeutic protein delivery it would be advantageous to be able to turn off the protein production or regulate the rate at which protein production occurs.
[0007]Thus, there is a need in the art for new therapeutic methods and compositions that provide these desired characteristics.
SUMMARY OF THE INVENTION
[0008]The present invention relates to methods and compositions for treating, ameliorating, or preventing a disease, disorder, or condition in a subject.
[0009]In one embodiment, the present invention provides a method for treating, ameliorating, or preventing a disease, disorder, or condition in a subject, comprising:
[0010](a) introducing into a subject (1) a first polynucleotide encoding a gene switch, where the gene switch comprises at least one transcription factor sequence encoding a ligand-dependent transcription factor through operable association with a therapeutic switch promoter, where the therapeutic switch promoter is constitutively active and (2) a second polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide in operable association with a factor-regulated promoter which is activated by said ligand-dependent transcription factor, where the first and second polynucleotides are introduced so as to permit their expression in the presence of ligand; and
[0011](b) administering ligand to the subject to induce expression of the therapeutic polypeptide or therapeutic polynucleotide.
[0012]A further embodiment of the invention provides a method for expressing a therapeutic polypeptide or therapeutic polynucleotide in a subject, comprising: [0013](a) introducing into a subject (1) a first polynucleotide encoding a gene switch, where the gene switch comprises at least one transcription factor sequence encoding a ligand-dependent transcription factor through operable association with a therapeutic switch promoter, where the therapeutic switch promoter is activated under conditions associated with the disease, disorder, or condition to be treated, and (2) a second polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide in operable association with a factor-regulated promoter which is activated by said ligand-dependent transcription factor, where the first and second polynucleotides are introduced so as to permit their expression in the subject under conditions associated with the disease, disorder, or condition; and [0014](b) administering ligand to the subject to induce expression of the therapeutic polypeptide or therapeutic polynucleotide.
[0015]A further embodiment of the invention provides a method for expressing a therapeutic polypeptide or therapeutic polynucleotide in a subject, comprising: [0016](a) introducing into a subject (1) a first polynucleotide encoding a gene switch, where the gene switch comprises at least one transcription factor sequence encoding a ligand-dependent transcription factor through operable association with a therapeutic switch promoter, where the therapeutic switch promoter is activated under conditions associated with a disease, disorder, or condition treatable by the therapeutic polypeptide or therapeutic polynucleotide, and (2) a second polynucleotide encoding the therapeutic polypeptide or therapeutic polynucleotide in operable association with a factor-regulated promoter which is activated by the ligand-dependent transcription factor, wherein said the and second polynucleotides are introduced so as to permit expression of the first polynucleotide under conditions associated with the disease, disorder, or condition; and [0017](b) administering ligand to the subject to induce expression of the therapeutic polypeptide or therapeutic polynucleotide.
[0018]In the methods described above, in one embodiment, the first polynucleotide encoding the therapeutic gene switch and the second polynucleotide encoding the therapeutic polypeptide or polynucleotide linked to a factor-regulated promoter are part of one larger polynucleotide, e.g., a vector. In another embodiment, the first polynucleotide encoding the therapeutic gene switch and the second polynucleotide encoding the therapeutic polypeptide or polynucleotide linked to a factor-regulated promoter are separate polynucleotides which may be administered as a nucleic acid composition.
[0019]The invention further relates to therapeutic gene switch constructs that are useful in the disclosed methods.
[0020]The invention additionally relates to vectors comprising the therapeutic gene switch constructs of the invention.
[0021]The invention further provides a method for expressing a therapeutic polypeptide or therapeutic polynucleotide in one or more modified cells, comprising: [0022](a) introducing into a cell (1) a first polynucleotide encoding a gene switch, where the gene switch comprises at least one transcription factor sequence encoding a ligand-dependent transcription factor through operable association with a therapeutic switch promoter which is activated under conditions associated with a disease, disorder, or condition, and (2) a second polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide through operable association with a factor-regulated promoter which is activated by the ligand-dependent transcription factor, thereby producing a modified cell; and [0023](b) administering ligand to the modified cell to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
[0024]The invention further relates to modified cells comprising the therapeutic gene switch constructs of the invention.
[0025]The invention also relates to bioreactor devices comprising modified cells of the invention either non-encapsulated, or encapsulated in such a way to shield the cells from the subject's immune system. Such bioreactors may take the form, for example, of coated cells, micro-encapsulated cells, or macro-encapsulated cells.
[0026]The invention also relates to kits for carrying out the methods of the invention, comprising, e.g., gene switch constructs, vectors, ligands, etc.
BRIEF DESCRIPTION OF THE DRAWINGS
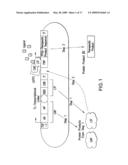
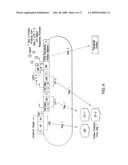
[0027]FIG. 1 shows an embodiment of the therapeutic gene switch of the invention in which two transcription factor sequences encoding two separate portions of a ligand-dependent transcription factor complex are under the control of a single promoter. "AD" represents a transactivation domain; "HP" represents a heterodimerization partner domain. The AD and HP domains are expressed as a fusion protein termed a "coactivation protein" or "CAP." "DBD" represents a DNA binding domain; "LBD" represents a ligand binding domain. The DBD and LBD domains are expressed as a fusion protein termed a "ligand-dependent transcription factor," or "LTF." "Transcriptional Linker" represents an IRES (Internal ribosomal entry site) or means of generating two separate protein products from a single open reading frame. "Therapeutic Product Sequence" represents a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide; "Therapeutic Product" represents a therapeutic polypeptide or therapeutic polynucleotide; and "TSP-1," represents either a constitutive therapeutic switch promoter, or a therapeutic switch promoter activated under conditions associated with a disease, disorder, or condition. CAP and LTF combine to form a ligand-dependent transcription factor complex (LDTFC) which in combination with ligand activates a factor-regulated promoter (FRP).
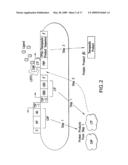
[0028]FIG. 2 shows an embodiment of the therapeutic gene switch of the invention in which two transcription factor sequences (CAP and LTF) encoding two separate portions of a ligand-dependent transcription factor complex are under the control of different promoters. The terms AD, HP, CAP, DBD, LBD, LTF, "Therapeutic Product Sequence," "Therapeutic Product," TSP, LDTFC, and FRP are defined in the legend to FIG. 1. "TSP-1" and "TSP-2" represent two different therapeutic switch promoters, each of which is, independently, either a constitutive promoter or a promoter activated under conditions associated with a disease, disorder, or condition. In one embodiment TSP-1 is a constitutive promoter and TSP-2 is a promoter activated under conditions associated with a disease, disorder, or condition. CAP and LTF combine to form a LDTFC which in combination with ligand activates a FRP.
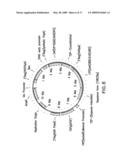
[0029]FIG. 3 shows an embodiment of the therapeutic gene switch of the invention in which three transcription factor sequences (CAP, LTF-1, and LTF-2), which may combine to form two separate LDTFCs under the control of different promoters. The terms AD, HP, CAP, DBD, LBD, LTF, "Therapeutic Product Sequence," "Therapeutic Product," TSP, LDTFC, and FRP are defined in the legend to FIG. 1. DBD-A represents a first DNA binding domain which is fused with an LBD to form LTF-1, DBD-B represents a second DNA binding domain which is fused with an LBD to form LTF-2. "Therapeutic Product A" represents a first therapeutic polypeptide or therapeutic polynucleotide; "Therapeutic Product B" represents a second therapeutic polypeptide or therapeutic polynucleotide; and TSP-1, TSP-2, and TSP-3 represent three different therapeutic switch promoters, each of which is, independently, either a constitutive promoter or a promoter activated under conditions associated with a disease, disorder, or condition. In one embodiment, TSP-1 is a constitutive therapeutic switch promoter and TSP-2, and TSP-3 are different therapeutic switch promoters, each of which is independently activated under conditions associated with a disease, disorder, or condition. CAP and LTF-1 combine to form LDTFC-1 which in combination with ligand activates FRP-1. CAP and LTF-2 combine to form LDTFC-2 which in combination with ligand activates FRP-2.
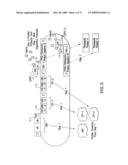
[0030]FIG. 4 shows an embodiment of the therapeutic gene switch of the invention in which three transcription factor sequences encoding CAP and two separate LTF portions of a ligand-dependent transcription factor complex are under the control of different promoters. The terms AD, HP, CAP, DBD, LBD, LTF, "Therapeutic Product Sequence," "Therapeutic Product," TSP, LDTFC, and FRP are defined in the legend to FIG. 1. TSP-1, TSP-2, and TSP-3 represent three different therapeutic switch promoters, each of which is, independently, either a constitutive promoter or a promoter activated under conditions associated with a disease, disorder, or condition. In one embodiment, TSP-1 is a constitutive promoter and TSP-2 and TSP-3 are different promoters, each of which is independently activated under conditions associated with a disease, disorder, or condition. Either LTF-1 or LTF-2 may combine with CAP to form LDTFC-1 or LDTFC-2. Either LDTFC-1 or LDTFC-2, in combination with ligand, activates FRP.
[0031]FIG. 5 is a diagram of a vector constructed under the scheme shown in FIG. 1, and engineered to express insulin growth factor-1 (IGF-1) under hypoxic conditions such as cardiac ischemia.
[0032]FIG. 6 is a diagram of a vector constructed under the scheme shown in FIG. 2, and engineered to express basic fibroblast growth factor (bFGF) under hypoxic conditions such as cardiac ischemia.
[0033]FIG. 7 is a diagram of a vector constructed under the scheme shown in FIG. 2, and engineered to express erythropoietin (EPO) under hypoxic conditions such as cardiac ischemia.
[0034]FIG. 8 is a diagram of a vector constructed under the scheme shown in FIG. 2, and engineered to express human B-type natriuretic peptide (BNP) under hypoxic conditions such as cardiac ischemia.
[0035]FIG. 9 is a diagram of a vector constructed under the scheme shown in FIG. 2, and engineered to express tissue plasminogen activator (tPA) under inflammatory conditions such as cardiac ischemia.
[0036]FIG. 10 is a diagram of a vector constructed under the scheme shown in FIG. 3, and engineered to express relaxin under inflammatory conditions and/or hepatocyte growth factor under hypoxic conditions, both conditions being associated with cardiac ischemia.
[0037]FIG. 11 is a diagram of a vector constructed under the scheme shown in FIG. 2, and engineered to express EPO under hypoxic conditions such as cardiac ischemia with expression being limited to cardiac myocytes.
[0038]FIG. 12 is a diagram of a vector constructed under the scheme shown in FIG. 4, and engineered to express IGF-1 under either inflammatory conditions or hypoxic conditions such as cardiac ischemia with expression being limited to cardiac myocytes.
[0039]FIG. 13 is a diagram of a vector constructed under the scheme shown in FIG. 1, and engineered to express tumor necrosis factor binding protein 2 (Enbrel®) under inflammatory conditions such as rheumatoid arthritis.
[0040]FIG. 14 is a diagram of a vector constructed under the scheme shown in FIG. 4, and engineered to express tumor necrosis factor binding protein 2 (Enbrel®) either in response to TNF alpha expression or under inflammatory conditions, both conditions associated with rheumatoid arthritis.
[0041]FIG. 15 is a diagram of a vector constructed under the scheme shown in FIG. 3, and engineered to express tumor necrosis factor binding protein 2 (Enbrel®) under inflammatory conditions and/or EPO under HIF-driven hypoxic conditions, both conditions being associated with rheumatoid arthritis.
[0042]FIG. 16 is a diagram of a vector constructed under the scheme shown in FIG. 1, and engineered to express human factor VIII:C constitutively.
[0043]FIG. 17 is a diagram of a vector constructed under the scheme shown in FIG. 2, and engineered to express human factor VIII:C under hypoxic conditions associated with hemophilia.
DETAILED DESCRIPTION OF THE INVENTION
[0044]The invention relates to methods and compositions for using a gene switch to express a therapeutic polypeptide or therapeutic polynucleotide in a cell. The methods and compositions may be used in vitro, ex vivo or in vivo. The invention further relates to methods and compositions for using a gene switch controlling expression of a therapeutic polypeptide or therapeutic polynucleotide for the treatment, amelioration, or prevention of diseases, disorders, or conditions in a subject. The methods of the invention can be carried out either ex vivo (by introducing the gene switch into isolated cells of a subject or non-autologous cells, and introducing the modified cells to the subject or into a different subject) or in vivo (by introducing the gene switch directly into cells of the subject). The methods of the invention involve the use of a gene switch in which expression of a ligand-dependent transcription factor is under the control of one or more therapeutic switch promoters. The methods also include, without limitation, applications of the gene switch technology in direct introduction into the subject to be treated, non-encapsulated and encapsulated cell therapies. The methods and compositions described herein provide a highly specific and tightly regulated therapeutic technique in which the level and timing of expression of a therapeutic product is controlled by administration of ligand to cells comprising the gene switch.
[0045]The following definitions are provided and should be helpful in understanding the scope and practice of the present invention.
[0046]The term "isolated" for the purposes of the present invention designates a biological material (cell, nucleic acid or protein) that has been removed from its original environment (the environment in which it is naturally present). For example, a polynucleotide present in the natural state in a plant or an animal is not isolated, however the same polynucleotide separated from the adjacent nucleic acids in which it is naturally present, is considered "isolated."
[0047]The term "purified," as applied to biological materials does not require the material to be present in a form exhibiting absolute purity, exclusive of the presence of other compounds. It is rather a relative definition.
[0048]"Nucleic acid," "nucleic acid molecule," "oligonucleotide," and "polynucleotide" are used interchangeably and refer to the phosphate ester polymeric form of ribonucleosides (adenosine, guanosine, uridine or cytidine; "RNA molecules") or deoxyribonucleosides (deoxyadenosine, deoxyguanosine, deoxythymidine, or deoxycytidine; "DNA molecules"), or any phosphoester analogs thereof, such as phosphorothioates and thioesters, in either single stranded form, or a double-stranded helix. Double stranded DNA-DNA, DNA-RNA and RNA-RNA helices are possible. The term nucleic acid molecule, and in particular DNA or RNA molecule, refers only to the primary and secondary structure of the molecule, and does not limit it to any particular tertiary forms. Thus, this term includes double-stranded DNA found, inter alia, in linear or circular DNA molecules (e.g., restriction fragments), plasmids, supercoiled DNA and chromosomes. In discussing the structure of particular double-stranded DNA molecules, sequences may be described herein according to the normal convention of giving only the sequence in the 5' to 3' direction along the non-transcribed strand of DNA (i.e., the strand having a sequence homologous to the mRNA). A "recombinant DNA molecule" is a DNA molecule that has undergone a molecular biological manipulation. DNA includes, but is not limited to, cDNA, genomic DNA, plasmid DNA, synthetic DNA, and semi-synthetic DNA. A "nucleic acid composition" of the invention comprises one or more nucleic acids as described herein.
[0049]The term "fragment," as applied to polynucleotide sequences, refers to a nucleotide sequence of reduced length relative to the reference nucleic acid and comprising, over the common portion, a nucleotide sequence identical to the reference nucleic acid. Such a nucleic acid fragment according to the invention may be, where appropriate, included in a larger polynucleotide of which it is a constituent. Such fragments comprise, or alternatively consist of, oligonucleotides ranging in length from at least 6, 8, 9, 10, 12, 15, 18, 20, 21, 22, 23, 24, 25, 30, 39, 40, 42, 45, 48, 50, 51, 54, 57, 60, 63, 66, 70, 75, 78, 80, 90, 100, 105, 120, 135, 150, 200, 300, 500, 720, 900, 1000, 1500, 2000, 3000, 4000, 5000, or more consecutive nucleotides of a nucleic acid according to the invention.
[0050]As used herein, an "isolated nucleic acid fragment" refers to a polymer of RNA or DNA that is single- or double-stranded, optionally containing synthetic, non-natural or altered nucleotide bases. An isolated nucleic acid fragment in the form of a polymer of DNA may be comprised of one or more segments of cDNA, genomic DNA or synthetic DNA.
[0051]A "gene" refers to a polynucleotide comprising nucleotides that encode a functional molecule, including functional molecules produced by transcription only (e.g., a bioactive RNA species) or by transcription and translation (e.g., a polypeptide). The term "gene" encompasses cDNA and genomic DNA nucleic acids. "Gene" also refers to a nucleic acid fragment that expresses a specific RNA, protein or polypeptide, including regulatory sequences preceding (5' non-coding sequences) and following (3' non-coding sequences) the coding sequence. "Native gene" refers to a gene as found in nature with its own regulatory sequences. "Chimeric gene" refers to any gene that is not a native gene, comprising regulatory and/or coding sequences that are not found together in nature. Accordingly, a chimeric gene may comprise regulatory sequences and coding sequences that are derived from different sources, or regulatory sequences and coding sequences derived from the same source, but arranged in a manner different than that found in nature. A chimeric gene may comprise coding sequences derived from different sources and/or regulatory sequences derived from different sources. "Endogenous gene" refers to a native gene in its natural location in the genome of an organism. A "foreign" gene or "heterologous" gene refers to a gene not normally found in the host organism, but that is introduced into the host organism by gene transfer. Foreign genes can comprise native genes inserted into a non-native organism, or chimeric genes. A "transgene" is a gene that has been introduced into the genome by a transformation procedure.
[0052]"Heterologous DNA" refers to DNA not naturally located in the cell, or in a chromosomal site of the cell. The heterologous DNA may include a gene foreign to the cell.
[0053]The term "genome" includes chromosomal as well as mitochondrial, chloroplast and viral DNA or RNA.
[0054]A nucleic acid molecule is "hybridizable" to another nucleic acid molecule, such as a cDNA, genomic DNA, or RNA, when a single stranded form of the nucleic acid molecule can anneal to the other nucleic acid molecule under the appropriate conditions of temperature and solution ionic strength. Hybridization and washing conditions are well known and exemplified in Sambrook et al. in Molecular Cloning: A Laboratory Manual, Second Edition, Cold Spring Harbor Laboratory Press, Cold Spring Harbor (1989), particularly Chapter 11 and Table 11.1 therein (entirely incorporated herein by reference). The conditions of temperature and ionic strength determine the "stringency" of the hybridization.
[0055]Stringency conditions can be adjusted to screen for moderately similar fragments, such as homologous sequences from distantly related organisms, to highly similar fragments, such as genes that duplicate functional enzymes from closely related organisms. For preliminary screening for homologous nucleic acids, low stringency hybridization conditions, corresponding to a Tm of 55°, can be used, e.g., 5×SSC, 0.1% SDS, 0.25% milk, and no formamide; or 30% formamide, 5×SSC, 0.5% SDS. Moderate stringency hybridization conditions correspond to a higher Tm, e.g., 40% formamide, with 5× or 6×SSC. High stringency hybridization conditions correspond to the highest Tm, e.g., 50% formamide, 5× or 6×SSC.
[0056]Hybridization requires that the two nucleic acids contain complementary sequences, although depending on the stringency of the hybridization, mismatches between bases are possible. The term "complementary" is used to describe the relationship between nucleotide bases that are capable of hybridizing to one another. For example, with respect to DNA, adenosine is complementary to thymine and cytosine is complementary to guanine. Accordingly, the present invention also includes isolated nucleic acid fragments that are complementary to the complete sequences as disclosed or used herein as well as those substantially similar nucleic acid sequences.
[0057]In one embodiment of the invention, polynucleotides are detected by employing hybridization conditions comprising a hybridization step at Tm of 55° C., and utilizing conditions as set forth above. In other embodiments, the Tm is 60° C., 63° C., or 65° C.
[0058]Post-hybridization washes also determine stringency conditions. One set of conditions uses a series of washes starting with 6×SSC, 0.5% SDS at room temperature for 15 minutes (min), then repeated with 2×SSC, 0.5% SDS at 45° C. for 30 min, and then repeated twice with 0.2×SSC, 0.5% SDS at 50° C. for 30 min. A preferred set of stringent conditions uses higher temperatures in which the washes are identical to those above except for the temperature of the final two 30 min washes in 0.2×SSC, 0.5% SDS is increased to 60° C. Another preferred set of highly stringent conditions uses two final washes in 0.1×SSC, 0.1% SDS at 65° C.
[0059]The appropriate stringency for hybridizing nucleic acids depends on the length of the nucleic acids and the degree of complementation, variables well known in the art. The greater the degree of similarity or homology between two nucleotide sequences, the greater the value of Tm for hybrids of nucleic acids having those sequences. The relative stability (corresponding to higher Tm) of nucleic acid hybridizations decreases in the following order: RNA:RNA, DNA:RNA, DNA:DNA. For hybrids of greater than 100 nucleotides in length, equations for calculating Tm have been derived (see Sambrook et al., supra, 9.50-0.51). For hybridization with shorter nucleic acids, i.e., oligonucleotides, the position of mismatches becomes more important, and the length of the oligonucleotide determines its specificity (see Sambrook et al., supra, 11.7-11.8).
[0060]In one embodiment of the invention, polynucleotides are detected by employing hybridization conditions comprising a hybridization step in less than 500 mM salt and at least 37° C., and a washing step in 2×SSPE at a temperature of at least 63° C. In another embodiment, the hybridization conditions comprise less than 200 mM salt and at least 37° C. for the hybridization step. In a further embodiment, the hybridization conditions comprise 2×SSPE and 63° C. for both the hybridization and washing steps.
[0061]In another embodiment, the length for a hybridizable nucleic acid is at least about 10 nucleotides. Preferably a minimum length for a hybridizable nucleic acid is at least about 15 nucleotides; e.g., at least about 20 nucleotides; e.g., at least 30 nucleotides. Furthermore, the skilled artisan will recognize that the temperature and wash solution salt concentration may be adjusted as necessary according to factors such as length of the probe.
[0062]The term "probe" refers to a single-stranded nucleic acid molecule that can base pair with a complementary single stranded target nucleic acid to form a double-stranded molecule.
[0063]As used herein, the term "oligonucleotide" refers to a short nucleic acid that is hybridizable to a genomic DNA molecule, a cDNA molecule, a plasmid DNA or an mRNA molecule. Oligonucleotides can be labeled, e.g., with 32P-nucleotides or nucleotides to which a label, such as biotin, has been covalently conjugated. A labeled oligonucleotide can be used as a probe to detect the presence of a nucleic acid. Oligonucleotides (one or both of which may be labeled) can be used as PCR primers, either for cloning full length or a fragment of a nucleic acid, for DNA sequencing, or to detect the presence of a nucleic acid. An oligonucleotide can also be used to form a triple helix with a DNA molecule. Generally, oligonucleotides are prepared synthetically, preferably on a nucleic acid synthesizer. Accordingly, oligonucleotides can be prepared with non-naturally occurring phosphoester analog bonds, such as thioester bonds, etc.
[0064]A "primer" refers to an oligonucleotide that hybridizes to a target nucleic acid sequence to create a double stranded nucleic acid region that can serve as an initiation point for DNA synthesis under suitable conditions. Such primers may be used in a polymerase chain reaction or for DNA sequencing.
[0065]"Polymerase chain reaction" is abbreviated PCR and refers to an in vitro method for enzymatically amplifying specific nucleic acid sequences. PCR involves a repetitive series of temperature cycles with each cycle comprising three stages: denaturation of the template nucleic acid to separate the strands of the target molecule, annealing a single stranded PCR oligonucleotide primer to the template nucleic acid, and extension of the annealed primer(s) by DNA polymerase. PCR provides a means to detect the presence of the target molecule and, under quantitative or semi-quantitative conditions, to determine the relative amount of that target molecule within the starting pool of nucleic acids.
[0066]"Reverse transcription-polymerase chain reaction" is abbreviated RT-PCR and refers to an in vitro method for enzymatically producing a target cDNA molecule or molecules from an RNA molecule or molecules, followed by enzymatic amplification of a specific nucleic acid sequence or sequences within the target cDNA molecule or molecules as described above. RT-PCR also provides a means to detect the presence of the target molecule and, under quantitative or semi-quantitative conditions, to determine the relative amount of that target molecule within the starting pool of nucleic acids.
[0067]A DNA "coding sequence" refers to a double-stranded DNA sequence that encodes a polypeptide and can be transcribed and translated into a polypeptide in a cell in vitro or in vivo when placed under the control of suitable regulatory sequences. "Suitable regulatory sequences" refers to nucleotide sequences located upstream (5' non-coding sequences), within, or downstream (3' non-coding sequences) of a coding sequence, and which influence the transcription, RNA processing or stability, or translation of the associated coding sequence. Regulatory sequences may include promoters, translation leader sequences, introns, polyadenylation recognition sequences, RNA processing sites, effector binding sites and stem-loop structures. The boundaries of the coding sequence are determined by a start codon at the 5' (amino) terminus and a translation stop codon at the 3' (carboxyl) terminus. A coding sequence can include, but is not limited to, prokaryotic sequences, cDNA from mRNA, genomic DNA sequences, and even synthetic DNA sequences. If the coding sequence is intended for expression in a eukaryotic cell, a polyadenylation signal and transcription termination sequence will usually be located 3' to the coding sequence.
[0068]"Open reading frame" is abbreviated ORF and refers to a length of nucleic acid sequence, either DNA, cDNA or RNA, that comprises a translation start signal or initiation codon, such as an ATG or AUG, and a termination codon and can be potentially translated into a polypeptide sequence.
[0069]The term "head-to-head" is used herein to describe the orientation of two polynucleotide sequences in relation to each other. Two polynucleotides are positioned in a head-to-head orientation when the 5' end of the coding strand of one polynucleotide is adjacent to the 5' end of the coding strand of the other polynucleotide, whereby the direction of transcription of each polynucleotide proceeds away from the 5' end of the other polynucleotide. The term "head-to-head" may be abbreviated (5')-to-(5') and may also be indicated by the symbols (→) or (3'→5'5'→3').
[0070]The term "tail-to-tail" is used herein to describe the orientation of two polynucleotide sequences in relation to each other. Two polynucleotides are positioned in a tail-to-tail orientation when the 3' end of the coding strand of one polynucleotide is adjacent to the 3' end of the coding strand of the other polynucleotide, whereby the direction of transcription of each polynucleotide proceeds toward the other polynucleotide. The term "tail-to-tail" may be abbreviated (3')-to-(3') and may also be indicated by the symbols (→) or (5→3'3'5').
[0071]The term "head-to-tail" is used herein to describe the orientation of two polynucleotide sequences in relation to each other. Two polynucleotides are positioned in a head-to-tail orientation when the 5' end of the coding strand of one polynucleotide is adjacent to the 3' end of the coding strand of the other polynucleotide, whereby the direction of transcription of each polynucleotide proceeds in the same direction as that of the other polynucleotide. The term "head-to-tail" may be abbreviated (5')-to-(3') and may also be indicated by the symbols (→→) or (5'→3'5'→3').
[0072]The term "downstream" refers to a nucleotide sequence that is located 3' to a reference nucleotide sequence. In particular, downstream nucleotide sequences generally relate to sequences that follow the starting point of transcription. For example, the translation initiation codon of a gene is located downstream of the start site of transcription.
[0073]The term "upstream" refers to a nucleotide sequence that is located 5' to a reference nucleotide sequence. In particular, upstream nucleotide sequences generally relate to sequences that are located on the 5' side of a coding sequence or starting point of transcription. For example, most promoters are located upstream of the start site of transcription.
[0074]The terms "restriction endonuclease" and "restriction enzyme" are used interchangeably and refer to an enzyme that binds and cuts within a specific nucleotide sequence within double stranded DNA.
[0075]"Homologous recombination" refers to the insertion of a foreign DNA sequence into another DNA molecule, e.g., insertion of a vector in a chromosome. Preferably, the vector targets a specific chromosomal site for homologous recombination. For specific homologous recombination, the vector will contain sufficiently long regions of homology to sequences of the chromosome to allow complementary binding and incorporation of the vector into the chromosome. Longer regions of homology, and greater degrees of sequence similarity, may increase the efficiency of homologous recombination.
[0076]Several methods known in the art may be used to propagate a polynucleotide according to the invention. Once a suitable host system and growth conditions are established, recombinant expression vectors can be propagated and prepared in quantity. As described herein, the expression vectors which can be used include, but are not limited to, the following vectors or their derivatives: human or animal viruses such as vaccinia virus or adenovirus; insect viruses such as baculovirus; yeast vectors; bacteriophage vectors (e.g., lambda), and plasmid and cosmid DNA vectors, to name but a few.
[0077]A "vector" refers to any vehicle for the cloning of and/or transfer of a nucleic acid into a host cell. A vector may be a replicon to which another DNA segment may be attached so as to bring about the replication of the attached segment. A "replicon" refers to any genetic element (e.g., plasmid, phage, cosmid, chromosome, virus) that functions as an autonomous unit of DNA replication in vivo, i.e., capable of replication under its own control. The term "vector" includes both viral and nonviral vehicles for introducing the nucleic acid into a cell in vitro, ex vivo or in vivo. A large number of vectors known in the art may be used to manipulate nucleic acids, incorporate response elements and promoters into genes, etc. Possible vectors include, for example, plasmids or modified viruses including, for example bacteriophages such as lambda derivatives, or plasmids such as pBR322 or pUC plasmid derivatives, or the Bluescript vector. Another example of vectors that are useful in the present invention is the UltraVector® Production System (Intrexon Corp., Blacksburg, Va.) as described in WO 2007/038276, incorporated herein by reference. For example, the insertion of the DNA fragments corresponding to response elements and promoters into a suitable vector can be accomplished by ligating the appropriate DNA fragments into a chosen vector that has complementary cohesive termini. Alternatively, the ends of the DNA molecules may be enzymatically modified or any site may be produced by ligating nucleotide sequences (linkers) into the DNA termini. Such vectors may be engineered to contain selectable marker genes that provide for the selection of cells that have incorporated the marker into the cellular genome. Such markers allow identification and/or selection of host cells that incorporate and express the proteins encoded by the marker.
[0078]Viral vectors, and particularly retroviral vectors, have been used in a wide variety of gene delivery applications in cells, as well as living animal subjects. Viral vectors that can be used include, but are not limited to, retrovirus, adeno-associated virus, pox, baculovirus, vaccinia, herpes simplex, Epstein-Barr, adenovirus, geminivirus, and caulimovirus vectors. Non-viral vectors include plasmids, liposomes, electrically charged lipids (cytofectins), DNA-protein complexes, and biopolymers. In addition to a nucleic acid, a vector may also comprise one or more regulatory regions, and/or selectable markers useful in selecting, measuring, and monitoring nucleic acid transfer results (transfer to which tissues, duration of expression, etc.).
[0079]The term "plasmid" refers to an extra-chromosomal element often carrying a gene that is not part of the central metabolism of the cell, and usually in the form of circular double-stranded DNA molecules. Such elements may be autonomously replicating sequences, genome integrating sequences, phage or nucleotide sequences, linear, circular, or supercoiled, of a single- or double-stranded DNA or RNA, derived from any source, in which a number of nucleotide sequences have been joined or recombined into a unique construction which is capable of introducing a promoter fragment and DNA sequence for a selected gene product along with appropriate 3' untranslated sequence into a cell.
[0080]A "cloning vector" refers to a "replicon," which is a unit length of a nucleic acid, preferably DNA, that replicates sequentially and which comprises an origin of replication, such as a plasmid, phage or cosmid, to which another nucleic acid segment may be attached so as to bring about the replication of the attached segment. Cloning vectors may be capable of replication in one cell type and expression in another ("shuttle vector"). Cloning vectors may comprise one or more sequences that can be used for selection of cells comprising the vector and/or one or more multiple cloning sites for insertion of sequences of interest.
[0081]The term "expression vector" refers to a vector, plasmid or vehicle designed to enable the expression of an inserted nucleic acid sequence following transformation into the host. The cloned gene, i.e., the inserted nucleic acid sequence, is usually placed under the control of control elements such as a promoter, a minimal promoter, an enhancer, or the like. Initiation control regions or promoters, which are useful to drive expression of a nucleic acid in the desired host cell are numerous and familiar to those skilled in the art. Virtually any promoter capable of driving expression of these genes can be used in an expression vector, including but not limited to, viral promoters, bacterial promoters, animal promoters, mammalian promoters, synthetic promoters, constitutive promoters, tissue specific promoters, pathogenesis or disease related promoters, developmental specific promoters, inducible promoters, light regulated promoters; CYC1, HIS3, GAL1, GAL4, GAL10, ADH1, PGK, PHO5, GAPDH, ADC1, TRP1, URA3, LEU2, ENO, TPI, alkaline phosphatase promoters (useful for expression in Saccharomyces); AOX1 promoter (useful for expression in Pichia); β-lactamase, lac, ara, tet, trp, IPL, IPR, T7, tac and trc promoters (useful for expression in Escherichia coli); light regulated-, seed specific-, pollen specific-, ovary specific-, cauliflower mosaic virus 35S, CMV 35S minimal, cassava vein mosaic virus (CsVMV), chlorophyll a/b binding protein, ribulose 1,5-bisphosphate carboxylase, shoot-specific, root specific, chitinase, stress inducible, rice tungro bacilliform virus, plant super-promoter, potato leucine aminopeptidase, nitrate reductase, mannopine synthase, nopaline synthase, ubiquitin, zein protein, and anthocyanin promoters (useful for expression in plant cells); animal and mammalian promoters known in the art including, but are not limited to, the SV40 early (SV40e) promoter region, the promoter contained in the 3' long terminal repeat (LTR) of Rous sarcoma virus (RSV), the promoters of the E1A or major late promoter (MLP) genes of adenoviruses (Ad), the cytomegalovirus (CMV) early promoter, the herpes simplex virus (HSV) thymidine kinase (TK) promoter, a baculovirus IE1 promoter, an elongation factor 1 alpha (EF1) promoter, a phosphoglycerate kinase (PGK) promoter, a ubiquitin (Ubc) promoter, an albumin promoter, the regulatory sequences of the mouse metallothionein-L promoter and transcriptional control regions, the ubiquitous promoters (HPRT, vimentin, α-actin, tubulin and the like), the promoters of the intermediate filaments (desmin, neurofilaments, keratin, GFAP, and the like), the promoters of therapeutic genes (of the MDR, CFTR or factor VIII type, and the like), pathogenesis or disease related-promoters, and promoters that exhibit tissue specificity and have been utilized in transgenic animals, such as the elastase I gene control region which is active in pancreatic acinar cells; insulin gene control region active in pancreatic beta cells, immunoglobulin gene control region active in lymphoid cells, mouse mammary tumor virus control region active in testicular, breast, lymphoid and mast cells; albumin gene, Apo AI and Apo AII control regions active in liver, alpha-fetoprotein gene control region active in liver, alpha 1-antitrypsin gene control region active in the liver, beta-globin gene control region active in myeloid cells, myelin basic protein gene control region active in oligodendrocyte cells in the brain, myosin light chain-2 gene control region active in skeletal muscle, and gonadotropic releasing hormone gene control region active in the hypothalamus, pyruvate kinase promoter, villin promoter, promoter of the fatty acid binding intestinal protein, promoter of the smooth muscle cell α-actin, and the like. In addition, these expression sequences may be modified by addition of enhancer or regulatory sequences and the like.
[0082]Vectors may be introduced into the desired host cells by methods known in the art, e.g., transfection, electroporation, microinjection, transduction, cell fusion, DEAE dextran, calcium phosphate precipitation, lipofection (lysosome fusion), use of a gene gun, or a DNA vector transporter (see, e.g., Wu et al., J. Biol. Chem. 267:963 (1992); Wu et al., J. Biol. Chem. 263:14621 (1988); and Hartmut et al., Canadian Patent Application No. 2,012,311).
[0083]A polynucleotide according to the invention can also be introduced in vivo by lipofection. For the past decade, there has been increasing use of liposomes for encapsulation and transfection of nucleic acids in vitro. Synthetic cationic lipids designed to limit the difficulties and dangers encountered with liposome-mediated transfection can be used to prepare liposomes for in vivo transfection of a gene encoding a marker (Felgner et al., Proc. Natl. Acad. Sci. USA. 84:7413 (1987); Mackey et al., Proc. Natl. Acad. Sci. USA 85:8027 (1988); and Ulmer et al., Science 259:1745 (1993)). The use of cationic lipids may promote encapsulation of negatively charged nucleic acids, and also promote fusion with negatively charged cell membranes (Felgner et al., Science 337:387 (1989)). Particularly useful lipid compounds and compositions for transfer of nucleic acids are described in WO95/18863, WO96/17823 and U.S. Pat. No. 5,459,127. The use of lipofection to introduce exogenous genes into the specific organs in vivo has certain practical advantages. Molecular targeting of liposomes to specific cells represents one area of benefit. It is clear that directing transfection to particular cell types would be particularly preferred in a tissue with cellular heterogeneity, such as pancreas, liver, kidney, and the brain. Lipids may be chemically coupled to other molecules for the purpose of targeting (Mackey et al. 1988, supra). Targeted peptides, e.g., hormones or neurotransmitters, and proteins such as antibodies, or non-peptide molecules could be coupled to liposomes chemically.
[0084]Other molecules are also useful for facilitating transfection of a nucleic acid in vivo, such as a cationic oligopeptide (e.g., WO95/21931), peptides derived from DNA binding proteins (e.g., WO96/25508), or a cationic polymer (e.g., WO95/21931).
[0085]It is also possible to introduce a vector in vivo as a naked DNA plasmid (see U.S. Pat. Nos. 5,693,622, 5,589,466 and 5,580,859). Receptor-mediated DNA delivery approaches can also be used (Curiel et al., Hum. Gene Ther. 3:147 (1992); and Wu et al., J. Biol. Chem. 262:4429 (1987)).
[0086]The term "transfection" refers to the uptake of exogenous or heterologous RNA or DNA by a cell. A cell has been "transfected" by exogenous or heterologous RNA or DNA when such RNA or DNA has been introduced inside the cell. A cell has been "transformed" by exogenous or heterologous RNA or DNA when the transfected RNA or DNA effects a phenotypic change. The transforming RNA or DNA can be integrated (covalently linked) into chromosomal DNA making up the genome of the cell.
[0087]"Transformation" refers to the transfer of a nucleic acid fragment into the genome of a host organism, resulting in genetically stable inheritance. Host organisms containing the transformed nucleic acid fragments are referred to as "transgenic" or "recombinant" or "transformed" organisms.
[0088]In addition, the recombinant vector comprising a polynucleotide according to the invention may include one or more origins for replication in the cellular hosts in which their amplification or their expression is sought, markers or selectable markers.
[0089]The term "selectable marker" refers to an identifying factor, usually an antibiotic or chemical resistance gene, that is able to be selected for based upon the marker gene's effect, i.e., resistance to an antibiotic, resistance to a herbicide, calorimetric markers, enzymes, fluorescent markers, and the like, wherein the effect is used to track the inheritance of a nucleic acid of interest and/or to identify a cell or organism that has inherited the nucleic acid of interest. Examples of selectable marker genes known and used in the art include: genes providing resistance to ampicillin, streptomycin, gentamycin, kanamycin, hygromycin, bialaphos herbicide, sulfonamide, and the like; and genes that are used as phenotypic markers, i.e., anthocyanin regulatory genes, isopentanyl transferase gene, and the like.
[0090]The term "reporter gene" refers to a nucleic acid encoding an identifying factor that is able to be identified based upon the reporter gene's effect, wherein the effect is used to track the inheritance of a nucleic acid of interest, to identify a cell or organism that has inherited the nucleic acid of interest, and/or to measure gene expression induction or transcription. Examples of reporter genes known and used in the art include: luciferase (Luc), green fluorescent protein (GFP), chloramphenicol acetyltransferase (CAT), β-galactosidase (LacZ), β-glucuronidase (Gus), and the like. Selectable marker genes may also be considered reporter genes.
[0091]"Promoter and "promoter sequence" are used interchangeably and refer to a DNA sequence capable of controlling the expression of a coding sequence or functional RNA. In general, a coding sequence is located 3' to a promoter sequence. Promoters may be derived in their entirety from a native gene, or be composed of different elements derived from different promoters found in nature, or even comprise synthetic DNA segments. It is understood by those skilled in the art that different promoters may direct the expression of a gene in different tissues or cell types, or at different stages of development, or in response to different environmental or physiological conditions. Promoters that cause a gene to be expressed in most cell types at most times are commonly referred to as "constitutive promoters." Promoters that cause a gene to be expressed in a specific cell type are commonly referred to as "cell-specific promoters" or "tissue-specific promoters." Promoters that cause a gene to be expressed at a specific stage of development or cell differentiation are commonly referred to as "developmentally-specific promoters" or "cell differentiation-specific promoters." Promoters that are induced and cause a gene to be expressed following exposure or treatment of the cell with an agent, biological molecule, chemical, ligand, light, or the like that induces the promoter are commonly referred to as "inducible promoters" or "regulatable promoters." It is further recognized that since in most cases the exact boundaries of regulatory sequences have not been completely defined, DNA fragments of different lengths may have identical promoter activity.
[0092]The promoter sequence is typically bounded at its 3' terminus by the transcription initiation site and extends upstream (5' direction) to include the minimum number of bases or elements necessary to initiate transcription at levels detectable above background. Within the promoter sequence will be found a transcription initiation site (conveniently defined for example, by mapping with nuclease S1), as well as protein binding domains (consensus sequences) responsible for the binding of RNA polymerase.
[0093]"Therapeutic switch promoter" ("TSP") refers to a promoter that controls expression of a gene switch component. Gene switches and their various components are described in detail elsewhere herein. In certain embodiments a TSP is constitutive, i.e., continuously active. A constitutive TSP may be either constitutive-ubiquitous (i.e., generally functions, without the need for additional factors or regulators, in any tissue or cell) or constitutive-tissue or cell specific (i.e., generally functions, without the need for additional factors or regulators, in a specific tissue type or cell type). In certain embodiments a TSP of the invention is activated under conditions associated with a disease, disorder, or condition. In certain embodiments of the invention where two or more TSPs are involved the promoters may be a combination of constitutive and activatable promoters. As used herein, a "promoter activated under conditions associated with a disease, disorder, or condition" includes, without limitation, disease-specific promoters, promoters responsive to particular physiological, developmental, differentiation, or pathological conditions, promoters responsive to specific biological molecules, and promoters specific for a particular tissue or cell type associated with the disease, disorder, or condition, e.g. tumor tissue or malignant cells. TSPs can comprise the sequence of naturally occurring promoters, modified sequences derived from naturally occurring promoters, or synthetic sequences (e.g., insertion of a response element into a minimal promoter sequence to alter the responsiveness of the promoter).
[0094]A coding sequence is "under the control" of transcriptional and translational control sequences in a cell when RNA polymerase transcribes the coding sequence into mRNA, which is then trans-RNA spliced (if the coding sequence contains introns) and translated into the protein encoded by the coding sequence.
[0095]"Transcriptional and translational control sequences" refer to DNA regulatory sequences, such as promoters, enhancers, terminators, and the like, that provide for the expression of a coding sequence in a host cell. In eukaryotic cells, polyadenylation signals are control sequences.
[0096]The term "response element" ("RE") refers to one or more cis-acting DNA elements which confer responsiveness on a promoter mediated through interaction with the DNA-binding domains of a transcription factor. This DNA element may be either palindromic (perfect or imperfect) in its sequence or composed of sequence motifs or half sites separated by a variable number of nucleotides. The half sites can be similar or identical and arranged as either direct or inverted repeats or as a single half site or multimers of adjacent half sites in tandem. The response element may comprise a minimal promoter isolated from different organisms depending upon the nature of the cell or organism into which the response element will be incorporated. The DNA binding domain of the transcription factor binds, in the presence or absence of a ligand, to the DNA sequence of a response element to initiate or suppress transcription of downstream gene(s) under the regulation of this response element. Examples of DNA sequences for response elements of the natural ecdysone receptor include: RRGG/TTCANTGAC/ACYY (SEQ ID NO: 1) (see Cherbas et. al., Genes Dev. 5:120 (1991)); AGGTCAN.sub.(n)AGGTCA, where N(n) can be one or more spacer nucleotides (SEQ ID NO: 2) (see D'Avino et al., Mol. Cell. Endocrinol. 113:1 (1995)); and GGGTTGAATGAATTT (SEQ ID NO: 3) (see Antoniewski et al., Mol. Cell. Biol. 14:4465 (1994)).
[0097]"Factor-regulated promoter" ("FRP") refers to a promoter comprising at least one response element that is recognized by the DNA binding domain of a ligand-dependent transcription factor encoded by a gene switch of the invention.
[0098]The terms "operably linked," "operably associated," "through operable association," and the like refer to the association of nucleic acid sequences on a single nucleic acid fragment so that the function of one is affected by the other. For example, a promoter is operably linked with a coding sequence when it is capable of affecting the expression of that coding sequence (i.e., that the coding sequence is under the transcriptional control of the promoter). Coding sequences can be operably linked to regulatory sequences in sense or antisense orientation.
[0099]The term "expression" as used herein refers to the transcription and stable accumulation of sense (mRNA) or antisense RNA derived from a nucleic acid or polynucleotide. Expression may also refer to translation of mRNA into a protein or polypeptide.
[0100]The terms "cassette," "expression cassette" and "gene expression cassette" refer to a segment of DNA that can be inserted into a nucleic acid or polynucleotide at specific restriction sites or by homologous recombination. The segment of DNA comprises a polynucleotide that encodes a polypeptide of interest, and the cassette and restriction sites are designed to ensure insertion of the cassette in the proper reading frame for transcription and translation. "Transformation cassette" refers to a specific vector comprising a polynucleotide that encodes a polypeptide of interest and having elements in addition to the polynucleotide that facilitate transformation of a particular host cell. Cassettes, expression cassettes, gene expression cassettes and transformation cassettes of the invention may also comprise elements that allow for enhanced expression of a polynucleotide encoding a polypeptide of interest in a host cell. These elements may include, but are not limited to: a promoter, a minimal promoter, an enhancer, a response element, a terminator sequence, a polyadenylation sequence, and the like.
[0101]For purposes of this invention, the term "gene switch" refers to the combination of a response element associated with a promoter, and a ligand-dependent transcription factor-based system which, in the presence of one or more ligands, modulates the expression of a gene into which the response element and promoter are incorporated.
[0102]The term "ecdysone receptor-based," with respect to a gene switch, refers to a gene switch comprising at least a functional part of a naturally occurring or synthetic ecdysone receptor ligand binding domain and which regulates gene expression in response to a ligand that binds to the ecdysone receptor ligand binding domain.
[0103]As used herein, the terms "bioreactor" or "bioreactor device" includes a cell or cells intended to secrete a therapeutic protein or therapeutic polynucleotide. In certain non-limiting embodiments, the bioreactor comprises modified cells as described elsewhere herein. In certain, but not all embodiments, bioreactor cells may be "immunoisolated." Bioreactor cells are considered "immunoisolated" from a subject when the cells are treated such that the cells, upon introduction or implantation into the subject, are protected from the subject's immune system. For example, immunoisolated bioreactor cells may be contained within a barrier system which allows dissemination of said therapeutic protein or therapeutic polynucleotide, but which prevents direct contact of bioreactor cells with cells of the subject's immune system. Immunoisolated cells may be, for example, coated or encapsulated. Immunoisolation methods include but are not limited to conformal coating of cells, microencapsulation where cells are suspended in a biocompatible material and separated into spherical masses, or macroencapsulation, where the cells are enclosed in devices composed of natural or synthetic polymers that are used to enclose cells.
[0104]The terms "modulate" and "modulates" mean to induce, reduce or inhibit nucleic acid or gene expression, resulting in the respective induction, reduction or inhibition of protein or polypeptide production.
[0105]The polynucleotides or vectors according to the invention may further comprise at least one promoter suitable for driving expression of a gene in a modified cell.
[0106]Enhancers that may be used in embodiments of the invention include but are not limited to: an SV40 enhancer, a cytomegalovirus (CMV) enhancer, an elongation factor 1 (EF1) enhancer, yeast enhancers, viral gene enhancers, and the like.
[0107]A "3'reg" as defined herein, is an expression modulating element situated 3' to a coding region of a gene or transcript. Such elements include, without limitation: primary transcript-encoded Splicing elements, UTR from processed transcript, a polyadenylation signal or a DNA-encoded Transcription termination domain.
[0108]Termination control regions, i.e., terminator or polyadenylation nucleotide sequences, may also be derived from various genes native to the preferred hosts. Optionally, a termination site may be unnecessary, however, it is most preferred if included. In a one embodiment of the invention, the termination control region may be comprised or be derived from a synthetic sequence, synthetic polyadenylation signal, an SV40 late polyadenylation signal, an SV40 polyadenylation signal, a bovine growth hormone (BGH) polyadenylation signal, viral terminator sequences, or the like.
[0109]The terms "3'non-coding sequences" or "3' untranslated region (UTR)" refer to DNA sequences located downstream (3') of a coding sequence and may comprise polyadenylation [poly(A)] recognition sequences and other sequences encoding regulatory signals capable of affecting mRNA processing or gene expression. The polyadenylation signal is usually characterized by affecting the addition of polyadenylic acid tracts to the 3' end of the mRNA precursor.
[0110]"Regulatory region" refers to a nucleic acid sequence that regulates the expression of a second nucleic acid sequence. A regulatory region may include sequences which are naturally responsible for expressing a particular nucleic acid (a homologous region) or may include sequences of a different origin that are responsible for expressing different proteins or even synthetic proteins (a heterologous region). In particular, the sequences can be sequences of prokaryotic, eukaryotic, or viral genes or derived sequences that stimulate or repress transcription of a gene in a specific or non-specific manner and in an inducible or non-inducible manner. Regulatory regions include origins of replication, RNA splice sites, promoters, enhancers, transcriptional termination sequences, and signal sequences which direct the polypeptide into the secretory pathways of the target cell.
[0111]A regulatory region from a "heterologous source" refers to a regulatory region that is not naturally associated with the expressed nucleic acid. Included among the heterologous regulatory regions are regulatory regions from a different species, regulatory regions from a different gene, hybrid regulatory sequences, and regulatory sequences which do not occur in nature, but which are designed by one having ordinary skill in the art.
[0112]"RNA transcript" refers to the product resulting from RNA polymerase-catalyzed transcription of a DNA sequence. When the RNA transcript is a perfect complementary copy of the DNA sequence, it is referred to as the primary transcript or it may be a RNA sequence derived from post-transcriptional processing of the primary transcript and is referred to as the mature RNA. "Messenger RNA (mRNA)" refers to the RNA that is without introns and that can be translated into protein by the cell. "cDNA" refers to a double-stranded DNA that is complementary to and derived from mRNA. "Sense" RNA refers to RNA transcript that includes the mRNA and so can be translated into protein by the cell. "Antisense RNA" refers to a RNA transcript that is complementary to all or part of a target primary transcript or mRNA and that blocks the expression of a target gene. The complementarity of an antisense RNA may be with any part of the specific gene transcript, i.e., at the 5'non-coding sequence, 3'non-coding sequence, or the coding sequence. "Functional RNA" refers to antisense RNA, ribozyme RNA, or other RNA that is not translated yet has an effect on cellular processes.
[0113]"Polypeptide," "peptide" and "protein" are used interchangeably and refer to a polymeric compound comprised of covalently linked amino acid residues.
[0114]An "isolated polypeptide," "isolated peptide" or "isolated protein" refer to a polypeptide or protein that is substantially free of those compounds that are normally associated therewith in its natural state (e.g., other proteins or polypeptides, nucleic acids, carbohydrates, lipids). "Isolated" is not meant to exclude artificial or synthetic mixtures with other compounds, or the presence of impurities which do not interfere with biological activity, and which may be present, for example, due to incomplete purification, addition of stabilizers, or compounding into a pharmaceutically acceptable preparation.
[0115]A "substitution mutant polypeptide" or a "substitution mutant" will be understood to mean a mutant polypeptide comprising a substitution of at least one wild-type or naturally occurring amino acid with a different amino acid relative to the wild-type or naturally occurring polypeptide. A substitution mutant polypeptide may comprise only one wild-type or naturally occurring amino acid substitution and may be referred to as a "point mutant" or a "single point mutant" polypeptide. Alternatively, a substitution mutant polypeptide may comprise a substitution of two or more wild-type or naturally occurring amino acids with two or more amino acids relative to the wild-type or naturally occurring polypeptide. According to the invention, a Group H nuclear receptor ligand binding domain polypeptide comprising a substitution mutation comprises a substitution of at least one wild-type or naturally occurring amino acid with a different amino acid relative to the wild-type or naturally occurring Group H nuclear receptor ligand binding domain polypeptide.
[0116]When the substitution mutant polypeptide comprises a substitution of two or more wild-type or naturally occurring amino acids, this substitution may comprise either an equivalent number of wild-type or naturally occurring amino acids deleted for the substitution, i.e., 2 wild-type or naturally occurring amino acids replaced with 2 non-wild-type or non-naturally occurring amino acids, or a non-equivalent number of wild-type amino acids deleted for the substitution, i.e., 2 wild-type amino acids replaced with 1 non-wild-type amino acid (a substitution+deletion mutation), or 2 wild-type amino acids replaced with 3 non-wild-type amino acids (a substitution+insertion mutation).
[0117]Substitution mutants may be described using an abbreviated nomenclature system to indicate the amino acid residue and number replaced within the reference polypeptide sequence and the new substituted amino acid residue. For example, a substitution mutant in which the twentieth (20th) amino acid residue of a polypeptide is substituted may be abbreviated as "x20z", wherein "x" is the amino acid to be replaced, "20" is the amino acid residue position or number within the polypeptide, and "z" is the new substituted amino acid. Therefore, a substitution mutant abbreviated interchangeably as "E20A" or "Glu20Ala" indicates that the mutant comprises an alanine residue (commonly abbreviated in the art as "A" or "Ala") in place of the glutamic acid (commonly abbreviated in the art as "E" or "Glu") at position 20 of the polypeptide.
[0118]A substitution mutation may be made by any technique for mutagenesis known in the art, including but not limited to, in vitro site-directed mutagenesis (Hutchinson et al., J. Biol. Chem. 253:6551 (1978); Zoller et al., DNA 3:479 (1984); Oliphant et al., Gene 44:177 (1986); Hutchinson et al., Proc. Natl. Acad. Sci. USA 83:710 (1986)), use of TAB® linkers (Pharmacia), restriction endonuclease digestion/fragment deletion and substitution, PCR-mediated/oligonucleotide-directed mutagenesis, and the like. PCR-based techniques are preferred for site-directed mutagenesis (see Higuchi, 1989, "Using PCR to Engineer DNA", in PCR Technology: Principles and Applications for DNA Amplification, H. Erlich, ed., Stockton Press, Chapter 6, pp. 61-70).
[0119]The term "fragment," as applied to a polypeptide, refers to a polypeptide whose amino acid sequence is shorter than that of the reference polypeptide and which comprises, over the entire portion with these reference polypeptides, an identical amino acid sequence. Such fragments may, where appropriate, be included in a larger polypeptide of which they are a part. Such fragments of a polypeptide according to the invention may have a length of at least 2, 3, 4, 5, 6, 8, 10, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 25, 26, 30, 35, 40, 45, 50, 100, 200, 240, or 300 or more amino acids.
[0120]A "variant" of a polypeptide or protein refers to any analogue, fragment, derivative, or mutant which is derived from a polypeptide or protein and which retains at least one biological property of the polypeptide or protein. Different variants of the polypeptide or protein may exist in nature. These variants may be allelic variations characterized by differences in the nucleotide sequences of the structural gene coding for the protein, or may involve differential splicing or post-translational modification. The skilled artisan can produce variants having single or multiple amino acid substitutions, deletions, additions, or replacements. These variants may include, inter alia: (a) variants in which one or more amino acid residues are substituted with conservative or non-conservative amino acids, (b) variants in which one or more amino acids are added to the polypeptide or protein, (c) variants in which one or more of the amino acids includes a substituent group, and (d) variants in which the polypeptide or protein is fused with another polypeptide such as serum albumin. The techniques for obtaining these variants, including genetic (suppressions, deletions, mutations, etc.), chemical, and enzymatic techniques, are known to persons having ordinary skill in the art. In one embodiment, a variant polypeptide comprises at least about 14 amino acids.
[0121]The term "homology" refers to the percent of identity between two polynucleotide or two polypeptide moieties. The correspondence between the sequence from one moiety to another can be determined by techniques known to the art. For example, homology can be determined by a direct comparison of the sequence information between two polypeptide molecules by aligning the sequence information and using readily available computer programs. Alternatively, homology can be determined by hybridization of polynucleotides under conditions that form stable duplexes between homologous regions, followed by digestion with single-stranded-specific nuclease(s) and size determination of the digested fragments.
[0122]As used herein, the term "homologous" in all its grammatical forms and spelling variations refers to the relationship between proteins that possess a "common evolutionary origin," including proteins from superfamilies (e.g., the immunoglobulin superfamily) and homologous proteins from different species (e.g., myosin light chain, etc.) (Reeck et al., Cell 50:667 (1987)). Such proteins (and their encoding genes) have sequence homology, as reflected by their high degree of sequence similarity. However, in common usage and in the present application, the term "homologous," when modified with an adverb such as "highly," may refer to sequence similarity and not a common evolutionary origin.
[0123]Accordingly, the term "sequence similarity" in all its grammatical forms refers to the degree of identity or correspondence between nucleic acid or amino acid sequences of proteins that may or may not share a common evolutionary origin (see Reeck et al., Cell 50:667 (1987)). In one embodiment, two DNA sequences are "substantially homologous" or "substantially similar" when at least about 50% (e.g., at least about 75%, 90%, or 95%) of the nucleotides match over the defined length of the DNA sequences. Sequences that are substantially homologous can be identified by comparing the sequences using standard software available in sequence data banks, or in a Southern hybridization experiment under, for example, stringent conditions as defined for that particular system. Defining appropriate hybridization conditions is within the skill of the art (see e.g., Sambrook et al., 1989, supra).
[0124]As used herein, "substantially similar" refers to nucleic acid fragments wherein changes in one or more nucleotide bases results in substitution of one or more amino acids, but do not affect the functional properties of the protein encoded by the DNA sequence. "Substantially similar" also refers to nucleic acid fragments wherein changes in one or more nucleotide bases do not affect the ability of the nucleic acid fragment to mediate alteration of gene expression by antisense or co-suppression technology. "Substantially similar" also refers to modifications of the nucleic acid fragments of the present invention such as deletion or insertion of one or more nucleotide bases that do not substantially affect the functional properties of the resulting transcript. It is therefore understood that the invention encompasses more than the specific exemplary sequences. Each of the proposed modifications is well within the routine skill in the art, as is determination of retention of biological activity of the encoded products.
[0125]Moreover, the skilled artisan recognizes that substantially similar sequences encompassed by this invention are also defined by their ability to hybridize, under stringent conditions (0.1×SSC, 0.1% SDS, 65° C. and washed with 2×SSC, 0.1% SDS followed by 0.1×SSC, 0.1% SDS), with the sequences exemplified herein. Substantially similar nucleic acid fragments of the present invention are those nucleic acid fragments whose DNA sequences are at least about 70%, 80%, 90% or 95% identical to the DNA sequence of the nucleic acid fragments reported herein.
[0126]Two amino acid sequences are "substantially homologous" or "substantially similar" when greater than about 40% of the amino acids are identical, or greater than 60% are similar (functionally identical). Preferably, the similar or homologous sequences are identified by alignment using, for example, the GCG (Genetics Computer Group, Program Manual for the GCG Package, Version 7, Madison, Wis.) pileup program.
[0127]The term "corresponding to" is used herein to refer to similar or homologous sequences, whether the exact position is identical or different from the molecule to which the similarity or homology is measured. A nucleic acid or amino acid sequence alignment may include spaces. Thus, the term "corresponding to" refers to the sequence similarity, and not the numbering of the amino acid residues or nucleotide bases.
[0128]A "substantial portion" of an amino acid or nucleotide sequence comprises enough of the amino acid sequence of a polypeptide or the nucleotide sequence of a gene to putatively identify that polypeptide or gene, either by manual evaluation of the sequence by one skilled in the art, or by computer-automated sequence comparison and identification using algorithms such as BLAST (Basic Local Alignment Search Tool; Altschul et al., J. Mol. Biol. 215:403 (1993)); available at ncbi.nlm.nih.gov/BLAST/). In general, a sequence of ten or more contiguous amino acids or thirty or more nucleotides is necessary in order to putatively identify a polypeptide or nucleic acid sequence as homologous to a known protein or gene. Moreover, with respect to nucleotide sequences, gene specific oligonucleotide probes comprising 20-30 contiguous nucleotides may be used in sequence-dependent methods of gene identification (e.g., Southern hybridization) and isolation (e.g., in situ hybridization of bacterial colonies or bacteriophage plaques). In addition, short oligonucleotides of 12-15 bases may be used as amplification primers in PCR in order to obtain a particular nucleic acid fragment comprising the primers. Accordingly, a "substantial portion" of a nucleotide sequence comprises enough of the sequence to specifically identify and/or isolate a nucleic acid fragment comprising the sequence.
[0129]The term "percent identity," as known in the art, is a relationship between two or more polypeptide sequences or two or more polynucleotide sequences, as determined by comparing the sequences. In the art, "identity" also means the degree of sequence relatedness between polypeptide or polynucleotide sequences, as the case may be, as determined by the match between strings of such sequences. "Identity" and "similarity" can be readily calculated by known methods, including but not limited to those described in: Computational Molecular Biology (Lesk, A. M., ed.) Oxford University Press, New York (1988); Biocomputing: Informatics and Genome Projects (Smith, D. W., ed.) Academic Press, New York (1993); Computer Analysis of Sequence Data, Part I (Griffin, A. M., and Griffin, H. G., eds.) Humana Press, New Jersey (1994); Sequence Analysis in Molecular Biology (von Heinje, G., ed.) Academic Press (1987); and Sequence Analysis Primer (Gribskov, M. and Devereux, J., eds.) Stockton Press, New York (1991). Preferred methods to determine identity are designed to give the best match between the sequences tested. Methods to determine identity and similarity are codified in publicly available computer programs. Sequence alignments and percent identity calculations may be performed using sequence analysis software such as the Megalign program of the LASERGENE bioinformatics computing suite (DNASTAR Inc., Madison, Wis.). Multiple alignment of the sequences may be performed using the Clustal method of alignment (Higgins et al., CABIOS. 5:151 (1989)) with the default parameters (GAP PENALTY=10, GAP LENGTH PENALTY=10). Default parameters for pairwise alignments using the Clustal method may be selected: KTUPLE 1, GAP PENALTY=3, WINDOW=5 and DIAGONALS SAVED=5.
[0130]The term "sequence analysis software" refers to any computer algorithm or software program that is useful for the analysis of nucleotide or amino acid sequences. "Sequence analysis software" may be commercially available or independently developed. Typical sequence analysis software includes, but is not limited to, the GCG suite of programs (Wisconsin Package Version 9.0, Genetics Computer Group (GCG), Madison, Wis.), BLASTP, BLASTN, BLASTX (Altschul et al., J. Mol. Biol. 215:403 (1990)), and DNASTAR (DNASTAR, Inc. 1228 S. Park St. Madison, Wis. 53715 USA). Within the context of this application it will be understood that where sequence analysis software is used for analysis, that the results of the analysis will be based on the "default values" of the program referenced, unless otherwise specified. As used herein "default values" will mean any set of values or parameters which originally load with the software when first initialized.
[0131]"Chemically synthesized," as related to a sequence of DNA, means that the component nucleotides were assembled in vitro. Manual chemical synthesis of DNA may be accomplished using well-established procedures, or automated chemical synthesis can be performed using one of a number of commercially available machines. Accordingly, the genes can be tailored for optimal gene expression based on optimization of nucleotide sequence to reflect the codon bias of the host cell. The skilled artisan appreciates the likelihood of successful gene expression if codon usage is biased towards those codons favored by the host. Determination of preferred codons can be based on a survey of genes derived from the host cell where sequence information is available.
[0132]As used herein, two or more individually operable gene regulation systems are said to be "orthogonal" when; a) modulation of each of the given systems by its respective ligand, at a chosen concentration, results in a measurable change in the magnitude of expression of the gene of that system, and b) the change is statistically significantly different than the change in expression of all other systems simultaneously operable in the cell, tissue, or organism, regardless of the simultaneity or sequentially of the actual modulation. Preferably, modulation of each individually operable gene regulation system effects a change in gene expression at least 2-fold greater than all other operable systems in the cell, tissue, or organism, e.g., at least 5-fold, 10-fold, 100-fold, or 500-fold greater. Ideally, modulation of each of the given systems by its respective ligand at a chosen concentration results in a measurable change in the magnitude of expression of the gene of that system and no measurable change in expression of all other systems operable in the cell, tissue, or organism. In such cases the multiple inducible gene regulation system is said to be "fully orthogonal." The present invention is useful to search for orthogonal ligands and orthogonal receptor-based gene expression systems such as those described in US 2002/0110861 A1, which is incorporated herein by reference in its entirety.
[0133]The term "exogenous gene" means a gene foreign to the subject, that is, a gene which is introduced into the subject through a transformation process, an unmutated version of an endogenous mutated gene or a mutated version of an endogenous unmutated gene. The method of transformation is not critical to this invention and may be any method suitable for the subject known to those in the art. Exogenous genes can be either natural or synthetic genes which are introduced into the subject in the form of DNA or RNA which may function through a DNA intermediate such as by reverse transcriptase. Such genes can be introduced into target cells, directly introduced into the subject, or indirectly introduced by the transfer of transformed cells into the subject.
[0134]The terms "therapeutic product" and "therapeutic molecule" as used herein refer to a therapeutic polypeptide ("TP", encoded by a "therapeutic proteins sequence" ("TPSQ")) or therapeutic polynucleotide which imparts a beneficial function to the subject to be treated. Therapeutic polypeptides may include, without limitation, peptides as small as three amino acids in length, single- or multiple-chain proteins, and fusion proteins. Therapeutic polynucleotides may include, without limitation, antisense oligonucleotides, small interfering RNAs, ribozymes, and RNA external guide sequences. Non-limiting examples of therapeutic products are disclosed elsewhere herein. The therapeutic product may comprise a naturally occurring sequence, a synthetic sequence or a combination of natural and synthetic sequences.
[0135]The term "ligand-dependent transcription factor complex" or "LDTFC" refers to a transcription factor comprising one or more protein subunits, which complex can regulate gene expression driven by a "factor-regulated promoter" as defined herein. A model LDTFC is an "ecdysone receptor complex" generally refers to a heterodimeric protein complex having at least two members of the nuclear receptor family, ecdysone receptor ("EcR") and ultraspiracle ("USP") proteins (see Yao et al., Nature 366:476 (1993)); Yao et al., Cell 71:63 (1992)). A functional LDTFC such as an EcR complex may also include additional protein(s) such as immunophilins. Additional members of the nuclear receptor family of proteins, known as transcriptional factors (such as DHR38, betaFTZ-1 or other insect homologs), may also be ligand dependent or independent partners for EcR and/or USP. A LDTFC such as an EcR complex can also be a heterodimer of EcR protein and the vertebrate homolog of ultraspiracle protein, retinoic acid-X-receptor ("RXR") protein or a chimera of USP and RXR. The terms "LDTFC" and "EcR complex" also encompass homodimer complexes of the EcR protein or USP, as well as single polypeptides or trimers, tetramer, and other multimers serving the same function.
[0136]A LDTFC such as an EcR complex can be activated by an active ecdysteroid or non-steroidal ligand bound to one of the proteins of the complex, inclusive of EcR, but not excluding other proteins of the complex. As used herein, the term "ligand," as applied to LDTFC-based gene switches e.g., EcD complex based gene switches, describes small and soluble molecules having the capability of activating a gene switch to stimulate expression of a polypeptide encoded therein. Examples of ligands include, without limitation, an ecdysteroid, such as ecdysone, 20-hydroxyecdysone, ponasterone A, muristerone A, and the like, 9-cis-retinoic acid, synthetic analogs of retinoic acid, N,N'-diacylhydrazines such as those disclosed in U.S. Pat. Nos. 6,013,836; 5,117,057; 5,530,028; and 5,378,726 and U.S. Published Application Nos. 2005/0209283 and 2006/0020146; oxadiazolines as described in U.S. Published Application No. 2004/0171651; dibenzoylalkyl cyanohydrazines such as those disclosed in European Application No. 461,809; N-alkyl-N,N'-diaroylhydrazines such as those disclosed in U.S. Pat. No. 5,225,443; N-acyl-N-alkylcarbonylhydrazines such as those disclosed in European Application No. 234,994; N-aroyl-N-alkyl-N'-aroylhydrazines such as those described in U.S. Pat. No. 4,985,461; amidoketones such as those described in U.S. Published Application No. 2004/0049037; each of which is incorporated herein by reference and other similar materials including 3,5-di-tert-butyl-4-hydroxy-N-isobutyl-benzamide, 8-O-acetylharpagide, oxysterols, 22(R) hydroxycholesterol, 24(S) hydroxycholesterol, 25-epoxycholesterol, T0901317, 5-alpha-6-alpha-epoxycholesterol-3-sulfate (ECHS), 7-ketocholesterol-3-sulfate, framesol, bile acids, 1,1-biphosphonate esters, juvenile hormone III, and the like. Examples of diacylhydrazine ligands useful in the present invention include RG-115819 (3,5-Dimethyl-benzoic acid N-(1-ethyl-2,2-dimethyl-propyl)-N'-(2-methyl-3-methoxy-benzoyl)-hydrazide- ), RG-115932 ((R)-3,5-Dimethyl-benzoic acid N-(1-tert-butyl-butyl)-N'-(2-ethyl-3-methoxy-benzoyl)-hydrazide), and RG-115830 (3,5-Dimethyl-benzoic acid N-(1-tert-butyl-butyl)-N'-(2-ethyl-3-methoxy-benzoyl)-hydrazide). See, e.g., U.S. patent application Ser. No. 12/155,111, and PCT Appl. No. PCT/US2008/006757, both of which are incorporated herein by reference in their entireties.
[0137]A LDTFC such as an EcR complex includes proteins which are members of the nuclear receptor superfamily wherein all members are characterized by the presence of one or more polypeptide subunits comprising an amino-terminal transactivation domain ("AD," "TD," or "TA," used interchangeably herein), a DNA binding domain ("DBD"), and a ligand binding domain ("LBD"). The AD may be present as a fusion with a "heterodimerization partner" or "HP." A fusion protein comprising an AD and HP of the invention is referred to herein as a "coactivation protein" or "CAP." The DBD and LBD may be expressed as a fusion protein, referred to herein as a "ligand-inducible transcription factor ("LTF"). The fusion partners may be separated by a linker, e.g., a hinge region. Some members of the LTF family may also have another transactivation domain on the carboxy-terminal side of the LBD. The DBD is characterized by the presence of two cysteine zinc fingers between which are two amino acid motifs, the P-box and the D-box, which confer specificity for ecdysone response elements. These domains may be either native, modified, or chimeras of different domains of heterologous receptor proteins.
[0138]The DNA sequences making up the exogenous gene, the response element, and the LDTFC, e.g., EcR complex, may be incorporated into archaebacteria, procaryotic cells such as Escherichia coli, Bacillus subtilis, or other enterobacteria, or eucaryotic cells such as plant or animal cells. However, because many of the proteins expressed by the gene are processed incorrectly in bacteria, eucaryotic cells are preferred. The cells may be in the form of single cells or multicellular organisms. The nucleotide sequences for the exogenous gene, the response element, and the receptor complex can also be incorporated as RNA molecules, preferably in the form of functional viral RNAs such as tobacco mosaic virus. Of the eucaryotic cells, vertebrate cells are preferred because they naturally lack the molecules which confer responses to the ligands of this invention for the EcR. As a result, they are "substantially insensitive" to the ligands of this invention. Thus, the ligands useful in this invention will have negligible physiological or other effects on transformed cells, or the whole organism. Therefore, cells can grow and express the desired product, substantially unaffected by the presence of the ligand itself.
[0139]The term "subject" means an intact insect, plant or animal. It is also anticipated that the ligands will work equally well when the subject is a fungus or yeast. When the subject is an intact animal, preferably the animal is a vertebrate, most preferably a mammal.
[0140]EcR ligands, when used with a LDTFC, e.g., an EcR complex, which in turn is bound to the response element linked to an exogenous gene (e.g., a reporter gene), provide the means for external temporal regulation of expression of the exogenous gene. The order in which the various components bind to each other, that is, ligand to receptor complex and receptor complex to response element, is not critical. Typically, modulation of expression of the exogenous gene is in response to the binding of a LDTFC, e.g., an EcR complex, to a specific control, or regulatory, DNA element. The EcR protein, like other members of the nuclear receptor family, possesses at least three domains, an AD, a DBD, and a LBD. This receptor, like a subset of the nuclear receptor family, also possesses less well-defined regions responsible for heterodimerization properties (referred to herein as a "heterodimerization partner" or "HP"). Binding of the ligand to the ligand binding domain of a LTF, e.g., an EcR protein, after heterodimerization with a CAP including, e.g., an AD and/or an HP, e.g., a USP or RXR protein, enables the DNA binding domains of the heterodimeric proteins to bind to the response element in an activated form, thus resulting in expression or suppression of the exogenous gene. This mechanism does not exclude the potential for ligand binding to individual subunits, e.g., LTF or CAP, e.g., an EcR or USP, and the resulting formation of active homodimer complexes (e.g. EcR+EcR or USP+USP). In one embodiment, one or more of the receptor domains can be varied producing a chimeric gene switch. Typically, one or more of the three domains may be chosen from a source different than the source of the other domains so that the chimeric receptor is optimized in the chosen host cell or organism for transactivating activity, complementary binding of the ligand, and recognition of a specific response element. In addition, the response element itself can be modified or substituted with response elements for other DNA binding protein domains such as the GAL-4 protein from yeast (see Sadowski et al., Nature 335:563 (1988) or LexA protein from E. coli (see Brent et al., Cell 43:729 (1985)) to accommodate chimeric LDTFCs, e.g., EcR complexes. Another advantage of chimeric systems is that they allow choice of a promoter used to drive the exogenous gene according to a desired end result. Such double control can be particularly important in areas of gene therapy, especially when cytotoxic proteins are produced, because both the timing of expression as well as the cells wherein expression occurs can be controlled. When exogenous genes, operatively linked to a suitable promoter, are introduced into the cells of the subject, expression of the exogenous genes is controlled by the presence of the ligand of this invention. Promoters may be constitutively or inducibly regulated or may be tissue-specific (that is, expressed only in a particular type of cell) or specific to certain developmental stages of the organism.
[0141]Numerous genomic and cDNA nucleic acid sequences coding for a variety of polypeptides, such as transcription factors and reporter genes, are well known in the art. Those skilled in the art have access to nucleic acid sequence information for virtually all known genes and can either obtain the nucleic acid molecule directly from a public depository, the institution that published the sequence, or employ routine methods to prepare the molecule.
[0142]For in vivo use, the ligands described herein may be taken up in pharmaceutically acceptable carriers, such as, for example, solutions, suspensions, tablets, capsules, ointments, elixirs, and injectable compositions. Pharmaceutical compositions may contain from 0.01% to 99% by weight of the ligand. Compositions may be either in single or multiple dose forms. The amount of ligand in any particular pharmaceutical composition will depend upon the effective dose, that is, the dose required to elicit the desired gene expression or suppression.
[0143]Suitable routes of administering the pharmaceutical preparations include oral, rectal, topical (including dermal, buccal and sublingual), vaginal, parenteral (including subcutaneous, intramuscular, intravenous, intradermal, intrathecal and epidural) and by naso-gastric tube. It will be understood by those skilled in the art that the preferred route of administration will depend upon the condition being treated and may vary with factors such as the condition of the recipient.
[0144]One embodiment of the invention comprises methods for treating, ameliorating, or preventing a disease, disorder, or condition in a subject, comprising: [0145](a) introducing into cells of said subject (1) a polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex, operably linked to a therapeutic switch promoter, wherein the promoter is activated during said disease, disorder, or condition, and (2) a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide linked to a promoter which is activated by said ligand-dependent transcription factor complex; and [0146](b) administering ligand to said subject to induce expression of said therapeutic polypeptide or therapeutic polynucleotide;
[0147]wherein said therapeutic polypeptide or therapeutic polynucleotide is expressed at a level sufficient to treat, ameliorate, or prevent said disease, disorder, or condition.
[0148]One embodiment of the invention comprises methods for treating, ameliorating, or preventing a disease, disorder, or condition in a subject, comprising:
[0149](a) introducing into a subject (1) a first polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex through operable association with a therapeutic switch promoter, and (2) a second polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide operably associated with a factor-regulated promoter which is activated by said ligand-dependent transcription factor complex, wherein said first and second polynucleotides are introduced so as to permit expression of said ligand-dependent transcription factor complex; and
[0150](b) administering ligand to said subject to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
[0151]One embodiment of the invention comprises methods for expressing a therapeutic polypeptide or therapeutic polynucleotide in a subject, comprising:
[0152](a) introducing into a subject (1) a first polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex through operable association with a therapeutic switch promoter, and (2) a second polynucleotide encoding said therapeutic polypeptide or therapeutic polynucleotide operably associated with a factor-regulated promoter which is activated by said ligand-dependent transcription factor complex, wherein said first and second polynucleotides are introduced so as to permit expression of said ligand-dependent transcription factor complex; and
[0153](b) administering ligand to said subject to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
[0154]In certain embodiments, the therapeutic switch promoter described in the methods is constitutive. In certain embodiments, the therapeutic switch promoter is activated under conditions associated with a disease, disorder, or condition, e.g., the promoter is activated in response to a disease, in response to a particular physiological, developmental, differentiation, or pathological condition, and/or in response to one or more specific biological molecules; and/or the promoter is activated in particular tissue or cell types. In certain embodiments, the disease, disorder, or condition is responsive to the therapeutic polypeptide or polynucleotide. For example in certain non-limiting embodiments the therapeutic polynucleotide or polypeptide is useful to treat, prevent, ameliorate, reduce symptoms, prevent progression, or cure the disease, disorder or condition, but need not accomplish any one or all of these things. In certain embodiments, the first and second polynucleotides are introduced so as to permit expression of the ligand-dependent transcription factor complex under conditions associated with a disease, disorder or condition. In one embodiment, the therapeutic methods are carried out such that the therapeutic polypeptide or therapeutic polynucleotide is expressed and disseminated through the subject at a level sufficient to treat, ameliorate, or prevent said disease, disorder, or condition. As used herein, "disseminated" means that the polypeptide is expressed and released from the modified cell sufficiently to have an effect or activity in the subject. Dissemination may be systemic, local or anything in between. For example, the therapeutic polypeptide or therapeutic polynucleotide might be systemically disseminated through the bloodstream or lymph system. Alternatively, the therapeutic polypeptide or therapeutic polynucleotide might be disseminated locally in a tissue or organ to be treated.
[0155]In one embodiment, the therapeutic methods are carried out by administering compositions of the invention, such as the first and second polynucleotides described above, directly to the subject to be treated, such that the polynucleotides are taken up, in vivo, by cells of the subject to be treated, and one or more therapeutic polypeptides or polynucleotides will be expressed by those cells under appropriate conditions, as described in detail elsewhere herein. Polynucleotides may be directly delivered to a subject to be treated by a variety of methods including, without limitation, viral vectors, e.g., retroviral vectors, adeno-associated virus vectors, pox virus vectors, e.g., vaccinia virus vectors, baculovirus vectors, herpes virus vectors, e.g., herpes simplex vectors or Epstein-Barr virus vectors, adenovirus vectors, geminivirus vectors, or caulimovirus vectors; non-viral vectors such as plasmids, which may be delivered, for example complexed with liposomes, electrically charged lipids (cytofectins), biopolymers or as DNA-protein complexes.
[0156]In another embodiment, the therapeutic methods are carried out by introducing the compositions of the invention, such as the first and second polynucleotides described above, into the subject to be treated contained in one or more modified cells. Following administration of the modified cells the one or more therapeutic polypeptides or polynucleotides are expressed by the modified cells under appropriate conditions, as described in detail elsewhere herein. The term "modified cell" refers to a cell or cells into which at least a first and second polynucleotide as described above have been inserted. As such, "a modified cell" refers to the cell harboring the first and second polynucleotides, which may or may not be a cell from, or related to, the subject to be treated. Such cells are included in the definition of "bioreactors" or "bioreactor devices" as described herein. As defined herein, however, a "bioreactor" or "bioreactor device" need not be not a modified cell, rather, a bioreactor or bioreactor device as defined herein is any cell or cells intended to secrete a therapeutic protein or therapeutic polynucleotide, whether or not the cell(s) are "modified cells."
[0157]In one embodiment, the therapeutic methods are carried out by introducing the compositions of the invention, such as the first and second polynucleotides described above, into cells that have been isolated from said subject, i.e., autologous cells, to produce modified cells, and the modified cells are re-introduced into said subject.
[0158]Alternatively, modified cells may be prepared by introducing the compositions of the invention, such as the first and second polynucleotides described above, into cells which are not isolated from the subject, i.e., they are non-autologous relative to the subject, to produce modified non-autologous (MNA) cells. Such MNA cells may be allogeneic relative to the subject to be treated, i.e., they are derived from a genetically non-identical member of the same species as the subject. For example, in treating a human subject, the cells would be human cells, but not directly derived from the subject to be treated. Alternatively, MNA cells may be xenogeneic relative to the subject to be treated. i.e., they are derived from a different species than the subject to be treated. For example, in treating a human subject the cells might be mouse cells, monkey cells, or pig cells.
[0159]MNA cells suitable for use in the present invention may be generated from any number of cells types, including, but not limited to immortalized cells, primary cells, and cells capable of terminal differentiation. Non-limiting examples of cells suitable for generating MNA modified cells for the present invention include C2C12 mouse myoblast cells, HEK293 human embryonic kidney cells, ARPE-19 cells, hMSC cells, pancreatic islet cells, MDCK cell, BHK cell, hybridoma cell CHO cell, an astrocyte derived cell, an oligodendrocyte derived cell, a myoblast derived cell, a parathyroid derived cell. In a specific embodiment where pancreatic islet cells are used to generate modified cells to treat a human subject, the pancreatic islet cells may be xenogeneic, e.g., porcine islet cells, or allogeneic, e.g., human islet cells derived from cadavers.
[0160]In one embodiment, the therapeutic methods are carried out in vivo.
[0161]In one embodiment, the polynucleotide encoding the gene switch and the polynucleotide encoding the therapeutic polypeptide or therapeutic polynucleotide linked to a promoter are part of one larger polynucleotide, e.g., a vector. In another embodiment, the polynucleotide encoding the gene switch and the polynucleotide encoding the therapeutic polypeptide or therapeutic polynucleotide linked to a promoter are separate polynucleotides, which may be combined to form a "nucleic acid composition.".
[0162]In certain embodiments, a bioreactor of the invention comprises modified or non-modified cells surrounded by a barrier (e.g., encapsulated) prior to being introduced into the subject. Such a bioreactor may be used with any subject instead of having to modify autologous cells from each individual. Cellular encapsulation methods have been used to immunoisolate cells while allowing, either selectively or unselectively, the release of desired biological materials. It may be desirable to provide encapsulation compositions and methods for making them, which are capable of providing improved structural characteristics and/or immune protection. Such compositions and methods may find use, where encapsulated cells can withstand mechanical, chemical or immune destruction within the subject to be treated, and would additionally provide for free permeability to nutrients, ions, oxygen, and other materials needed to both maintain the tissue and support normal metabolic functions, but impermeable to bacteria, lymphocytes, and large proteins of the type responsible for immunochemical reactions. Barriers suitable for use in the present invention allow dissemination of a therapeutic protein or therapeutic polynucleotide expressed by modified or non-modified cells contained within the barrier, but prevent direct contact of the cells with cells of the subject's immune system. The barrier may also function to prevent non-autologous or autologous modified or non-modified cells from escaping from the site of introduction, e.g., rogue cells that might cause harm to the subject if allowed to circulate. In one embodiment the barrier is a selectively permeable barrier, e.g., a barrier that is permeable to small molecules such as hormones and small peptides but impermeable to larger polypeptides such as antibodies. For example, the barrier may be impermeable to molecules with a molecular weight greater than about 100,000, about 50,000, about 25,000, about 10,000, about 5,000 or about 1,000 daltons.
[0163]Any number of barrier systems are suitable for use in the present invention. In one embodiment, for example, the barrier comprises a conformal coating which encases one or more cells. Typically a conformal coating is made of a polymer material, e.g., polyethylene glycol or hydroxyethyl methacrylate-methyl methacrylate (HEMA-MMA). Conformal coatings typically enclose a small number of modified cells, e.g., 1-10 cells, 1-20 cells, 1-30 cells 1-50 cells 1-70 cells or 1-90 cells. See, e.g., Shoichet M S, Winn S R., Adv Drug Delivery Rev. 42:81-102 (2000), which is incorporated herein by reference in its entirety.
[0164]In other embodiments, a barrier system suitable for use in the present invention comprises a bioreactor, which comprises encapsulated cells. Two non-limiting encapsulation methods, microencapsulation and macroencapsulation, are known in the art. Typically, in microencapsulation, the cells are suspended in a biologically compatible encapsulation material which is then shaped into bead-like structures, whereas in macroencapsulation the device is generally manufactured prior to the addition of cells and can be composed of one or more synthetic membranes. As compared to conformal coatings, barrier systems comprising encapsulated cells tend to be more uniform in size, and tend to have uniform pore size allowing better control of protein dissemination. For encapsulation, living cells and other sensitive materials may be treated under sufficiently mild conditions allowing the cells or biomaterial to remain substantially unaffected by the encapsulation process, yet permitting the formation of a capsule of sufficient strength to exist over long periods of time.
[0165]Living cell(s) can be encapsulated and the resulting encapsulated cell(s) maintain long term in vivo activity by encapsulating the cells within a biocompatible semi-permeable membrane. One way to increase biocompatibility is to add an outer surface of biocompatible negatively-charged material. The term "biocompatible" as used herein refers collectively to both the intact capsule and its contents. Specifically, it refers to the capability of the implanted intact encapsulated cell to avoid detrimental effects of the body's various protective systems, such as immune system or foreign body fibrotic response, and remain functional for a significant period of time.
[0166]Bioreactors comprising encapsulated cells which are suitable for use in the present invention are especially useful for the administration of cells to an animal, wherein the immune response of the animal towards the cell is to be minimized. Cells which produce antibodies, enzymes, and other bioactive materials can also be administered. The small size of the resulting encapsulated cells within the subject of the invention facilitate administration of the microcapsules by injection, implantation or transplantation into a subject.
[0167]Living cells can be encapsulated in a variety of gels, e.g., alginate, to form implantable bead-like structures, e.g., microbeads or microspheres to physically isolate the cells once implanted into a subject to be treated. To prevent entry of smaller molecular weight substances such as antibodies and complement (with a molecular weight of about 150 kDa) into these bead-like structures, they can be coated with a material such as poly-L-lysine, chitosan, or PAN-PVC, which provides an outer shell with a controlled pore size or they can be treated by e.g., cross-linking, to control their internal porosity. Additional examples of useful materials include conventional biocompatible materials made up of natural or synthetic polymers or co-polymers, such as poly-L-lysine-alginate, collagen, gelatin, laminin, methyl methacrylate, hydroxyethyl methacrylate, MATRIGEL, VIRTOGEN, polyvinylalcohol, agarose, polyethylene glycol, hydrogels, polylactic acid, polyglycolic acid, poly(lactide-co-glycolide), polyhydroxybutyrate-polyhydroxyvalerate, copolymer, poly(lactide-co-caprolactone), polyesteramides, polyorthoesters, poly 13-hydroxybutyric acid, polyanhydrides, polyethylene terephthalate, polyetrafluoroethylene, pllyacrylates (including acrylic copolymers), polyvinylidenes, polyvinyl chloride copolymers, polyurethanes, polystyrenes, polyamides, cellulose acetates, cellulose nitrates, polysulfones (including polyether sulfones), polyphosphazenes, polyacrylonitriles, and poly(acrylonitrile/covinyl chloride).
[0168]One form of encapsulation is microencapsulation, which involves suspension of the cells in a liquid or gelatinous encapsulation material, which is then formed into a supporting particulate matrix, e.g., a hydrogel matrix to form a bead-like structure, which serves as a core of an implantable device. The core maintains a proper cell distribution, provides strength, and enhances cell viability, longevity, and function. The core can also contribute to immunoisolation. It also protects the internal cells contained in the bead-like structures from direct cell-cell interactions that can elicit an undesirable immune response in the subject to be treated.
[0169]A barrier system may contain multiple layers, e.g., where each layer serves a different purpose (e.g., support, control of permeability). Barriers may comprise contrast agents or other properties that render the barrier imageable (e.g., by x-ray, sonography, etc.) to ensure proper positioning of the implanted cells. Examples of barrier systems useful for cell implantation are described in U.S. Pat. Nos. 7,226,978, RE39,542 (agarose), 6,960,351, 6,916,640, 6,911,227 (polyethylene glycol), 6,818,018, 6,808,705, 6,783,964, 6,762,959, 6,727,322, 6,610,668 (poly-14-N-acetylglucosamine (p-GlcNAc) polysaccharide), 6,558,665, RE38,027, 6,495,161, 6,368,612, 6,365,385, 6,337,008, 6,306,454 (polyalkylene), 6,303,355, 6,287,558 (gel super matrix), 6,281,015, 6,264,941, 6,258,870, 6,180,007, 6,126,936 (polyamine acid), 6,123,700, 6,083,523, 6,020,200, 5,916,790, 5,912,005, 5,908,623, 5,902,745, 5,858,746, 5,846,530 (polysaccaharides), 5,843,743, 5,837,747, 5,837,234, 5,834,274, 5,834,001, 5,801,033, 5,800,829, 5,800,828, 5,798,113, 5,788,988, 5,786,216, 5,773,286, 5,759,578, 5,700,848, 5,656,481, 5,653,975, 5,648,099, 5,550,178, 5,550,050, 4,806,355, 4,689,293, 4,680,174, 4,673,566, 4,409,331, 4,352,883, and U.S. Patent Application Publications 2006/0263405 (alginate/polymer) and 2004/0005302 (alignate-poly-L-lysine), each incorporated herein by references in its entirety.
[0170]In certain embodiments, a barrier system suitable for use in the present invention comprises microencapsulated cells. Microencapsulation generates approximately spherical and relatively uniform bead-like structures comprising encapsulated cells, where the bead-like structures are about 100-700 μm in diameter, e.g., about 100, 200, 300, 400, 500, 600 or 700 μm in diameter. Microencapsulated cells of the invention may be produced using a variety of encapsulation materials as described above. In one embodiment, the encapsulation material comprises a hydrogel. In another embodiment the encapsulation material comprises a polymer. Suitable polymers include, without limitation, cellulose, e.g., cellulose sulfate, and alginate. For example, one microcapsule of the invention comprises polyanionic alginate and a poly-cationic polymer to interact and form a physical permselective membrane barrier. An alternative method of microencapsulation comprises the formation of poly (L-lactide) acid (PLLA) or a poly-L-ornithine (PLO) alginate microspheres. See, e.g., Darrabie, M. D. et al. Biomaterials 26:6846-6852 (2005) and Blasi, P. et al. Int J. Pharm. 324:27-36 (2006). Alginate based microencapsulation materials may further contain ultra high viscosity (UHV) polymers, which may also be biodegradable. See, e.g., Zimmermann, U. et al. Ann N Y Acad. Sci. 944:199-215 (2001).
[0171]Bioreactors of the present invention comprising microencapsulated cells typically comprise at least one up to about 1000 cells per "bead," e.g., modified or non-modified cells intended to secrete a desired therapeutic polypeptide or polynucleotide as described herein. For example, a bioreactor of the invention which comprises microencapsulated cells may result in at least 50, at least 100, at least 200, at least 400, at least 500, at least 800 to about 1000 or more cells per "bead."
[0172]In certain embodiments, a bioreactor suitable for use in the present invention comprises cells enclosed in a macroencapsulation device. As compared to bioreactors comprising microencapsulated cells, bioreactors comprising macroencapsulation devices are typically larger and often non-spherical encapsulated cell entities, and may be composed of one or more synthetic membranes, e.g., one, two, three, four, 8, 10, or more membranes, which may be the same composition of different compositions. As denoted by the name, macrocapsulated cell devices are of a size such that individual entities may be easily manipulated. For example, a typical macroencapsulation device may be an oblong shape, about 3 mm, 4 mm, 5 mm, 6 mm, 7 mm, 8 mm, 9 mm, 10 mm or more long and about 1 mm, 2 mm, 3 mm, 4 mm, or 5 mm or more in diameter. An exemplary but non-limiting macroencapsulation device of the invention is about 6 mm long and about 1 mm in diameter.
[0173]In certain embodiments a macroencapsulation device suitable for use in the present invention comprises two or more synthetic membranes, where the synthetic membranes have different pore sizes so as to regulate transit of therapeutic molecules through the device and their dissemination into the environment. In certain embodiments, a macroencapsulation device of the invention comprises a semi-permeable polymer outer membrane and an internal scaffold to support the cells. In non-limiting examples, the outer membrane comprises pores of about 15 nm to allow exchange of nutrients of therapeutic molecules. The internal scaffold may comprise any number of materials. In one non-limiting example the scaffold comprises poly (ethylene terephthalate) yarn (available from Neurotech (www.neurotechusa.com)).
[0174]In another non-limiting example, a macroencapsulaton device suitable for use in the invention comprises a polymeric membrane bilayer, where the bilayer comprises an outer layer of 5 μm poly(tetrafluoroethylene) (PTFE) membrane laminated onto an inner tighter pore 0.45 uM PTFE immunobarrier layer (available from Theracyte (www.theracyte.com)). Such a macroencapsulation device may further comprise a non-woven poly mesh layer exterior to said polymeric membrane bilayer. In yet another non-limiting example, a macroencapsulation device suitable for use in the invention is composed of polyethersulfone (PES) hollow fibers. See, e.g., Li, Y., et al. J. Membrane Sci. 245:53-60 (2004).
[0175]Macroencapsulation devices suitable for use in the present invention may optionally have additional structures to allow convenient implantation into and retrieval from a subject to be treated. For example, a macroencapsulation device may comprise, without limitation, a suture clip, a loading port, a tether, or other structure for ease of use.
[0176]The interior space of macroencapsulation devices of the invention is typically suitable to comprise at least one up to about 105 cells, e.g., modified or non-modified cells intended to secrete a desired therapeutic polypeptide or polynucleotide. For example, a macroencapsulation device of the invention may comprise at least 500, at least 1,000, at least 2,000, at least 4,000, at least 5,000, at least 8,000 to about 10,000 or more cells.
[0177]Some bioreactor devices, e.g., encapsulated or coated modified or non-modified cells of the present invention intended to secrete a desired therapeutic polypeptide or polynucleotide, may further comprise protective cells, e.g., within the barrier or capsule, where the protective cells are capable of providing protection to the modified or non-modified cells intended to secrete a desired therapeutic polypeptide or polynucleotide. Non-limiting examples of such protective cells include modified or non-modified sertoli cells and erythrocytes. Additionally, some bioreactor devices, e.g., encapsulated or coated modified or non-modified cells of the present invention intended to secrete a desired therapeutic polypeptide or polynucleotide, may further comprise an outer coating capable of creating a more compatible or protective micro-environment. Exemplary, non-limiting micro-environments which may be created include an anti-inflammatory micro-environment and a pro-angiogenic micro-environment.
[0178]In still other embodiments, bioreactor devices of the present invention may include modified cells with a "safety-shutoff" mechanism. For example, modified cells contained in a bioreactor device may comprise a regulated suicide gene which encodes a lethal polypeptide where the gene, upon activation, would induce destruction of the modified cell itself. For example, a modified cell might be programmed to die if it escapes from a barrier system, or if it undergoes oncogenic conversion. Non-limiting examples of lethal polypeptides suitable for use in the present invention are described in more detail below.
[0179]The subject on which the therapeutic methods are carried out may be any subject for which treatment or prevention is desired. For example, the subject may be one that is exhibiting one or more symptoms of a disease, disorder, or condition. The subject may also be one that is predisposed to a disease, disorder, or condition, e.g., due to genetics, family history, or environmental exposure. The subject may be a member of the general public, e.g., as part of a preventative immunization against a disease, disorder, or condition in a population.
[0180]The disease, disorder, or condition to be treated or prevented by the methods of the invention may be any disease, disorder, or condition for which one or more therapeutic switch promoters is available. Examples of diseases or disorders which may be treated or prevented by the methods of the invention include, without limitation, hyperproliferative diseases, disorders, or conditions (e.g., cancer), cardiovascular diseases, disorders, or conditions, neural diseases, disorders, or conditions, autoimmune diseases, disorders, or conditions, bone diseases, disorders, or conditions, gastrointestinal diseases, disorders, or conditions, blood diseases, disorders, or conditions, metabolic diseases, disorders, or conditions, inflammatory diseases, disorders, or conditions, and infectious diseases, disorders, or conditions.
[0181]The therapeutic switch promoters of the invention may be any promoter that is useful for treating, ameliorating, or preventing a specific disease, disorder, or condition. Examples include, without limitation, promoters of genes that exhibit increased expression only during a specific disease, disorder, or condition and promoters of genes that exhibit increased expression under specific cell conditions (e.g., proliferation, apoptosis, change in pH, oxidation state, oxygen level). In some embodiments where the gene switch comprises more than one transcription factor sequence, the specificity of the therapeutic methods can be increased by combining a disease- or condition-specific promoter with a tissue- or cell type-specific promoter to limit the tissues in which the therapeutic product is expressed. Thus, tissue- or cell type-specific promoters are encompassed within the definition of therapeutic switch promoter.
[0182]As an example of disease-specific promoters, useful promoters for treating cancer include the promoters of oncogenes. Examples of classes of oncogenes include, but are not limited to, growth factors, growth factor receptors, protein kinases, programmed cell death regulators and transcription factors. Specific examples of oncogenes include, but are not limited to, sis, erb B, erb B-2, ras, abl, myc and bcl-2 and TERT. Examples of other cancer-related genes include tumor associated antigen genes and other genes that are overexpressed in neoplastic cells (e.g., MAGE-1, carcinoembryonic antigen, tyrosinase, prostate specific antigen, prostate specific membrane antigen, p53, MUC-1, MUC-2, MUC-4, HER-2/neu, T/Tn, MART-1, gp100, GM2, Tn, sTn, and Thompson-Friedenreich antigen (TF)).
[0183]Examples of promoter sequences and other regulatory elements (e.g., enhancers) that are known in the art and are useful as therapeutic switch promoters in the present invention are disclosed in the references listed in Tables 1 and 2, along with the disease/disorder (Table 1) or tissue specificity (Table 2) associated with each promoter. The promoter sequences disclosed in these references are herein incorporated by reference in their entirety.
TABLE-US-00001 TABLE 1 Patent/Published Promoter Sequence Disease/Disorder Application No. Her-2/neu (ERBB2/c-erbB-2) cancer 5,518,885 osteocalcin calcified tumors 5,772,993 stromelysin-1 cancer 5,824,794 prostate specific antigen prostate cancer 5,919,652 human sodium-iodide symporter thyroid carcinoma 6,015,376 H19, IF-1, IGF-2 cancer 6,306,833 thymosin β15 breast, pancreatic, prostate 6,489,463 cancer T cell factor cancer 6,608,037 cartilage-derived retinoic acid-sensitive chondrosarcoma, 6,610,509 protein mammary tumor insulin pancreatic cancer 6,716,824 PEG-3 cancer 6,737,523 telomerase reverse transcriptase cancer 6,777,203 melanoma differentiation associated cancer 6,841,362 gene-7 prostasin cancer 6,864,093 telomerase catalytic subunit; cancer 6,936,595 cyclin-A midkine; c-erbB-2 cancer 7,030,099 prostate-specific membrane antigen prostate cancer 7,037,647 p51 cancer 7,038,028 telomerase RNA cancer 7,084,267 prostatic acid phosphatase prostate cancer 7,094,533 PCA3dd3 prostate cancer 7,138,235 DF3/MUC1 cancer 7,247,297 hex II cancer 2001/0011128 cyclooxygenase-2 cancer 2002/0107219 super PSA prostate cancer 2003/0078224 skp2 cancer 2003/0109481 PRL-3 metastatic colon cancer 2004/0126785 CA125/M17S2 ovarian cancer 2004/0126824 IAI.3B ovarian cancer 2005/0031591 CRG-L2 liver cancer 2005/0124068 TRPM4 prostate cancer 2006/0188990 RTVP glioma 2006/0216731 TARP prostate cancer, breast 2007/0032439 cancer telomere reverse transcriptase cancer 2007/0059287 A4 amyloid protein Alzheimer's disease 5,151,508 amyloid β-protein precursor Alzheimer's disease 5,643,726 precursor of the Alzheimer's Disease Alzheimer's disease 5,853,985 A4 amyloid protein neuropeptide FF CNS disorders 6,320,038 endoplasmic reticulum stress stress 7,049,132 elements urocortin II psychopathologies 7,087,385 tyrosine hydroxylase neurological disorders 7,195,910 complement factor 3; serum amyloid inflammation 5,851,822 A3 tissue inhibitor of metalloproteinase- rheumatism, cancer, 5,854,019 3 (TIMP-3) autoimmune disease, inflammation p75 tumor necrosis factor receptor autoimmune disease 5,959,094 tumor necrosis factor-α inflammation 6,537,784 peroxisome proliferator activated inflammation 6,870,044 receptor/IIA-1 nonpancreatic secreted phospholipase A2 SOCS-3 growth disorders, 2002/0174448 autoimmune disease, inflammation SR-BI lipid disorders 5,965,790 Ob obesity 5,698,389 site-1 protease obesity, diabetes 7,045,294 TIGR glaucoma 7,138,511 VL30 anoxia 5,681,706 excitatory amino acid transporter-2 nervous system ischemia 2004/0171108 MDTS9 renal failure 2006/0014931 LIM, pyrroline 5-carboxylate prostate disorders 2006/0134688 reductase, SIM2 Bax apoptosis 5,744,310 fas apoptosis 5,888,764 bbc3 apoptosis 7,202,024 PINK-1 PI-3 kinase/Akt pathway 2006/0228776 disorders
TABLE-US-00002 TABLE 2 Patent/Published Promoter Sequence Tissue Specificity Application No. troponin T skeletal muscle 5,266,488 myoD muscle 5,352,595 actin muscle 5,374,544 smooth muscle 22α arterial smooth muscle 5,837,534 utrophin muscle 5,972,609 myostatin muscle 6,284,882 smooth muscle myosin heavy chain smooth muscle 6,780,610 cardiac ankyrin repeat protein cardiac muscle 7,193,075 MLP muscle 2002/0042057 smoothelin smooth muscle 2003/0157494 MYBPC3 cardiomyocytes 2004/0175699 Tα1 α-tubulin neurons 5,661,032 intercellular adhesion molecule-4 neurons 5,753,502 (ICAM-4) γ-aminobutyric acid type A receptor β1 hippocampus 6,066,726 subunit neuronal nicotinic acetylcholine neurons 6,177,242 receptor β2-subunit presenilin-1 neurons 6,255,473 calcium-calmodulin-dependent forebrain 6,509,190 kinase IIα CRF2α receptor brain 7,071,323 nerve growth factor neurons 2003/159159 GLP-2 receptor gut, brain 2002/0045173 type I transglutaminase keratinocytes 5,643,746 K14 keratinocytes 6,596,515 stearoyl-CoA desaturase skin 2002/0151018 megsin renal cells 6,790,617 prolactin pituitary 5,082,779 GDF-9 ovary, testes, 7,227,013 hypothalamus, pituitary, placenta PSP94 prostate 2003/0110522 NRL; NGAL mammary gland 5,773,290 long whey acidic protein mammary gland 5,831,141 mammary associated amyloid A mammary ductal epithelial 2005/0107315 cells endothelin-1 endothelial cells 5,288,846 serglycin hematopoietic cells 5,340,739 platelet-endothelial cell adhesion platelets, leukocytes, 5,668,012 molecule-1 (PECAM-1) endothelial cells Tie receptor tyrosine kinase endothelial cells, bone 5,877,020 marrow KDR/flk-1 endothelial cells 5,888,765 endoglin endothelial cells 6,103,527 CCR5 myeloid and lymphoid 6,383,746 cells CD11d myeloid cells 6,881,834 platelet glycoprotein IIb hematopoietic cells 6,884,616 preproendothelin-1 endothelial cells 7,067,649 interleukin-18 binding protein mononuclear cells 2006/0239984 CD34 hematopoietic stem cells 5,556,954 Tec tyrosine kinase hematopoietic stem cells, 6,225,459 liver
[0184]Other genes that exhibit changes in expression levels during specific diseases or disorders and therefore may provide promoters that are useful in the present invention include, without limitation, the genes (along with the associated disease/disorder) listed in Table 3.
TABLE-US-00003 TABLE 3 Patent/Published Gene Disease/Disorder Application No. MLH1, MSH2, MSH6, PMS1, APC Colorectal cancer 7,148,016 LEF-1 Colon cancer 2002/0169300 F2 receptor Colon cancer 2002/0187502 TGF-β type II receptor Colon cancer 2004/0038284 EYA4 Colon cancer 2005/0003463 PCA3 Prostate cancer 7,138,235 K2 Prostate cancer 6,303,361 PROST 03 Prostate cancer metastases 2002/0009455 PCAM-1 Prostate cancer 2002/0042062 PCADM-1 Prostate cancer 2003/0100033 PCA3dd3 Prostate cancer 2003/0165850 PCAV Prostate cancer 2006/0275747 PAcP Androgen-insensitive 2006/0294615 prostate cancer SEQ ID NO: 1 of the U.S. Pat. No. Liver cancer 5,866,329 5,866,329, incorporated by reference herein SEQ ID NOS: 1, 3 of the U.S. patent Hepatocellular cancer 2002/0115094 application publication 2002/0115094, incorporated by reference herein SEQ ID NO: 1 of the patent U.S. Hepatocellular carcinoma 2005/0037372 application publication 2005/0037372, incorporated by reference herein ATB0 Hepatocellular carcinoma 2006/0280725 SEQ ID NOS: 1, 3 of the U.S. patent Liver cancer 2007/0042420 application publication 2007/0042420, incorporated by reference herein CSA-1 Chondrosarcoma 2001/0016649 SEQ ID NOS: 1-15 of the U.S. patent Pancreatic cancer 2001/0016651 application publication 2001/0016651, incorporated by reference herein SEQ ID NOS: 1-15 of the U.S. patent Pancreatic cancer 2003/0212264 application publication 2003/0212264, incorporated by reference herein SYG972 Breast cancer 2002/0055107 Urb-ctf Breast cancer 2003/0143546 BCU399 Breast cancer 2003/0180728 TBX2 Breast cancer 2004/0029185 Cyr61 Breast cancer 2004/0086504 DIAPH3 Breast cancer 2005/0054826 SEQ ID NOS: 1-24 of the U.S. patent Breast cancer 2007/0134669 application publication 2007/0134669, incorporated by reference herein Human aspartyl (asparaginyl) beta- CNS cancer 2002/0102263 hydroxylase BEHAB CNS cancer 2003/0068661 IL-8 Kaposi's Sarcoma 2003/0096781 SEQ ID NOS: 1-278 of the U.S. Hematological cancers 2002/0198362 patent application publication 2002/0198362, incorporated by reference herein BLSA B-cell cancer 2003/0147887 BP1 Leukemia 2003/0171273 DAP-kinase, HOXA9 Non-small cell lung cancer 2003/0224509 ARP Clear cell renal carcinoma, 2004/0010119 inflammatory disorders Nbk Renal cancer 2005/0053931 CD43 Ovarian cancer 2006/0216231 SEQ ID NOS: 1-84 of the U.S. patent Ovarian cancer 2007/0054268 application publication 2007/0054268, incorporated by reference herein β7-hcG, β6-hCG, β6e-hCG, Uterine tumors 2006/0292567 β5-hCG, β8-hcG, β3-hCG MTA1s Hormone insensitive 2006/0204957 cancer Old-35, Old-64 Tumor proliferation 2003/0099660 LAGE-1 Cancer 6,794,131 CIF150/hTAF.sub.II150 Cancer 6,174,679 P65 oncofetal protein Cancer 5,773,215 Telomerase Cancer 2002/0025518 CYP1B1 Cancer 2002/0052013 14-3-3σ Cancer 2002/0102245 NES1 Cancer 2002/0106367 CAR-1 Cancer 2002/0119541 HMGI, MAG Cancer 2002/0120120 ELL2 Cancer 2002/0132329 Ephrin B2 Cancer 2002/0136726 WAF1 Cancer 2002/0142442 CIF130 Cancer 2002/0143154 C35 Cancer 2002/0155447 BMP2 Cancer 2002/0159986 BUB3 Cancer 2002/0160403 Polymerase kappa Cancer 2003/0017573 EAG1, EAG2 Cancer 2003/0040476 SEQ ID NOS: 18, 20, 22 of the U.S. Cancer 2003/0044813 patent application publication 2003/0044813, incorporated by reference herein HMG I Cancer 2003/0051260 HLTF Cancer 2003/0082526 Barx2 Cancer 2003/0087243 SEQ ID NOS: 18, 20, 22, 32, 34, Cancer 2003/0108920 36 of the U.S. patent application publication 2003/0108920, incorporated by reference herein Cables Cancer 2003/0109443 Pp 32r1 Cancer 2003/0129631 BMP4 Cancer 2003/0134790 TS10q23.3 Cancer 2003/0139324 Nuclear spindle-associating protein Cancer 2003/0157072 PFTAIRE Cancer 2003/0166217 SEMA3B Cancer 2003/0166557 MOGp Cancer, multiple sclerosis, 2003/0166898 inflammatory disease Fortilin Cancer 2003/0172388 SEQ ID NO: 1 of the U.S. patent Cancer 2003/0215833 application publication 2003/0215833, incorporated by reference herein IGFBP-3 Cancer 2004/0005294 Polyhomeotic 2 Cancer 2004/0006210 PNQALRE Cancer 2004/0077009 SEQ ID NOS: 1, 3 of the U.S. patent Cancer 2004/0086916 application publication 2004/0086916, incorporated by reference herein SCN5A Cancer 2004/0146877 miR15, miR16 Cancer 2004/0152112 Headpin Cancer 2004/0180371 PAOh1/SMO Cancer 2004/0229241 Hippo, Mst2 Cancer 2005/0053592 PSMA-like Cancer, neurological 2005/0064504 disorders JAB1 Cancer 2005/0069918 NF-AT Cancer 2005/0079496 P28ING5 Cancer 2005/0097626 MTG16 Cancer 2005/0107313 ErbB-2 Cancer 2005/0123538 HDAC9 Cancer 2005/0130146 GPBP Cancer 2005/0130227 MG20 Cancer 2005/0153352 KLF6 Cancer 2005/0181374 ARTS1 Cancer 2005/0266443 Dock 3 Cancer 2006/0041111 Annexin 8 Cancer 2006/0052320 MH15 Cancer 2006/0068411 DELTA-N p73 Cancer 2006/0088825 RapR6 Cancer 2006/099676 StarD10 Cancer 2006/0148032 Ciz1 Cancer 2006/0155113 HLJ1 Cancer 2006/0194235 RapR7 Cancer 2006/0240021 A34 Cancer 2006/0292154 Sef Cancer 2006/0293240 Killin Cancer 2007/0072218 SGA-1M Cancer 2007/0128593 TGFβ Type II receptor Cancer 2002/0064786 GCA-associated genes Giant cell arteritis 6,743,903 PRV-1 Polycythemia vera 6,686,153 SEQ ID NOS: 2, 4 of the U.S. Pat. No. Ischemia 5,948,637 5,948,637, incorporated by reference herein Vezf1 Vascular disorders 2002/0023277 MLP Dilatative cardiomyopathy 2002/0042057 VEGI Pathological angiogenesis 2002/0111325 PRO256 Cardiovascular disorders 2002/0123091 AOP2 Atherosclerosis 2002/0142417 Remodelin Arterial restenosis, fibrosis 2002/0161211 Phosphodiesterase 4D Stroke 2003/0054531 Prostaglandin receptor subtype EP3 Peripheral arterial 2003/0157599 occlusive disease CARP Heart disorders 2004/0014706 HOP Congenital heart disease 2004/0029158 SEQ ID NOS: 1-4 of the U.S. patent Apoplexy 2004/0087784 application publication 2004/0087784, incorporated by reference herein PLTP Atherosclerosis, vascular 2006/0252787 disease, hypercholesterolemia, Tangier's disease, familial HDL deficiency disease SEQ ID NOS: 1, 3-8, 15, 16 of the Thrombosis 2007/0160996 U.S. patent application publication 2007/0160996, incorporated by reference herein UCP-2 Stroke 2002/0172958 FLJ11011 Fanconi's Anemia 2006/0070134 Codanin-1 Anemia 2006/0154331 SEQ ID NOS: 1, 6, 8 of the U.S. Insulin-dependent diabetes 5,763,591 Pat. No. 5,763,591, incorporated by mellitus reference herein Resistin Type II diabetes 2002/0161210 Archipelin Diabetes 2003/0202976 SEQ ID NOS: 2, 7, 16, 27 of the U.S. Diabetes, hyperlipidemia 2004/0053397 patent application publication 2004/0053397, incorporated by reference herein Neuronatin Metabolic disorders 2004/0259777 Ncb5or Diabetes 2005/0031605 7B2 Endocrine disorders 2005/0086709 PTHrP, PEX Metabolic bone diseases 2005/0113303 KChIPl Type II diabetes 2005/0196784 SLIT-3 Type II diabetes 2006/0141462 CX3CR1 Type II diabetes 2006/0160076 SMAP-2 Diabetes 2006/0210974 SEQ ID NOS: 2, 8, 12, 16, 22, 26, Type II diabetes 2006/0228706 28, 32 of the U.S. patent application publication 2006/0228706, incorporated by reference herein IC-RFX Diabetes 2006/0264611 E2IG4 Diabetes, insulin 2007/0036787 resistance, obesity SEQ ID NOS: 2, 8, 10, 14, 18, 24, Diabetes 2007/0122802 26, 30, 34, 38, 44, 50, 54, 60, 62, 68, 74, 80, 86, 92, 98, 104, 110 of the U.S. patent application publication 2007/0122802, incorporated by reference herein UCP2 Body weight disorders 2002/0127600 Ob receptor Body weight disorders 2002/0182676 Ob Body weight disorders 2004/0214214 Dp1 Neurodegenerative 2001/0021771 disorders NRG-1 Schizophrenia 2002/0045577 Synapsin III Schizophrenia 2002/0064811 NRG1AG1 Schizophrenia 2002/0094954 AL-2 Neuronal disorders 2002/0142444 Proline dehydrogenase Bipolar disorder, major 2002/0193581 depressive disorder, schizophrenia, obsessive compulsive disorder MNR2 Chronic neurodegenerative 2002/0197678 disease ATM Ataxia-telangiectasia 2004/0029198 Ho-1 Dementing diseases 2004/0033563 CON202 Schizophrenia 2004/0091928 Ataxin-1 Neurodegenerative 2004/0177388 disorders NR3B Motor neuron disorders 2005/0153287 NIPA-1 Hereditary spastic 2005/0164228 paraplegia DEPP, adrenomedullin, csdA Schizophrenia 2005/0227233 Inf-20 Neurodegenerative 2006/0079675 diseases EOPA Brain development and 2007/0031830 degeneration disorders
SERT Autism 2007/0037194 FRP-1 Glaucoma 2002/0049177 Serum amyloid A Glaucoma 2005/0153927 BMP2 Osteoporosis 2002/0072066 BMPR1A Juvenile polyposis 2003/0072758 ACLP Gastroschisis 2003/0084464 Resistin-like molecule β Familial adenomatous 2003/0138826 polyposis, diabetes, insulin resistance, colon cancer, inflammatory bowel disorder Dlg5 Inflammatory bowel 2006/0100132 disease SEQ ID NOS: 1-82 of the U.S. patent Osteoarthritis 2002/0119452 application publication 2002/0119452, incorporated by reference herein TRANCE Immune system disorders 2003/0185820 Matrilin-3 Osteoarthritis 2003/0203380 Synoviolin Rheumatoid arthritis 2004/0152871 SEQ ID NOS: 9, 35 of the U.S. Osteoarthritis 2007/0028314 patent application publication 2007/0028314, incorporated by reference herein HIV LTR HIV infection 5,627,023 SHIVA HIV infection 2004/0197770 EBI 1, EBI 2, EBI 3 Epstein Barr virus infection 2002/0040133 NM23 family Skin/intestinal disorders 2002/0034741 SEQ ID NO: 1 of the U.S. patent Psoriasis 2002/0169127 application publication 2002/0169127, incorporated by reference herein Eps8 Skin disorders, wound 2003/0180302 healing Beta-10 Thyroid gland pathology 2002/0015981 SEQ ID NO: 2 of the U.S. patent Thyroid conditions 2003/0207403 application publication 2003/0207403, incorporated by reference herein SEQ ID NO: 3 of the U.S. patent Thyroid disorders 2007/0020275 application publication 2007/0020275, incorporated by reference herein Hair follicle growth factor Alopecia 2003/0036174 Corneodesmosin Alopecia 2003/0211065 GCR9 Asthma, lymphoma, 2003/0166150 leukemia SEQ ID NO: 1-71 of the U.S. patent Asthma 2004/0002084 application publication 2004/0002084, incorporated by reference herein Bg Chediak-Higashi syndrome 2002/0115144 SEQ ID NOS: 1-16 of the U.S. patent Endometriosis 2002/0127555 application publication 2002/0127555, incorporated by reference herein FGF23 Hypophosphatemic 2005/0156014 disorders BBSR Bardet-Biedl syndrome 2003/0152963 MIC-1 Fetal abnormalities, cancer, 2004/0053325 inflammatory disorders, miscarriage, premature birth MIA-2 Liver damage 2004/0076965 IL-17B Cartilage degenerative 2004/0171109 disorders Formylglycine generating enzyme Multiple sulfatase 2004/0229250 deficiency LPLA2 Pulmonary alveolar 2006/0008455 proteinosis CXCL1O Respiratory illnesses 2006/0040329 SEQ ID NOS: 1, 2 of the U.S. patent Nephropathy 2006/0140945 application publication 2006/0140945, incorporated by reference herein HFE2A Iron metabolism disease 2007/0166711
[0185]Once a gene with an expression pattern that is modulated during a disease, disorder, or condition is identified, the promoter of the gene may be used in the gene switch of the invention. The sequence of many genes, including the promoter region, is known in the art and available in public databases, e.g., GenBank. Thus, once an appropriate gene is identified, the promoter sequence can be readily identified and obtained. Another aspect of the present invention is directed towards identifying suitable genes whose promoter can be isolated and placed into a gene switch. The identity of the gene, therefore, may not be critical to specific embodiments of the present invention, provided the promoter can be isolated and used in subsequent settings or environments. The current invention thus includes the use of promoters from genes that are yet to be identified. Once suitable genes are identified, it is a matter of routine skill or experimentation to determine the genetic sequences needed for promoter function. Indeed, several commercial protocols exist to aid in the determination of the promoter region of genes of interest. By way of example, Ding et al. recently elucidated the promoter sequence of the novel Sprouty4 gene (Am. J. Physiol. Lung Cell. Mol. Physiol. 287: L52 (2004), which is incorporated by reference) by progressively deleting the 5'-flanking sequence of the human Sprouty4 gene. Briefly, once the transcription initiation site was determined, PCR fragments were generated using common PCR primers to clone segments of the 5'-flanking segment in a unidirectional manner. The generated segments were cloned into a luciferase reporter vector and luciferase activity was measured to determine the promoter region of the human Sprouty4 gene.
[0186]Another example of a protocol for acquiring and validating gene promoters includes the following steps: (1) acquire diseased and non-diseased cell/tissue samples of similar/same tissue type; (2) isolate total RNA or mRNA from the samples; (3) perform differential microarray analysis of diseased and non-diseased RNA; (4) identify candidate disease-specific transcripts; (5) identify genomic sequences associated with the disease-specific transcripts; (6) acquire or synthesize DNA sequence upstream and downstream of the predicted transcription start site of the disease-specific transcript; (7) design and produce promoter reporter vectors using different lengths of DNA from step 6; and (8) test promoter reporter vectors in diseased and non-diseased cells/tissues, as well as in unrelated cells/tissues.
[0187]The source of the promoter that is inserted into the gene switch can be natural or synthetic, and the source of the promoter should not limit the scope of the invention described herein. In other words, the promoter may be directly cloned from cells, or the promoter may have been previously cloned from a different source, or the promoter may have been synthesized.
Gene Switch Systems
[0188]The gene switch may be any gene switch that regulates gene expression by addition or removal of a specific ligand. In one embodiment, the gene switch is one in which the level of gene expression is dependent on the level of ligand that is present. Examples of ligand-dependent transcription factor complexes that may be used in the gene switches of the invention include, without limitation, members of the nuclear receptor superfamily activated by their respective ligands (e.g., glucocorticoid, estrogen, progestin, retinoid, ecdysone, and analogs and mimetics thereof) and rTTA activated by tetracycline. In one aspect of the invention, the gene switch is an EcR-based gene switch. Examples of such systems include, without limitation, the systems described in U.S. Pat. Nos. 6,258,603, 7,045,315, U.S. Published Patent Application Nos. 2006/0014711, 2007/0161086, and International Published Application No. WO 01/70816. Examples of chimeric ecdysone receptor systems are described in U.S. Pat. No. 7,091,038, U.S. Published Patent Application Nos. 2002/0110861, 2004/0033600, 2004/0096942, 2005/0266457, and 2006/0100416, and International Published Application Nos. WO 01/70816, WO 02/066612, WO 02/066613, WO 02/066614, WO 02/066615, WO 02/29075, and WO 2005/108617, each of which is incorporated by reference in its entirety. An example of a non-steroidal ecdysone agonist-regulated system is the RheoSwitch® Mammalian Inducible Expression System (New England Biolabs, Ipswich, Mass.). In another aspect of the invention, the gene switch is based on heterodimerization of FK506 binding protein (FKBP) with FKBP rapamycin associated protein (FRAP) and is regulated through rapamycin or its non-immunosuppressive analogs. Examples of such systems, include, without limitation, the ARGENT® Transcriptional Technology (ARIAD Pharmaceuticals, Cambridge, Mass.) and the systems described in U.S. Pat. Nos. 6,015,709, 6,117,680, 6,479,653, 6,187,757, and 6,649,595.
[0189]In one embodiment, the gene switch comprises a single transcription factor sequence encoding a ligand-dependent transcription factor complex under the control of a therapeutic switch promoter. The transcription factor sequence may encode a ligand-dependent transcription factor complex that is a naturally occurring or an artificial ligand-dependent transcription factor complex. An artificial transcription factor is one in which the natural sequence of the transcription factor has been altered, e.g., by mutation of the sequence or by the combining of domains from different transcription factors. In one embodiment, the transcription factor comprises a Group H nuclear receptor ligand binding domain. In one embodiment, the Group H nuclear receptor ligand binding domain is from an ecdysone receptor, a ubiquitous receptor (UR), an orphan receptor 1 (OR-1), a steroid hormone nuclear receptor 1 (NER-1), a retinoid X receptor interacting protein-15 (RIP-15), a liver X receptor β (LXRP), a steroid hormone receptor like protein (RLD-1), a liver X receptor (LXR), a liver X receptor α (LXRα), a farnesoid X receptor (FXR), a receptor interacting protein 14 (RIP-14), or a farnesol receptor (HRR-1). In another embodiment, the Group H nuclear receptor LBD is from an ecdysone receptor.
[0190]A. Ecdysone-Based Gene Switch
[0191]The EcR and the other Group H nuclear receptors are members of the nuclear receptor superfamily wherein all members are generally characterized by the presence of an amino-terminal transactivation domain (AD, also referred to interchangeably as "TA" or "TD"), optionally fused to a heterodimerization partner (HP) to form a coactivation protein (CAP), a DNA binding domain (DBD), and a LBD fused to the DBD via a hinge region to form a ligand-dependent transcription factor (LTF). As used herein, the term "DNA binding domain" comprises a minimal polypeptide sequence of a DNA binding protein, up to the entire length of a DNA binding protein, so long as the DNA binding domain functions to associate with a particular response element. Members of the nuclear receptor superfamily are also characterized by the presence of four or five domains: A/B, C, D, E, and in some members F (see U.S. Pat. No. 4,981,784 and Evans, Science 240:889 (1988)). The "A/B" domain corresponds to the transactivation domain, "C" corresponds to the DNA binding domain, "D" corresponds to the hinge region, and "E" corresponds to the ligand binding domain. Some members of the family may also have another transactivation domain on the carboxy-terminal side of the LBD corresponding to "F".
[0192]The following polypeptide sequence was reported as a polypeptide sequence of Ecdysone receptor (Ecdysteroid receptor) (20-hydroxy-ecdysone receptor) (20E receptor) (EcRH) (Nuclear receptor subfamily 1 group H member 1) and has the accession number P34021 in Genbank.
TABLE-US-00004 Ecdysone receptor (878 aa) from Drosophila melanogaster (Fruit fly) (SEQ ID NO: 5) 1 mkrrwsnngg fmrlpeesss evtsssnglv lpsgvnmsps sldshdycdq dlwlcgnesg 61 sfggsnghgl sqqqqsvitl amhgcsstlp aqttiiping nangnggstn gqyvpgatnl 121 galangmlng gfngmqqqiq nghglinstt pstpttplhl qqnlggaggg giggmgilhh 181 angtpnglig vvgggggvgl gvggggvggl gmqhtprsds vnsissgrdd lspssslngy 241 sanescdakk skkgpaprvq eelclvcgdr asgyhynalt cegckgffrr svtksavycc 301 ktgracemdm ymrrkcqecr lkkclavgmr pecvvpenqc amkrrekkaq kekdkmttsp 361 ssqhggngsl asgggqdtvk keildlmtce ppqhatipll pdeilakcqa rnipsltynq 421 laviykliwy qdgyeqpsee dlrrimsqpd enesqtdvsf rhiteitilt vqlivefakg 481 lpattkipqe dqitllkacs sevmmlrmar rydhssdsif fannrsytrd sykmagmadn 541 iedllhfcrq mtsmkvdnve yalltaivif sdrpglekaq lveaiqsyyi dtlriyilnr 601 hcgdsmslvf yakllsilte lrtlgnqnae mcfslklknr klpkfleeiw dvhaippsvq 661 shlqitqeen erleraermr asvggaitag idcdsastsa aaaaaqhqpq pqpqpqpssl 721 tqndsqhqtq pqlqpqlppq lqgqlqpqlq pqlqtqlqpq iqpqpqllpv sapvpasvta 781 pgslsavsts seymggsaai gpitpattss itaavtasst tsavpmgngv gvgvgvggnv 841 smyanaqtam almgvalhsh qeqliggvav ksehstta
[0193]The DBD is characterized by the presence of two cysteine zinc fingers between which are two amino acid motifs, the P-box and the D-box, which confer specificity for response elements. These domains may be either native, modified, or chimeras of different domains of heterologous receptor proteins. The EcR, like a subset of the nuclear receptor family, also possesses less well-defined regions responsible for heterodimerization properties. Because the domains of nuclear receptors are modular in nature, the LBD, DBD, and AD may be interchanged.
[0194]In another embodiment, the transcription factor comprises a AD, a DBD that recognizes a response element associated with the therapeutic protein or therapeutic polynucleotide whose expression is to be modulated; and a Group H nuclear receptor LBD. In certain embodiments, the Group H nuclear receptor LBD comprises a substitution mutation.
[0195]In another embodiment, the gene switch comprises a first transcription factor sequence, e.g., a CAP, under the control of a first therapeutic switch promoter (TSP-1) and a second transcription factor sequence, e.g., a LTF, under the control of a second therapeutic switch promoter (TSP-2), wherein the proteins encoded by said first transcription factor sequence and said second transcription factor sequence interact to form a protein complex (LDTFC), i.e., a "dual switch"- or "two-hybrid"-based gene switch. The first and second TSPs may be the same or different. In this embodiment, the presence of two different TSPs in the gene switch that are required for therapeutic molecule expression enhances the specificity of the therapeutic method (see FIG. 2). FIG. 2 also demonstrates the ability to modify the therapeutic gene switch to treat any disease, disorder, or condition simply by inserting the appropriate TSPs.
[0196]In a further embodiment, both the first and the second transcription factor sequence, e.g., a CAP or a LTF, are under the control of a single therapeutic switch promoter (e.g. TSP-1 in FIG. 1). Activation of this promoter will generate both CAP and LTF with a single open reading frame. This can be achieved with the use of a transcriptional linker such as an IRES (internal ribosomal entry site). In this embodiment, both portions of the ligand-dependent transcription factor complex will be synthesized upon activation of TSP-1. TSP-1 can be a constitutive promoter or only activated under conditions associated with the disease, disorder, or condition.
[0197]In a further embodiment, one transcription factor sequence, e.g. a LTF, is under the control of a therapeutic switch promoter only activated under conditions associated with the disease, disorder, or condition (e.g., TSP-2 or TSP-3 in FIG. 4) and the other transcription factor sequence, e.g., CAP, is under the control of a constitutive therapeutic switch promoter (e.g., TSP-1 in FIG. 4). In this embodiment, one portion of the ligand-dependent transcription factor complex will be constitutively present while the second portion will only be synthesized under conditions associated with the disease, disorder, or condition.
[0198]In another embodiment, one transcription factor sequence, e.g., CAP, is under the control of a first TSP (e.g., TSP-1 in FIG. 3) and two or more different second transcription factor sequences, e.g., LTF-1 and LTF-2 are under the control of different TSPs (e.g., TSP-2 and TSP-3 in FIG. 3). In this embodiment, each of the LTFs may have a different DBD that recognizes a different factor-regulated promoter sequence (e.g., DBD-A binds to a response element associated with factor-regulated promoter-1 (FRP-1) and DBD-B binds to a response element associated with factor-regulated promoter-2 (FRP-2). Each of the factor-regulated promoters may be operably linked to a different therapeutic gene. In this manner, multiple treatments may be provided simultaneously.
[0199]In one embodiment, the first transcription factor sequence encodes a polypeptide comprising a AD, a DBD that recognizes a response element associated with the therapeutic product sequence whose expression is to be modulated; and a Group H nuclear receptor LBD, and the second transcription factor sequence encodes a transcription factor comprising a nuclear receptor LBD selected from the group consisting of a vertebrate retinoid X receptor (RXR), an invertebrate RXR, an ultraspiracle protein (USP), or a chimeric nuclear receptor comprising at least two different nuclear receptor ligand binding domain polypeptide fragments selected from the group consisting of a vertebrate RXR, an invertebrate RXR, and a USP (see WO 01/70816 A2 and US 2004/0096942 A1). The "partner" nuclear receptor ligand binding domain may further comprise a truncation mutation, a deletion mutation, a substitution mutation, or another modification.
[0200]In another embodiment, the gene switch comprises a first transcription factor sequence encoding a first polypeptide comprising a nuclear receptor LBD and a DBD that recognizes a response element associated with the therapeutic product sequence whose expression is to be modulated, and a second transcription factor sequence encoding a second polypeptide comprising an AD and a nuclear receptor LBD, wherein one of the nuclear receptor LBDs is a Group H nuclear receptor LBD. In a preferred embodiment, the first polypeptide is substantially free of an AD and the second polypeptide is substantially free of a DBD. For purposes of the invention, "substantially free" means that the protein in question does not contain a sufficient sequence of the domain in question to provide activation or binding activity.
[0201]In another aspect of the invention, the first transcription factor sequence encodes a protein comprising a heterodimerization partner and an AD (a "CAP") and the second transcription factor sequence encodes a protein comprising a DBD and a LBD (a "LTF").
[0202]When only one nuclear receptor LBD is a Group H LBD, the other nuclear receptor LBD may be from any other nuclear receptor that forms a dimer with the Group H LBD. For example, when the Group H nuclear receptor LBD is an EcR LBD, the other nuclear receptor LBD "partner" may be from an EcR, a vertebrate RXR, an invertebrate RXR, an ultraspiracle protein (USP), or a chimeric nuclear receptor comprising at least two different nuclear receptor LBD polypeptide fragments selected from the group consisting of a vertebrate RXR, an invertebrate RXR, and a USP (see WO 01/70816 A2, International Patent Application No. PCT/US02/05235 and US 2004/0096942 A1, incorporated herein by reference in their entirety). The "partner" nuclear receptor ligand binding domain may further comprise a truncation mutation, a deletion mutation, a substitution mutation, or another modification.
[0203]In one embodiment, the vertebrate RXR LBD is from a human Homo sapiens, mouse Mus musculus, rat Rattus norvegicus, chicken Gallus gallus, pig Sus scrofa domestica, frog Xenopus laevis, zebrafish Danio rerio, tunicate Polyandrocarpa misakiensis, or jellyfish Tripedalia cysophora RXR.
[0204]In one embodiment, the invertebrate RXR ligand binding domain is from a locust Locusta migratoria ultraspiracle polypeptide ("LmUSP"), an ixodid tick Amblyomma americanum RXR homolog 1 ("AmaRXR1"), an ixodid tick Amblyomma americanum RXR homolog 2 ("AmaRXR2"), a fiddler crab Celuca pugilator RXR homolog ("CpRXR"), a beetle Tenebrio molitor RXR homolog ("TmRXR"), a honeybee Apis mellifera RXR homolog ("AmRXR"), an aphid Myzus persicae RXR homolog ("MpRXR"), or a non-Dipteran/non-Lepidopteran RXR homolog.
[0205]In one embodiment, the chimeric RXR LBD comprises at least two polypeptide fragments selected from the group consisting of a vertebrate species RXR polypeptide fragment, an invertebrate species RXR polypeptide fragment, and a non-Dipteran/non-Lepidopteran invertebrate species RXR homolog polypeptide fragment. A chimeric RXR ligand binding domain for use in the present invention may comprise at least two different species RXR polypeptide fragments, or when the species is the same, the two or more polypeptide fragments may be from two or more different isoforms of the species RXR polypeptide fragment.
[0206]In one embodiment, the chimeric RXR ligand binding domain comprises at least one vertebrate species RXR polypeptide fragment and one invertebrate species RXR polypeptide fragment.
[0207]In another embodiment, the chimeric RXR ligand binding domain comprises at least one vertebrate species RXR polypeptide fragment and one non-Dipteran/non-Lepidopteran invertebrate species RXR homolog polypeptide fragment.
[0208]The ligand, when combined with the LBD of the nuclear receptor(s), which in turn are bound to the response element of a FRP associated with a therapeutic product sequence, provides external temporal regulation of expression of the therapeutic product sequence. The binding mechanism or the order in which the various components of this invention bind to each other, that is, for example, ligand to LBD, DBD to response element, AD to promoter, etc., is not critical.
[0209]In a specific example, binding of the ligand to the LBD of a Group H nuclear receptor and its nuclear receptor LBD partner enables expression of the therapeutic product sequence. This mechanism does not exclude the potential for ligand binding to the Group H nuclear receptor (GHNR) or its partner, and the resulting formation of active homodimer complexes (e.g. GHNR+GHNR or partner+partner). Preferably, one or more of the receptor domains is varied producing a hybrid gene switch. Typically, one or more of the three domains, DBD, LBD, and AD, may be chosen from a source different than the source of the other domains so that the hybrid genes and the resulting hybrid proteins are optimized in the chosen host cell or organism for transactivating activity, complementary binding of the ligand, and recognition of a specific response element. In addition, the response element itself can be modified or substituted with response elements for other DNA binding protein domains such as the GAL-4 protein from yeast (see Sadowski et al., Nature 335:563 (1988)) or LexA protein from Escherichia coli (see Brent et al., Cell 43:729 (1985)), or synthetic response elements specific for targeted interactions with proteins designed, modified, and selected for such specific interactions (see, for example, Kim et al., Proc. Natl. Acad. Sci. USA, 94:3616 (1997)) to accommodate hybrid receptors. Another advantage of two-hybrid systems is that they allow choice of a promoter used to drive the gene expression according to a desired end result. Such double control may be particularly important in areas of gene therapy, especially when cytotoxic proteins are produced, because both the timing of expression as well as the cells wherein expression occurs may be controlled. When genes, operably linked to a suitable promoter, are introduced into the cells of the subject, expression of the exogenous genes is controlled by the presence of the system of this invention. Promoters may be constitutively or inducibly regulated or may be tissue-specific (that is, expressed only in a particular type of cells) or specific to certain developmental stages of the organism.
[0210]The DNA binding domain of the first hybrid protein binds, in the presence or absence of a ligand, to the DNA sequence of a response element to initiate or suppress transcription of downstream gene(s) under the regulation of this response element.
[0211]The functional LDTFC, e.g., an EcR complex, may also include additional protein(s) such as immunophilins. Additional members of the nuclear receptor family of proteins, known as transcriptional factors (such as DHR38 or betaFTZ-1), may also be ligand dependent or independent partners for EcR, USP, and/or RXR. Additionally, other cofactors may be required such as proteins generally known as coactivators (also termed adapters or mediators). These proteins do not bind sequence-specifically to DNA and are not involved in basal transcription. They may exert their effect on transcription activation through various mechanisms, including stimulation of DNA-binding of activators, by affecting chromatin structure, or by mediating activator-initiation complex interactions. Examples of such coactivators include RIP140, TIF1, RAP46/Bag-1, ARA70, SRC-1/NCoA-1, TIF2/GRIP/NCoA-2, ACTR/AIB1/RAC3/pCIP as well as the promiscuous coactivator C response element B binding protein, CBP/p300 (for review see Glass et al., Curr. Opin. Cell Biol. 9:222 (1997)). Also, protein cofactors generally known as corepressors (also known as repressors, silencers, or silencing mediators) may be required to effectively inhibit transcriptional activation in the absence of ligand. These corepressors may interact with the unliganded EcR to silence the activity at the response element. Current evidence suggests that the binding of ligand changes the conformation of the receptor, which results in release of the corepressor and recruitment of the above described coactivators, thereby abolishing their silencing activity. Examples of corepressors include N-CoR and SMRT (for review, see Horwitz et al., Mol. Endocrinol. 10:1167 (1996)). These cofactors may either be endogenous within the cell or organism, or may be added exogenously as transgenes to be expressed in either a regulated or unregulated fashion.
[0212]B. Rapamycin Based Gene Switch
[0213]The present invention further provides a gene switch system which utilizes FK506 binding protein as the ligand-dependent transcription factor complex and rapamycin as the ligand. In one embodiment, the construct encoding the gene switch comprises [0214](a) a first polynucleotide encoding a first chimeric protein which binds to rapamycin or an analog thereof and which comprises at least one FK506-binding protein (FKBP) domain and at least one protein domain heterologous thereto, wherein the FKBP domain comprises a peptide sequence selected from: [0215](1) a naturally occurring FKBP [0216](2) a variant of a naturally occurring FKBP in which up to 10 amino acid residues have been deleted, inserted, or replaced with substitute amino acids, and [0217](3) an FKBP encoded by a DNA sequence which selectively hybridizes to a DNA sequence encoding an FKBP of (1) or (2); [0218](b) a second polynucleotide encoding a second chimeric protein which forms a complex with both (a) rapamycin or a rapamycin analog and (b) the first chimeric protein, and which comprises at least one FKBP:rapamycin binding (FRB) domain and at least one protein domain heterologous thereto, wherein the FRB domain comprises a peptide sequence selected from: [0219](4) a naturally occurring FRB domain, [0220](5) a variant of a naturally occurring FRB domain in which up to 10 amino acid residues have been deleted, inserted, or replaced with substitute amino acids, and [0221](6) an FRB domain encoded by a DNA sequence which selectively hybridizes to a DNA sequence encoding an FRB of (4) or (5).
[0222]In this gene switch system, each of the first polynucleotide and the second polynucleotide are under the control of one or more therapeutic switch promoters as described elsewhere herein. Furthermore, in certain embodiments, at least one protein domain heterologous to the FKBP and/or FRB domains in the first and second chimeric protein may be one or more "action" or "effector" domains. Effector domains may be selected from a wide variety of protein domains including DNA binding domains, transcription activation domains, cellular localization domains and signaling domains (i.e., domains which are capable upon clustering or multimerization, of triggering cell growth, proliferation, differentiation, apoptosis, gene transcription, etc.).
[0223]In certain embodiments, one fusion protein contains at least one DNA binding domain (e.g., a GAL4 or ZFHD1 DNA-binding domain) and another fusion protein contains at least one transcription activation domain (e.g., a VP16 or p65 transcription activation domain). Ligand-mediated association of the fusion proteins represents the formation of a transcription factor complex and leads to initiation of transcription of a target gene linked to a DNA sequence recognized by (i.e., capable of binding with) the DNA-binding domain on one of the fusion proteins. Information regarding the gene expression system as well as the ligand is disclosed in U.S. Pat. Nos. 6,187,757 B1, 6,649,595 B1, 6,509,152 B1, 6,479,653 B1, and 6,117,680 B1.
[0224]In other embodiments, the present invention provides a gene switch system which comprises polynucleotides encoding two fusion proteins which self-aggregate in the absence of a ligand, wherein (a) the first fusion protein comprises a conditional aggregation domain which binds to a selected ligand and a transcription activation domain, and (b) the second fusion protein comprising a conditional aggregation domain which binds to a selected ligand and a DNA binding domain, and (c) in the absence of ligand, the cells express a gene operably linked to regulatory DNA to which said DNA binding domain binds. Modified cells comprising the gene switch system are expanded in the presence of the ligand in an amount sufficient for repression of the gene. Ligand removal induces expression of the encoded protein that causes cell death. The nucleic acids encoding the two fusion proteins are under the control of at least one conditional promoter. The gene expression system utilizing conditional aggregation domains is disclosed in U.S. Publication No. 2002/0048792.
[0225]C. Procaryotic Repressor/Operator based Gene Switch System
[0226]In one embodiment, the present invention provides gene switch system comprising (a) a first polynucleotide coding for a transactivator fusion protein comprising a prokaryotic tetracycline ("tet") repressor and a eucaryotic transcriptional activator protein domain; and (b) a second polynucleotide coding for a therapeutic protein or therapeutic polypeptide, wherein said second polynucleotide is operably linked to a minimal promoter and at least one tet operator sequence. The first polynucleotide coding for a transactivator fusion protein may comprise therapeutic switch promoter as described elsewhere herein. The expression of the lethal protein is up-regulated in the absence of tetracycline. (see, e.g., Gossen et al. (1992) Proc. Natl. Acad. Sci. 89: 5547-5551; Gossen et al. (1993) TIBS 18: 471-475; Furth et al. (1994) Proc. Natl. Acad. Sci. 91: 9302-9306; and Shockett et al. (1995) Proc. Natl. Acad. Sci. 92: 6522-6526). The TetO expression system is disclosed in U.S. Pat. No. 5,464,758 B1.
[0227]In another embodiment, the gene switch system comprises the lactose ("Lac") repressor-operator systems from the bacterium Escherichia coli. The gene switch system of the present invention may also comprise (a) a first polynucleotide coding for a transactivator fusion protein comprising a prokaryotic lac 1 repressor and a eucaryotic transcriptional activator protein domain; and (b) a second polynucleotide coding for a therapeutic protein or therapeutic polypeptide, wherein said second polynucleotide is operably linked to a therapeutic switch promoter. In the Lac system, a lac operon is inactivated in the absence of lactose, or synthetic analogs such as isopropyl-b-D-thiogalactoside.
[0228]Additional gene switch systems include those described in the following: U.S. Pat. No. 7,091,038; WO2004078924; EP1266015; US20010044151; US20020110861; US20020119521; US20040033600; US20040197861; US20040235097; US20060020146; US20040049437; US20040096942; US20050228016; US20050266457; US20060100416; WO2001/70816; WO2002/29075; WO2002/066612; WO2002/066613; WO2002/066614; WO2002/066615; WO2005/108617; U.S. Pat. No. 6,258,603; US20050209283; US20050228016; US20060020146; EP0965644; U.S. Pat. No. 7,304,162; U.S. Pat. No. 7,304,161; MX234742; KR10-0563143; AU765306; AU2002-248500; and AU2002-306550.
[0229]D. Combination of the Gene Switch Systems
[0230]The present invention provides nucleic acid compositions, modified cells, and bioreactors comprising two or more gene switch systems comprising different ligand-dependent transcription factor complexes which are activated by an effective amount of one or more ligands, wherein the two or more gene switch systems comprise a first gene switch and a second gene switch, both of which selectively induce expression of one or more therapeutic polypeptides or therapeutic polynucleotides, upon binding to one or more ligands. Within the scope of the present invention are any numbers of and/or combinations of gene switch systems.
[0231]In one embodiment, the present invention provides a nucleic acid composition comprising:
(c) a first gene switch system which comprises: [0232]i. a first gene expression cassette comprising a polynucleotide encoding a first hybrid polypeptide which comprises: [0233]1. a transactivation domain, which activates a factor-regulated promoter operably associated with a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide; and [0234]2. a heterodimer partner domain, [0235]ii. a second gene expression cassette comprising a polynucleotide encoding a second hybrid polypeptide which comprises: [0236]1. a DNA-binding domain, which recognizes a factor-regulated promoter operably associated with a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide; and [0237]2. a ligand binding domain; and [0238]iii. a third gene expression cassette comprising a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide comprising: [0239]1. a factor-regulated promoter, which is activated by the transactivation domain of the second hybrid polypeptide; and, [0240]2. a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide, andb. a second gene expression system which comprises: [0241]i. a first gene expression cassette comprising a polynucleotide encoding a first hybrid polypeptide which comprises: [0242]1. a transactivation domain, which activates a factor-regulated promoter operably associated with a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide; and [0243]2. a heterodimer partner domain, [0244]ii. a second gene expression cassette comprising a polynucleotide encoding a second hybrid polypeptide which comprises: [0245]1. a DNA-binding domain, which recognizes a factor-regulated promoter operably associated with a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide; and [0246]2. a ligand binding domain; and [0247]iii. a third gene expression cassette comprising a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide comprising: [0248]1. a factor-regulated promoter, which is activated by the transactivation domain of the second hybrid polypeptide; and, [0249]2. a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide.
[0250]The multiple inducible gene expression systems provide for expression of a given therapeutic polynucleotide or therapeutic polypeptide under conditions associated with different diseases, disorders or conditions, or expression of multiple therapeutic polypeptides or therapeutic polynucleotides either under the same conditions associated with the same disease disorder or condition, or under different conditions associated with different diseases, disorders, or conditions.
[0251]In certain embodiments, the combination of two or more gene switch systems may be (1) a dual-switch ecdysone receptor based gene expression system and (2) a single-switch ecdysone receptor based gene switch. In other embodiments, the combination may be (1) an single- or dual-switch ecdysone receptor based gene switch and (2) a rapamycin based gene switch. Alternatively, the combination of gene switch systems may be two identical rapamycin based gene switch systems disclosed above. Any possible combinations of the gene switch systems are within the scope of the invention.
Ligands
[0252]The ligand for a ligand-dependent transcription factor complex of the invention binds to the protein complex comprising one or more of the ligand binding domain, the heterodimer partner domain, the DNA binding domain, and the transactivation domain. The choice of ligand to activate the ligand-dependent transcription factor complex depends on the type of the gene switch utilized.
[0253]For example, a ligand for the edysone receptor based gene switch may be selected from any suitable ligands. Both naturally occurring ecdysone or ecdyson analogs (e.g., 20-hydroxyecdysone, muristerone A, ponasterone A, ponasterone B, ponasterone C, 26-iodoponasterone A, inokosterone or 26-mesylinokosterone) and non-steroid inducers may be used as a ligand for gene switch of the present invention. U.S. Pat. No. 6,379,945 B1, describes an insect steroid receptor isolated from Heliothis virescens ("HEcR") which is capable of acting as a gene switch responsive to both steroid and certain non-steroidal inducers. Non-steroidal inducers have a distinct advantage over steroids, in this and many other systems which are responsive to both steroids and non-steroid inducers, for a number of reasons including, for example: lower manufacturing cost, metabolic stability, absence from insects, plants, or mammals, and environmental acceptability. U.S. Pat. No. 6,379,945 B1 describes the utility of two dibenzoylhydrazines, 1,2-dibenzoyl-1-tert-butyl-hydrazine and tebufenozide (N-(4-ethylbenzoyl)-N'-(3,5-dimethylbenzoyl)-N'-tert-butyl-hydrazine) as ligands for an ecdysone-based gene switch. Also included in the present invention as a ligand are other dibenzoylhydrazines, such as those disclosed in U.S. Pat. No. 5,117,057 B1. Use of tebufenozide as a chemical ligand for the ecdysone receptor from Drosophila melanogaster is also disclosed in U.S. Pat. No. 6,147,282. Additional, non-limiting examples of ecdysone ligands are 3,5-di-tert-butyl-4-hydroxy-N-isobutyl-benzamide, 8-O-acetylharpagide, a 1,2-diacyl hydrazine, an N'-substituted-N,N'-disubstituted hydrazine, a dibenzoylalkyl cyanohydrazine, an N-substituted-N-alkyl-N,N-diaroyl hydrazine, an N-substituted-N-acyl-N-alkyl, carbonyl hydrazine or an N-aroyl-N'-alkyl-N'-aroyl hydrazine. (See U.S. Pat. No. 6,723,531).
[0254]In one embodiment, the ligand for an ecdysone based gene switch system is a diacylhydrazine ligand or chiral diacylhydrazine ligand. The ligand used in the gene switch system may be compounds of Formula I
##STR00001##
[0255]wherein
[0256]A is alkoxy, arylalkyloxy or aryloxy;
[0257]B is optionally substituted aryl or optionally substituted heteroaryl; and
[0258]R1 and R2 are independently optionally substituted alkyl, arylalkyl, hydroxyalkyl, haloalkyl, optionally substituted cycloalkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted heterocyclo, optionally substituted aryl or optionally substituted heteroaryl;
[0259]or pharmaceutically acceptable salts, hydrates, crystalline forms or amorphous forms thereof.
[0260]In another embodiment, the ligand may be enantiomerically enriched compounds of Formula II
##STR00002##
[0261]wherein [0262]A is alkoxy, arylalkyloxy, aryloxy, arylalkyl, optionally substituted aryl or optionally substituted heteroaryl;
[0263]B is optionally substituted aryl or optionally substituted heteroaryl; and
[0264]R1 and R2 are independently optionally substituted alkyl, arylalkyl, hydroxyalkyl,
[0265]haloalkyl, optionally substituted cycloalkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted heterocyclo, optionally substituted aryl or optionally substituted heteroaryl;
[0266]with the proviso that R1 does not equal R2;
[0267]wherein the absolute configuration at the asymmetric carbon atom bearing R1 and R2 is predominantly S;
[0268]or pharmaceutically acceptable salts, hydrates, crystalline forms or amorphous forms thereof.
[0269]In certain embodiments, the ligand may be enantiomerically enriched compounds of Formula III
##STR00003##
[0270]wherein
[0271]A is alkoxy, arylalkyloxy, aryloxy, arylalkyl, optionally substituted aryl or optionally substituted heteroaryl;
[0272]B is optionally substituted aryl or optionally substituted heteroaryl; and
[0273]R1 and R2 are independently optionally substituted alkyl, arylalkyl, hydroxyalkyl, haloalkyl, optionally substituted cycloalkyl, optionally substituted alkenyl, optionally substituted alkynyl, optionally substituted heterocyclo, optionally substituted aryl or optionally substituted heteroaryl;
[0274]with the proviso that R1 does not equal R2;
[0275]wherein the absolute configuration at the asymmetric carbon atom bearing R1 and R2 is predominantly R;
[0276]or pharmaceutically acceptable salts, hydrates, crystalline forms or amorphous forms thereof.
[0277]In one embodiment, a ligand may be (R)-3,5-dimethyl-benzoic acid N-(1-tert-butyl-butyl)-N'-(2-ethyl-3-methoxy-benzoyl)-hydrazide having an enantiomeric excess of at least 95% or a pharmaceutically acceptable salt, hydrate, crystalline form or amorphous form thereof.
[0278]The diacylhydrazine ligands of Formula I and chiral diacylhydrazine ligands of Formula II or III, when used with an ecdysone-based gene switch system, provide the means for external temporal regulation of expression of a therapeutic polypeptide or therapeutic polynucleotide of the present invention.
[0279]The ligands used in the present invention may form salts. The term "salt(s)" as used herein denotes acidic and/or basic salts formed with inorganic and/or organic acids and bases. In addition, when a compound of Formula I, II or III contains both a basic moiety and an acidic moiety, zwitterions ("inner salts") may be formed and are included within the term "salt(s)" as used herein. Pharmaceutically acceptable (i.e., non-toxic, physiologically acceptable) salts are used, although other salts are also useful, e.g., in isolation or purification steps which may be employed during preparation. Salts of the compounds of Formula I, II or III may be formed, for example, by reacting a compound with an amount of acid or base, such as an equivalent amount, in a medium such as one in which the salt precipitates or in an aqueous medium followed by lyophilization.
[0280]The ligands which contain a basic moiety may form salts with a variety of organic and inorganic acids. Exemplary acid addition salts include acetates (such as those formed with acetic acid or trihaloacetic acid, for example, trifluoroacetic acid), adipates, alginates, ascorbates, aspartates, benzoates, benzenesulfonates, bisulfates, borates, butyrates, citrates, camphorates, camphorsulfonates, cyclopentanepropionates, digluconates, dodecylsulfates, ethanesulfonates, fumarates, glucoheptanoates, glycerophosphates, hemisulfates, heptanoates, hexanoates, hydrochlorides (formed with hydrochloric acid), hydrobromides (formed with hydrogen bromide), hydroiodides, 2-hydroxyethanesulfonates, lactates, maleates (formed with maleic acid), methanesulfonates (formed with methanesulfonic acid), 2-naphthalenesulfonates, nicotinates, nitrates, oxalates, pectinates, persulfates, 3-phenylpropionates, phosphates, picrates, pivalates, propionates, salicylates, succinates, sulfates (such as those formed with sulfuric acid), sulfonates (such as those mentioned herein), tartrates, thiocyanates, toluenesulfonates such as tosylates, undecanoates, and the like.
[0281]The ligands which contain an acidic moiety may form salts with a variety of organic and inorganic bases. Exemplary basic salts include ammonium salts, alkali metal salts such as sodium, lithium, and potassium salts, alkaline earth metal salts such as calcium and magnesium salts, salts with organic bases (for example, organic amines) such as benzathines, dicyclohexylamines, hydrabamines (formed with N,N-bis(dehydroabietyl)ethylenediamine), N-methyl-D-glucamines, N-methyl-D-glucamides, t-butyl amines, and salts with amino acids such as arginine, lysine and the like.
[0282]Non-limiting examples of the ligands for the inducible gene expression system utilizing the FK506 binding domain are FK506, Cyclosporin A, or Rapamycin. FK506, rapamycin, and their analogs are disclosed in U.S. Pat. Nos. 6,649,595 B2 and 6,187,757. See also U.S. Pat. Nos. 7,276,498 and 7,273,874.
[0283]The ligands described herein may be administered alone or as part of a pharmaceutical composition comprising a pharmaceutically acceptable carrier. In one embodiment, the pharmacetical compoistion are in the form of solutions, suspensions, tablets, capsules, ointments, elixirs, or injectable compositions.
Pharmaceutical Compositions
[0284]Pharmaceutically acceptable carriers include fillers such as saccharides, for example lactose or sucrose, mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for example tricalcium phosphate or calcium hydrogen phosphate, as well as binders such as starch paste, using, for example, maize starch, wheat starch, rice starch, potato starch, gelatin, tragacanth, methyl cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose, and/or polyvinyl pyrrolidone. If desired, disintegrating agents may be added such as the above-mentioned starches and also carboxymethyl-starch, cross-linked polyvinyl pyrrolidone, agar, or alginic acid or a salt thereof, such as sodium alginate. Auxiliaries are flow-regulating agents and lubricants, for example, silica, talc, stearic acid or salts thereof, such as magnesium stearate or calcium stearate, and/or polyethylene glycol. In one embodiment, dragee cores are provided with suitable coatings which, if desired, are resistant to gastric juices. For this purpose, concentrated saccharide solutions may be used, which may optionally contain gum arabic, talc, polyvinyl pyrrolidone, polyethylene glycol and/or titanium dioxide, lacquer solutions and suitable organic solvents or solvent mixtures. In order to produce coatings resistant to gastric juices, solutions of suitable cellulose preparations such as acetylcellulose phthalate or hydroxypropylmethyl-cellulose phthalate, are used. Dye stuffs or pigments may be added to the tablets or dragee coatings, for example, for identification or in order to characterize combinations of active compound doses.
[0285]Other pharmaceutical preparations which can be used orally include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer such as glycerol or sorbitol. The push-fit capsules can contain the active compounds in the form of granules or nanoparticles which may optionally be mixed with fillers such as lactose, binders such as starches, and/or lubricants such as talc or magnesium stearate and, optionally, stabilizers. In one embodiment, the is dissolved or suspended in suitable liquids, such as fatty oils, or liquid paraffin, optionally with stabilizers.
[0286]Fatty oils may comprise mono-, di- or triglycerides. Mono-, di- and triglycerides include those that are derived from C6, C8, C10, C12, C14, C16, C18, C20 and C22 acids. Exemplary diglycerides include, in particular, diolein, dipalmitolein, and mixed caprylin-caprin diglycerides. Triglycerides include vegetable oils, fish oils, animal fats, hydrogenated vegetable oils, partially hydrogenated vegetable oils, synthetic triglycerides, modified triglycerides, fractionated triglycerides, medium and long-chain triglycerides, structured triglycerides, and mixtures thereof. Exemplary triglycerides include: almond oil; babassu oil; borage oil; blackcurrant seed oil; canola oil; castor oil; coconut oil; corn oil; cottonseed oil; evening primrose oil; grapeseed oil; groundnut oil; mustard seed oil; olive oil; palm oil; palm kernel oil; peanut oil; rapeseed oil; safflower oil; sesame oil; shark liver oil; soybean oil; sunflower oil; hydrogenated castor oil; hydrogenated coconut oil; hydrogenated palm oil; hydrogenated soybean oil; hydrogenated vegetable oil; hydrogenated cottonseed and castor oil; partially hydrogenated soybean oil; partially soy and cottonseed oil; glyceryl tricaproate; glyceryl tricaprylate; glyceryl tricaprate; glyceryl triundecanoate; glyceryl trilaurate; glyceryl trioleate; glyceryl trilinoleate; glyceryl trilinolenate; glyceryl tricaprylate/caprate; glyceryl tricaprylate/caprate/laurate; glyceryl tricaprylate/caprate/linoleate; and glyceryl tricaprylate/caprate/stearate.
[0287]In one embodiment, the triglyceride is the medium chain triglyceride available under the trade name LABRAFAC CC. Other triglycerides include neutral oils, e.g., neutral plant oils, in particular fractionated coconut oils such as known and commercially available under the trade name MIGLYOL, including the products: MIGLYOL 810; MIGLYOL 812; MIGLYOL 818; and CAPTEX 355. Other triglycerides are caprylic-capric acid triglycerides such as known and commercially available under the trade name MYRITOL, including the product MYRITOL 813. Further triglycerides of this class are CAPMUL MCT, CAPTEX 200, CAPTEX 300, CAPTEX 800, NEOBEE M5 and MAZOL 1400.
[0288]Pharmaceutical compositions comprising triglycerides may further comprise lipophilic and/or hydrophilic surfactants which may form clear solutions upon dissolution with an aqueous solvent. One such surfactant is tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS). Examples of such compositions are described in U.S. Pat. No. 6,267,985.
[0289]In another embodiment, the pharmaceutically acceptable carrier comprises LABRASOL (Gattefosse SA), which is PEG-8 caprylic/capric glycerides. In another embodiment, the pharmaceutically acceptable carrier comprises PL90G, vitamin E TPGS, and Miglyol 812N.
[0290]Possible pharmaceutical preparations which can be used rectally include, for example, suppositories, which consist of a combination of one or more of the ligands with a suppository base. Suitable suppository bases are, for example, natural or synthetic triglycerides, or paraffin hydrocarbons. In addition, it is also possible to use gelatin rectal capsules which consist of a combination of the ligand with a base. Possible base materials include, for example, liquid triglycerides, polyethylene glycols, or paraffin hydrocarbons.
[0291]Suitable formulations for parenteral administration include aqueous solutions of the ligand in water-soluble form, for example, water-soluble salts and alkaline solutions. In addition, suspensions of the ligand as appropriate oily injection suspensions may be administered. Suitable lipophilic solvents or vehicles include fatty oils, for example, sesame oil, or synthetic fatty acid esters, for example, ethyl oleate or triglycerides or polyethylene glycol-400. Aqueous injection suspensions may contain substances which increase the viscosity of the suspension include, for example, sodium carboxymethyl cellulose, sorbitol, and/or dextran. Optionally, the suspension may also contain stabilizers.
[0292]The topical compositions may be formulated as oils, creams, lotions, ointments and the like by choice of appropriate carriers. Suitable carriers include vegetable or mineral oils, white petrolatum (white soft paraffin), branched chain fats or oils, animal fats and high molecular weight alcohol (greater than C12). Emulsifiers, stabilizers, humectants and antioxidants may also be included as well as agents imparting color or fragrance, if desired. Additionally, transdermal penetration enhancers can be employed in these topical formulations. Examples of such enhancers can be found in U.S. Pat. Nos. 3,989,816 and 4,444,762.
[0293]Creams may be formulated from a mixture of mineral oil, self-emulsifying beeswax and water in which ligand, dissolved in a small amount of an oil such as almond oil, is admixed. A typical example of such a cream is one which includes about 40 parts water, about 20 parts beeswax, about 40 parts mineral oil and about 1 part almond oil.
[0294]Ointments may be formulated by mixing a suspension of the ligand in a vegetable oil such as almond oil with warm soft paraffin and allowing the mixture to cool. A typical example of such an ointment is one which includes about 30% almond oil and about 70% white soft paraffin by weight.
[0295]Lotions may be conveniently prepared by preparing a suspension of the ligand in a suitable high molecular weight alcohol such as propylene glycol or polyethylene glycol.
[0296]Examples of antioxidants which may be added to the pharmaceutical compositions include BHA and BHT.
[0297]In one embodiment, the pharmaceutical composition comprises 30 mg ligand per mL LABRASOL in a solid gelatin capsule. In another embodiment, the capsule contains 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95 or 100 mg ligand.
[0298]Pharmaceutical compositions may contain from 0.01% to 99% by weight of the ligand. Compositions may be either in single or multiple dose forms. The amount of ligand in any particular pharmaceutical composition will depend upon the effective dose, that is, the dose required to elicit the desired gene expression or suppression. In one embodiment, 0.1 to 7.5 mg/kg is administered to the subject. In another embodiment, 0.1 to 3 mg/kg is administered to the subject. In another embodiment, 0.1 to 3 mg/kg is administered.
[0299]Suitable routes of administering the pharmaceutical compositions include oral, rectal, topical (including dermal, buccal and sublingual), vaginal, parenteral (including subcutaneous, intramuscular, intravenous, intradermal, intrathecal, intra-tumoral and epidural) and by naso-gastric tube. It will be understood by those skilled in the art that the route of administration will depend upon the condition being treated and may vary with factors such as the condition of the recipient. The pharmaceutical compositions may be administered one or more times daily.
Therapeutic Molecules
[0300]The therapeutic molecule, e.g., the polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide may be any sequence that encodes a polypeptide or polynucleotide that is useful for the treatment, amelioration, or prevention of a disease, disorder, or condition. Therapeutic polypeptides may be any polypeptide known to be effective for treating, ameliorating, or preventing a disease, disorder, or condition. Examples of classes of therapeutic polypeptides that may be used in the invention include, without limitation, cytokines, chemokines, hormones, antibodies, engineered immunoglobulin-like molecules, single chain antibodies, fusion proteins, enzymes, immune co-stimulatory molecules, immunomodulatory molecules, transdominant negative mutants of target proteins, toxins, conditional toxins, antigens, tumor suppressor proteins, growth factors, membrane proteins, vasoactive proteins and peptides, anti-viral proteins or variants thereof. Therapeutic polynucleotides include, without limitation, antisense sequences, small interfering RNAs, ribozymes, and RNA external guide sequences. Therapeutic polynucleotides may be targeted to any transcript associated with a particular disease, disorder, or condition and for which it is desired to decrease or eliminate expression. Numerous genes exhibiting elevated expression during a disease, disorder, or condition are known in the art, including the genes listed in Tables 1-3 above.
[0301]The polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide is operably linked to or operably associated with a factor-regulated promoter comprising at least one response element that is recognized by the DBD of the ligand-dependent transcription factor complex encoded by the gene switch. In one embodiment, the promoter comprises 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more copies of the response element. Promoters comprising the desired response elements may be naturally occurring promoters or artificial promoters created using techniques that are well known in the art, e.g., one or more response elements operably linked to a minimal promoter.
[0302]Specific therapeutic polypeptides which may be expressed using a therapeutic gene-switch include, but are not limited to antibodies, including monoclonal antibodies, minimal antibodies, fusion proteins, endogenous protein mimetics, enzymes, hormones, cytokines, chemokines, growth factors, and fragments, variants or derivatives of any such polypeptides. Non-limiting representative therapeutic molecules are described below. All references to these molecules, including patent publications, scientific literature, and polynucleotide and polypeptide sequence accession numbers, are hereby incorporated by reference in their entireties.
Monoclonal Antibodies
[0303]Therapeutic gene-switch constructs of the present invention may be used to express therapeutic monoclonal antibodies, or fragments, variants or analogs thereof (collectively "monoclonal antibodies"). Such monoclonal antibodies are useful for treatment of diseases and disorders including, without limitation, cancer, autoimmune diseases (e.g., MS, Crohn's disease, rheumatoid arthritis), cancer, infectious diseases, inflammatory diseases, allergies, heart disases, and transplantation rejection. Antibodies for use in the present invention include any known therapeutic monoclonal antibodies including, but not limited to those listed below, monoclonal antibodies which bind to the same epitope or target as any known monoclonal antibodies. Monoclonal antibody constructs suitable for expression via therapeutic gene switch constructs include multispecific, human, humanized, primatized, or chimeric antibodies, single chain antibodies, epitope-binding fragments, e.g., Fab, Fab' and F(ab')2, Fd, Fvs, single-chain Fvs (scFv), single-chain antibodies, disulfide-linked Fvs (sdFv), and fragments comprising either a VL or VH domain. ScFv molecules are known in the art and are described, e.g., in U.S. Pat. No. 5,892,019. Immunoglobulin or antibody molecules of the invention can be of any type (e.g., IgG, IgE, IgM, IgD, IgA, and IgY), class (e.g., IgG1, IgG2, IgG3, IgG4, IgA1 and IgA2) or subclass of immunoglobulin molecule. Antibody fragments, including single-chain antibodies, may comprise the variable region(s) alone or in combination with the entirety or a portion of the following: hinge region, CH1, CH2, and CH3 domains. Also included in the invention are antigen-binding fragments also comprising any combination of variable region(s) with a hinge region, CH1, CH2, and CH3 domains.
[0304]In certain embodiments, the present invention includes therapeutic gene switch constructs which encode monoclonal antibodies against antigens including, but not limited to CTLA4, CD25, HER-2/neu (ErbB2), CD20, TNFα, EGFR, and VEGF.
[0305]Anti-CTLA4 antibodies employable in the present invention, and methods of producing them, are described in the International Application No. PCT/US99/30895, published on Jun. 29, 2000 as WO 00/37504 (e.g., ticilimumab, also known as 11.2.1 and CP-675,206), European Patent Appl. No. EP 1262193 A1, published Apr. 12, 2002, U.S. patent application Ser. No. 09/472,087, now issued as U.S. Pat. No. 6,682,736, U.S. patent application Ser. No. 09/948,939, now published as U.S. Pat. App. Pub. No. 2002/0086014 (e.g., ipilimumab, also known as 10D1 and MDX-010, Medarex, Princeton, N.J.),
[0306]Anti-CD25 antibodies employable in the present invention include, without limitation, Daclizumab. See, e.g., U.S. Pat. No. 5,530,101. Daclizumab (brand name: Zenapax®, marketed by Roche) is a humanized IgG1 monoclonal antibody directed against CD25 (IL-2 receptor). Functioning as an IL-2 receptor antagonist, it binds with high affinity to the Tac subunit of the high-affinity IL-2 receptor complex. Daclizumab is indicated for the prophylaxis of acute organ rejection in renal transplant patients when used in combination with cyclosporine and corticosteroids.
[0307]Anti-HER-2/neu (ErbB2) antibodies employable in the invention include, without limitation, Trastuzumab. See, e.g., U.S. Pat. No. 5,677,171. Trastuzumab (brand name: Herceptin®, marketed by Genentech) is a humanized, monoclonal antibody targeted against the extracellular domain of the c-erbB2/HER2/neu protein, a transmembrane receptor protein (structurally related to the Epidermal Growth Factor receptor) which is overexpressed in certain types of breast cancer. As a mediator of antibody-dependent cellular cytotoxicity, trastuzumab is preferentially toxic to HER2-expressing cancer cells.
[0308]Anti-CD20 antibodies employable in the invention include, without limitation rituximab (see, e.g., U.S. Pat. No. 5,736,137). Rituximab (brand name: Rituxan®, marketed by Biogen Idec and Genentech) is a chimeric (murine/human) monoclonal IgG1κ antibody. Rituximab was initially designed and licensed for treatment of non-Hodgkin's lymphoma, and more recently has been licensed for treatment of anti-TNF refractory rheumatoid arthritis.
[0309]Anti-TNFα antibodies employable in the invention include, without limitation, Adalimumab (see, e.g., U.S. Pat. No. 7,223,394), and Infliximab (see, e.g., U.S. Pat. No. 7,138,118). Adalimumab, (brand name: Humira®, marketed by Abbott) is a recombinant human IgG1κ monoclonal antibody which binds specifically to TNFα, thereby blocking interaction of TNFα with the p55 and p75 surface TNF receptors. Adalimumab is licensed for use in rheumatoid arthritis, and juvenile idiopathic arthritis.
[0310]Additional indications for adalimumab include Crohn's disease, plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis. Infliximab (brand name: Remicade, marketed by Centocor) is a recombinant chimeric IgG1κ monoclonal antibody which binds specifically to TNFα, thereby blocking interaction of TNFα with the p55 and p75 surface TNF receptors. Infliximab is licensed for use in Crohn's disease. Additional indications include rheumatoid arthritis, psoriatic arthritis, severe chronic plaque psoriasis, and ankylosing spondylitis.
[0311]Anti EGFR (Epidermal Growth Factor Receptor) antibodies employable in the invention include, without limitation, Cetuximab (see, e.g., U.S. Pat. No. 6,217,866). Cetuximab (brand name: Erbitux®, marketed by Imclone and Bristol-Meyers Squibb (North America) and by Merck KGaA (other areas) wis a chimeric monoclonal antibody which binds specifically to EGFR. Cetuximab is indicated for metastatic colorectal cancer; and head and neck cancer.
[0312]Anti-VEGF antibodies employable in the invention include, without limitation, Bevacizumab (see, e.g., U.S. Pat. No. 6,383,486). Bevacizumab (brand name: Avastin®, marketed by Genentech) is a human monoclonal antibody that inhibits the function of vascular endothelial growth factor (VEGF), thus inhibiting tumor neoangiogenesis. Bevacizumab is indicated for treatment in combination with other anti-cancer chemotherapeutics for the first- and second-line treatment of patients with metastatic colorectal cancer and first-line treatment of patients with recurrent or metastatic non-squamous non-small cell lung cancer (NSCLC).
Fusion Proteins
[0313]Therapeutic gene-switch constructs of the present invention may be used to express therapeutic fusion proteins, such as a chimeric TNFα binding protein 2. Tumor necrosis factor binding protein 2 (Enbrel) is produced from the membrane form by proteolytic processing. Enbrel is a recombinant fusion protein consisting of two soluble TNF receptors joined by the Fc fragment of a human IgG1 molecule. It binds to TNF-alpha and blocks TNF-alpha interaction with its receptor. Enbrel is used to treat moderate to severe rheumatoid arthritis. The amino acid sequence coding for Enbrel is available from public database as accession number P20333.
[0314]The polynucleotide sequences of Enbrel are available from public databases as accession numbers DD292498 and DD 292499, sequences of which are incorporated by reference herein.
Enzymes
[0315]Therapeutic gene-switch constructs of the present invention may be used to express therapeutic enzymes, including tissue plasminogen activator. Plasminogen activator, tissue type isoform 3 preproprotein (tPA) is a secreted serine protease which converts the proenzyme plasminogen to plasmin, a fibrinolytic enzyme. This enzyme plays a role in cell migration and tissue remodeling. The amino acid sequences coding for tPA are available from public databases as accession numbers NP--127509 and NP--000921 (both human), sequences of which are incorporated by reference herein.
[0316]The polynucleotide sequences coding for tPA are available from public databases as accession numbers NM--033011 and NM--000930 (both human), sequences of which are incorporated by reference herein.
Endogenous Protein Mimetics
[0317]Therapeutic gene-switch constructs of the present invention may be used to express therapeutic mimetics of endogenous proteins, such as the following.
[0318]Alphanate (Coagulation factor III), along with calcium and phospholipid, acts as a cofactor for factor IXa when it converts factor X to the activated form of factor Xa. Alphanate is purified Factor VIII (also know as Antihemophilic factor) and von Willebrand factor. Alphanate is approved for the prevention and control of bleeding in patients with Factor VIII deficiency due to hemophilia A or acquired Factor VIII deficiency. The amino acid sequences coding for factor VIII are available from public databases as accession numbers AAA52485 (human); and AAA37385 (mouse), sequences of which are incorporated by reference herein.
[0319]The polynucleotide sequences coding for factor VIII are available from public databases as accession numbers M14113 (human); and L05573 (mouse), sequences of which are incorporated by reference herein.
[0320]Aralast (Alpha-1 proteinase inhibitor) amino acid sequences are available from public databases as accession numbers AAB59375 (human alpha 1-antitrypsin); AAC28869 (mouse alpha-1 protease inhibitor); and AAA40788 (rat alpha-1-antitrypsin), sequences of which are incorporated by reference herein.
[0321]The polynucleotide sequences coding for alpha-1 proteinase inhibitor are available from public databases as accession numbers K01396 (human); M75721 (mouse); and M32247 (rat), sequences of which are incorporated by reference herein.
[0322]Nesiritide (Natrecor®) is a recombinant form of human B-type natriuretic peptide (hBNP) that has been approved for the intravenous treatment of patients with acute decompensated congestive heart failure (CHF) who have dyspnea at rest or with minimal activity. The amino acid sequence coding for Brain natriuretic peptide is available from public database as accession number NP--002512, sequence of which is incorporated by reference herein.
[0323]The polynucleotide sequence coding for brain natriuretic peptide is available from public database as accession number NM--002521, sequence of which is incorporated by reference herein.
[0324]The amino acid sequence coding for human insulin is available from public database as accession number AAH05255, sequence of which is incorporated by reference herein.
[0325]The polynucleotide sequence coding for human insulin is available from public database as accession number BC005255, sequence of which is incorporated by reference herein.
[0326]Granulocyte/macrophage colony-stimulating factor (GM-CSF) is a cytokine that functions as a white blood cell growth factor, stimulates stems cells to produce granulocytes (neutrophils, eosinophils, and basophils) and monocytes. The amino acid sequences coding for granulocyte/macrophage colony-stimulating factor (GM-CSF) are available from public databases as accession numbers AAA52122 (human); NP--034099 (mouse); NP--001032749 (rat Csf2ra); and NP-598239 (Csf2rb), sequences of which are incorporated by reference herein.
[0327]The polynucleotide sequences coding for GM-CSF are available from public databases as accession numbers M11734 (human); NM--009969 (mouse); NM--001037660 (rat Csf2ra); and NM--133555 (rat Csf2rb), sequences of which are incorporated by reference herein.
[0328]The amino acid sequences coding for erythropoietin are available from public databases as accession numbers AAH93628 (human); AA119266 (mouse); and BAA01593 (rat), sequences of which are incorporated by reference herein.
[0329]The polynucleotide sequences coding for erythropoietin are available from public databases as accession numbers BC093628 (human); BC119265 (mouse); and D10763 (rat), sequences of which are incorporated by reference herein.
[0330]The amino acid sequences coding for growth hormone are available from public databases as accession numbers AAA98618 (human); NP--032143 (mouse); and NP--001030020 (rat), sequences of which are incorporated by reference herein.
[0331]The polynucleotide sequences coding for growth hormone are available from public databases as accession numbers M13438 (human); NM--008117 (mouse); and NM--001034848 (rat), sequences of which are incorporated by reference herein.
Recombinant Protein
[0332]Therapeutic gene-switch constructs of the present invention may be used to express therapeutic recombinant proteins, such as the botulinum toxin. The botulinum toxin inhibits neurotransmitter acetylcholine release at nerve terminals, and is available under the name BOTOX for the treatment of strabismus and blepharospasm associated with dystonia and cervical dystonia. BOTOX is also used for the treatment of hemifacial spasm and a number of other neurological disorders characterized by abnormal muscle contraction. The amino acid sequence coding for botulinum neurotoxin type A precursor (BoNT/A) (Bontoxilysin-A) (BOTOX) are available from public databases as accession numbers P10845.
Treatment of Cardiovascular Diseases
[0333]The present invention is further directed to a method of treating, ameliorating, or preventing cardiovascular disease, comprising administering to a subject in need of such treatment a therapeutic gene product which ameliorates, prevents, or treats cardiovascular related diseases under control of the switch proteins referenced earlier. Such treatment may be delivered directly to the subject to be treated, or via a bioreactor containing encapsulated or non-encapsulated non-modified or genetically modified cells which secrete one or more therapeutic proteins or therapeutic polypeptides as described elsewhere herein. According to this embodiment, the cell will express one or more therapeutic gene products effective in treating cardiovascular disease when transplanted into a subject, e.g., into an infarct zone of a cardiovascular disease patient. Examples of such therapeutic gene products are described in more detail below. In certain embodiments, a genetically modified cell of the present invention expresses the one or more therapeutic gene products constitutively, i.e., one or more heterologous therapeutic gene products are expressed in the cell continuously. Alternatively, expression of one, two, three, or more heterologous therapeutic gene products expressed by the cell is controlled by a therapeutic gene switch. In certain aspects, bioreactors for treatment, amelioration, or prevention of cardiovascular disease comprise encapsulated cells, e.g., the cells are encapsulated in an alginate-based formulation. Examples and methods of cell encapsulation, to provide, e.g., a physical or immunological barrier from the subject being treated, are described in detail elsewhere herein.
[0334]The invention further provides a nucleic acid composition comprising one or more polynucleotides which express therapeutic gene products, e.g., therapeutic polypeptides and/or therapeutic polynucleotides, useful for the treatment, amelioration, or prevention of cardiovascular disease through operable association with a promoter. In certain embodiments a promoter controlling expression of a therapeutic gene product is activated by a ligand-dependent transcription factor complex, where at least a portion of the transcription factor is expressed via operable linkage to one or more therapeutic switch promoters, where the activity of the therapeutic switch promoters is constitutive and/or is modulated under conditions associated with a tissue type or associated with a disease, disorder, or condition. In embodiments relating to the treatment, amelioration, or prevention of cardiovascular disease, a therapeutic switch promoter could be, for example, a heart-specific promoter, or a promoter which is activated during conditions such as congestive heart failure, ischemic heart disease, hypertensive heart disease, coronary artery disease, peripheral vascular disease and ischemic cardiac events, e.g., myocardial infarction, heart attack, heart failure, arrhythmia, myocardial rupture, pericarditis, and cardiogenic shock. Exemplary promoters are presented in Tables 1-3. Additional promoters are described elsewhere herein, for example in Examples 1-8. Additional promoters can also be easily identified via methods described herein.
[0335]Examples of therapeutic switch promoters useful for regulated gene switch expression in cardiac cells or under conditions related to cardiac diseases, disorders, or conditions include, without limitation: the S100A6 promoter, which is tissue-specific for cardiac myocytes (Tsoporis et al., J. Biol. Chem. (2008) (Epub ahead of print; PMID: 18753141)); Atrial Naturetic Factor (ANF) promoter, Alpha-myosin heavy chain promoter, c-fos promoter, BNP promoter, or alpha actins promoter, all of which are tissue-specific for cardiomyocytes (Nelson et al., J. Mol. Cell. Cardiol. 39(3):479 (2005)); Erythropoietin promoter, which is activated in myocardium under ischemic conditions (Su et al., Proc. Natl. Acad. Sci. U.S.A. 99(14):9480 (2002)); AlphaB-Crystallin (CRYAB) promoter including, for example, a BRG1-response element, which is tissue-specific for vertebrate eye lens (Duncan B. and Zhao K. DNA Cell. Biol. 26(10):745 (2007)); AlphaB-Crystallin (CRYAB) promoter with cis-acting regulatory elements, e.g., alpha BE-1, alpha BE-2, alpha BE-3, and MRF, which is tissue-specific for skeletal muscle (Gopal-Srivastava et al., J. Mol. Cell. Biol. 15(12):7081 (1995)); NCX1 promoter, which is tissue-specific for cardiomyocytes (Xu et al., J. Biol. Chem. 281(45):34430 (2006)); Beta myosin heavy chain promoter, which is tissue-specific for cardiomyocytes (Nelson et al., J. Mol. Cell. Cardiol. 39(3):479 (2005), Ross et al., Development. 122(6):1799 (1996), and Lee et al., Mol. Cell. Biol. 14(2):1220 (1994)), Myosin light chain-2 ventricular promoter including an HF-1a/HF-1b/MEF-2 combinatorial element (Ross et al., Development. 122(6):1799 (1996)) or an HF-1a/HF-1b element and an HF-3 regulatory element, (Lee et al., Mol. Cell. Biol. 14(2):1220 (1994)), which is tissue-specific for cardiac ventricles; Myosin light chain promoters, e.g., MLC1F and MLC3F, which are differentially activated during skeletal muscle development (Kelly et al., J. Cell. Biol. 129(2):383 (1995)); Myosin light chain 2v (MLC-2v) promoter, which is tissue-specific for cardiac muscles (Su et al., Proc. Natl. Acad. Sci. USA. 101(46): 16280 (2004)); and Cardiac troponin I (TnIc) promoter, which is tissue-specific and developmental stage-specific in cardiac muscles (Bhavsar et al., J. Mol. Cell. Cardiol. 32(1):95 (2000)).
[0336]The invention further provides one or more vectors comprising the aforementioned nucleic acid composition, and one or more genetically modified cells comprising such vectors. Such cells may be allogeneic, autologous, or xenogeneic relative to the subject to be treated. The invention further provides one or more encapsulation methodologies for the treatment, amelioration, or prevention of cardiovascular disease, comprising the aforementioned modified cells, where the cells have been treated in such a way as to be protected from a subject's immune system upon introduction into the subject. Such treatments include, without limitation, provision of a conformal coating, microencapsulation, or macroencapsulation.
[0337]Cardiovascular diseases include, but are not limited to congestive heart failure, ischemic heart disease, hypertensive heart disease, coronary artery disease, peripheral vascular disease and ischemic cardiac events, e.g., myocardial infarction, heart attack, heart failure, arrhythmia, myocardial rupture, pericarditis, and cardiogenic shock. Causes of such events include, without limitation, thrombosis, embolism, atherosclerosis, and stenosis. Populations predisposed include, without limitation, smokers, persons with diabetes, hypertension, or dyslipidemia.
[0338]Suitable therapeutic molecules for the treatment, amelioration, or prevention of cardiovascular disease include, without limitation, pro-angiogenic factors, cardioprotective factors, and cardioregenerative factors.
[0339]The therapeutic molecules useful for the present invention to prevent, treat, or ameliorate cardiovascular diseases include, without limitation, the atrial natriuretic factor (ANF), carperitide, brain Natriuretic factor (BNP), nesiritide, relaxin, vascular endothelial growth factor (VEGF165), hepatocyte growth factor (HGF), Angiopoietin-1 (Ang-1), basic fibroblast growth factor (bFGF), fibroblast growth factor 4 (FGF-4), insulin-like growth factor 1 (IGF-1), hypoxia-inducible factor1-alpha (HIF1-alpha), erythropoietin, tissue plasminogen activator (tPA), growth hormone, Stromal-Derived Factor-1 (SDF-1), sarco-endoplasmic reticulum Ca2+-ATPase (SERCA2a), adenylycyclase type VI (AC6), S100A1, parvalbumin, phosphatase inhibitor 2, and phosphatase inhibitor 1. These molecules are known to exert the effects on cardiac tissues through various mechanisms, e.g., hemodynamics, angiogenesis, cardiac regeneration, anti-fibrosis, and/or cardiac repair. These therapeutic molecules may provide multiple therapeutic actions and may be used in combination with each other or other molecules that are known in public.
[0340]In one embodiment, pro-angiogenic gene therapy clinical trials for the treatment, amelioration, or prevention of cardiovascular disease are currently being performed using therapeutic proteins useful for promoting neo-vascularization. These include, without limitation, pro-angiogenic factors such as VEGF, HGF, bFGF, Ang-1, FGF-4, IGF-1, and HIF 1-alpha as well as fragments, variants and derivatives thereof. Identification of suitable molecules for promoting neo-vascularization are well within the capabilities of a person of ordinary skill in the art Such pro-angiogenic factors stimulate neo-angiogenesis to supply oxygen and nutrients within the infarct zone. This will limit infarct zone expansion and sustain any cardiac progenitors that migrate into the infarct.
[0341]Indeed, pro-angionic factor VEGF165 is known to induce neovascularization (Benest et al., Microcirculation. 13(6):423 (2006); Riley et al., Biomaterials. 27(35):5935 (2006); Shyu et al., Life Sci. 73(5):563 (2003); Arsic et al., Mol. Ther. 7(4):450 (2003); Ye et al., J. Heart Lung Transplant. 24(9):1393 (2005); Lubiatowski et al., Plast. Reconstr. Surg. 110(1):149 (2002) (Erratum in: Plast. Reconstr. Surg. 111(3):1380 (2003)); Kim et al., Ann. Thorac. Surg. 83(2):640 (2007) (Comment in: Ann. Thorac. Surg. 83(2):646 (2007)); Thurston G., J. Anat. 200(6):575 (2002); Ryu et al., Mol. Ther. 13(4):705 (2006); Chae et al., Arterioscler. Thromb. Vasc. Biol. 20(12):2573 (2000); and Chen et al., Acta. Pharmacol. Sin. 28(4):493 (2007)). The shortcomings of early clinical trials in therapeutic neovascularization have been partly attributed to the single administration of high doses of growth factor. See Zacchigna et al., Hum. Gene Ther. 18(6):515 (2007) and Yla-Herttuala et al., J Am Coll Cardiol. 49(10):1015 (2007) (Comment in: J Am Coll Cardiol. 50(2):186 (2007)). Since then, preclinical data on VEGF expression and release has suggested that prolonged exposure results in the formation of stable vessels, whereas short-term delivery merely produces leaky vessels that regress easily. High local concentrations caused, for example, by VEGF-A-producing myoblasts results in leaky and abnormal vessels, whereas moderate amounts of the growth factor initiated the growth of healthy vessels. See Arsic et al., Mol. Ther. 7(4):450 (2003); Benest et al., Microcirculation. 13(6):423 (2006); Yamauchi et al., J Gene Med. 5(11):994 (2003); Jiang et al., Acta Cardiol. 61(2):145 (2006); Ozawa et al., J Clin Invest. 113(4):516 (2004). Additionally, the combination of VEGF (initiation of angiogenesis) and Ang-1 (maturation of vessels) has been shown to result in more stable vascular growth. See Thurston G., J Anat. 200(6):575 (2002); Jiang et al., Acta Cardiol. 61(2):145 (2006); Benest et al., Microcirculation. 13(6):423 (2006); Zhou et al., Gene Ther. 12(3):196 (2005) (Erratum in: Gene Ther. 12(6):552 (2005); Liu et al., Scand Cardiovasc J. 41(2):95 (2007); Shyu et al., Life Sci. 73(5):563 (2003); Yamauchi et al., J Gene Med. 5(11):994 (2003); Arsic et al., Mol. Ther. 7(4):450 (2003); Ye et al., J Heart Lung Transplant. 24(9):1393 (2005); Ye et al., Eur J Heart Fail. 9(1):15 (2007); Lubiatowski et al., Plast Reconstr Surg. 110(1): 149 (2002) (Erratum in: Plast Reconstr Surg. 111(3):1380 (2003)); Ryu et al., Mol. Ther. 13(4):705 (2006); Chen et al., Eur J. Pharmacol. 568(1-3):222 (2007); Chae et al., Arterioscler Thromb Vasc Biol. 20(12):2573 (2000); and Chen et al., Acta Pharmacol Sin. 28(4):493 (2007).
[0342]Therefore, pro-angiogenic factor VEGF165 is known to prevent, treat or ameliorate various cardiovascular disease (Yamauchi et al., J Gene Med. 5(11):994 (2003) and Xu et al., Cytotherapy. 6(3):204 (2004) (Comment in: Cytotherapy. 7(1):74 (2005))) including, without limitation, myocardial infarction (Zhou et al., Gene Ther. 12(3):196 (2005) (Erratum in: Gene Ther. 12(6):552 (2005); Ye et al., Circulation. 116(11 Suppl):1113 (2007); Liu et al., Scand Cardiovasc J. 41(2):95 (2007); Ye et al., Eur. J. Heart Fail. 7(6):945 (2005); Zhang et al., Cell Transplant. 14(10):787 (2005); Bonaros et al., Interact. Cardiovasc. Thorac. Surg. 7(2):249 (2008); Shyu et al., J. Biomed. Sci. 13(1):47 (2006); Ventura et al., J Biol. Chem. 282(19):14243 (2007); Sugimoto et al., Jpn. J. Thorac. Cardiovasc. Surg. 51(5):192 (2003); Yau et al., Ann. Thorac. Surg. 83(3):1110 (2007) (Comment in: Ann Thorac Surg. 83(3):1119 (2007)); Rong et al., Chin. Med. J. (Engl). 121(4):347 (2008); Yang et al., Cardiology. 107(1):17 (2007); Wang et al., J. Mol. Cell. Cardiol. 40(5):736 (2006); Chen et al., Eur J Clin Invest. 35(11):677 (2005); Suzuki et al., Circulation. 104(12 Suppl 1):1207 (2001); Ye et al., Ann. Acad. Med. Singapore. 32(5 Suppl):S21 (2003); and Haider et al., J Mol. Med. 82(8):539 (2004) (Comment in: J Mol Med. 82(8):485 (2004))) or ischemia or reperfusion injury (Becker et al., Int J. Cardiol. 113(3):348 (2006); Gao et al., Can. J. Cardiol. 23(11):891 (2007); Ye et al. Eur J Heart Fail. 9(1):15 (2007); Chen et al., Eur. J. Pharmacol. 568(1-3):222 (2007); and Jiang et al., Acta Cardiol. 61(2):145 (2006)).
[0343]Furthermore, another pro-angiogenic factor HGF (human nucleotide sequence accession No.: M29145, human amino acid sequence accession No.: NP--000592.3), which provides multipotent actions, are useful for the present invention. HGF, mediated by c-Met receptor, provides a pro-angiogenic effect through mitogenic activity on endothelial cells, a cardioprotective anti-apoptotic effect on cardiomyocytes, an anti-fibrotic effect through suppression of TGF-beta1 signaling, and is a type I collagen regenerative factor through mobilization of CD117(+)/c-Met(+) stem cells into ischemic myocardium. See Li et al., Chin Med J (Engl) 121(4):336 (2008); Guo et al., Arch. Med. Res. 39(2):179 (2008); Ventura et al., J. Biol. Chem. 282(19):14243 (2007); Yang et al., Gene Ther. 13(22):1564 (2006); Tambara et al., Circulation. 112(9 Suppl):1129 (2005); Zhang et al., Tissue Eng. Part A. 14(6):1025 (2008); and Sakaguchi et al., Ann. Thorac. Surg. 79(5):1627 (2005).
[0344]Similarly, bFGF (amino acid sequence accession no. NP--001997) has been shown to have the added effect of cardioprotection by promoting angiogenesis, neovascularization, and tissue regeneration. (Doi et al., Heart Vessels. 22(2):104 (2007); Fujita et al., J. Surg. Res. 126(1):27 (2005); Fujita et al., Wound Repair Regen. 15(1):58 (2007); Hosaka et al., Circulation. 110(21):3322 (2004); Iwakura et al., Heart Vessels. 18(2):93 (2003); Lai et al., Tissue Eng. 12(9):2499 (2006); Nakajima et al., J. Artif. Organs. 7(2):58 (2004); Perets et al., J. Biomed. Mater. Res. A. 65(4):489 (2003); Pike et al., Biomaterials. 27(30):5242 (2006); Sakakibara et al., J Thorac Cardiovasc Surg. 124(1):50 (2002); Sakakibara et al., Eur J Cardiothorac Surg. 24(1):105 (2003); Shao et al., Circ J. 70(4):471 (2006); Tabata Y. and Ikada Y., Biomaterials. 20(22):2169 (1999); Yamamoto et al., Artif. Organs. 27(2):181 (2003); Yamamoto et al., Jpn. Circ. J. 65(5):439 (2001); Yang et al., Ophthalmic Res. 32(1):19 (2000); and Zhu et al., Chin. Med. Sci. J. 15(4):210 (2000)) In certain embodiments, bFGF may be used to prevent, treat, or ameliorate osteoarthritis. See Inoue et al., Arthritis Rheum. 54(1):264 (2006);
[0345]IGF-1 (human amino acid sequence accession No.: NP--001104753.1) is also known to exert multipotent function of protecting cardiomyocytes from apoptosis and enhancing neovascularization (Su et al., Am J Physiol Heart Circ Physiol. 284(4):H1429 (2003); Chao et al., J. Gene Med. (4):277 (2003); Rabinovsky E. D. and Draghia-Akli R., Mol. Ther. 9(1):46 (2004); and Barton et al., Circulation. 112(9 Suppl):146 (2005)) and may be used in the present invention.
[0346]In addition, FGF-4 may be used as a therapeutic molecule to prevent, treat, or ameliorate chronic ischemic heart disease by inducing myocardial angio-/arteriogenesis. (Kapur N. K. and Rade J. J., Trends Cardiovasc. Med. 18(4):133 (2008); Henry et al., J. Am. Coll. Cardiol. 50(11):1038 (2007); Grines et al., Am. J. Cardiol. 92(9B):24N (2003); (no author listed) BioDrugs. 16(1):75 (2002)).
[0347]Furthermore, HIF1-alpha gene therapy, e.g., HIF1-alpha (aa 1-390)/VP16 (aa 413-490), is known to treat, prevent, or ameliorate ischemic disease by enhancing BNP gene expression (Rajagopalan et al., Circulation. 115(10):1234 (2007) (Comment in: Circulation. 115(10):1180 (2007)); Wilhide M. E. and Jones W. K., Mol. Pharmacol. 69(6):1773 (2006) (Comment on: Mol. Pharmacol. 69(6):1953 (2006)); Luo et al., Mol. Pharmacol. 69(6):1953 (2006) (Comment in: Mol. Pharmacol. 69(6):1773 (2006)) or improve angiogenesis in myocardial infarction (Shyu et al., Cardiovasc Res. 54(3):576 (2002); Vincent et al., Circulation. 102(18):2255 (2000)).
[0348]In certain embodiments, cardioprotective factors for the treatment, amelioration, or prevention of cardiovascular diseases are provided, either alone, or in combination with angiogenic factors and/or cardioregenerative factors. Cardioprotective molecules provide anti-fibrotic, anti-apoptotic signal to resident cardiomyocytes, limiting infact zone size and supplying survival signals to migrating stem cells. In certain embodiments, the cardioprotective factor is erythropoietin alfa (EPO) (human amino acid accession no. CAA26095.1), e.g., human erythropoietin alfa or EPOGEN®, manufactured by Amgen. Erythropoietin has been shown to have cardioprotective, angiogenic and neuroprotective effects (Ben-Dor et al., Cardiovasc Drugs Ther. 21(5):339 (2007); Lin et al., Circ J. 71(1): 132 (2007); Prunier et al., Am J Physiol Heart Circ Physiol. 292(1):H522 (2007)).
[0349]Other cardioprotective hormones demonstrated to be protective against experimental myocardial ischemia-reperfusion injury include, without limitation, adrenomedullin, bradykinin, relaxin, ANF, also known as atrial natriuretic peptide (ANP, human nucleotide sequence accession No.: NM--006172, human amino acid sequence accession No.: NP--006163), BNP, also known as B-type natriuretic peptide or GC-B (human amino acid sequence accession No.: NP--002512.1; human nucleotide sequence accession No.: M25296), C-type natriuretic peptide (CNP), carperitide, tissue plasminogen activator (tPA) and urocortins. Many have also been shown to reduce fibrosis or mediate hemodynamics. Nesiritide (brand name Natrecor®, marketed by Scios), a recombinant form of human B-type natriuretic peptide, ANF, and Carperitide are used in the treatment, amelioration, or prevention of acute decompensated heart failure, and may also be used in the present invention (Burnett J. C. Jr., J. Cardiol. 48(5):235 (2006)).
[0350]Relaxin (human amino acid accession no. NP--604390.1), known for its effects on the female reproductive system, is also a potent vasodilator of the systemic and coronary circulation by a mechanism of action involving nitric oxide, and influences cardiac beating rate. Relaxin is also known as a cardiovascular drug that may prevent, treat, or ameliorate ischemic heart disease (acute and chronic myocardial infarction), cardiac fibrosis, and obliterative peripheral arterial disease and restore cardiac function in cell transplantation. (Nistri et al., Pharmacol. Res. 57(1):43 (2008); Samuel et al., Adv. Exp. Med. Biol. 612:88 (2007); Du X J., J. Cell Mol. Med. 11(5):1101 (2007); Formigli et al., J. Cell Mol. Med. 11(5):1087 (2007); Bathgate et al., Mol. Cell. Endocrinol. 280(1-2):30 (2008); Nistri et al., Cardiovasc. Hematol. Agents Med. Chem. 5(2):101 (2007); Moore et al., Endocrinology. 148(4):1582 (2007); Lekgabe et al., Endocrinology. 147(12):5575 (2006); Samuel et al., Pharmacol. Ther. 112(2):529 (2006); Zhang et al., Peptides. 26(9):1632 (2005); Perna et al., Ann. N.Y. Acad. Sci. 1041:431 (2005); Perna et al., FASEB J. 19(11):1525 (2005); Samuel et al., Endocrinology. 145(9):4125 (2004); Masini et al., Br J. Pharmacol. 137(3):337 (2002); Ndisang et al., Inflamm. Res. 50 Suppl. 2:S122-3 (2001); Dschietzig et al., FASEB. J. 15(12):2187 (2001); Bani et al., Am J. Pathol. 152(5):1367 (1998); and Masini et al., Inflamm. Res. 45 Suppl 1:S27 (1996))
[0351]In certain embodiments, therapeutic proteins of the invention useful for the treatment, amelioration, or prevention of cardiovascular diseases have multiple therapeutic benefits. For example, in the early phase after myocardial infarction, elevated (SDF-1, human nucleotide sequence accession No.: U16752, human amino acid sequence accession No.: NP--954637) levels have been reported in the infarct zone. This provides the required stimulus for mobilization of stem cells from BM niches to the damaged site as part of a natural repair process. SDF-1 recruits bone marrow haematopoietic stem cells (primarily CD31.sup.+, C-kit.sup.+ and CD34.sup.+ cells) to the infarcted heart resulting in both neoangiogenic and cardioprotective activities. Furthermore, SDF-1 activates the cell-survival factor protein kinase B (PKB/Akt) via the G protein-coupled receptor CXCR4 regenerative factors. See also U.S. Patent Appl. Publ. No. 20060111290 A1; Elmadbouh et al., J Mol Cell Cardiol. 42(4):792 (2007); Bonaros et al., Interact Cardiovasc Thorac Surg. 7(2):249 (2008); Zhang et al., J Mol Cell Cardiol. 44(2):281 (2008); Ma et al., Basic Res Cardiol. 100(3):217 (2005); and Zhang et al., Tissue Eng. 13(8):2063 (2007).
[0352]In addition, tPA (human amino acid accession no. 28274638), e.g., human tissue Plasminogen Activator or Retavase®, manufactured by PDL BioPharma, Inc. is known to prevent, treat, or ameliorate post cardiac transplant complications by inhibiting graft atherosclerosis (Scholl et al., J Heart Lung Transplant. 20(3):322 (2001); Dunn et al., Circulation. 93(7):1439 (1996) (Comment in: Circulation. 93(7):1319 (1996)); and Gong et al., Gene Ther. 14(21):1537 (2007)). Furthermore, the growth hormone is also known to prevent, treat, or ameliorate cardiovascular disease and may be used in the present invention (Isgaard J. and Bergh C. H., BioDrugs. 12(4):245 (1999); Fazio et al., J. Clin. Endocrinol. Metab. 92(11):4218 (2007); Climent et al., Curr Med. Chem. 14(13):1399 (2007); Perez-Berbel et al., Int J. Cardiol. 124(3):393 (2008) (Comment on: Int J. Cardiol. 110(3):313 (2006)); and Le Corvoisier et al., J Clin Endocrinol Metab. 92(1):180 (2007)) by promoting angiogenesis and attenuate apoptosis (Rong et al., Chin Med J (Engl). 121(4):347 (2008)).
[0353]In other embodiments, the therapeutic molecules that restore cardiac function are included in the present invention. Cardiac repair molecules include, but are not limited to, SERCA2a, AC6, S100A1, parvalbumin, phosphatase inhibitor 2 and phosphatase inhibitor 1. For example, SERCA2a is known to improve cardiac contractility in vivo and in vitro and cardiac function in heart failure (Asahi et al., Proc Natl Acad Sci USA. 101(25):9199 (2004); Cavagna et al., J Physiol. 528 Pt 1:53 (2000); Chaudhri et al., Mol Cell Biochem. 251(1-2):103 (2003); Davia et al., J Mol Cell Cardiol. 33(5):1005 (2001); del Monte et al., Circulation. 100(23):2308 (1999) (Comment in: Circulation. 100(23):2303 (1999); del Monte et al., Circulation. 104(12):1424 (2001); Hajjar et al., Circ Res. 81(2):145 (1997) (Comment in: Circ Res. 88(4):373 (2001) and Circulation. 101(7):790 (2000)); Kawase et al., J Am Coll Cardiol. 51(11):1112 (2008); Maier et al., Cardiovasc Res. 67(4):636 (2005) (Comment in: Cardiovasc Res. 67(4):581 (2005); Meyer M. and Dillmann W. H., Cardiovasc Res. 37(2):360 (1998); Miyamoto et al., Proc Natl Acad Sci USA. 97(2):793 (2000); Muller et al., Cardiovasc Res. 59(2):380 (2003); Sakata et al., J Mol Cell Cardiol. 42(4):852 (2007); Sakata et al., Am J Physiol Heart Circ Physiol. 292(2):H1204 (2007); Schmidt et al., Circulation. 101(7):790 (2000) (Comment in: Circulation. 101(7):738 (2000), Circ Res. 81(2):145 (1997), Circ Res. 83(9):889 (1998), and Circulation. 95(2):423 (1997)) Suarez et al., Am J Physiol Heart Circ Physiol. 287(5):H2164 (2004); Terracciano et al., Cell Calcium. 31(6):299 (2002); Trost et al., Diabetes. 51(4):1166 (2002); and Vetter et al., FASEB J. 16(12):1657 (2002))
[0354]Furthermore, AC6 is known to restore affinity of SERCA2a to calcium and maximum velocity of cardiac calcium uptake by sarcoplasmic reticulum in cardiomyopathy (Gao et al., Proc Natl Acad Sci US A. 95(3):1038 (1998); Roth et al., Circulation. 99(24):3099 (1999); Lai et al., Circulation. 102(19):2396 (2000); Roth et al., Circulation. 105(16):1989 (2002) (Comment in: Circulation. 105(16):1876 (2002)); Gao et al., Cardiovasc Res. 56(2):197 (2002) (Comment in: Cardiovasc Res. 56(2):181 (2002)); Roth et al., Basic Res Cardiol. 98(6):380 (2003); Roth et al., Am J Physiol Heart Circ Physiol. 287(1):H172 (2004); Gao et al., J Biol. Chem. 279(37):38797 (2004); Tang et al., Am J Physiol Heart Circ Physiol. 287(5):H1906 (2004); Lai et al., Circulation. 110(3):330 (2004) (Comment in: Circulation. 110(3):242 (2004); Roth et al., Hum Gene Ther. 15(10):989 (2004); Timofeyev et al., J Mol Cell Cardiol. 41(1):170 (2006) (Comment in: J Mol Cell Cardiol. 41(3):424 (2006); Takahashi et al., Circulation. 114(5):388 (2006) (Erratum in: Circulation. 114(11):e497 (2006); Comment in: Circulation. 114(5):365 (2006); Sastry et al., J Am Coll Cardiol. 48(3):559 (2006); Rebolledo et al., Hum Gene Ther. 17(10):1043 (2006); Hammond H. K., Ann N Y Acad Sci. 1080:426 (2006); Phan et al., Trends Cardiovasc Med. 17(7):215 (2007); Tang et al., Circulation. 117(1):61 (2008); and Lai et al., J Am Coll Cardiol. 51(15):1490 (2008)).
[0355]In certain embodiments, the Ca2+-binding protein S100A1 may restore cardiac function and therefore be used in the present invention. S100A1 is known to increase myocardial contraction in vivo and reduce propensity toward heart failure after myocardial infarction. (Most et al., J Clin Invest. 114(11):1550 (2004); Most et al., Circulation. 114(12):1258 (2006); Pleger et al., Mol. Ther. 12(6):1120 (2005); Pleger et al., Eur J Med. Res. 11(10):418 (2006); Remppis et al., J Gene Med. 6(4):387 (2004); Most et al., Am J Physiol Regul Integr Comp Physiol. 293(2):R568 (2007); Remppis et al., Basic Res Cardiol. 97 Suppl 1:156 (2002); Pleger et al., Circulation. 115(19):2506 (2007); and Most et al., J Biol. Chem. 278(36):33809 (2003)). Other non-limiting examples of the therapeutic molecules that improve or restore cardiac function are: paralbumin (Hirsch et al., Am J Physiol Heart Circ Physiol. 286(6):H2314 (2004); Michele et al., Mol. Ther. 10(2):399 (2004); and Sakata et al., J Mol Cell Cardiol. 42(4):852 (2007)), phosphatase inhibitor 2 (Yamada et al., FASEB J. 20(8):1197 (2006); Gupta et al., Mol Cell Biochem. 269(1-2):49 (2005); and Kirchhefer et al., Cardiovasc Res. 68(1):98 (2005)) and phosphatase inhibitor 1 (Gupta et al., Mol Cell Biochem. 269(1-2):49 (2005) and Gupta et al., Am J Physiol Heart Circ Physiol. 285(6):H2373 (2003))
[0356]Additional therapeutic molecules that may be useful for the present invention to prevent, treat, or ameliorate a disease or disorder include, but are not limited to, monoclonal antibodies (e.g., HERCEPTIN®-HC, HERCEPTIN®-LC, TICILIMUMAB®-HC, TICILIMUMAB®-LC, ZENAPAX®-HC, ZENAPAX®-LC, HUMIRA®-HC, HUMIRA®-LC, RITUXAN®-HC, RITUXAN®-LC, IPILIMUMAB®-HC, IPILIMUMAB®-LC, AVASTIN®-HC, AVASTIN®-LC, Erbitux®-HC, and ERBITUX®-LC), recombinant enzymes (e.g., RETAVASE®, ACTRAPID®-A chain, ACTRAPID®-B chain, NEULASTA®, pre-pro insulin, EPOGEN®, and NORDITROPIN®), fusion protein (e.g., ENBREL®), or any purified proteins (e.g., ALPHANATE® and ARALAST®). In addition, identification of suitable therapeutic molecules for preventing, treating, or ameliorating a particular disease or disorder is well within the capabilities of a person of ordinary in the art.
Vectors and Host Cells
[0357]To introduce the polynucleotides into the cells, a vector can be used. The vector may be, for example, a plasmid vector or a single- or double-stranded RNA or DNA viral vector. Such vectors may be introduced into cells by well-known techniques for introducing DNA and RNA into cells. Viral vectors may be replication competent or replication defective. In the latter case, viral propagation generally will occur only in complementing viral competent cells.
[0358]Thus, at a minimum, the vectors must include the polynucleotides of the invention. Other components of the vector may include, but are not limited to, selectable markers, chromatin modification domains, additional promoters driving expression of other polypeptides that may also be present on the vector (e.g., a lethal polypeptide), genomic integration sites, recombination sites, and molecular insertion pivots. The vectors may comprise any number of these additional elements, either within or not within the polynucleotides, such that the vector can be tailored to the specific goals of the therapeutic methods desired.
[0359]In one embodiment of the present invention, the vectors that are introduced into the cells further comprise a "selectable marker gene" which, when expressed, indicates that the therapeutic gene switch construct of the present invention has been integrated into the genome of the modified cell. In this manner, the selector gene can be a positive marker for the genome integration. While not critical to the methods of the present invention, the presence of a selectable marker gene allows the practitioner to select for a population of live cells where the vector construct has been integrated into the genome of the cells. Thus, certain embodiments of the present invention comprise selecting cells where the vector has successfully been integrated. As used herein, the term "select" or variations thereof, when used in conjunction with cells, is intended to mean standard, well-known methods for choosing cells with a specific genetic make-up or phenotype. Typical methods include, but are not limited to, culturing cells in the presence of antibiotics, such as G418, neomycin and ampicillin. Other examples of selectable marker genes include, but are not limited to, genes that confer resistance to dihydrofolate reductase, hygromycin, or mycophenolic acid. Other methods of selection include, but are not limited to, a selectable marker gene that allows for the use of thymidine kinase, hypoxanthine-guanine phosphoribosyltransferase or adenine phosphoribosyltransferase as selection agents. Cells comprising a vector construct comprising an antibiotic resistance gene or genes would then be capable of tolerating the antibiotic in culture. Likewise, cells not comprising a vector construct comprising an antibiotic resistance gene or genes would not be capable of tolerating the antibiotic in culture.
[0360]As used herein, a "chromatin modification domain" (CMD) refers to nucleotide sequences that interact with a variety of proteins associated with maintaining and/or altering chromatin structure, such as, but not limited to, DNA insulators. See Ciavatta et al., Proc. Nat'l Acad. Sci. U.S.A., 103:9958 (2006), which is incorporated by reference herein. Examples of CMDs include, but are not limited to, the chicken β-globulin insulator and the chicken hypersensitive site 4 (cHS4). The use of different CMD sequences between one or more gene programs (i.e., a promoter, coding sequence, and 3' regulatory region), for example, can facilitate the use of the differential CMD DNA sequences as "mini homology arms" in combination with various microorganism or in vitro recombineering technologies to "swap" gene programs between existing multigenic and monogenic shuttle vectors. Other examples of chromatin modification domains are known in the art or can be readily identified.
[0361]Particular vectors for use with the present invention are expression vectors that code for polypeptides or polynucleotides. Generally, such vectors comprise cis-acting control regions effective for expression in a modified cell, operatively linked to the polynucleotide to be expressed. Appropriate trans-acting factors are supplied by the modified cell, supplied by a complementing vector or supplied by the vector itself upon introduction into the cell.
[0362]A great variety of expression vectors can be used to express polypeptides or polynucleotides. Such vectors include chromosomal, episomal and virus-derived vectors, e.g., vectors derived from bacterial plasmids, from bacteriophage, from yeast episomes, from yeast chromosomal elements, from viruses such as adeno-associated viruses, lentiviruses, baculoviruses, papova viruses, such as SV40, vaccinia viruses, adenoviruses, fowl pox viruses, pseudorabies viruses and retroviruses, and vectors derived from combinations thereof, such as those derived from plasmid and bacteriophage genetic elements, such as cosmids and phagemids. All may be used for expression in accordance with this aspect of the present invention. Generally, any vector suitable to maintain, propagate or express polynucleotides or polypeptides in a cell may be used for expression in this regard.
[0363]The polynucleotide sequence in the expression vector is operatively linked to appropriate expression control sequence(s) including, for instance, a promoter to direct mRNA transcription. Representatives of additional promoters include, but are not limited to, constitutive promoters and tissue specific or inducible promoters. Examples of constitutive eukaryotic promoters include, but are not limited to, the promoter of the mouse metallothionein I gene (Hamer et al., J. Mol. Appl. Gen. 1:273 (1982)); the TK promoter of Herpes virus (McKnight, Cell 31:355 (1982)); the SV40 early promoter (Benoist et al., Nature 290:304 (1981)); and the vaccinia virus promoter. All of the above listed references are incorporated by reference herein. Additional examples of the promoters that could be used to drive expression of a protein or polynucleotide include, but are not limited to, tissue-specific promoters and other endogenous promoters for specific proteins, such as the albumin promoter (hepatocytes), a proinsulin promoter (pancreatic beta cells) and the like. In general, expression constructs will contain sites for transcription, initiation and termination and, in the transcribed region, a ribosome binding site for translation. The coding portion of the mature transcripts expressed by the constructs may include a translation initiating AUG at the beginning and a termination codon (UAA, UGA or UAG) appropriately positioned at the end of the polypeptide to be translated.
[0364]In addition, the constructs may contain control regions that regulate, as well as engender expression. Generally, such regions will operate by controlling transcription, such as repressor binding sites and enhancers, among others.
[0365]Examples of eukaryotic vectors include, but are not limited to, pW-LNEO, pSV2CAT, pOG44, pXT1 and pSG available from Stratagene; pSVK3, pBPV, pMSG and pSVL available from Amersham Pharmacia Biotech; and pCMVDsRed2-express, pIRES2-DsRed2, pDsRed2-Mito, and pCMV-EGFP available from Clontech. Many other vectors are well-known and commercially available.
[0366]Particularly useful vectors, which comprise molecular insertion pivots for rapid insertion and removal of elements of gene programs, are described in United States Published Patent Application No. 2004/0185556, U.S. patent application Ser. No. 11/233,246 and International Published Application Nos. WO 2005/040336 and WO 2005/116231, all of which are incorporated by reference. An example of such vectors is the UltraVector® Production System (Intrexon Corp., Blacksburg, Va.), as described in WO 2007/038276, incorporated herein by reference. As used herein, a "gene program" is a combination of genetic elements comprising a promoter (P), an expression sequence (E) and a 3' regulatory sequence (3), such that "PE3" is a gene program. The elements within the gene program can be easily swapped between molecular pivots that flank each of the elements of the gene program. A molecular pivot, as used herein, is defined as a polynucleotide comprising at least two non-variable rare or uncommon restriction sites arranged in a linear fashion. In one embodiment, the molecular pivot comprises at least three non-variable rare or uncommon restriction sites arranged in a linear fashion. Typically any one molecular pivot would not include a rare or uncommon restriction site of any other molecular pivot within the same gene program. Cognate sequences of greater than 6 nucleotides upon which a given restriction enzyme acts are referred to as "rare" restriction sites. There are, however, restriction sites of 6 bp that occur more infrequently than would be statistically predicted, and these sites and the endonucleases that cleave them are referred to as "uncommon" restriction sites. Examples of either rare or uncommon restriction enzymes include, but are not limited to, AsiS I, Pac I, Sbf I, Fse I, Asc I, Mlu I, SnaB I, Not I, Sal I, Swa I, Rsr II, BSiW I, Sfo I, Sgr AI, AflII, Pvu I, Ngo MIV, Ase I, Flp I, Pme I, Sda I, Sgf I, Srf I, and Sse8781 I.
[0367]The vector may also comprise restriction sites for a second class of restriction enzymes called homing endonuclease (HE) enzymes. HE enzymes have large, asymmetric restriction sites (12-40 base pairs), and their restriction sites are infrequent in nature. For example, the HE known as I-Scel has an 18 bp restriction site (5'TAGGGATAACAGGGTAAT3' (SEQ ID NO:4)), predicted to occur only once in every 7×1010 base pairs of random sequence. This rate of occurrence is equivalent to only one site in a genome that is 20 times the size of a mammalian genome. The rare nature of HE sites greatly increases the likelihood that a genetic engineer can cut a gene program without disrupting the integrity of the gene program if HE sites were included in appropriate locations in a cloning vector plasmid.
[0368]Selection of appropriate vectors and promoters for expression in a host cell is a well-known procedure, and the requisite techniques for vector construction and introduction into the host cell, as well as its expression in the host cell are routine skills in the art.
[0369]The introduction of the polynucleotides into the cells can be a transient transfection, stable transfection, or can be a locus-specific insertion of the vector.
[0370]Transient and stable transfection of the vectors into the host cell can be effected by calcium phosphate transfection, DEAE-dextran mediated transfection, cationic lipid-mediated transfection, electroporation, transduction, infection, or other methods. Such methods are described in many standard laboratory manuals, such as Davis et al., Basic Methods in Molecular Biology (1986); Keown et al., 1990, Methods Enzymol. 185: 527-37; Sambrook et al., 2001, Molecular Cloning, A Laboratory Manual, Third Edition, Cold Spring Harbor Laboratory Press, N.Y., which are hereby incorporated by reference. These stable transfection methods result in random insertion of the vector into the genome of the cell. Further, the copy number and orientation of the vectors are also, generally speaking, random.
[0371]In another embodiment, locus-specific insertion may be carried out by recombinase-site specific gene insertion. Briefly, bacterial recombinase enzymes, such as, but not limited to, PhiC31 integrase can act on "pseudo" recombination sites within the human genome. These pseudo recombination sites can be targets for locus-specific insertion using the recombinases. Recombinase-site specific gene insertion is described in Thyagarajan et al., Mol. Cell. Biol. 21:3926 (2001), which is hereby incorporated by reference. Other examples of recombinases and their respective sites that may be used for recombinase-site specific gene insertion include, but are not limited to, serine recombinases such as R4 and TP901-1 and recombinases described in WO 2006/083253, incorporated herein by reference.
[0372]In order to stably integrate the one or more gene expression systems in the genome of a modified cell, any known methods of integration may be used for the purpose of this invention. In one embodiment, locus-specific insertion may be carried out by recombinase-site specific gene insertion. Briefly, bacterial recombinase enzymes, such as, but not limited to, PhiC31 integrase may act on "pseudo" recombination sites within the human genome. See US publication No. 2004/0003420 A1; Groth et al., Proc. Natl. Acad. Science, 97, 5995-6000 (2000). These pseudo recombination sites may be targets for locus-specific insertion using the recombinases. Recombinase-site specific gene insertion is described in Thyagarajan, B. et al., Mol. Cell. Biol. 21(12):3926-34 (2001).
[0373]In certain embodiments, the first inducible gene expression system further comprises an integrase, which will stably integrate the first gene switch system into pseudo-sites within the genome of the targeted cells. A second gene switch system may also comprise an integrase, which will integrate the second gene switch system into the pseudo-sites within the genome of the targeted cells. The first gene switch system may further comprise an integrase acceptor site, which may allow integration of the second inducible gene switch system in the pre-positioned acceptor site within the genome of the targeted cells.
[0374]The following polypeptide sequence was reported as a polypeptide sequence encoding the Streptomyces phase PhiC31 integrase polypeptide sequence and has the accession number NP--047974 in Genbank.
TABLE-US-00005 Streptomyces phage PhiC31 integrase (605 aa) (SEQ ID NO: 6) 1 mdtyagaydr qsrerenssa aspatqrsan edkaadlqre verdggrfrf vghfseapgt 61 safgtaerpe ferilnecra grlnmiivyd vsrfsrlkvm daipivsell algvtivstq 121 egvfrqgnvm dlihlimrld ashkesslks akildtknlq relggyvggk apygfelvse 181 tkeitrngrm vnvvinklah sttpltgpfe fepdvirwww reikthkhlp fkpgsqaaih 241 pgsitglckr mdadavptrg etigkktass awdpatvmri lrdpriagfa aeviykkkpd 301 gtpttkiegy riqrdpitlr pveldcgpii epaewyelqa wldgrgrgkg lsrgqailsa 361 mdklycecga vmtskrgees ikdsyrcrrr kvvdpsapgq hegtcnvsma aldkfvaeri 421 fnkirhaegd eetlallwea arrfgkltea peksgeranl vaeradalna leelyedraa 481 gaydgpvgrk hfrkqqaalt lrqqgaeerl aeleaaeapk lpldqwfped adadptgpks 541 wwgrasvddk rvfvglfvdk ivvtksttgr gqgtpiekra sitwakpptd ddeddaqdgt 601 edvaa
[0375]Other examples of recombinases and their respective sites that may be used for recombinase-site specific gene insertion include, but are not limited to, serine recombinases such as R4 and TP901-1. Site-specific recombinases (SSRs), such as the bacteriophage P1-derived Cre recombinase recognize specific DNA sequences ("recognition sites," "recognition sequences," or "integrase acceptor site") and catalyze recombination between two recognition sites. Cre recombinase, for example, recognizes the 34 base pair (bp) loxP motif (Austin et al., Cell 25, 729-736 (1981)). If the two sites are located on the same DNA molecule in the same orientation, the intervening DNA sequence is excised by the recombinase from the parental molecule as a closed circle, leaving one recognition site on each of the reaction products. If the two sites are in inverted orientation, the recognition-site flanked region is inverted through recombinase mediated recombination. Alternatively, if the two recognition sites are located on different molecules, recombinase-mediated recombination will lead to integration of a circular molecule or translocation between two linear molecules.
[0376]In addition to Cre, a few recombinases have been shown to exhibit some activity in mammalian cells. The best characterized examples are the yeast derived FLP and Kw recombinases, which exhibit optimal activity at 30° C. and are unstable at 37° C. (Buchholz et al., Nature Biotech., 16, 657-662 (1998); Ringrose et al., Eur. J. Biochem., 248, 903-912). Other recombinases that show some activity in mammalian cells include a mutant integrase of phage lambda, the integrases of phage HK022, mutant gamma delta-resolvase and beta-recombinase (Lorbach et al., J. Mol. Biol., 296, 1175-81 (2000); Kolot et al., Mol. Biol. Rep. 26, 207-213 (1999); Schwikardi et al., FEBS Lett., 471, 147-150 (2000); Diaz et al., J. Biol. Chem., 274, 6634-6640 (1999)). Moreover, an improved version of the phiC31 integrase has been developed. This modified C31-Int (C31-Int (CNLS)) carries a C-terminal nuclear localization signal (NLS) and displays a recombination efficiency in mammalian cells that is significantly enhanced over the wild type form and is comparable to that of Cre recombinase (EP 1205490; US Publication No. 2004/0003420 A1). This makes the C31-Int a valuable tool for mammalian genome modification.
[0377]In one embodiment of the invention, the vector is inserted into a bio-neutral site in the genome. A bio-neutral site is a site in the genome where insertion of the polynucleotides interferes very little, if any, with the normal function of the cell. Bio-neutral sites may be analyzed using available bioinformatics. Many bio-neutral sites are known in the art, e.g., the ROSA-equivalent locus. Other bio-neutral sites may be identified using routine techniques well known in the art. Characterization of the genomic insertion site(s) is performed using methods known in the art. To control the location, copy number and/or orientation of the polynucleotides when introducing the vector into the cells, methods of locus-specific insertion may be used. Methods of locus-specific insertion are well-known in the art and include, but are not limited to, homologous recombination and recombinase-mediated genome insertion. Of course, if locus-specific insertion methods are to be used in the methods of the present invention, the vectors may comprise elements that aid in this locus-specific insertion, such as, but not limited to, homologous recombination. For example, the vectors may comprise one, two, three, four or more genomic integration sites (GISs). As used herein, a "genomic integration site" is defined as a portion of the vector sequence which nucleotide sequence is identical or nearly identical to portions of the genome within the cells that allows for insertion of the vector in the genome. In particular, the vector may comprise two genomic insertion sites that flank at least the polynucleotides. Of course, the GISs may flank additional elements, or even all elements present on the vector.
[0378]In a further embodiment, the vector may comprise a chemo-resistance gene, e.g., the multidrug resistance gene mdr1, dihydrofolate reductase, or O6-alkylguanine-DNA alkyltransferase. The chemo-resistance gene may be under the control of a constitutive (e.g., CMV) or inducible (e.g., RheoSwitch®) promoter. In this embodiment, if it is desired to treat a disease in a subject while maintaining the modified cells within the subject, a clinician may apply a chemotherapeutic agent to destroy diseased cells while the modified cells would be protected from the agent due to expression of a suitable chemo-resistance gene and may continue to be used for treatment, amelioration, or prevention of a disease, disorder, or condition. By placing the chemo-resistance gene under an inducible promoter, the unnecessary expression of the chemo-resistance gene can be avoided, yet it will still be available in case continued treatment is needed. If the modified cells themselves become diseased, they could still be destroyed by inducing expression of a lethal polypeptide as described below.
[0379]The methods of the invention are carried out by introducing the polynucleotides encoding the gene switch and the therapeutic polypeptide or therapeutic polynucleotide into cells of a subject. Any method known for introducing a polynucleotide into a cell known in the art, such as those described above, can be used.
[0380]When the polynucleotides are to be introduced into cells ex vivo, the cells may be obtained from a subject by any technique known in the art, including, but not limited to, biopsies, scrapings, and surgical tissue removal. The isolated cells may be cultured for a sufficient amount of time to allow the polynucleotides to be introduced into the cells, e.g., 2, 4, 6, 8, 10, 12, 18, 24, 36, 48, hours or more. Methods for culturing primary cells for short periods of time are well known in the art. For example, cells may be cultured in plates (e.g., in microwell plates) either attached or in suspension.
[0381]For ex vivo therapeutic methods, cells are isolated from a subject and cultured under conditions suitable for introducing the polynucleotides into the cells. Once the polynucleotides have been introduced into the cells, the cells are incubated for a sufficient period of time to allow the ligand-dependent transcription factor complex to be expressed, e.g., 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 18, or 24 hours or more. At some point after the introduction of the polynucleotides into the cells (either before or after significant levels of the ligand-dependent transcription factor complex is expressed), the cells are introduced back into the subject. Reintroduction may be carried out by any method known in the art, e.g., intravenous infusion or direct injection into a tissue or cavity. In one embodiment, the presence of the polynucleotides in the cells is determined prior to introducing the cells back into the subject. In another embodiment, cells containing the polynucleotides are selected (e.g., based on the presence of a selectable marker in the polynucleotides) and only those cells containing the polynucleotides are reintroduced into the subject. After the cells are reintroduced to the subject, ligand is administered to the subject to induce expression of the therapeutic polypeptide or therapeutic polynucleotide. In an alternative embodiment, the ligand may be added to the cells even before the cells are reintroduced to the subject such that the therapeutic polypeptide or therapeutic polynucleotide is expressed prior to reintroduction of the cells. The ligand may be administered by any suitable method, either systemically (e.g., orally, intravenously) or locally (e.g., intraperitoneally, intrathecally, intraventricularly, direct injection into the tissue or organ where the cells were reintroduced). The optimal timing of ligand administration can be determined for each type of cell and disease, disorder, or condition using only routine techniques.
[0382]In a different embodiment, the ex vivo therapeutic methods may be carried out using non-autologous cells, e.g., cells that are allogeneic or xenogeneic to the subject, instead of autologous cells from the subject. The polynucleotides may be introduced into the non-autologous cells ex vivo to produce modified cells and the modified cells may then be introduced into the subject. The non-autologous cells may be any cells that are viable after transplantation into a subject, including, without limitation, stem cells (such as embryonic stem cells or hematopoietic stem cells) and fibroblasts.
[0383]The in vivo therapeutic methods of the invention involve direct in vivo introduction of the polynucleotides into the cells of the subject. The polynucleotides may be introduced into the subject systemically or locally (e.g., at the site of the disease, disorder, or condition). Once the polynucleotides have been introduced to the subject, the ligand may be administered to induce expression of the therapeutic polypeptide or therapeutic polynucleotide. The ligand may be administered by any suitable method, either systemically (e.g., orally, intravenously) or locally (e.g., intraperitoneally, intrathecally, intraventricularly, direct injection into the tissue or organ where the disease, disorder, or condition is occurring). The optimal timing of ligand administration can be determined for each type of cell and disease, disorder, or condition using only routine techniques.
[0384]In one embodiment, the ligand may be administered to the subject continuously or intermittently, and the pattern of ligand administration may be altered as necessary depending on the status of the disease, disorder, or condition. The level of expression of the therapeutic polypeptide or therapeutic polynucleotide can be modulated both by the schedule of ligand administration and the amount of ligand that is administered, permitting careful control of the therapeutic treatment.
[0385]The therapeutic methods of the invention may also be coupled with diagnostic technologies in order to improve treatment outcomes in various approaches that are known in the art as pharmacodiagnostics or theranostics. For example, administration of the ligand may be coordinated with monitoring of the status or progression of the disease, disorder, or condition. In one embodiment, the polynucleotides of the invention are introduced into a cell together with one or more polynucleotides designed to diagnose or monitor a disease, disorder, or condition. In another embodiment, the diagnostic polynucleotides are present on the same vector comprising the polynucleotides of the invention. In this embodiment, the therapeutic treatment and the diagnostic test for monitoring effectiveness of the treatment are administered together in one unit, ensuring that all cells that receive the treatment also receive the diagnostic test. In one embodiment, the diagnostic polynucleotides comprise a diagnostic switch promoter (i.e., a promoter whose activity is modulated during a disease, disorder, or condition) operably linked to a reporter gene, and monitoring of the status of the disease, disorder, or condition involves detecting the level of expression of the reporter gene.
[0386]In another theranostic embodiment of the invention, the level of expression of a therapeutic polypeptide or therapeutic polynucleotide is monitored through detecting the level of expression of a reporter gene, wherein the level of expression of the reporter directly correlates with the level of expression of the therapeutic polypeptide or therapeutic polynucleotide. For example, the level of expression of a therapeutic protein such as interleukin-12 may be monitored non-invasively in various tissues through a bioneutral reporter such as the human type 2 somatostatin receptor, which may be imaged with a radiolabeled somatostatin analog (see, e.g., Zinn et al., J. Nucl. Med. 41:887-895 (2000)). The reporter may be linked to the same promoter as the therapeutic polypeptide or polynucleotide, or may be placed under a different promoter that is modulated by the therapeutic polypeptide or polynucleotide.
[0387]An additional embodiment of the invention relates to methods for expressing a therapeutic polypeptide or therapeutic polynucleotide in a subject, comprising: [0388](a) introducing into cells of said subject (1) a polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex, operably linked to a therapeutic switch promoter, wherein the promoter is activated during said disease, disorder, or condition, and (2) a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide linked to a promoter which is activated by said ligand-dependent transcription factor complex, to produce modified cells; and [0389](b) administering ligand to said subject to induce expression of said therapeutic polypeptide or therapeutic polynucleotide. [0390]In one embodiment, the methods for expressing a therapeutic polypeptide or therapeutic polynucleotide in a subject may be carried out using laboratory animals (e.g., mice, rats, cats, dogs, monkeys) or farm animals (e.g., pigs, sheep, cows). For example, methods of expressing therapeutic products in animals may be carried out for research purposes or for the large scale production of therapeutic polypeptides or therapeutic polynucleotides.
[0391]A further embodiment of the invention relates to methods for expressing a therapeutic polypeptide or therapeutic polynucleotide in a cell, comprising: [0392](a) introducing into said cell (1) a polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex, operably linked to a therapeutic switch promoter, wherein the promoter is activated during said disease, disorder, or condition, and (2) a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide linked to a promoter which is activated by said ligand-dependent transcription factor complex, to produce a modified cell; and [0393](b) administering ligand to said modified cell to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
[0394]Another embodiment of the invention is a method for expressing a therapeutic polypeptide or therapeutic polynucleotide in one or more modified cells, comprising: [0395](a) introducing into a cell (1) a first polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex through operable association with a therapeutic switch promoter, wherein said therapeutic switch promoter is activated under conditions associated with a disease, disorder, or condition, and (2) a second polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide operably associated with a factor-regulated promoter which is activated by said ligand-dependent transcription factor complex, thereby producing a modified cell; and [0396](b) administering ligand to said modified cell to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
[0397]In one embodiment, the methods for expressing a therapeutic polypeptide or therapeutic polynucleotide in a cell may be carried out in vitro, e.g., in cells in culture. For example, in vitro methods of therapeutic product expression may be carried out for research use or for the large scale production of therapeutic polypeptides or therapeutic polynucleotides.
[0398]In any embodiments described herein, the polynucleotides or vector comprising the polynucleotides may comprise a sequence encoding a lethal polypeptide that can be turned on to express a product that will kill a cell containing the polynucleotides or vector. Lethal polypeptide expression can be used to eliminate the modified cells from a subject, either because treatment is no longer needed or because of a problem with the modified cells (e.g., hyperproliferation or toxicity). A lethal polypeptide, as used herein, is a polypeptide that, when expressed, is lethal to the cell that expresses the polypeptide, either because the polypeptide itself is lethal or the polypeptide produces a compound that is lethal. As used herein, a lethal polypeptide includes polypeptides that induce cell death in any fashion, including but not limited to, necrosis, apoptosis and cytotoxicity. Examples of lethal polypeptides include, but are not limited to, apoptosis inducing tumor suppressor genes such as, but not limited to, p53, Rb and BRCA-1, toxins such as diphtheria toxin (DTA), shigella neurotoxin, botulism toxin, tetanus toxin, cholera toxin, CSE-V2 and other variants of scorpion protein toxins to name a few, suicide genes such as cytosine deaminase and thymidine kinase, and cytotoxic genes, e.g., tumor necrosis factor, interferon-alpha. The present invention is not limited by the identity of the lethal polypeptide, provided that the polypeptide is capable of being lethal to the cell in which it is expressed. If the modified cells are short-lived cells (e.g., cells with a limited lifespan (e.g., about 10 days or less, such as dendritic cells), it may not be necessary to include a lethal polypeptide in the polynucleotides or vector as the cells will be naturally removed over a short period of time.
[0399]For each of the methods described above, in one embodiment, the polynucleotide encoding the gene switch and the polynucleotide encoding the therapeutic polypeptide or therapeutic polynucleotide linked to a promoter are part of one larger polynucleotide, e.g., a vector. In another embodiment, the polynucleotide encoding the gene switch and the polynucleotide encoding the therapeutic polypeptide or therapeutic polynucleotide linked to a promoter are separate polynucleotides, which may be combined to form a "nucleic acid composition."
[0400]In one aspect, the invention relates to polynucleotides that may be used in the methods of the invention. In one embodiment, the polynucleotide encodes a gene switch, the gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor complex, operably linked to a therapeutic switch promoter, wherein the activity of the promoter is modulated during said disease, disorder, or condition. In another embodiment, the polynucleotide further encodes a therapeutic polypeptide or therapeutic polynucleotide linked to a factor-regulated promoter which is activated by said ligand-dependent transcription factor complex. In one embodiment, the gene switch is an EcR-based gene switch. In another embodiment, the gene switch comprises a first transcription factor sequence under the control of a first therapeutic switch promoter and a second transcription factor sequence under the control of a second therapeutic switch promoter, wherein the proteins encoded by said first transcription factor sequence and said second transcription factor sequence interact to form a protein complex which functions as a ligand-dependent transcription factor complex. In one embodiment, the first therapeutic switch promoter and the second therapeutic switch promoter are different. In another embodiment, the first therapeutic switch promoter and the second therapeutic switch promoter are the same. In another embodiment, the first transcription factor sequence encodes a protein comprising a heterodimer partner and a transactivation domain and the second transcription factor sequence encodes a protein comprising a DNA binding domain and a ligand-binding domain. In a further embodiment, the polynucleotide also encodes a lethal polypeptide operably linked to an inducible promoter.
[0401]Another aspect of the invention relates to vectors comprising any of the polynucleotides described above. In one embodiment, the vector is a plasmid vector or a viral vector.
[0402]In another aspect, the invention provides kits that may be used in conjunction with methods of the invention. Kits according to this aspect of the invention may comprise one or more containers, which may contain one or more components selected from the group consisting of one or more nucleic acid molecules, restriction enzymes and one or more cells comprising such nucleic acid molecules. Kits of the invention may further comprise one or more containers containing supporting cells suitable for supporting the cells of the invention in culture, one or more containers containing cell culture media suitable for culturing cells of the invention, one or more containers containing antibiotics suitable for use in culturing cells of the invention, one or more containers containing buffers, one or more containers containing transfection reagents, one or more containers containing substrates for enzymatic reactions, and/or one or more ligands for gene switch activation.
[0403]Kits of the invention may contain a wide variety of nucleic acid molecules that can be used with the invention. Examples of nucleic acid molecules that can be supplied in kits of the invention include those that contain promoters, sequences encoding gene switches, enhancers, repressors, selection markers, transcription signals, translation signals, primer hybridization sites (e.g., for sequencing or PCR), recombination sites, restriction sites and polylinkers, sites that suppress the termination of translation in the presence of a suppressor tRNA, suppressor tRNA coding sequences, sequences that encode domains and/or regions, origins of replication, telomeres, centromeres, and the like. In one embodiment, kits may comprise a polynucleotide comprising a gene switch without any therapeutic switch promoters, the polynucleotide being suitable for insertion of any therapeutic switch promoters of interest. Nucleic acid molecules of the invention may comprise any one or more of these features in addition to polynucleotides as described above.
[0404]Kits of the invention may comprise containers containing one or more recombination proteins. Suitable recombination proteins include, but are not limited to, Cre, Int, IHF, Xis, Flp, Fis, Hin, Gin, Cin, Tn3 resolvase, φC31, TndX, XerC, and XerD. Other suitable recombination sites and proteins are those associated with the GATEWAY® Cloning Technology available from Invitrogen Corp., Carlsbad, Calif., and described in the product literature of the GATEWAY® Cloning Technology (version E, Sep. 22, 2003), the entire disclosures of which are incorporated herein by reference.
[0405]Kits of the invention can also be supplied with primers. These primers will generally be designed to anneal to molecules having specific nucleotide sequences. For example, these primers can be designed for use in PCR to amplify a particular nucleic acid molecule. Sequencing primers may also be supplied with the kit.
[0406]One or more buffers (e.g., one, two, three, four, five, eight, ten, fifteen) may be supplied in kits of the invention. These buffers may be supplied at working concentrations or may be supplied in concentrated form and then diluted to the working concentrations. These buffers will often contain salt, metal ions, co-factors, metal ion chelating agents, etc. for the enhancement of activities or the stabilization of either the buffer itself or molecules in the buffer. Further, these buffers may be supplied in dried or aqueous forms. When buffers are supplied in a dried form, they will generally be dissolved in water prior to use.
[0407]Kits of the invention may contain virtually any combination of the components set out above or described elsewhere herein. As one skilled in the art would recognize, the components supplied with kits of the invention will vary with the intended use for the kits. Thus, kits may be designed to perform various functions set out in this application and the components of such kits will vary accordingly.
EXAMPLES
[0408]The examples which follow further illustrate the invention, but should not be construed to limit the scope of the invention in any way. The practice of the present invention, including the following examples will employ, unless otherwise indicated, conventional techniques of cell biology, cell culture, molecular biology, transgenic biology, microbiology, and recombinant DNA, which are within the skill of the art. Such techniques are explained fully in the literature. See, for example, Molecular Cloning: A Laboratory Manual (3-Volume Set), J. Sambrook, D. W. Russell, Cold Spring Harbor Laboratory Press (2001); Genes VIII, B. Lewin, Prentice Hall (2003); PCR Primer, C. W. Dieffenbach and G. S. Dveksler, CSHL Press (2003); DNA Cloning, D. N. Glover ed., Volumes I and II (1985); Oligonucleotide Synthesis: Methods and Applications (Methods in Molecular Biology), P. Herdewijn (Ed.), Humana Press (2004); Culture of Animal Cells: A Manual of Basic Technique, 4th edition, R. I. Freshney, Wiley-Liss (2000); Oligonucleotide Synthesis, M. J. Gait (Ed.), (1984); Mullis et. al U.S. Pat. No. 4,683,195; Nucleic Acid Hybridization, B. D. Hames & S. J. Higgins eds. (1984); Nucleic Acid Hybridization, M. L. M. Anderson, Springer (1999); Animal Cell Culture and Technology, 2nd edition, M. Butler, BIOS Scientific Publishers (2004); Immobilized Cells and Enzymes: A Practical Approach (Practical Approach Series), J. Woodward, IRL Press (1992); Transcription And Translation, B. D. Hames & S. J. Higgins (Eds.) (1984); Culture Of Animal Cells, R. I. Freshney, Alan R. Liss, Inc., (1987); A Practical Guide To Molecular Cloning, 3rd edition, B. Perbal, John Wiley & Sons Inc. (1988); Gene Transfer Vectors For Mammalian Cells, J. H. Miller and M. P. Calos eds., Cold Spring Harbor Laboratory (1987); Methods In Enzymology, Vols. 154 and 155, Wu et. al (Eds.); Immunochemical Methods In Cell And Molecular Biology, Mayer and Walker, (Eds.), Academic Press, London (1987); and in Ausubel et. al, Current Protocols in Molecular Biology, John Wiley and Sons, Baltimore, Md. (1989).
Example 1
[0409]This example describes a gene therapy vector useful for the treatment of ischemic heart disease through the promotion of angiogenesis. Insulin like growth factor 1 is a hormone that may be useful in the treatment of ischemic heart disease. (IGF-1, GenBank Accession No.: NP--001104753.1, SEQ ID NO:20). Use of IGF-1 in preclinical models is associated with improved cardiac function, anti-apoptosis, neo-vascularization and cardiac muscle regeneration (reviewed in Santini, M. P., et al. Novartis Found Symp. 274:228-38 (2006); discussion 239-43, 272-6; and Saetrum Opgaard, O., and Wang, P. H. Growth Horm IGF Res. 15:89-94 (2005)). For this purpose, an example of inducible IGF-1 expression, in response to ischemia and/or inflammation is given. An inducible expression system for the expression if IGF-1 upon administration of ligand, under hypoxic conditions which occur in ischemic tissue is shown in FIG. 5.
TABLE-US-00006 SEQ ID NO: 20: MGKISSLPTQLFKCCFCDFLKVKMHTMSSSHLFYLALCLLTFTSSATAGPETLCGAELVD ALQFVCGDRGFYFNKPTGYGSSSRRAPQTGIVDECCFRSCDLRRLEMYCAPLKPAKSAR SVRAQRHTDMPKTQKYQPPSTNKNTKSQRRKGSTFEERK
[0410]The complete nucleotide sequence of the construct shown in FIG. 5 is presented as SEQ ID NO:7. The nucleotide coordinates for salient elements of the construct are shown in Table 4.
TABLE-US-00007 TABLE 4 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 197 455 Neo reverse 795 462 1256 SV40 early promoter reverse 278 1446 1723 3'Reg(SV40pA) reverse 221 1830 2050 LTF[Gal4(DBD):EcR(LBD)] reverse 1467 2057 3523 TL (cMyc ires) reverse 408 3536 3943 CAP[VP16(AD):RXR(HP)] reverse 975 3950 4924 TSP-1 (Cardiac hypoxia-inducible) reverse 578 4958 5535 FRP[6xGalRE:Minimal Promoter] forward 189 5946 6134 Insulin like growth factor (IGF-1) forward 477 6348 6824 Coding Region 3'Reg(hGH PolyA) forward 627 6897 7523 Replication Origin reverse 589 7957 8545 AmpR reverse 858 8920 9777 bla Promoter reverse 39 9811 9849
[0411]The vector shown in FIG. 5 is modeled according to the gene switch system shown in FIG. 1. Under this system, both the CAP subunit, and the LTF subunit of the ligand-dependent transcription factor complex (LDTFC) are expressed through operable association with a single therapeutic switch promoter (TSP-1) via use of an internal ribosome entry site (IRES). The promoter utilized in this system is a UV-conformed synthetic hypoxia-inducible promoter.
[0412]The coding region for the therapeutic product, IGF-1, is operably associated with a factor-regulated promoter (FRP) which is activated upon contact with the LDTFC in the presence of ligand.
[0413]The construct shown in FIG. 5 is inserted into a suitable vector system, for example, a viral vector, for delivery to a subject in need of treatment for ischemic heart disease.
[0414]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of gene therapy vectors are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and the transcription factor may be expressed under the appropriate physiological conditions. The LTF encoded by the vector will be expressed under hypoxic conditions associated with, e.g., cardiac ischemia. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of IGF-1 under control of the factor-regulated promoter. IGF-1 expression in turn promotes targeted angiogenesis in the ischemic tissue.
Example 2
[0415]This example describes a bioreactor/cell therapy vector useful for the treatment of ischemic cardiovascular disease through the promotion of angiogenesis and cardioprotection. The vector, shown in FIG. 6, will confer expression of human basic fibroblast growth factor (bFGF, GenBank Accession No.: NP--001997, SEQ ID NO:21) upon administration of ligand, under hypoxic conditions which occur in ischemic tissue.
TABLE-US-00008 SEQ ID NO: 21: MVGVGGGDVEDVTPRPGGCQISGRGARGCNGIPGAAAWEAALPRRRPRRHPSVNPRSR AAGSPRTRGRRTEERPSGSRLGDRGRGRALPGGRLGGRGRGRAPERVGGRGRGRGTAA PRAAPAARGSRPGPAGTMAAGSITTLPALPEDGGSGAFPPGHFKDPKRLYCKNGGFFLRI HPDGRVDGVREKSDPHIKLQLQAEERGVVSIKGVCANRYLAMKEDGRLLASKCVTDEC FFFERLESNNYNTYRSRKYTSWYVALKRTGQYKLGSKTGPGQKAILFLPMSAKS
[0416]The complete nucleotide sequence of the construct shown in FIG. 6 is presented as SEQ ID NO:8. The nucleotide coordinates for salient elements of the construct are shown in Table 5.
TABLE-US-00009 TABLE 5 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 SV40 early promoter reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 TSP-1 (constitutive) reverse 571 3026 3596 3'Reg(SV40pA) reverse 221 3719 3939 LTF[Gal4(DBD):EcR(LBD)] reverse 1467 3946 5412 TSP-2(hypoxia-inducible) reverse 870 5446 6315 FRP[6x GalRE:Minimal forward 189 6648 6836 Promoter] TSPq(bFGF) forward 867 7050 7916 3'Reg(hGH PolyA) forward 627 7989 8615 Replication Origin reverse 589 9049 9637 AmpR reverse 858 9882 10739 bla Promoter reverse 39 10773 10811
[0417]The vector shown in FIG. 6 is modeled according to the gene switch system shown in FIG. 2. Under this system, the CAP subunit of the LDTFC is expressed through operable association with a first, constitutive therapeutic switch promoter, TSP-1, and the LTF subunit of the LDTFC is expressed through operable association with a second, inducible therapeutic switch promoter (TSP-2). The promoter used in this construct is the hypoxia-inducible control promoter-1.
[0418]The coding region for the therapeutic product, bFGF, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0419]The construct shown in FIG. 6 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for ischemic heart disease.
[0420]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of cell-based therapies are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and the LTF encoded by the vector will be expressed under hypoxic conditions associated with, e.g., cardiac ischemia. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of bFGF under control of the FRP. bFGF expression in turn promotes targeted angiogenesis and/or cardioprotection in the ischemic tissue.
Example 3
[0421]This example describes a bioreactor/cell therapy vector useful for the treatment of ischemic cardiovascular disease through the promotion of cardioprotection. The vector, shown in FIG. 7, will confer expression of human erythropoietin (EPO, GenBank Accession No.: CAA26095.1, SEQ ID NO:22) upon administration of ligand, under hypoxic conditions which occur in ischemic tissue. Erythropoietin has been shown to function in cardioprotection and anti-remodeling, in response to ischemia.
TABLE-US-00010 SEQ ID NO: 22: MGVHECPAWLWLLLSLLSLPLGLPVLGAPPRLICDSRVLQRYLLEAKEAENITTGCAEHC SLNENITVPDTKVNFYAWKRMEVGQQAVEVWQGLALLSEAVLRGQALLVNSSQPWEP LQLHVDKAVSGLRSLTTLLRALGAQKEAISPPDAASAAPLRTITADTFRKLFRVYSNFLR GKLKLYTGEACRTGDR
[0422]The complete nucleotide sequence of the construct shown in FIG. 7 is presented as SEQ ID NO:9. The nucleotide coordinates for salient elements of the construct are shown in Table 6.
TABLE-US-00011 TABLE 6 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 SV40 early promoter reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 TSP-1(constitutive) reverse 571 3026 3596 3'Reg(SV40pA) reverse 221 3719 3939 LTF[Gal4(DBD):EcR(LBD)] reverse 1467 3946 5412 TSP2(Hypoxia-inducible) reverse 870 5446 6315 FRP(6x GalRE:minimal forward 189 6648 6836 promoter) TSPQ(Epo) forward 582 7050 7631 3'Reg(hGH PolyA) forward 627 7704 8330 Replication Origin reverse 589 8764 9352 AmpR reverse 858 9597 10454 bla Promoter reverse 39 10488 10526
[0423]The vector shown in FIG. 7 is modeled according to the gene switch system shown in FIG. 2. Under this system, the CAP subunit of the LDTFC is expressed through operable association with a first, constitutive therapeutic switch promoter (TSP-1), and the LTF subunit of the LDTFC is expressed through operable association with a second, inducible therapeutic switch promoter (TSP-2). The inducible therapeutic switch promoter used in this vector is the hypoxia-inducible control promoter-1.
[0424]The coding region for the therapeutic product, EPO, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0425]The construct shown in FIG. 7 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for ischemic heart disease.
[0426]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of cell-based therapies are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and LTF encoded by the vector will be expressed under hypoxic conditions associated with, e.g., cardiac ischemia. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of EPO under control of the FRP. EPO expression in turn promotes targeted cardioprotection in the ischemic tissue.
Example 4
[0427]This example describes a bioreactor/cell therapy vector useful for the treatment of ischemic cardiovascular disease through the promotion of antiogenesis and hemodynamics. The vector, shown in FIG. 8, will confer expression of human brain natriuretic factor (BNP, GenBank Accession No.: NP--002512, SEQ ID NO:23) upon administration of ligand, under hypoxic conditions which occur in ischemic tissue. BNP, as well as other natriuretic peptides, such as relaxin, ANF, CNP and adrenomodulin, has been shown to function in cardioprotection, vasodilation and anti-remodeling, in the heart. For this purpose, an example of inducible expression of BNP, in response to ischemia is given.
TABLE-US-00012 SEQ ID NO: 23: MDPQTAPSRALLLLLFLHLAFLGGRSHPLGSPGSASDLETSGLQEQRNHLQGKLSELQVE QTSLEPLQESPRPTGVWKSREVATEGIRGHRKMVLYTLRAPRSPKMVQGSGCFGRKMD RISSSSGLGCKVLRRH
[0428]The complete nucleotide sequence of the construct shown in FIG. 8 is presented as SEQ ID NO:10. The nucleotide coordinates for salient elements of the construct are shown in Table 7.
TABLE-US-00013 TABLE 7 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 SV40 early promoter reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 TSP-1(constitutive) reverse 571 3026 3596 3'Reg(SV40pA) reverse 221 3719 3939 LTF[GAL4(DBD):EcR(LBD)] reverse 1467 3946 5412 TSP-2(Hypoxia-inducible) reverse 870 5446 6315 FRP(6x GalRE:Minimal forward 189 6648 6836 Promoter) TSPQ(BNP) forward 405 7050 7454 3'Reg(hGH PolyA) forward 627 7527 8153 Replication Origin reverse 589 8587 9175 AmpR reverse 858 9420 10277 bla Promoter reverse 39 10311 10349
[0429]The vector shown in FIG. 8 is modeled according to the gene switch system shown in FIG. 2. Under this system, the CAP subunit of the LDTFC is expressed through operable association with a first, constitutive therapeutic switch promoter (TSP-1), and the LTF subunit of the LDTFC is expressed through operable association with a second, inducible therapeutic switch promoter (TSP-2). The inducible TSP-2 used in this vector is the hypoxia-inducible control promoter-1.
[0430]The coding region for the therapeutic product, BNP, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0431]The construct shown in FIG. 8 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for ischemic heart disease.
[0432]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of cell-based therapies are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and the LTF encoded by the vector will be expressed under hypoxic conditions associated with, e.g., cardiac ischemia. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of BNP under control of the FRP. BNP expression in turn promotes targeted cardioprotection, vasodilation and anti-remodeling in the ischemic tissue.
Example 5
[0433]This example describes a bioreactor/cell therapy vector useful for the treatment of ischemic cardiovascular disease through the breakdown of fibrin deposition in the heart. The vector, shown in FIG. 9, will confer expression of human tissue plasminogen activator (tPA, GenBank Accession No.: AAO34406, SEQ ID NO:24) upon administration of ligand, under inflammatory conditions which occur in ischemic tissue. Tissue plasminogen activator is a serine protease that catalyzes the conversion of plasminogen to the activated enzyme plasmin, that degrades fibrin. The use of recombinant tPA has been proven effective as a thrombolytic, for the breakdown of fibrin clots, in diseases such as pulmonary embolism, myocardial infarction and stroke. In addition to clot formation, excess fibrin deposition in the heart and vasculature is associated with insulin resistant diabetes, atherosclerosis and myocardial infarction in response to inflammation. For this purpose, an example of inducible expression of tPA, in response to ischemia is given.
TABLE-US-00014 SEQ ID NO: 24: MDAMKRGLCCVLLLCGAVFVSPSQEIHARFRRGARSYQVICRDEKTQMIYQQHQSWLR PVLRSNRVEYCWCNSGRAQCHSVPVKSCSEPRCFNGGTCQQALYFSDFVCQCPEGFAG KCCEIDTRATCYEDQGISYRGTWSTAESGAECTNWNSSALAQKPYSGRRPDAIRLGLGN HNYCRNPDRDSKPWCYVFKAGKYSSEFCSTPACSEGNSDCYFGNGSAYRGTHSLTESGA SCLPWNSMILIGNVYTAQNPSAQALGLGKHNYCRNPDGDAKPWCHVLKNRRLTWEYC DVPSCSTCGLRQYSQPQFRIKGGLFADIASHPWQAAIFAKHRRSPGERFLCGGILISSCWIL SAAHCFQERFPPHHLTVILGRTYRVVPGEEEQKFEVEKYIVHKEFDDDTYDNDIALLQLK SDSSRCAQESSVVRTVCLPPADLQLPDWTECELSGYGKHEALSPFYSERLKEAHVRLYPS SRCTSQHLLNRTVTDNMLCAGDTRSGGPQANLHDACQGDSGGPLVCLNDGRMTLVGII SWGLGCGQKDVPGVYTKVTNYLDWIRDNMRP
[0434]The complete nucleotide sequence of the construct shown in FIG. 9 is presented as SEQ ID NO:11. The nucleotide coordinates for salient elements of the construct are shown in Table 8.
TABLE-US-00015 TABLE 8 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 SV40 early promoter reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 TSP-1 (constitutive) reverse 571 3026 3596 3'Reg(SV40pA) reverse 221 3719 3939 LTF[GAL4(DBD):EcR(LBD)] reverse 1467 3946 5412 TSP-2 (hypoxia inducible) reverse 770 5446 6215 FRP[6xGalRE:Minimal forward 189 6548 6736 Promoter] TPSQ (Reteplase, tPA) forward 1689 6950 8638 3'Reg(hGH PolyA) forward 627 8711 9337 Replication Origin reverse 589 9771 10359 AmpR reverse 858 10604 11461 bla Promoter reverse 39 11495 11533
[0435]The vector shown in FIG. 9 is modeled according to the gene switch system shown in FIG. 2. Under this system, the CAP subunit of the LDTFC is expressed through operable association with a first, constitutive therapeutic switch promoter (TSP-1), and the LTF subunit of the LDTFC is expressed through operable association with a second, inducible therapeutic switch promoter (TSP-2). The inducible therapeutic switch promoter used in this vector is the human Plexin D1 promoter.
[0436]The coding region for the therapeutic product, tPA, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0437]The construct shown in FIG. 9 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for ischemic heart disease.
[0438]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of cell-based therapies are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and the LTF encoded by the vector will be expressed in the event of an inflammatory response associated with, e.g., cardiac ischemia. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of tPA under control of the FRP. tPA expression in turn promotes targeted break-up of fibrin deposition in the ischemic tissue.
Example 6
[0439]This example describes a bioreactor/cell therapy vector useful for the treatment of ischemic cardiovascular disease through the promotion of cardioprotection, antiogenesis and hemodynamics. The vector, shown in FIG. 10, will confer expression of two therapeutic polypeptides, human relaxin (GenBank Accession No.: NP--604390.1, SEQ ID NO:25) and human hepatocyte growth factor (HGF, GenBank Accession No.: NP--000592.3, SEQ ID NO:26) upon administration of ligand, under inflammatory conditions and/or hypoxia, respectively, both of which occur in ischemic tissue. Relaxin is a potent vasodilator of the systemic and coronary circulation by a mechanism of action involving nitric oxide, and influences cardiac beating rate. HGF provides a pro-angiogenic effect, a cardioprotective anti-apoptotic effect, an anti-fibrotic effect, and is a type I collagen regenerative factor in ischemic myocardium. For this purpose, an example of separately controlled inducible expression of relaxin and HGF in response to ischemia is given.
TABLE-US-00016 SEQ ID NO: 25: MPRLFFFHLLGVCLLLNQFSRAVADSWMEEVIKLCGRELVRAQIAICGMSTWSKRSLSQ EDAPQTPRPVAEIVPSFINKDTETINMMSEFVANLPQELKLTLSEMQPALPQLQQHVPVL KDSSLLFEEFKKLIRNRQSEAADSSPSELKYLGLDTHSRKKRQLYSALANKCCHVGCTKR SLARFC SEQ ID NO: 26: MWVTKLLPALLLQHVLLHLLLLPIAIPYAEGQRKRRNTIHEFKKSAKTTLIKIDPALKIKT KKVNTADQCANRCTRNKGLPFTCKAFVFDKARKQCLWFPFNSMSSGVKKEFGHEFDLY ENKDYIRNCIIGKGRSYKGTVSITKSGIKCQPWSSMIPHEHSFLPSSYRGKDLQENYCRNP RGEEGGPWCFTSNPEVRYEVCDIPQCSEVECMTCNGESYRGLMDHTESGKICQRWDHQ TPHRHKFLPERYPDKGFDDNYCRNPDGQPRPWCYTLDPHTRWEYCAIKTCADNTMNDT DVPLETTECIQGQGEGYRGTVNTIWNGIPCQRWDSQYPHEHDMTPENFKCKDLRENYC RNPDGSESPWCFTTDPNIRVGYCSQIPNCDMSHGQDCYRGNGKNYMGNLSQTRSGLTCS MWDKNMEDLHRHIFWEPDASKLNENYCRNPDDDAHGPWCYTGNPLIPWDYCPISRCE GDTTPTIVNLDHPVISCAKTKQLRVVNGIPTRTNIGWMVSLRYRNKHICGGSLIKESWVL TARQCFPSRDLKDYEAWLGIHDVHGRGDEKCKQVLNVSQLVYGPEGSDLVLMKLARP AVLDDFVSTIDLPNYGCTIPEKTSCSVYGWGYTGLINYDGLLRVAHLYIMGNEKCSQHH RGKVTLNESEICAGAEKIGSGPCEGDYGGPLVCEQHKMRMVLGVIVPGRGCAIPNRPGIF VRVAYYAKWIHKIILTYKVPQS
[0440]The complete nucleotide sequence of the construct shown in FIG. 10 is presented as SEQ ID NO:13. The nucleotide coordinates for salient elements of the construct are shown in Table 9.
TABLE-US-00017 TABLE 9 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 197 455 NEO reverse 804 462 1265 SV40e Promoter reverse 280 1385 1664 3'Reg(Synthetic PolyA) reverse 49 1782 1830 CAP[VP16(AD):RxR(HP)] reverse 975 1837 2811 TSP-1(ubiquitous) reverse 571 2845 3415 3'Reg(SV40pA) reverse 221 3538 3758 LTF-1[EcR(LBD):Gal4(DBD)] reverse 1467 3765 5231 TSP-2(inflammatory inducible) reverse 770 5265 6034 FRP-1[6x GalRE:Minimal forward 189 6405 6593 Promoter] TPSQ-1(Relaxin) forward 558 6807 7364 3'Reg(hGH PolyA) forward 627 7437 8063 FRP-2[8X LexA RE:Minimal forward 216 8437 8652 Promoter] TPSQ-2(HGF) forward 2187 8866 11052 3'Reg(SV40 early pA) forward 135 11112 11246 TSP-3(Hypoxia-inducible) forward 870 11478 12347 LTF-2[LexA(DBD) forward 1629 12381 14009 CfEcR-DEF(LBD)] EcR-LBD (DEF) forward 1014 12996 14009 3'Reg(Human S100 CABP) forward 765 14016 14780 Replication Origin reverse 589 15114 15702 AmpR reverse 858 16038 16895 bla Promoter reverse 39 16929 16967
[0441]The vector shown in FIG. 10 is modeled according to the gene switch system shown in FIG. 3. Under this system, the CAP subunit of the LDTFC is expressed through operable association with a first, constitutive therapeutic switch promoter (TSP-1), a first LTF (LTF-1) subunit is expressed through operable association with a second, inducible therapeutic switch promoter (TSP-2), and a second LTF (LTF-2) subunit is expressed through operable association with a third, inducible therapeutic switch promoter (TSP-3). TSP-2 in this vector is the human Plexin D1 promoter and TSP-3 in this vector is the hypoxia-inducible control promoter 1.
[0442]The coding region for the first therapeutic product, relaxin, is operably associated with a first FRP (FRP-1) having response elements which recognize the first DNA binding domain DBD-A of LTF-1, and the coding region for the second therapeutic product, HGF, is operably associated with a second FRP (FRP-2) having response elements which recognize the second DNA binding domain (DBD-B) of LTF-2. Both FRPs are activated upon contact with the respective LDTFC in the presence of ligand.
[0443]The construct shown in FIG. 10 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for ischemic heart disease.
[0444]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of cell-based therapies are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and the LTF-1 and/or LTF-2 will be expressed in the event of an inflammatory response and/or hypoxia associated with, e.g., cardiac ischemia. One or more ligands will be administered to the subject to be treated which will combine with the expressed LDTFC(s) to drive expression of relaxin and/or HGF under control of FRPs.
Example 7
[0445]This example describes a bioreactor/cell therapy vector useful for the treatment of ischemic cardiovascular disease through the promotion of cardiac repair and cardioprotection. The vector, shown in FIG. 11, will confer expression of human EPO (see Example 3) upon administration of ligand, under hypoxic conditions which occur in ischemic tissue. EPO has been shown to function in cardioprotection and anti-remodeling, in response to ischemia. In this example, EPO expression is specifically limited to cardiac tissue.
[0446]The vector shown in FIG. 11 is modeled according to the gene switch system shown in FIG. 2. Under this system, the CAP subunit is expressed through operable association with a promoter which is specific for cardiac tissue (Nxc1 cardiomyocyte-specific promoter), and the LTF subunit is expressed through operable association with the hypoxia-inducible control promoter-1.
[0447]The coding region for the therapeutic product, EPO, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0448]The complete nucleotide sequence of the construct shown in FIG. 11 is presented as SEQ ID NO: 12. The nucleotide coordinates for salient elements of the construct are shown in Table 10.
TABLE-US-00018 TABLE 10 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 SV40 early promoter reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 TSP-1(Cardiac Specific) reverse 240 3026 3265 3'Reg(SV40pA) reverse 221 3388 3608 LTF[Gal4(DBD):EcR(LBD)] reverse 1467 3615 5081 TSP-2(ischemic, inducible) reverse 870 5115 5984 FRP[6x GalRE:Minimal forward 189 6317 6505 Promoter] TPSQ (Epo) forward 582 6719 7300 3'Reg(hGH PolyA) forward 627 7373 7999 Replication Origin reverse 589 8433 9021 AmpR reverse 858 9266 10123 bla Promoter reverse 39 10157 10195
[0449]The construct shown in FIG. 11 is inserted into a suitable vector system, for example, a viral vector, for delivery to a subject in need of treatment for ischemic heart disease.
[0450]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of gene therapy vectors are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and the LDTFC may be expressed under the appropriate physiological conditions. The LDTFC encoded by the vector will be expressed specifically in cardiomyocytes under hypoxic conditions associated with, e.g., cardiac ischemia. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of EPO under control of the FRP. EPO expression in turn promotes targeted cardioprotection in the ischemic tissue.
Example 8
[0451]This example describes a gene therapy vector useful for the treatment of ischemic heart disease through the promotion of cardiac repair and angiogenesis. The vector, shown in FIG. 12, will confer expression of human IGF-1 (see Example 1) upon administration of ligand, under either hypoxic conditions in an inflammatory response, both of which may occur in ischemic tissue. For this purpose, an example of inducible IGF-1 expression, in response to hypoxia and/or an inflammatory response is given. In this example, inducible expression if IGF-1 is specifically limited to cardiomyocytes.
[0452]The vector shown in FIG. 12 is modeled according to the gene switch system shown in FIG. 4. Under this system, the CAP subunit is expressed through operable association with a promoter which is specific for cardiac tissue (Nxc1 cardiomyocyte-specific promoter), and LTF subunits (LTF-1 and LTF-2) of the LDTFC are expressed through either of two inducible TSPs, the first through operable association with the hypoxia-inducible control promoter-1, and the second through operable association with the human plexin D1 promoter.
[0453]The coding region for the therapeutic product, IGF-1, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0454]The complete nucleotide sequence of the construct shown in FIG. 12 is presented as SEQ ID NO:14. The nucleotide coordinates for salient elements of the construct are shown in Table 11.
TABLE-US-00019 TABLE 11 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 SV40 early promoter reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 Transactivation Domain reverse 261 2732 2992 TSP-1(cardiac-specific) reverse 240 3026 3265 3'Reg(SV40pA) reverse 221 3388 3608 LTF-1[Gal4(DBD):EcR(LBD)] reverse 1467 3615 5081 TSP-2(Hypoxia-inducible) reverse 870 5115 5984 FRP[6xGalRE:Minimal forward 189 6317 6505 promoter] Minimal promoter forward 60 6446 6505 TPSQ (IGF-1) forward 477 6719 7195 3'Reg(hGH PolyA) forward 627 7268 7894 TSP-3(Inflammatory inducible) forward 770 8040 8809 LTF-2[Gal42(DBD):EcR(LBD)] forward 1467 8843 10309 CfEcR-LBD(2) forward 1014 9296 10309 3'Reg(Human S100 Calcium forward 765 10316 11080 Binding Protein pA) Replication Origin reverse 589 11414 12002 AmpR reverse 858 12247 13104 bla Promoter reverse 39 13138 13176
[0455]The construct shown in FIG. 12 is inserted into a suitable vector system, for example, a viral vector, for delivery to a subject in need of treatment for ischemic heart disease.
[0456]The vector may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to cardiac tissue, e.g., via angioplasty. Methods for systemic and/or local administration of gene therapy vectors are well known in the art. Upon delivery the vector will be taken up by cells, e.g., cardiac cells, and the LDTFC may be expressed under the appropriate physiological conditions. The LDTFC encoded by the vector will be expressed specifically in cardiomyocytes under hypoxic conditions, and/or in the event of an inflammatory response, associated with, e.g., cardiac ischemia. Ligand will be administered to the subject to be treated which will combine with the expressed transcription factor to drive expression of IGF-1 under control of the FRP. IGF-1 expression in turn promotes targeted angiogenesis in the ischemic tissue.
Example 9
[0457]This example describes a bioreactor/cell therapy vector useful for the treatment of rheumatoid arthritis, active ankylosing spondylitis or plaque psoriasis or for inhibition of structural damage by the active arthritis ("RA or related diseases"). Conventional treatment of RA and related diseases includes traditional Disease Modifying Anti-Rheumatic Drugs (DMARDs) as well as biologic DMARDs such as etanercept, infliximab, and adalimumab. For example, etanercept (Enbrel®), manufactured by Amgen, is a fusion protein that contains two extracellular domains of human TNF-alpha receptor 2 fused to a Fc portion by a hinge peptide. See U.S. Pat. No. 7,276,477 (incorporated herein by reference in its entirety). Etanercept should be administered s.c. once or twice a week. Use of the etanercept gene switch system utilizing inflammation or cytokine response promoters may therefore increase convenience and safety by limiting any production of etanercept in the absence of TNF activation. The complete nucleotide sequence of the construct shown in FIG. 13 is presented as SEQ ID NO: 16. The nucleotide coordinates for salient elements of the construct are shown in Table 12.
TABLE-US-00020 TABLE 12 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 197 455 Neo reverse 795 462 1256 SV40 early promoter reverse 278 1446 1723 3'Reg(SV40pA) reverse 221 1830 2050 LTF[GAL4(DBD):EcR(LBD)] reverse 1467 2057 3523 TL(cMyc ires) reverse 408 3536 3943 CAP[VP16(AD):RxR(HP)] reverse 975 3950 4924 TSP-1(TNF-responsive reverse 800 4958 5757 inflammatory inducible) FRP[6x GalRE:Minimal forward 189 6168 6356 Promoter] TPSQ(entanercept) forward 1407 6570 7976 3'Reg(hGH PolyA) forward 627 8049 8675 Replication Origin reverse 589 9109 9697 AmpR reverse 858 10072 10929 bla Promoter reverse 39 10963 11001
[0458]The vector shown in FIG. 13 is modeled according to the gene switch system shown in FIG. 1. Under this system, both the CAP subunit and the LTF subunit of the LDTFC are expressed through operable association with a single TSP-1 via use of an internal ribosome entry site (IRES). The promoter utilized in this system is a vascular cell adhesion molecule (VCAM1) promoter, which is activated by TNF-alpha. Another example of the TNF-alpha regulated promoters that may be used for the invention is human pentraxin 3(PTX3) promoter, which is responsive to TNF-alpha or Interleukin (IL)-1 beta. See Basile et al., J. Biol. Chem. 272(13): 8172 (1997).
[0459]The coding region for the therapeutic product, etanercept, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0460]The construct shown in FIG. 13 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for RA.
[0461]The cells may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to joints. Systemic and/or local administration of gene therapy cells are well known in the art. Upon delivery of the cells, the LDTFC may be expressed under the appropriate physiological conditions. The LDTFC encoded by the vector will be expressed in the presence of TNF-alpha associated with, e.g., RA. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of etanercept under control of the TNF-alpha regulated promoter. Etanercept expression in turn captures TNF-alpha and reduces the TNF-alpha concentration in the tissues.
Example 10
[0462]This example describes a bioreactor/cell therapy vector useful for the treatment of RA and related disease through reducing the TNF-alpha level. The vector shown in FIG. 14 will confer expression of etanercept upon administration of ligand, under either the presence of TNF-alpha and/or severe inflammation, both of which may occur in RA or related diseases. For this purpose, an example of inducible etanercept expression, in response to the presence of TNF-alpha and/or an inflammatory response is given.
[0463]The vector shown in FIG. 14 is modeled according to the gene switch system shown in FIG. 4. Under this system, the CAP subunit is expressed through operable association with a constitutive promoter (TSP-1), and a LTF subunit of a LDTFC is expressed by either of two inducible transcription cassettes, the first (LTF-1) through operable association with human plasminogen activator inhibitor type-2 (PAT2) promoter (TSP-2), and the second (LTF-2) through operable association with the human serum amyloid A1 (SAA1) promoter (TSP-3). The human PA12 promoter is activated in the presence of TNF-alpha. See Mahony et al. Eur. J. Biochem. 263(3) (1999) and Matsuo et al., Biochem. J. 405: 605 (2007). The SAA1 promoter is upregulated not directly by proinflammatory cytokines such as TNF-alpha, but by other acute inflammatory signals such as glucocorticoid. See Kumon et al., Scandinavian J. Immunol. 56: 504 (2002).
[0464]The coding region for the therapeutic product, etanercept, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0465]The complete nucleotide sequence of the construct shown in FIG. 14 is presented as SEQ ID NO: 15. The nucleotide coordinates for salient elements of the construct are shown in Table 13.
TABLE-US-00021 TABLE 13 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 SV40 early promoter reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 Transactivation Domain reverse 261 2732 2992 TSP-1(constitutive) reverse 571 3026 3596 3'Reg(SV40pA) reverse 221 3719 3939 LTF-1[Gal4(DBD):EcR(LBD)] reverse 1467 3946 5412 TSP-2(TNF-responsive reverse 252 5446 5697 Inflammatory inducible) FRP[6x GalRE:Minimal forward 189 6030 6218 Promoter] TPSQ(etanercept) forward 1407 6432 7838 3'Reg(hGH PolyA) forward 627 7911 8537 TSP-3(Inflammatory inducible) forward 253 8683 8935 LTF- forward 1467 8969 10435 2(Gal4(DBD):CfEcR(LBD)) EcRLBD(2) forward 1014 9422 10435 3'Reg(Human S100 CABP) forward 765 10442 11206 Replication Origin reverse 589 11540 12128 AmpR reverse 858 12373 13230 bla Promoter reverse 39 13264 13302
[0466]The construct shown in FIG. 14 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for RA.
[0467]The cells may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to joints. Methods for systemic and/or local administration of gene therapy cells are well known in the art. Upon delivery of the cells, the LDTFC may be expressed under the appropriate physiological conditions. The LDTFC encoded by the vector will be expressed specifically in the administered cells under the presence of TNF-alpha and/or severe inflammation. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of etanercept under control of the FRP. Etanercept expression in turn captures TNF-alpha and reduces the TNF-alpha concentration in the tissues.
Example 11
[0468]This example describes a bioreactor/cell therapy vector useful for the treatment of RA. The vector will confer expression of two therapeutic polypeptides, etanercept and human erythropoietin (EPO) upon administration of ligand, under the presence of TNF-alpha and/or inflammatory conditions, respectively, both of which occur in RA patients. EPO induces erythrogenesis in anemic RA patients. See Mercuriali et al. Transfusion 34(6): 501 (2003). For this purpose, an example of separately controlled inducible expression of etanercept and EPO in response to RA and anemia, respectively, is given.
[0469]The complete nucleotide sequence of the construct shown in FIG. 15 is presented as SEQ ID NO: 17. The nucleotide coordinates for salient elements of the construct are shown in Table 14.
TABLE-US-00022 TABLE 14 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 197 455 NEO reverse 804 462 1265 SV40e Promoter reverse 280 1385 1664 3'Reg(Synthetic PolyA) reverse 49 1782 1830 CAP[VP16(AD):RxR(HP)] reverse 975 1837 2811 TSP-1(constitutive) reverse 571 2845 3415 3'Reg(SV40pA) reverse 221 3538 3758 LTF-1[Gal4(DBD):EcR(LBD)] reverse 1467 3765 5231 TSP-2(Inflammatory-inducible) reverse 253 5265 5517 FRP-1[6x GalRE:Minimal forward 189 5888 6076 promoter] TPSQ-1(etanercept) forward 1407 6290 7696 3'Reg(hGH PolyA) forward 627 7769 8395 FRP-2[8X LexA:Minimal forward 216 8769 8984 Promoter] TPSQ-2(Epo) forward 582 9198 9779 3'Reg(SV40 early pA) forward 135 9839 9973 TSP-3(Hypoxia-inducible) forward 870 10205 11074 LTF2[LexA(DBD):CfEcR- forward 1629 11108 12736 DEF(LBD)] 3'Reg(Human S100 Calcium forward 765 12743 13507 Binding Protein pA) Replication Origin reverse 589 13841 14429 AmpR reverse 858 14765 15622 bla Promoter reverse 39 15656 15694
[0470]The vector shown in FIG. 15 is modeled according to the gene switch system shown in FIG. 3. Under this system, the CAP subunit of the LDTFC is expressed through operable association with a first, constitutive TSP-1, a first LTF subunit of a LDTFC (LTF-1) is expressed through operable association with a second, inducible TSP-2, and a second LTF subunit of a LDTFC (LTF-2) is expressed through operable association with a third, inducible TSP-3. The second inducible TSP-2 used in this vector is the human serum amyloid A1 (SAA1) promoter and the third inducible TSP-3 used in this vector is the hypoxia-inducible control promoter 1.
[0471]The coding region for the first therapeutic product, etanercept, is operably associated with a first FPR-1 having response elements which recognize the first DNA binding domain (DBD-A) associated with LTF-1, and the coding region for the second therapeutic product, EPO, is operably associated with a second FPR-2 having response elements which recognize the second DNA binding domain (DBD-B) associated with LTF-2. Both factor-regulated promoters are activated upon contact with the respective LDTFC in the presence of ligand.
[0472]The construct shown in FIG. 15 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for RA.
[0473]The cells may be delivered to a subject systemically, for example, via intravenous infusion, or may be delivered directly to joints. Methods for systemic and/or local administration of gene therapy cells are well known in the art. Upon delivery of the cells, the LDTFC(s) may be expressed under the appropriate condition. One or more ligands will be administered to the subject to be treated which will combine with the expressed LDTFC(s) to drive expression of etanercept and/or EPO under control of FRP-1 or FRP-2. Etanercept expression in turn captures TNF-alpha and reduces the TNF-alpha concentration in the tissues, and EPO expression induces erythrogenesis and improves anemia.
Example 12
[0474]This example describes a bioreactor/cell therapy vector useful for the treatment of hemophilia. Hemophilia is caused by lack of either Factor VIII or Factor IX. Deficiency of Factor VIII is called hemophilia A, and deficiency of Factor IX is called hemophilia B.
[0475]Hemophilia A or B may be treated by administering recombinantly produced Factor VIII or IX, respectively. See Garcia-Martin et al., J. Gene Med. 4(2): 215 (2002). For example, recombinantly produced Factor VIII that may be used in the present invention includes, without limitation, full length Factor VIII such as RECOMBINATE® (markted by Baxter), BIOCLATE® (marketed by Aventis), KOGENATE® (marketed by Bayer), HELIXATE® (marketed by Aventis), or ADVATE® (marketed by Baxter), B-domain deleted Factor VIII such as REFACTO® and XYNTHA® (both marked by Genetics Institute and Wyeth), or Factor VIII and von Willebrand Factor complex such as ALPHANATE® (marketed by Grifols Biologicals, Inc.). For this purpose, an example of inducible ALPRANATE®) expression for a bioreactor/cell therapy in response to administration of ligand is shown in FIG. 16. Use of bioreactor/cell therapy improves problems of stability and continuous infusion. See Pipe S. W., J. Thromb. Haemost. 3(8): 1692 (2005).
[0476]The complete nucleotide sequence of the construct shown in FIG. 16 is presented as SEQ ID NO: 18. The nucleotide coordinates for salient elements of the construct are shown in Table 15.
TABLE-US-00023 TABLE 15 Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 318 576 Neo reverse 795 583 1377 3'Reg(SV40 early promoter) reverse 278 1567 1844 3'Reg(Synthetic PolyA) reverse 49 1963 2011 CAP[VP16(AD):RxR(HP)] reverse 975 2018 2992 TSP-1(constitutive) reverse 571 3026 3596 3'Reg(SV40pA) reverse 221 3719 3939 LTF[Gal4(DBD):EcR(LBD)] reverse 1467 3946 5412 TSP-2(constitutive) reverse 1417 5446 6862 FRP[6xGalRE:Minimal forward 189 7195 7383 promoter] TSPQ(Hu Factor VIII) forward 7002 7597 14598 3'Reg(hGH PolyA) forward 627 14671 15297 Replication Origin reverse 589 15731 16319 AmpR reverse 858 16564 17421 bla Promoter reverse 39 17455 17493
[0477]The vector shown in FIG. 16 is modeled according to the gene switch system shown in FIG. 2. Under this system, the CAP subunit is expressed through operable association with a first, constitutive promoter (TSP-1), and the LTF subunit of the LDTFC is expressed through operable association with a second, constitutive promoter (TSP-2). The promoter utilized for the first constitutive promoter is UbC (short) promoter, and the promoter utilized for the second constitutive promoter is UbB (short) promoter.
[0478]The coding region for the therapeutic product, ALPHANATE®), is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0479]The construct shown in FIG. 16 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for hemophilia.
[0480]The cells may be delivered to a subject systemically, for example, via intravenous infusion. Systemic and/or local administration of gene therapy cells are well known in the art. Upon delivery the cells, the LDTFC may be expressed constitutively. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of ALPHANATE® under control of the factor regulated promoter. ALPHANATE® expression in turn treats the symptoms of hemophilia.
Example 13
[0481]This example describes a bioreactor/cell therapy vector useful for the treatment of hemophilia. The vector shown in FIG. 17 is modeled according to the gene switch system shown in FIG. 1. Under this system, both CAP subunit, and the LTF subunit of the LDTFC are expressed through operable association with a single constitutive promoter (TSP-1) via use of an internal ribosome entry site (IRES). The constitutive promoter is UbC (short) promoter.
[0482]The coding region for the therapeutic product, ALPHANATE®, is operably associated with a FRP which is activated upon contact with the LDTFC in the presence of ligand.
[0483]The complete nucleotide sequence of the construct shown in FIG. 17 is presented as SEQ ID NO: 19. The nucleotide coordinates for salient elements of the construct are shown in Table 16.
TABLE-US-00024 TABLE 16 (MOD 8361) Label Direction Length Start End 3'Reg(HSVTKpA) reverse 259 197 455 Neo reverse 795 462 1256 SV40 early promoter reverse 278 1446 1723 3'Reg(SV40pA) reverse 221 1830 2050 LTF[Gal4(DBD):EcR(LBD)] reverse 1467 2057 3523 TL(cMyc ires) reverse 408 3536 3943 CAP[VP16(AD):RxR(HP)] reverse 975 3950 4924 TSP-1(constitutive) reverse 571 4958 5528 FRP[6x GalRE:Minimal forward 189 5939 6127 Promoter] TSPQ(Human Factor VIII) forward 7002 6341 13342 3'Reg(hGH PolyA) forward 627 13415 14041 Replication Origin reverse 589 14475 15063 AmpR reverse 858 15438 16295 bla Promoter reverse 39 16329 16367
[0484]The construct shown in FIG. 17 may be prepared in a vector suitable for introduction into cells prior to introduction into the subject to be treated. The cells may be autologous cells removed from the subject to be treated or non-autologous allogeneic or xenogeneic cells, either primary cells or cell-lines maintained in culture. The vector is introduced into the cells via any standard method, e.g., transfection, transduction, lipofection, or electroporation, to produce modified cells. Following introduction of the vector, the modified cells may optionally be treated to produce a barrier system, e.g., the cells may be coated or encapsulated so as to provide immunoisolation. The modified cells will then be formulated as a bioreactor for administration to a subject in need of treatment for hemophilia.
[0485]The cells may be delivered to a subject systemically, for example, via intravenous infusion. Systemic and/or local administration of gene therapy cells are well known in the art. Upon delivery the cells, the LDTFC may be expressed constitutively. Ligand will be administered to the subject to be treated which will combine with the expressed LDTFC to drive expression of ALPHANATE® under control of the factor regulated promoter. ALPHANATE® expression in turn treats the symptoms of hemophilia.
[0486]Additional embodiments of the invention include the following:
[0487]E1. A method for treating, ameliorating, or preventing a disease or disorder in a subject, comprising: [0488](a) introducing into cells of said subject (1) a polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor, operably linked to a therapeutic switch promoter, wherein the promoter is activated during said disease or disorder, and (2) a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide linked to a promoter which is activated by said ligand-dependent transcription factor, to produce modified cells; and [0489](b) administering ligand to said subject to induce expression of said therapeutic polypeptide or therapeutic polynucleotide; [0490]wherein said therapeutic polypeptide or therapeutic polynucleotide is expressed at a level sufficient to treat, ameliorate, or prevent said disease or disorder.
[0491]E2. A method for expressing a therapeutic polypeptide or therapeutic polynucleotide in a subject, comprising: [0492](a) introducing into cells of said subject (1) a polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor, operably linked to a therapeutic switch promoter, wherein the promoter is activated during said disease or disorder, and (2) a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide linked to a promoter which is activated by said ligand-dependent transcription factor, to produce modified cells; and [0493](b) administering ligand to said subject to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
[0494]E3. The method of E1 or E2, wherein said polynucleotides are introduced into cells that have been isolated from said subject to produce modified cells, and the modified cells are re-introduced into said subject.
[0495]E4. The method of E1 or E2, wherein said method is carried out in vivo.
[0496]E5. The method of E1 or E2, wherein said gene switch is an ecdysone receptor (EcR)-based gene switch.
[0497]E6. The method of E5, wherein said ligand binds to the EcR ligand binding domain.
E7. The method of E6, wherein said ligand is a diacylhydrazine.
[0498]E8. The method of E7, wherein said ligand is selected from the group consisting of RG-115819, RG-15932, and RG-115830.
E9. The method of E6, wherein said ligand is an amidoketone or oxadiazoline.
[0499]E10. The method of E1 or E2, wherein said gene switch comprises a first transcription factor sequence under the control of a first therapeutic switch promoter and a second transcription factor sequence under the control of a second therapeutic switch promoter, wherein the proteins encoded by said first transcription factor sequence and said second transcription factor sequence interact to form a protein complex which functions as a ligand-dependent transcription factor.
[0500]E11. The method of E10, wherein said first therapeutic switch promoter and said second therapeutic switch promoter are different.
[0501]E12. The method of E10, wherein said first therapeutic switch promoter and said second therapeutic switch promoter are the same.
[0502]E13. The method of E10, wherein said first transcription factor sequence encodes a protein comprising a heterodimer partner and a transactivation domain.
[0503]E14. The method of E10, wherein said second transcription factor sequence encodes a protein comprising a DNA binding domain and a ligand-binding domain.
[0504]E15. The method of E1 or E2, wherein one of said polynucleotides further encodes a lethal polypeptide operably linked to an inducible promoter.
[0505]E16. A method for expressing a therapeutic polypeptide or therapeutic polynucleotide in a cell, comprising: [0506](a) introducing into said cell (1) a polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor, operably linked to a therapeutic switch promoter, wherein the promoter is activated during said disease or disorder, and (2) a polynucleotide encoding a therapeutic polypeptide or therapeutic polynucleotide linked to a promoter which is activated by said ligand-dependent transcription factor, to produce a modified cell; and [0507](b) administering ligand to said modified cell to induce expression of said therapeutic polypeptide or therapeutic polynucleotide.
[0508]E17. The method of E16, wherein said method is carried out in vitro.
[0509]E18. The method of E16, wherein said method is carried out ex vivo in a cell that has been isolated from a subject.
[0510]E19. The method of E16, wherein said method is carried out in vivo.
[0511]E20. The method of E16, wherein said gene switch is an EcR-based gene switch.
[0512]E21. The method of E20, wherein said ligand binds to the EcR ligand binding domain.
[0513]E22. The method of E21, wherein said ligand is a diacylhydrazine.
[0514]E23. The method of E22, wherein said ligand is selected from the group consisting of RG-115819, RG-115932, and RG-115830.
[0515]E24. The method of E21, wherein said ligand is an amidoketone or oxadiazoline.
[0516]E25. The method of E16, wherein said gene switch comprises a first transcription factor sequence under the control of a first therapeutic switch promoter and a second transcription factor sequence under the control of a second therapeutic switch promoter, wherein the proteins encoded by said first transcription factor sequence and said second transcription factor sequence interact to form a protein complex which functions as a ligand-dependent transcription factor.
[0517]E26. The method of E25, wherein said first therapeutic switch promoter and said second therapeutic switch promoter are different.
[0518]E27. The method of E25, wherein said first therapeutic switch promoter and said second therapeutic switch promoter are the same.
[0519]E28. The method of E25, wherein said first transcription factor sequence encodes a protein comprising a heterodimer partner and a transactivation domain.
[0520]E29. The method of E25, wherein said second transcription factor sequence encodes a protein comprising a DNA binding domain and a ligand-binding domain.
[0521]E30. The method of E16, wherein one of said polynucleotides further encodes a lethal polypeptide operably linked to an inducible promoter.
[0522]E31. A polynucleotide encoding a gene switch, said gene switch comprising at least one transcription factor sequence, wherein said at least one transcription factor sequence encodes a ligand-dependent transcription factor, operably linked to a therapeutic switch promoter, wherein the activity of the promoter is modulated during said disease or disorder.
[0523]E32. The polynucleotide of E31, further encoding a reporter gene linked to a promoter which is activated by said ligand-dependent transcription factor.
[0524]E33. The polynucleotide of E31, wherein said gene switch is an EcR-based gene switch.
[0525]E34. The polynucleotide of E31, wherein said gene switch comprises a first transcription factor sequence under the control of a first therapeutic switch promoter and a second transcription factor sequence under the control of a second therapeutic switch promoter, wherein the proteins encoded by said first transcription factor sequence and said second transcription factor sequence interact to form a protein complex which functions as a ligand-dependent transcription factor.
[0526]E35. The polynucleotide of E34, wherein said first therapeutic switch promoter and said second therapeutic switch promoter are different.
[0527]E36. The polynucleotide of E34, wherein said first therapeutic switch promoter and said second therapeutic switch promoter are the same.
[0528]E37. The polynucleotide of E34, wherein said first transcription factor sequence encodes a protein comprising a heterodimer partner and a transactivation domain.
[0529]E38. The polynucleotide of E34, wherein said second transcription factor sequence encodes a protein comprising a DNA binding domain and a ligand-binding domain.
[0530]E39. The polynucleotide of E31, wherein said polynucleotide further encodes a lethal polypeptide operably linked to an inducible promoter.
[0531]E40. A vector comprising the polynucleotide of E31.
[0532]E41. The vector of E40, which is a plasmid vector.
[0533]E42. The vector of E40, which is a viral vector.
[0534]E43. A kit comprising the polynucleotide of E31.
[0535]E44. A kit comprising the vector of E42.
[0536]The present invention further relates to instructions for performing one or more methods of the invention. Such instructions can instruct a user of conditions suitable for performing methods of the invention. Instructions of the invention can be in a tangible form, for example, written instructions (e.g., typed on paper), or can be in an intangible form, for example, accessible via a computer disk or over the internet.
[0537]It will be recognized that a full text of instructions for performing a method of the invention or, where the instructions are included with a kit, for using the kit, need not be provided. One example of a situation in which a kit of the invention, for example, would not contain such full length instructions is where the provided directions inform a user of the kits where to obtain instructions for practicing methods for which the kit can be used. Thus, instructions for performing methods of the invention can be obtained from internet web pages, separately sold or distributed manuals or other product literature, etc. The invention thus includes kits that direct a kit user to one or more locations where instructions not directly packaged and/or distributed with the kits can be found. Such instructions can be in any form including, but not limited to, electronic or printed forms.
[0538]Having now fully described the invention, it will be understood by those of ordinary skill in the art that the same can be performed within a wide and equivalent range of conditions, formulations and other parameters without affecting the scope of the invention or any embodiment thereof. All patents, patent applications and publications cited herein are fully incorporated by reference herein in their entirety.
Sequence CWU
1
26117DNAArtificial SequenceSynthetic ecdysone receptor response element
1rrggttcant gacacyy
17213DNAArtificial SequenceSynthetic ecdysone receptor response element
2aggtcanagg tca
13315DNAArtificial SequenceSynthetic ecdysone receptor response element
3gggttgaatg aattt
15418DNAArtificial SequenceSynthetic I-SceI homing endonuclease
restriction site 4tagggataac agggtaat
185878PRTDrosophila melanogasterEcdysone receptor 5Met Lys
Arg Arg Trp Ser Asn Asn Gly Gly Phe Met Arg Leu Pro Glu1 5
10 15Glu Ser Ser Ser Glu Val Thr Ser
Ser Ser Asn Gly Leu Val Leu Pro20 25
30Ser Gly Val Asn Met Ser Pro Ser Ser Leu Asp Ser His Asp Tyr Cys35
40 45Asp Gln Asp Leu Trp Leu Cys Gly Asn Glu
Ser Gly Ser Phe Gly Gly50 55 60Ser Asn
Gly His Gly Leu Ser Gln Gln Gln Gln Ser Val Ile Thr Leu65
70 75 80Ala Met His Gly Cys Ser Ser
Thr Leu Pro Ala Gln Thr Thr Ile Ile85 90
95Pro Ile Asn Gly Asn Ala Asn Gly Asn Gly Gly Ser Thr Asn Gly Gln100
105 110Tyr Val Pro Gly Ala Thr Asn Leu Gly
Ala Leu Ala Asn Gly Met Leu115 120 125Asn
Gly Gly Phe Asn Gly Met Gln Gln Gln Ile Gln Asn Gly His Gly130
135 140Leu Ile Asn Ser Thr Thr Pro Ser Thr Pro Thr
Thr Pro Leu His Leu145 150 155
160Gln Gln Asn Leu Gly Gly Ala Gly Gly Gly Gly Ile Gly Gly Met
Gly165 170 175Ile Leu His His Ala Asn Gly
Thr Pro Asn Gly Leu Ile Gly Val Val180 185
190Gly Gly Gly Gly Gly Val Gly Leu Gly Val Gly Gly Gly Gly Val Gly195
200 205Gly Leu Gly Met Gln His Thr Pro Arg
Ser Asp Ser Val Asn Ser Ile210 215 220Ser
Ser Gly Arg Asp Asp Leu Ser Pro Ser Ser Ser Leu Asn Gly Tyr225
230 235 240Ser Ala Asn Glu Ser Cys
Asp Ala Lys Lys Ser Lys Lys Gly Pro Ala245 250
255Pro Arg Val Gln Glu Glu Leu Cys Leu Val Cys Gly Asp Arg Ala
Ser260 265 270Gly Tyr His Tyr Asn Ala Leu
Thr Cys Glu Gly Cys Lys Gly Phe Phe275 280
285Arg Arg Ser Val Thr Lys Ser Ala Val Tyr Cys Cys Lys Phe Gly Arg290
295 300Ala Cys Glu Met Asp Met Tyr Met Arg
Arg Lys Cys Gln Glu Cys Arg305 310 315
320Leu Lys Lys Cys Leu Ala Val Gly Met Arg Pro Glu Cys Val
Val Pro325 330 335Glu Asn Gln Cys Ala Met
Lys Arg Arg Glu Lys Lys Ala Gln Lys Glu340 345
350Lys Asp Lys Met Thr Thr Ser Pro Ser Ser Gln His Gly Gly Asn
Gly355 360 365Ser Leu Ala Ser Gly Gly Gly
Gln Asp Phe Val Lys Lys Glu Ile Leu370 375
380Asp Leu Met Thr Cys Glu Pro Pro Gln His Ala Thr Ile Pro Leu Leu385
390 395 400Pro Asp Glu Ile
Leu Ala Lys Cys Gln Ala Arg Asn Ile Pro Ser Leu405 410
415Thr Tyr Asn Gln Leu Ala Val Ile Tyr Lys Leu Ile Trp Tyr
Gln Asp420 425 430Gly Tyr Glu Gln Pro Ser
Glu Glu Asp Leu Arg Arg Ile Met Ser Gln435 440
445Pro Asp Glu Asn Glu Ser Gln Thr Asp Val Ser Phe Arg His Ile
Thr450 455 460Glu Ile Thr Ile Leu Thr Val
Gln Leu Ile Val Glu Phe Ala Lys Gly465 470
475 480Leu Pro Ala Phe Thr Lys Ile Pro Gln Glu Asp Gln
Ile Thr Leu Leu485 490 495Lys Ala Cys Ser
Ser Glu Val Met Met Leu Arg Met Ala Arg Arg Tyr500 505
510Asp His Ser Ser Asp Ser Ile Phe Phe Ala Asn Asn Arg Ser
Tyr Thr515 520 525Arg Asp Ser Tyr Lys Met
Ala Gly Met Ala Asp Asn Ile Glu Asp Leu530 535
540Leu His Phe Cys Arg Gln Met Phe Ser Met Lys Val Asp Asn Val
Glu545 550 555 560Tyr Ala
Leu Leu Thr Ala Ile Val Ile Phe Ser Asp Arg Pro Gly Leu565
570 575Glu Lys Ala Gln Leu Val Glu Ala Ile Gln Ser Tyr
Tyr Ile Asp Thr580 585 590Leu Arg Ile Tyr
Ile Leu Asn Arg His Cys Gly Asp Ser Met Ser Leu595 600
605Val Phe Tyr Ala Lys Leu Leu Ser Ile Leu Thr Glu Leu Arg
Thr Leu610 615 620Gly Asn Gln Asn Ala Glu
Met Cys Phe Ser Leu Lys Leu Lys Asn Arg625 630
635 640Lys Leu Pro Lys Phe Leu Glu Glu Ile Trp Asp
Val His Ala Ile Pro645 650 655Pro Ser Val
Gln Ser His Leu Gln Ile Thr Gln Glu Glu Asn Glu Arg660
665 670Leu Glu Arg Ala Glu Arg Met Arg Ala Ser Val Gly
Gly Ala Ile Thr675 680 685Ala Gly Ile Asp
Cys Asp Ser Ala Ser Thr Ser Ala Ala Ala Ala Ala690 695
700Ala Gln His Gln Pro Gln Pro Gln Pro Gln Pro Gln Pro Ser
Ser Leu705 710 715 720Thr
Gln Asn Asp Ser Gln His Gln Thr Gln Pro Gln Leu Gln Pro Gln725
730 735Leu Pro Pro Gln Leu Gln Gly Gln Leu Gln Pro
Gln Leu Gln Pro Gln740 745 750Leu Gln Thr
Gln Leu Gln Pro Gln Ile Gln Pro Gln Pro Gln Leu Leu755
760 765Pro Val Ser Ala Pro Val Pro Ala Ser Val Thr Ala
Pro Gly Ser Leu770 775 780Ser Ala Val Ser
Thr Ser Ser Glu Tyr Met Gly Gly Ser Ala Ala Ile785 790
795 800Gly Pro Ile Thr Pro Ala Thr Thr Ser
Ser Ile Thr Ala Ala Val Thr805 810 815Ala
Ser Ser Thr Thr Ser Ala Val Pro Met Gly Asn Gly Val Gly Val820
825 830Gly Val Gly Val Gly Gly Asn Val Ser Met Tyr
Ala Asn Ala Gln Thr835 840 845Ala Met Ala
Leu Met Gly Val Ala Leu His Ser His Gln Glu Gln Leu850
855 860Ile Gly Gly Val Ala Val Lys Ser Glu His Ser Thr
Thr Ala865 870 8756605PRTStreptomyces
sp.Streptomyces phage phiC31 integrase 6Met Asp Thr Tyr Ala Gly Ala Tyr
Asp Arg Gln Ser Arg Glu Arg Glu1 5 10
15Asn Ser Ser Ala Ala Ser Pro Ala Thr Gln Arg Ser Ala Asn
Glu Asp20 25 30Lys Ala Ala Asp Leu Gln
Arg Glu Val Glu Arg Asp Gly Gly Arg Phe35 40
45Arg Phe Val Gly His Phe Ser Glu Ala Pro Gly Thr Ser Ala Phe Gly50
55 60Thr Ala Glu Arg Pro Glu Phe Glu Arg
Ile Leu Asn Glu Cys Arg Ala65 70 75
80Gly Arg Leu Asn Met Ile Ile Val Tyr Asp Val Ser Arg Phe
Ser Arg85 90 95Leu Lys Val Met Asp Ala
Ile Pro Ile Val Ser Glu Leu Leu Ala Leu100 105
110Gly Val Thr Ile Val Ser Thr Gln Glu Gly Val Phe Arg Gln Gly
Asn115 120 125Val Met Asp Leu Ile His Leu
Ile Met Arg Leu Asp Ala Ser His Lys130 135
140Glu Ser Ser Leu Lys Ser Ala Lys Ile Leu Asp Thr Lys Asn Leu Gln145
150 155 160Arg Glu Leu Gly
Gly Tyr Val Gly Gly Lys Ala Pro Tyr Gly Phe Glu165 170
175Leu Val Ser Glu Thr Lys Glu Ile Thr Arg Asn Gly Arg Met
Val Asn180 185 190Val Val Ile Asn Lys Leu
Ala His Ser Thr Thr Pro Leu Thr Gly Pro195 200
205Phe Glu Phe Glu Pro Asp Val Ile Arg Trp Trp Trp Arg Glu Ile
Lys210 215 220Thr His Lys His Leu Pro Phe
Lys Pro Gly Ser Gln Ala Ala Ile His225 230
235 240Pro Gly Ser Ile Thr Gly Leu Cys Lys Arg Met Asp
Ala Asp Ala Val245 250 255Pro Thr Arg Gly
Glu Thr Ile Gly Lys Lys Thr Ala Ser Ser Ala Trp260 265
270Asp Pro Ala Thr Val Met Arg Ile Leu Arg Asp Pro Arg Ile
Ala Gly275 280 285Phe Ala Ala Glu Val Ile
Tyr Lys Lys Lys Pro Asp Gly Thr Pro Thr290 295
300Thr Lys Ile Glu Gly Tyr Arg Ile Gln Arg Asp Pro Ile Thr Leu
Arg305 310 315 320Pro Val
Glu Leu Asp Cys Gly Pro Ile Ile Glu Pro Ala Glu Trp Tyr325
330 335Glu Leu Gln Ala Trp Leu Asp Gly Arg Gly Arg Gly
Lys Gly Leu Ser340 345 350Arg Gly Gln Ala
Ile Leu Ser Ala Met Asp Lys Leu Tyr Cys Glu Cys355 360
365Gly Ala Val Met Thr Ser Lys Arg Gly Glu Glu Ser Ile Lys
Asp Ser370 375 380Tyr Arg Cys Arg Arg Arg
Lys Val Val Asp Pro Ser Ala Pro Gly Gln385 390
395 400His Glu Gly Thr Cys Asn Val Ser Met Ala Ala
Leu Asp Lys Phe Val405 410 415Ala Glu Arg
Ile Phe Asn Lys Ile Arg His Ala Glu Gly Asp Glu Glu420
425 430Thr Leu Ala Leu Leu Trp Glu Ala Ala Arg Arg Phe
Gly Lys Leu Thr435 440 445Glu Ala Pro Glu
Lys Ser Gly Glu Arg Ala Asn Leu Val Ala Glu Arg450 455
460Ala Asp Ala Leu Asn Ala Leu Glu Glu Leu Tyr Glu Asp Arg
Ala Ala465 470 475 480Gly
Ala Tyr Asp Gly Pro Val Gly Arg Lys His Phe Arg Lys Gln Gln485
490 495Ala Ala Leu Thr Leu Arg Gln Gln Gly Ala Glu
Glu Arg Leu Ala Glu500 505 510Leu Glu Ala
Ala Glu Ala Pro Lys Leu Pro Leu Asp Gln Trp Phe Pro515
520 525Glu Asp Ala Asp Ala Asp Pro Thr Gly Pro Lys Ser
Trp Trp Gly Arg530 535 540Ala Ser Val Asp
Asp Lys Arg Val Phe Val Gly Leu Phe Val Asp Lys545 550
555 560Ile Val Val Thr Lys Ser Thr Thr Gly
Arg Gly Gln Gly Thr Pro Ile565 570 575Glu
Lys Arg Ala Ser Ile Thr Trp Ala Lys Pro Pro Thr Asp Asp Asp580
585 590Glu Asp Asp Ala Gln Asp Gly Thr Glu Asp Val
Ala Ala595 600 60579861DNAArtificial
SequenceSynthetic hypoxia-inducible gene switch constructor
expressing IGF-1 7taaacaaata ggggttccgc gcacatttcc ccgaaaagtg ccacctgacg
tactgaggac 60gccagggttt tcccagtcac gacgttgtaa aacgacggcc agtagcagac
aagcccgtca 120gggcgcgtca gcgggtgttg gcgggtgtcg gggatttagg tgacactata
ggctgagcgc 180cgcacaggca tctagaggct atggcagggc ctgccgcccc gacgttggct
gcgagccctg 240ggccttcacc cgaacttggg gggtggggtg gggaaaagga agaaacgcgg
gcgtattggc 300cccaatgggg tctcggtggg gtatcgacag agtgccagcc ctgggaccga
accccgcgtt 360tatgaacaaa cgacccaaca ccgtgcgttt tattctgtct ttttattgcc
gtcatagcgc 420gggttccttc cggtattgtc tccttccgtg tttcaatcga ttcaaaagaa
ctcgtccagc 480agacggtaaa aagcaatgcg ttgagaatcc ggtgcagcaa tgccatacag
caccagaaag 540cgatcagccc attcaccgcc cagttcttca gcaatgtcac gggttgccag
tgcgatgtcc 600tgatagcgat cagccacgcc caggcgaccg cagtcgataa agccggagaa
acggccgttt 660tccaccataa tgtttggcag acaagcatcg ccgtgggtca caaccaggtc
ctcgccatct 720ggcatacgtg ctttcaggcg tgcgaacagt tctgccggtg ccagaccctg
atgttcctcg 780tccaggtcat cctgatcaac caggccagct tccatgcgag tgcgtgcgcg
ctcgatacgg 840tgtttagctt ggtgatcgaa tgggcaagta gctgggtcca gggtatgcag
acggcgcata 900gcatcagcca tgatggaaac cttttctgcc ggtgccagat gagaggacag
cagatcctgg 960cctggaacct cgcccagcag cagccagtcg cggccagcct cggtcacaac
atccagcaca 1020gctgcgcatg gaacgccggt agtagccagc caggacagac gagctgcttc
atcttgcagt 1080tcgttcagtg cgccggacag atcggtctta acaaacagca ccggacggcc
ttgagcggac 1140agacggaaca cagctgcgtc ggagcaaccg atagtctgtt gagcccagtc
atagccaaac 1200agacgttcca cccaagcagc cggagaacca gcgtgcagac cgtcttgttc
aatcatggtg 1260gcaattgggt gtctgagcga tgtggctcgg ctggcgacgc aaaagaagat
gcggctgact 1320gtcgaacagg aggagcagag agcgaagcgg gaggctgcgg gctcaatttg
catgctttag 1380ttcctcacct tgtcgtatta tactatgccg atatactatg ccgatgatta
attgtcaacg 1440tatacggaat agctctgagg ccgaggcagc ttcggcctct gcataaataa
aaaaaattag 1500tcagccatgg ggcggagaat gggcggaact gggcggagtt aggggcggga
tgggcggagt 1560taggggcggg actatggttg ctgactaatt gagatgcttg ctttgcatac
ttctgcctgc 1620tggggagcct ggggactttc cacacctggt tgctgactaa ttgagatgct
tgctttgcat 1680acttctgcct gctggggagc ctggggactt tccacaccct aacctcgagg
ccatcgtggc 1740acgccagggt tttcccagtc acgacgttgt aaaacgacgg ccagtgctct
tctcccccgc 1800gggaggtttt ataaatccga ctgtctagat accacatttg tagaggtttt
acttgcttta 1860aaaaacctcc cacatctccc cctgaacctg aaacataaaa tgaatgcaaa
tgttttatta 1920acttgtttat tgcagcttat aatggttaca aattaagcaa tagcatcaca
aatttcacaa 1980attaagcatt tttttcactg cattctagtt gtggtttgtc caaactcatc
aatgtatctt 2040atcatgtcta atcgattcac aggttggtgg ggctctccag gatggggggg
ggctgggtgt 2100ggctcatgtc ggccacgtcc caaatctcct ccaggaaggg gggcagcttc
ctgttcttca 2160gcttcaggct gatacacata ttgctgttct gcattcccag ggtcctcagc
tcgctcagga 2220tgctcaggat cttgccgtag atcacgctgc tcctggcgct gccgctcagc
tggttcagga 2280tgtagatcct cagggtgttc aggtagtacc tctggatctc ctccaccagc
tggggctgct 2340ccaggccggg cctgtcgctg aagatcacca cggcggtcag cagggcgtag
tggatgttgt 2400ccagggccat gctgtacata catctgcaga agtgcaggag gtcctcgatc
acctcggcca 2460tgccagcctt cctgtagttg tccctggtgt aagcctggtt gttggcgaac
aggatgctgt 2520cgctggcggc gtcgtacctc ctggccaccc tcagcatcat cacctcgctg
ctgcaagcct 2580tcagcagggt gatctggtcg ggctggctga tcttggcgaa tccgggcagg
cccttggcga 2640actccacgat cagctgcacg gtcaggatgg tcatctcggt gatctgcctg
aagggggtgt 2700cgctctcctc gttctcgtcg tcggcctgct gccaggtctg ggtgatcctt
ttcaggtcct 2760cgtcgctggg ctgctcgtag ccgtcctgat accagatcag cctggcgatc
aggaactgct 2820ggttggcggt cagctggggg atgttcttct gcctgttggt caccagcagc
ttgtcgctca 2880ggaacctggg cacgacctcg tgaatcctgg cggcctcggg gggggggggc
tcgcactgca 2940tgatgggggg catgtggtca tcgacggtgg tggtgctcac gggcagcttg
tccttctcct 3000tctgggcctt cttctccttc cttttcatgg cgcactgggt ctcgggcacc
acgcactcgg 3060gcctgatccc gggaaactcg gggctcacgg tcagctgcct ctggcccttg
ttgctgctct 3120cctcgctgct gctggtggcg ctgatcctgt gctgcctcag ggtcaggggc
atgtcggtct 3180ccacgctggc cagcctgtcg gtcacggcgt ccttgttcac gttgtcctgc
acgaacaggc 3240cggtcagcag ggccttgatg tcttgcaggc tgtccatctt caggatcatg
tccaggtcct 3300ccctggggaa gatcagcagg aacagctgct ccagcctctc cagcctgctc
tccacctcgg 3360tcaggtgggc cctggtcagg gggctcctct tggtcttggg gctgtatctg
cactcccagt 3420tgttcttcag gcacttggcg cacttgggct tctccttgct gcacttcagc
ttcttcagcc 3480tgcagatgtc gcaagcctgc tcgatgctgc tcagcagctt catggtggca
gatctggtcg 3540cgggaggctg ctggttttcc actacccgaa aaaaatccag cgtctaagca
gctgcaagga 3600gagcctttca gagaagcggg tcctggcagc ggcggggaag tgtccccaaa
tgggcagaat 3660agcctccccg cgtcgggaga gtcgcgtcct tgctcgggtg ttgtaagttc
cagtgcaaag 3720tgcccgcccg ctgctatggg caaagtttcg tggatgcggc tagggttgcg
caccgctggc 3780tgggggatca gcgggagggc tgggccagag gcgaagcccc ctattcgctc
cggatctccc 3840ttcccaggac gcccgcagcg cagctctgct cgccgggctc ctccacccta
gcccgccgcc 3900cgctcgctcc ctctgcctct cgctggaatt actacagcga gttgccggct
cagctgtcgc 3960tggggctctc cagcatctcc atcaggaagg tgtcgatggg cacgtcgccg
atcagcctga 4020agaagaacag gtgctccagg cacttcaggc cgatgctcct caggctgggc
agcctcagca 4080gcagcttggc gaatctgccg ggctcgtcgg ggtgggtggt cctggtgtac
tcctccaggg 4140cggcgtacac cttctccctc agcagctcca cctcctgggc gcttttcagg
cccctcacct 4200cggggttgaa caggatgatg gccctcaggc agcccagctc ggtcttgtcc
atcctcatgt 4260ccctcatctt gctcaccagc tcggtcagca ccctgtcgaa gatggcgccc
actccggcgc 4320tgtgggcgct gttcctatgg acgtgcaggc cggtggccag caggatgccg
tccctcacgt 4380cgatgctcct gtggctgaag ctggcgatca gcagctcgtt ccatccggcc
ctcagcagga 4440tcacctggtc gtccaggggc aggctgctga agtggggaat cctcttggcc
cactccacca 4500gggtgaacag ctgcttgtcg gcggcctggc agatgttggt cacggggtcg
ttggggctgc 4560tgccgctgcc gccggttccg ccggggccct ccacgccctg gtcgcttttc
tgctccacgg 4620cgagttcggc ctccagaatc ctgtccacgg gcatctccat atggccgccg
tactcgtcga 4680tgcccagggc gtcggtgaac atctgctcga actcgaagtc ggccatgtcc
agggcgccgt 4740agggggcgct gtcgtggggg gtgaagccgg ggccggggct gtcgccgtcg
cccagcatgt 4800ccaggtcgaa gtcgtccagg gcgtcggcgt gggccatggc cacgtcctcg
ccgtccaggt 4860gcagctcgtc gcccaggctc acgtcggtgg ggggggccac cttccttttc
ttcttggggc 4920ccatggtggc caattgcccc cccccccccc cgcatgcggc tggctgctgc
ctctgaagaa 4980ttcaaggagc ctgctggtcg gcccctgctg tggaacaata aatacctctt
ccctggggtt 5040aaaaataacc ccatgaccac ttttggcagt tgtaggtgag gcagaggcca
cctaagcccc 5100cccaccccat gccgttcttc tgaagtaaga gccgttcaaa gtccgacacc
cgcctgggtg 5160gacacgtgag acccaaaaat gccttcaagg ttaaagagac agtggctggg
gcctggcctg 5220ggaacgatgc tctgtgctct gggtcctgcc attgccgaca ggcgctgtgt
gagacagcac 5280gtagggcgac aggcgctgtg tgagacagca cgtagggcga caggcgctgt
gtgagacagc 5340acgtagggcg acaggcgctg tgtgagacag cacgtagggc gacaggcgct
gtgtgagaca 5400gcacgtaggg cgacaggcgc tgtgtgagac agcacgtagg gcgacaggcg
ctgtgtgaga 5460cagcacgtag ggcgacaggc gctgtgtgag acagcacgta gggcgacagg
cgctgtgtga 5520gacagcacgt agggcctcga gtaggcgaga ccaatgggtg cgccatgggc
tcttccaaaa 5580atttaggtga cactataggg caccgctcgc acctgcgcac aggcataagc
caaatggaac 5640tacgagacct gcatcgtggt gtaactataa cggtcctaag gtagcgaccg
cggagactag 5700gtgtatttat ctaagcgatc gcttaattaa ggccggccgc cgcaataaaa
tatctttatt 5760ttcattacat ctgtgtgttg gttttttgtg tgaatccata gtactaacat
acgctctcca 5820tcaaaacaaa acgaaacaaa acaaactagc aaaataggct gtccccagtg
caagtccagg 5880tgccagaaca tttctctatc cataatgcag gggtaccggg tgatgacggt
gaaaacctcc 5940aattgcggag tactgtcctc cgagcggagt actgtcctcc gagcggagta
ctgtcctccg 6000agcggagtac tgtcctccga gcggagtact gtcctccgag cggagtactg
tcctccgagc 6060ggagagtccc cggggaccta gagggtatat aatgggtgcc ttagctggtg
tgtgacctca 6120tcttcctgta cgcccctgca ggggcgcgcc acgcgtcgaa gaaggtgagt
aatcttaaca 6180tgctcttttt tttttttttt gctaatccct tttgtgtgct gatgttagga
tgacatttac 6240aacaaatgtt tgttcctgac aggaaaaacc ttgctgggta ccttcgttgc
cggacacttc 6300ttgtcctcta ctttggaaaa aaggaattga gagccgctag cgccaccatg
ggaaaaatca 6360gcagcctgcc cacccaatta tttaagtgct gcttttgtga tttcttgaag
gtgaagatgc 6420acaccatgtc ctcctcgcat ctgttctacc tggcgctgtg cctgctgacc
ttcaccagct 6480ctgccacggc tggaccggag accctctgcg gggccgagct ggtggatgcc
ctgcaattcg 6540tgtgtggaga caggggcttc tacttcaaca agcccacagg gtatggctcc
agcagtcgga 6600gagcccccca gacaggcatc gtggatgagt gctgcttcag aagctgtgat
ctaaggaggc 6660tggagatgta ttgcgcaccc ctcaagcctg ccaagtcagc tcgctctgtc
cgtgcccagc 6720gccacaccga catgcccaag acccagaagt atcagccccc atctaccaac
aagaacacga 6780agtctcagag aaggaaagga agtacatttg aagaacgcaa gtagatcgat
tgcgcaaagc 6840tttcgcgata ggcgagacca atgggtgtgt acgtagcggc cgcgtcgacg
atagcttgat 6900gggtggcatc cctgtgaccc ctccccagtg cctctcctgg ccctggaagt
tgccactcca 6960gtgcccacca gccttgtcct aataaaatta agttgcatca ttttgtctga
ctaggtgtcc 7020ttctataata ttatggggtg gaggggggtg gtatggagca aggggcaagt
tgggaagaca 7080acctgtaggg cctgcggggt ctattgggaa ccaagctgga gtgcagtggc
acaatcttgg 7140ctcactgcaa tctccgcctc ctgggttcaa gcgattctcc tgcctcagcc
tcccgagttg 7200ttgggattcc aggcatgcat gaccaggctc agctaatttt tgtttttttg
gtagagacgg 7260ggtttcacca tattggccag gctggtctcc aactcctaat ctcaggtgat
ctacccacct 7320tggcctccca aattgctggg attacaggcg tgaaccactg ctcccttccc
tgtccttctg 7380attttaaaat aactatacca gcaggaggac gtccagacac agcataggct
acctggccat 7440gcccaaccgg tgggacattt gagttgcttg cttggcactg tcctctcatg
cgttgggtcc 7500actcagtaga tgcctgttga attctgattt aaatcggtcc gcgtacggcg
tggtaggtcc 7560gaacgaatcc atggattacc ctgttatccc tactcaagga catcatccct
ttagtgaggg 7620ttaattcacg cagtgggtac ggaactaaag gcagcacaca tcgtgtaatc
atggtcatag 7680ctgtttcctg tgtgaaattg ttatccgcta cgtctctccc ccgcagtaag
ggctagatta 7740actcgtctcg tgaatatccg gaactccctt tagtgagggt taattgcgtt
gcgctcactg 7800cccgctttcc agtcgggaaa cctgtcgtgc cagcttaatc atggtcatag
ctgtttcctg 7860tgtgaaattg ttatccgcta ccggaaacgc ttccttcatg tgagcaaaag
gccagcaaaa 7920ggccaggaac cgtaaaaagg ccgcgttgct ggcgtttttc cataggctcc
gcccccctga 7980cgagcatcac aaaaatcgac gctcaagtca gaggtggcga aacccgacag
gactataaag 8040ataccaggcg tttccccctg gaagctccct cgtgcgctct cctgttccga
ccctgccgct 8100taccggatac ctgtccgcct ttctcccttc gggaagcgtg gcgctttctc
atagctcacg 8160ctgtaggtat ctcagttcgg tgtaggtcgt tcgctccaag ctgggctgtg
tgcacgaacc 8220ccccgttcag cccgaccgct gcgccttatc cggtaactat cgtcttgagt
ccaacccggt 8280aagacacgac ttatcgccac tggcagcagc cactggtaac aggattagca
gagcgaggta 8340tgtaggcggt gctacagagt tcttgaagtg gtggcctaac tacggctaca
ctagaagaac 8400agtatttggt atctgcgctc tgctgaagcc agttaccttc ggaaaaagag
ttggtagctc 8460ttgatccggc aaacaaacca ccgctggtag cggtggtttt tttgtttgca
agcagcagat 8520tacgcgcaga aaaaaaggat ctcaagaaga tcctttgatc ttttctacgg
ggtctgacgc 8580tcagtggaac gaaaactcac gttaagggat tttggtcatg cctaggacga
aaggaggtcg 8640tgaaatggat aaaaaaatac agcgtttttc atgtacaact atactagttg
tagtgcctaa 8700ataatgcttt taaaacttaa aaataatcta tgtcgggtgc ggagaaagag
gtaatgaaat 8760ggcaaatcaa tgtcctcagc gaaatggcat acgagtaaac ttggtctgac
accgctgcat 8820gagattatca aaaaggatct tcacctagat ccttttaaat taaaaatgaa
gttttaaatc 8880aatctaaagt atatatgagt aaacttggtc tgacagttac caatgcttaa
tcagtgaggc 8940acctatctca gcgatctgtc tatttcgttc atccatagtt gcctgactcc
ccgtcgtgta 9000gataactacg atacgggagg gcttaccatc tggccccagt gctgcaatga
taccgcgaga 9060cccacgctca ccggctccag atttatcagc aataaaccag ccagccggaa
gcgccgagcg 9120cagaagtggt cctgcaactt tatccgcctc catccagtct attaactgtt
gccgggaagc 9180tagagtaagt agttcgccag ttaatagttt gcggagcgtt gttgccattg
ctacaggcat 9240cgtggtgtca cgctcgtcgt ttggtatggc ttcattcagc tccggttccc
aacgatcaag 9300gcgagttaca tgatccccca tgttgtgcaa aaaagcggtt agctccttcg
gtcctccgat 9360ggttgtcaga agtaagttgg ccgcagtgtt atcactcatg gttatggcag
cactgcataa 9420ttctcttact gtcatgccat ccgtaagatg cttttctgtg actggtgagt
attcaaccaa 9480gtcattctga gaatagtgta tgcggcgacc gagttgctct tgcccggcgt
caatacggga 9540taataccgcg ccacatagca gaactttaaa agtgctcatc attgggaagc
gttcttcggg 9600gcgaaaactc tcaaggatct taccgctgtt gagatccagt tcgatgtaac
ccacacgagc 9660acccaactga tcttcagcat cttttacttt caccagcgtt tctgggtgag
caaaaacagg 9720aaggcaaaat gccgcaaaaa agggaataag ggcgacacgg aaatgttgaa
tactcatacg 9780cttccttttt caatagtatt gaagcattta tcagggttat tgtctcggga
gcgaatacat 9840atttgaatgt atttagaaaa a
9861810823DNAArtificial SequenceSynthetic ubiquitous and
hypoxia inducible promoter gene switch construct expressing bFGF
8taaacaaata ggggttccgc gcacatttcc ccgaaaagtg ccacctgacg tactgaggac
60gccagggttt tcccagtcac gacgttgtaa aacgacggcc agtagcagac aagcccgtca
120gggcgcgtca gcgggtgttg gccctaggac gaaaggaggt cgtgaaatgg ataaaaaaat
180acagcgtttt tcatgtacaa ctatactagt tgtagtgcct aaataatgct tttaaaactt
240aaaaataatc aatgtcctca gcggggtgtc ggggatttag gtgacactat aggctgagcg
300ccgcacaggc atctagaggc tatggcaggg cctgccgccc cgacgttggc tgcgagccct
360gggccttcac ccgaacttgg ggggtggggt ggggaaaagg aagaaacgcg ggcgtattgg
420ccccaatggg gtctcggtgg ggtatcgaca gagtgccagc cctgggaccg aaccccgcgt
480ttatgaacaa acgacccaac accgtgcgtt ttattctgtc tttttattgc cgtcatagcg
540cgggttcctt ccggtattgt ctccttccgt gtttcaatcg attcaaaaga actcgtccag
600cagacggtaa aaagcaatgc gttgagaatc cggtgcagca atgccataca gcaccagaaa
660gcgatcagcc cattcaccgc ccagttcttc agcaatgtca cgggttgcca gtgcgatgtc
720ctgatagcga tcagccacgc ccaggcgacc gcagtcgata aagccggaga aacggccgtt
780ttccaccata atgtttggca gacaagcatc gccgtgggtc acaaccaggt cctcgccatc
840tggcatacgt gctttcaggc gtgcgaacag ttctgccggt gccagaccct gatgttcctc
900gtccaggtca tcctgatcaa ccaggccagc ttccatgcga gtgcgtgcgc gctcgatacg
960gtgtttagct tggtgatcga atgggcaagt agctgggtcc agggtatgca gacggcgcat
1020agcatcagcc atgatggaaa ccttttctgc cggtgccaga tgagaggaca gcagatcctg
1080gcctggaacc tcgcccagca gcagccagtc gcggccagcc tcggtcacaa catccagcac
1140agctgcgcat ggaacgccgg tagtagccag ccaggacaga cgagctgctt catcttgcag
1200ttcgttcagt gcgccggaca gatcggtctt aacaaacagc accggacggc cttgagcgga
1260cagacggaac acagctgcgt cggagcaacc gatagtctgt tgagcccagt catagccaaa
1320cagacgttcc acccaagcag ccggagaacc agcgtgcaga ccgtcttgtt caatcatggt
1380ggcaattggg tgtctgagcg atgtggctcg gctggcgacg caaaagaaga tgcggctgac
1440tgtcgaacag gaggagcaga gagcgaagcg ggaggctgcg ggctcaattt gcatgcttta
1500gttcctcacc ttgtcgtatt atactatgcc gatatactat gccgatgatt aattgtcaac
1560gtatacggaa tagctctgag gccgaggcag cttcggcctc tgcataaata aaaaaaatta
1620gtcagccatg gggcggagaa tgggcggaac tgggcggagt taggggcggg atgggcggag
1680ttaggggcgg gactatggtt gctgactaat tgagatgctt gctttgcata cttctgcctg
1740ctggggagcc tggggacttt ccacacctgg ttgctgacta attgagatgc ttgctttgca
1800tacttctgcc tgctggggag cctggggact ttccacaccc taacctcgag gccatcgtgg
1860cacgccaggg ttttcccagt cacgacgttg taaaacgacg gccagtgctc ttctcccccg
1920cgggaggttt tataaatccg actgtctaga ttgttgttaa atcacacaaa aaaccaacac
1980acagatgtaa tgaaaataaa gatattttat tatcgattca gctgtcgctg gggctctcca
2040gcatctccat caggaaggtg tcgatgggca cgtcgccgat cagcctgaag aagaacaggt
2100gctccaggca cttcaggccg atgctcctca ggctgggcag cctcagcagc agcttggcga
2160atctgccggg ctcgtcgggg tgggtggtcc tggtgtactc ctccagggcg gcgtacacct
2220tctccctcag cagctccacc tcctgggcgc ttttcaggcc cctcacctcg gggttgaaca
2280ggatgatggc cctcaggcag cccagctcgg tcttgtccat cctcatgtcc ctcatcttgc
2340tcaccagctc ggtcagcacc ctgtcgaaga tggcgcccac tccggcgctg tgggcgctgt
2400tcctatggac gtgcaggccg gtggccagca ggatgccgtc cctcacgtcg atgctcctgt
2460ggctgaagct ggcgatcagc agctcgttcc atccggccct cagcaggatc acctggtcgt
2520ccaggggcag gctgctgaag tggggaatcc tcttggccca ctccaccagg gtgaacagct
2580gcttgtcggc ggcctggcag atgttggtca cggggtcgtt ggggctgctg ccgctgccgc
2640cggttccgcc ggggccctcc acgccctggt cgcttttctg ctccacggcg agttcggcct
2700ccagaatcct gtccacgggc atctccatat ggccgccgta ctcgtcgatg cccagggcgt
2760cggtgaacat ctgctcgaac tcgaagtcgg ccatgtccag ggcgccgtag ggggcgctgt
2820cgtggggggt gaagccgggg ccggggctgt cgccgtcgcc cagcatgtcc aggtcgaagt
2880cgtccagggc gtcggcgtgg gccatggcca cgtcctcgcc gtccaggtgc agctcgtcgc
2940ccaggctcac gtcggtgggg ggggccacct tccttttctt cttggggccc atggtggcca
3000attgcccccc cccccccccg catgccgtct aacaaaaaag ccaaaaacgg ccagaattta
3060gcggacaatt tactagtcta acactgaaaa ttacatattg acccaaatga ttacatttca
3120aaaggtgcct aaaaaacttc acaaaacaca ctcgccaacc ccgagcgcac gtacccagcc
3180cagcagcccg ctactcacca agtgacgatc acagcgatcc acaaacaaga accgcgaccc
3240aaatcccggc tgcgacggaa ctagctgtgc cacacccggc gcgtccttat ataatcatcg
3300gcgttcaccg ccccacggag atccctccgc agaatcgccg agaagggact acttttcctc
3360gcctgttccg ctctctggaa agaaaaccag tgccctagag tcacccaagt cccgtcctaa
3420aatgtccttc tgctgatact ggggttctaa ggccgagtct tatgagcagc gggccgctgt
3480cctgagcgtc cgggcggaag gatcaggacg ctcgctgcgc ccttcgtctg acgtggcagc
3540gctcgccgtg aggagggggg cgcccgcggg aggcgccaaa acccggcgcg gaggccctcg
3600agtaggcgag accaatgggt gcgccatggg ctcttccaaa aatttaggtg acactatagg
3660gcaccgctcg cacctgcgca caggcccgcg gctacaaact acgaacgatc attctagata
3720ccacatttgt agaggtttta cttgctttaa aaaacctccc acatctcccc ctgaacctga
3780aacataaaat gaatgcaaat gttttattaa cttgtttatt gcagcttata atggttacaa
3840attaagcaat agcatcacaa atttcacaaa ttaagcattt ttttcactgc attctagttg
3900tggtttgtcc aaactcatca atgtatctta tcatgtctaa tcgattcaca ggttggtggg
3960gctctccagg atgggggggg gctgggtgtg gctcatgtcg gccacgtccc aaatctcctc
4020caggaagggg ggcagcttcc tgttcttcag cttcaggctg atacacatat tgctgttctg
4080cattcccagg gtcctcagct cgctcaggat gctcaggatc ttgccgtaga tcacgctgct
4140cctggcgctg ccgctcagct ggttcaggat gtagatcctc agggtgttca ggtagtacct
4200ctggatctcc tccaccagct ggggctgctc caggccgggc ctgtcgctga agatcaccac
4260ggcggtcagc agggcgtagt ggatgttgtc cagggccatg ctgtacatac atctgcagaa
4320gtgcaggagg tcctcgatca cctcggccat gccagccttc ctgtagttgt ccctggtgta
4380agcctggttg ttggcgaaca ggatgctgtc gctggcggcg tcgtacctcc tggccaccct
4440cagcatcatc acctcgctgc tgcaagcctt cagcagggtg atctggtcgg gctggctgat
4500cttggcgaat ccgggcaggc ccttggcgaa ctccacgatc agctgcacgg tcaggatggt
4560catctcggtg atctgcctga agggggtgtc gctctcctcg ttctcgtcgt cggcctgctg
4620ccaggtctgg gtgatccttt tcaggtcctc gtcgctgggc tgctcgtagc cgtcctgata
4680ccagatcagc ctggcgatca ggaactgctg gttggcggtc agctggggga tgttcttctg
4740cctgttggtc accagcagct tgtcgctcag gaacctgggc acgacctcgt gaatcctggc
4800ggcctcgggg ggggggggct cgcactgcat gatggggggc atgtggtcat cgacggtggt
4860ggtgctcacg ggcagcttgt ccttctcctt ctgggccttc ttctccttcc ttttcatggc
4920gcactgggtc tcgggcacca cgcactcggg cctgatcccg ggaaactcgg ggctcacggt
4980cagctgcctc tggcccttgt tgctgctctc ctcgctgctg ctggtggcgc tgatcctgtg
5040ctgcctcagg gtcaggggca tgtcggtctc cacgctggcc agcctgtcgg tcacggcgtc
5100cttgttcacg ttgtcctgca cgaacaggcc ggtcagcagg gccttgatgt cttgcaggct
5160gtccatcttc aggatcatgt ccaggtcctc cctggggaag atcagcagga acagctgctc
5220cagcctctcc agcctgctct ccacctcggt caggtgggcc ctggtcaggg ggctcctctt
5280ggtcttgggg ctgtatctgc actcccagtt gttcttcagg cacttggcgc acttgggctt
5340ctccttgctg cacttcagct tcttcagcct gcagatgtcg caagcctgct cgatgctgct
5400cagcagcttc atggtggcca attgcccccc cccccccccg catgcacagg aagatgagct
5460cacacaccag ctaaggcacc cattatatac cctctaggtc ttcgcggacg tgcgggaacc
5520cacgtggggg ctccgtcacg tactccgagt cccgcacgca ctcagcgtcg ctctggggcc
5580ccacgtccgg ccctgacgtg cggcttcgtt tgtcacgtcg cggacgtgcg ggaacccacg
5640tgggggctcc gtcacgtact ccgagtcccg cacgcactca gcgtcgctct ggggccccac
5700gtccggccct gacgtgcggc ttcgtttgtc acgtcgcgga cgtgcgggaa cccacgtggg
5760ggctccgtca cgtactccga gtcccgcacg cactcagcgt cgctctgggg ccccacgtcc
5820ggccctgacg tgcggcttcg tttgtcacgt cgcggacgtg cgggaaccca cgtgggggct
5880ccgtcacgta ctccgagtcc cgcacgcact cagcgtcgct ctggggcccc acgtccggcc
5940ctgacgtgcg gcttcgtttg tcacgtcgcg gacgtgcggg aacccacgtg ggggctccgt
6000cacgtactcc gagtcccgca cgcactcagc gtcgctctgg ggccccacgt ccggccctga
6060cgtgcggctt cgtttgtcac gtcgcggacg tgcgggaacc cacgtggggg ctccgtcacg
6120tactccgagt cccgcacgca ctcagcgtcg ctctggggcc ccacgtccgg ccctgacgtg
6180cggcttcgtt tgtcacgtcg cggacgtgcg ggaacccacg tgggggctcc gtcacgtact
6240ccgagtcccg cacgcactca gcgtcgctct ggggccccac gtccggccct gacgtgcggc
6300ttcgtttgtc acgtcctcga gtggtaatac aatggccggt tcccatggac ctgcatcgtg
6360gtgtaactat aacggtccta aggtagcgac cgcggagact aggtgtattt atctaagcga
6420tcgcttaatt aaggccggcc gccgcaataa aatatcttta ttttcattac atctgtgtgt
6480tggttttttg tgtgaatcca tagtactaac atacgctctc catcaaaaca aaacgaaaca
6540aaacaaacta gcaaaatagg ctgtccccag tgcaagtcca ggtgccagaa catttctcta
6600tccataatgc aggggtaccg ggtgatgacg gtgaaaacct ccaattgcgg agtactgtcc
6660tccgagcgga gtactgtcct ccgagcggag tactgtcctc cgagcggagt actgtcctcc
6720gagcggagta ctgtcctccg agcggagtac tgtcctccga gcggagagtc cccggggacc
6780tagagggtat ataatgggtg ccttagctgg tgtgtgacct catcttcctg tacgcccctg
6840caggggcgcg ccacgcgtcg aagaaggtga gtaatcttaa catgctcttt tttttttttt
6900ttgctaatcc cttttgtgtg ctgatgttag gatgacattt acaacaaatg tttgttcctg
6960acaggaaaaa ccttgctggg taccttcgtt gccggacact tcttgtcctc tactttggaa
7020aaaaggaatt gagagccgct agcgccacca tggtgggtgt ggggggtgga gatgtagaag
7080atgtgacgcc caggcccggc gggtgccaga ttagcggacg cggtgccaga ggctgcaacg
7140gcatccccgg cgcagccgcc tgggaagccg ctctccccag gcggcgtccc agaaggcacc
7200catccgtgaa ccccaggagc agagccgccg gatcgccgcg caccaggggc agaagaacag
7260aggaacggcc gagcggcagc agactggggg acagaggcag aggcagagcg ctgccgggcg
7320ggaggctggg gggccggggc cggggccgtg ccccggagcg ggtcggaggc cggggccggg
7380gccgggggac ggcggctccc cgcgcggctc cagcggctcg gggctccaga cccggccccg
7440cagggacaat ggccgccggg agcatcacca cgctgcccgc cttgcccgag gatggcggca
7500gcggcgcttt cccgcccggc cacttcaagg accccaagcg gctgtactgc aaaaacgggg
7560gcttcttcct gcgcatccac cccgacggcc gagttgacgg ggtccgggag aagtccgacc
7620ctcacatcaa gctacaactt caagcagagg agagaggagt tgtgtctatc aaaggagtgt
7680gtgctaaccg ttacctggct atgaaggaag atggaagatt actggcttct aaatgtgtta
7740cggatgagtg tttctttttt gaacgattgg aatctaataa ctacaatact tacagaagca
7800ggaaatacac cagttggtat gtggcactga aacgaactgg gcagtataaa cttggcagca
7860aaacaggacc tgggcagaaa gctatacttt ttcttccaat gtctgctaag agctgaatcg
7920attgcgcaaa gctttcgcga taggcgagac caatgggtgt gtacgtagcg gccgcgtcga
7980cgatagcttg atgggtggca tccctgtgac ccctccccag tgcctctcct ggccctggaa
8040gttgccactc cagtgcccac cagccttgtc ctaataaaat taagttgcat cattttgtct
8100gactaggtgt ccttctataa tattatgggg tggagggggg tggtatggag caaggggcaa
8160gttgggaaga caacctgtag ggcctgcggg gtctattggg aaccaagctg gagtgcagtg
8220gcacaatctt ggctcactgc aatctccgcc tcctgggttc aagcgattct cctgcctcag
8280cctcccgagt tgttgggatt ccaggcatgc atgaccaggc tcagctaatt tttgtttttt
8340tggtagagac ggggtttcac catattggcc aggctggtct ccaactccta atctcaggtg
8400atctacccac cttggcctcc caaattgctg ggattacagg cgtgaaccac tgctcccttc
8460cctgtccttc tgattttaaa ataactatac cagcaggagg acgtccagac acagcatagg
8520ctacctggcc atgcccaacc ggtgggacat ttgagttgct tgcttggcac tgtcctctca
8580tgcgttgggt ccactcagta gatgcctgtt gaattctgat ttaaatcggt ccgcgtacgg
8640cgtggtaggt ccgaacgaat ccatggatta ccctgttatc cctactcaag gacatcatcc
8700ctttagtgag ggttaattca cgcagtgggt acggaactaa aggcagcaca catcgtgtaa
8760tcatggtcat agctgtttcc tgtgtgaaat tgttatccgc tacgtctctc ccccgcagta
8820agggctagat taactcgtct cgtgaatatc cggaactccc tttagtgagg gttaattgcg
8880ttgcgctcac tgcccgcttt ccagtcggga aacctgtcgt gccagcttaa tcatggtcat
8940agctgtttcc tgtgtgaaat tgttatccgc taccggaaac gcttccttca tgtgagcaaa
9000aggccagcaa aaggccagga accgtaaaaa ggccgcgttg ctggcgtttt tccataggct
9060ccgcccccct gacgagcatc acaaaaatcg acgctcaagt cagaggtggc gaaacccgac
9120aggactataa agataccagg cgtttccccc tggaagctcc ctcgtgcgct ctcctgttcc
9180gaccctgccg cttaccggat acctgtccgc ctttctccct tcgggaagcg tggcgctttc
9240tcatagctca cgctgtaggt atctcagttc ggtgtaggtc gttcgctcca agctgggctg
9300tgtgcacgaa ccccccgttc agcccgaccg ctgcgcctta tccggtaact atcgtcttga
9360gtccaacccg gtaagacacg acttatcgcc actggcagca gccactggta acaggattag
9420cagagcgagg tatgtaggcg gtgctacaga gttcttgaag tggtggccta actacggcta
9480cactagaaga acagtatttg gtatctgcgc tctgctgaag ccagttacct tcggaaaaag
9540agttggtagc tcttgatccg gcaaacaaac caccgctggt agcggtggtt tttttgtttg
9600caagcagcag attacgcgca gaaaaaaagg atctcaagaa gatcctttga tcttttctac
9660ggggtctgac gctcagtgga acgaaaactc acgttaaggg attttggtca tgatctatgt
9720cgggtgcgga gaaagaggta atgaaatggc atacgagtaa acttggtctg acaccgctgc
9780atgagattat caaaaaggat cttcacctag atccttttaa attaaaaatg aagttttaaa
9840tcaatctaaa gtatatatga gtaaacttgg tctgacagtt accaatgctt aatcagtgag
9900gcacctatct cagcgatctg tctatttcgt tcatccatag ttgcctgact ccccgtcgtg
9960tagataacta cgatacggga gggcttacca tctggcccca gtgctgcaat gataccgcga
10020gacccacgct caccggctcc agatttatca gcaataaacc agccagccgg aagcgccgag
10080cgcagaagtg gtcctgcaac tttatccgcc tccatccagt ctattaactg ttgccgggaa
10140gctagagtaa gtagttcgcc agttaatagt ttgcggagcg ttgttgccat tgctacaggc
10200atcgtggtgt cacgctcgtc gtttggtatg gcttcattca gctccggttc ccaacgatca
10260aggcgagtta catgatcccc catgttgtgc aaaaaagcgg ttagctcctt cggtcctccg
10320atggttgtca gaagtaagtt ggccgcagtg ttatcactca tggttatggc agcactgcat
10380aattctctta ctgtcatgcc atccgtaaga tgcttttctg tgactggtga gtattcaacc
10440aagtcattct gagaatagtg tatgcggcga ccgagttgct cttgcccggc gtcaatacgg
10500gataataccg cgccacatag cagaacttta aaagtgctca tcattgggaa gcgttcttcg
10560gggcgaaaac tctcaaggat cttaccgctg ttgagatcca gttcgatgta acccacacga
10620gcacccaact gatcttcagc atcttttact ttcaccagcg tttctgggtg agcaaaaaca
10680ggaaggcaaa atgccgcaaa aaagggaata agggcgacac ggaaatgttg aatactcata
10740cgcttccttt ttcaatagta ttgaagcatt tatcagggtt attgtctcgg gagcgaatac
10800atatttgaat gtatttagaa aaa
10823910538DNAArtificial SequenceSynthetic ubiquitous and hypoxia
inducible gene switch constructexpressing EPO 9taaacaaata
ggggttccgc gcacatttcc ccgaaaagtg ccacctgacg tactgaggac 60gccagggttt
tcccagtcac gacgttgtaa aacgacggcc agtagcagac aagcccgtca 120gggcgcgtca
gcgggtgttg gccctaggac gaaaggaggt cgtgaaatgg ataaaaaaat 180acagcgtttt
tcatgtacaa ctatactagt tgtagtgcct aaataatgct tttaaaactt 240aaaaataatc
aatgtcctca gcggggtgtc ggggatttag gtgacactat aggctgagcg 300ccgcacaggc
atctagaggc tatggcaggg cctgccgccc cgacgttggc tgcgagccct 360gggccttcac
ccgaacttgg ggggtggggt ggggaaaagg aagaaacgcg ggcgtattgg 420ccccaatggg
gtctcggtgg ggtatcgaca gagtgccagc cctgggaccg aaccccgcgt 480ttatgaacaa
acgacccaac accgtgcgtt ttattctgtc tttttattgc cgtcatagcg 540cgggttcctt
ccggtattgt ctccttccgt gtttcaatcg attcaaaaga actcgtccag 600cagacggtaa
aaagcaatgc gttgagaatc cggtgcagca atgccataca gcaccagaaa 660gcgatcagcc
cattcaccgc ccagttcttc agcaatgtca cgggttgcca gtgcgatgtc 720ctgatagcga
tcagccacgc ccaggcgacc gcagtcgata aagccggaga aacggccgtt 780ttccaccata
atgtttggca gacaagcatc gccgtgggtc acaaccaggt cctcgccatc 840tggcatacgt
gctttcaggc gtgcgaacag ttctgccggt gccagaccct gatgttcctc 900gtccaggtca
tcctgatcaa ccaggccagc ttccatgcga gtgcgtgcgc gctcgatacg 960gtgtttagct
tggtgatcga atgggcaagt agctgggtcc agggtatgca gacggcgcat 1020agcatcagcc
atgatggaaa ccttttctgc cggtgccaga tgagaggaca gcagatcctg 1080gcctggaacc
tcgcccagca gcagccagtc gcggccagcc tcggtcacaa catccagcac 1140agctgcgcat
ggaacgccgg tagtagccag ccaggacaga cgagctgctt catcttgcag 1200ttcgttcagt
gcgccggaca gatcggtctt aacaaacagc accggacggc cttgagcgga 1260cagacggaac
acagctgcgt cggagcaacc gatagtctgt tgagcccagt catagccaaa 1320cagacgttcc
acccaagcag ccggagaacc agcgtgcaga ccgtcttgtt caatcatggt 1380ggcaattggg
tgtctgagcg atgtggctcg gctggcgacg caaaagaaga tgcggctgac 1440tgtcgaacag
gaggagcaga gagcgaagcg ggaggctgcg ggctcaattt gcatgcttta 1500gttcctcacc
ttgtcgtatt atactatgcc gatatactat gccgatgatt aattgtcaac 1560gtatacggaa
tagctctgag gccgaggcag cttcggcctc tgcataaata aaaaaaatta 1620gtcagccatg
gggcggagaa tgggcggaac tgggcggagt taggggcggg atgggcggag 1680ttaggggcgg
gactatggtt gctgactaat tgagatgctt gctttgcata cttctgcctg 1740ctggggagcc
tggggacttt ccacacctgg ttgctgacta attgagatgc ttgctttgca 1800tacttctgcc
tgctggggag cctggggact ttccacaccc taacctcgag gccatcgtgg 1860cacgccaggg
ttttcccagt cacgacgttg taaaacgacg gccagtgctc ttctcccccg 1920cgggaggttt
tataaatccg actgtctaga ttgttgttaa atcacacaaa aaaccaacac 1980acagatgtaa
tgaaaataaa gatattttat tatcgattca gctgtcgctg gggctctcca 2040gcatctccat
caggaaggtg tcgatgggca cgtcgccgat cagcctgaag aagaacaggt 2100gctccaggca
cttcaggccg atgctcctca ggctgggcag cctcagcagc agcttggcga 2160atctgccggg
ctcgtcgggg tgggtggtcc tggtgtactc ctccagggcg gcgtacacct 2220tctccctcag
cagctccacc tcctgggcgc ttttcaggcc cctcacctcg gggttgaaca 2280ggatgatggc
cctcaggcag cccagctcgg tcttgtccat cctcatgtcc ctcatcttgc 2340tcaccagctc
ggtcagcacc ctgtcgaaga tggcgcccac tccggcgctg tgggcgctgt 2400tcctatggac
gtgcaggccg gtggccagca ggatgccgtc cctcacgtcg atgctcctgt 2460ggctgaagct
ggcgatcagc agctcgttcc atccggccct cagcaggatc acctggtcgt 2520ccaggggcag
gctgctgaag tggggaatcc tcttggccca ctccaccagg gtgaacagct 2580gcttgtcggc
ggcctggcag atgttggtca cggggtcgtt ggggctgctg ccgctgccgc 2640cggttccgcc
ggggccctcc acgccctggt cgcttttctg ctccacggcg agttcggcct 2700ccagaatcct
gtccacgggc atctccatat ggccgccgta ctcgtcgatg cccagggcgt 2760cggtgaacat
ctgctcgaac tcgaagtcgg ccatgtccag ggcgccgtag ggggcgctgt 2820cgtggggggt
gaagccgggg ccggggctgt cgccgtcgcc cagcatgtcc aggtcgaagt 2880cgtccagggc
gtcggcgtgg gccatggcca cgtcctcgcc gtccaggtgc agctcgtcgc 2940ccaggctcac
gtcggtgggg ggggccacct tccttttctt cttggggccc atggtggcca 3000attgcccccc
cccccccccg catgccgtct aacaaaaaag ccaaaaacgg ccagaattta 3060gcggacaatt
tactagtcta acactgaaaa ttacatattg acccaaatga ttacatttca 3120aaaggtgcct
aaaaaacttc acaaaacaca ctcgccaacc ccgagcgcac gtacccagcc 3180cagcagcccg
ctactcacca agtgacgatc acagcgatcc acaaacaaga accgcgaccc 3240aaatcccggc
tgcgacggaa ctagctgtgc cacacccggc gcgtccttat ataatcatcg 3300gcgttcaccg
ccccacggag atccctccgc agaatcgccg agaagggact acttttcctc 3360gcctgttccg
ctctctggaa agaaaaccag tgccctagag tcacccaagt cccgtcctaa 3420aatgtccttc
tgctgatact ggggttctaa ggccgagtct tatgagcagc gggccgctgt 3480cctgagcgtc
cgggcggaag gatcaggacg ctcgctgcgc ccttcgtctg acgtggcagc 3540gctcgccgtg
aggagggggg cgcccgcggg aggcgccaaa acccggcgcg gaggccctcg 3600agtaggcgag
accaatgggt gcgccatggg ctcttccaaa aatttaggtg acactatagg 3660gcaccgctcg
cacctgcgca caggcccgcg gctacaaact acgaacgatc attctagata 3720ccacatttgt
agaggtttta cttgctttaa aaaacctccc acatctcccc ctgaacctga 3780aacataaaat
gaatgcaaat gttttattaa cttgtttatt gcagcttata atggttacaa 3840attaagcaat
agcatcacaa atttcacaaa ttaagcattt ttttcactgc attctagttg 3900tggtttgtcc
aaactcatca atgtatctta tcatgtctaa tcgattcaca ggttggtggg 3960gctctccagg
atgggggggg gctgggtgtg gctcatgtcg gccacgtccc aaatctcctc 4020caggaagggg
ggcagcttcc tgttcttcag cttcaggctg atacacatat tgctgttctg 4080cattcccagg
gtcctcagct cgctcaggat gctcaggatc ttgccgtaga tcacgctgct 4140cctggcgctg
ccgctcagct ggttcaggat gtagatcctc agggtgttca ggtagtacct 4200ctggatctcc
tccaccagct ggggctgctc caggccgggc ctgtcgctga agatcaccac 4260ggcggtcagc
agggcgtagt ggatgttgtc cagggccatg ctgtacatac atctgcagaa 4320gtgcaggagg
tcctcgatca cctcggccat gccagccttc ctgtagttgt ccctggtgta 4380agcctggttg
ttggcgaaca ggatgctgtc gctggcggcg tcgtacctcc tggccaccct 4440cagcatcatc
acctcgctgc tgcaagcctt cagcagggtg atctggtcgg gctggctgat 4500cttggcgaat
ccgggcaggc ccttggcgaa ctccacgatc agctgcacgg tcaggatggt 4560catctcggtg
atctgcctga agggggtgtc gctctcctcg ttctcgtcgt cggcctgctg 4620ccaggtctgg
gtgatccttt tcaggtcctc gtcgctgggc tgctcgtagc cgtcctgata 4680ccagatcagc
ctggcgatca ggaactgctg gttggcggtc agctggggga tgttcttctg 4740cctgttggtc
accagcagct tgtcgctcag gaacctgggc acgacctcgt gaatcctggc 4800ggcctcgggg
ggggggggct cgcactgcat gatggggggc atgtggtcat cgacggtggt 4860ggtgctcacg
ggcagcttgt ccttctcctt ctgggccttc ttctccttcc ttttcatggc 4920gcactgggtc
tcgggcacca cgcactcggg cctgatcccg ggaaactcgg ggctcacggt 4980cagctgcctc
tggcccttgt tgctgctctc ctcgctgctg ctggtggcgc tgatcctgtg 5040ctgcctcagg
gtcaggggca tgtcggtctc cacgctggcc agcctgtcgg tcacggcgtc 5100cttgttcacg
ttgtcctgca cgaacaggcc ggtcagcagg gccttgatgt cttgcaggct 5160gtccatcttc
aggatcatgt ccaggtcctc cctggggaag atcagcagga acagctgctc 5220cagcctctcc
agcctgctct ccacctcggt caggtgggcc ctggtcaggg ggctcctctt 5280ggtcttgggg
ctgtatctgc actcccagtt gttcttcagg cacttggcgc acttgggctt 5340ctccttgctg
cacttcagct tcttcagcct gcagatgtcg caagcctgct cgatgctgct 5400cagcagcttc
atggtggcca attgcccccc cccccccccg catgcacagg aagatgagct 5460cacacaccag
ctaaggcacc cattatatac cctctaggtc ttcgcggacg tgcgggaacc 5520cacgtggggg
ctccgtcacg tactccgagt cccgcacgca ctcagcgtcg ctctggggcc 5580ccacgtccgg
ccctgacgtg cggcttcgtt tgtcacgtcg cggacgtgcg ggaacccacg 5640tgggggctcc
gtcacgtact ccgagtcccg cacgcactca gcgtcgctct ggggccccac 5700gtccggccct
gacgtgcggc ttcgtttgtc acgtcgcgga cgtgcgggaa cccacgtggg 5760ggctccgtca
cgtactccga gtcccgcacg cactcagcgt cgctctgggg ccccacgtcc 5820ggccctgacg
tgcggcttcg tttgtcacgt cgcggacgtg cgggaaccca cgtgggggct 5880ccgtcacgta
ctccgagtcc cgcacgcact cagcgtcgct ctggggcccc acgtccggcc 5940ctgacgtgcg
gcttcgtttg tcacgtcgcg gacgtgcggg aacccacgtg ggggctccgt 6000cacgtactcc
gagtcccgca cgcactcagc gtcgctctgg ggccccacgt ccggccctga 6060cgtgcggctt
cgtttgtcac gtcgcggacg tgcgggaacc cacgtggggg ctccgtcacg 6120tactccgagt
cccgcacgca ctcagcgtcg ctctggggcc ccacgtccgg ccctgacgtg 6180cggcttcgtt
tgtcacgtcg cggacgtgcg ggaacccacg tgggggctcc gtcacgtact 6240ccgagtcccg
cacgcactca gcgtcgctct ggggccccac gtccggccct gacgtgcggc 6300ttcgtttgtc
acgtcctcga gtggtaatac aatggccggt tcccatggac ctgcatcgtg 6360gtgtaactat
aacggtccta aggtagcgac cgcggagact aggtgtattt atctaagcga 6420tcgcttaatt
aaggccggcc gccgcaataa aatatcttta ttttcattac atctgtgtgt 6480tggttttttg
tgtgaatcca tagtactaac atacgctctc catcaaaaca aaacgaaaca 6540aaacaaacta
gcaaaatagg ctgtccccag tgcaagtcca ggtgccagaa catttctcta 6600tccataatgc
aggggtaccg ggtgatgacg gtgaaaacct ccaattgcgg agtactgtcc 6660tccgagcgga
gtactgtcct ccgagcggag tactgtcctc cgagcggagt actgtcctcc 6720gagcggagta
ctgtcctccg agcggagtac tgtcctccga gcggagagtc cccggggacc 6780tagagggtat
ataatgggtg ccttagctgg tgtgtgacct catcttcctg tacgcccctg 6840caggggcgcg
ccacgcgtcg aagaaggtga gtaatcttaa catgctcttt tttttttttt 6900ttgctaatcc
cttttgtgtg ctgatgttag gatgacattt acaacaaatg tttgttcctg 6960acaggaaaaa
ccttgctggg taccttcgtt gccggacact tcttgtcctc tactttggaa 7020aaaaggaatt
gagagccgct agcgccacca tgggggtgca tgaatgtcct gcctggctgt 7080ggcttctcct
gtccctgctg tcgctccctc tgggcctccc agtcctgggc gctccaccac 7140gcctcatctg
tgacagccga gtcctggaga gatacctgtt ggaggccaag gaagccgaga 7200atatcacgac
gggctgtgct gaacactgct ccttgaatga gaatatcact gtcccagaca 7260ccaaagttaa
tttctatgcc tggaagcgga tggaggtcgg gcagcaggcc gtagaagtct 7320ggcagggcct
ggccctgctg tcggaagctg tcctgcgggg ccaggccctg ttggtcaaca 7380gcagccagcc
gtgggagccc ctgcaactgc atgtggataa agccgtcagt ggccttcgca 7440gcctcaccac
tctgcttcgg gctctgcgag cccagaagga agccatctcc cctccagatg 7500cggccagcgc
cgctccactc cgaacaatca ctgctgacac tttccgcaaa ctgttccgag 7560tctactccaa
tttcctccgg ggaaagctga agctgtacac aggggaggct tgcaggacag 7620gggacagatg
aatcgattgc gcaaagcttt cgcgataggc gagaccaatg ggtgtgtacg 7680tagcggccgc
gtcgacgata gcttgatggg tggcatccct gtgacccctc cccagtgcct 7740ctcctggccc
tggaagttgc cactccagtg cccaccagcc ttgtcctaat aaaattaagt 7800tgcatcattt
tgtctgacta ggtgtccttc tataatatta tggggtggag gggggtggta 7860tggagcaagg
ggcaagttgg gaagacaacc tgtagggcct gcggggtcta ttgggaacca 7920agctggagtg
cagtggcaca atcttggctc actgcaatct ccgcctcctg ggttcaagcg 7980attctcctgc
ctcagcctcc cgagttgttg ggattccagg catgcatgac caggctcagc 8040taatttttgt
ttttttggta gagacggggt ttcaccatat tggccaggct ggtctccaac 8100tcctaatctc
aggtgatcta cccaccttgg cctcccaaat tgctgggatt acaggcgtga 8160accactgctc
ccttccctgt ccttctgatt ttaaaataac tataccagca ggaggacgtc 8220cagacacagc
ataggctacc tggccatgcc caaccggtgg gacatttgag ttgcttgctt 8280ggcactgtcc
tctcatgcgt tgggtccact cagtagatgc ctgttgaatt ctgatttaaa 8340tcggtccgcg
tacggcgtgg taggtccgaa cgaatccatg gattaccctg ttatccctac 8400tcaaggacat
catcccttta gtgagggtta attcacgcag tgggtacgga actaaaggca 8460gcacacatcg
tgtaatcatg gtcatagctg tttcctgtgt gaaattgtta tccgctacgt 8520ctctcccccg
cagtaagggc tagattaact cgtctcgtga atatccggaa ctccctttag 8580tgagggttaa
ttgcgttgcg ctcactgccc gctttccagt cgggaaacct gtcgtgccag 8640cttaatcatg
gtcatagctg tttcctgtgt gaaattgtta tccgctaccg gaaacgcttc 8700cttcatgtga
gcaaaaggcc agcaaaaggc caggaaccgt aaaaaggccg cgttgctggc 8760gtttttccat
aggctccgcc cccctgacga gcatcacaaa aatcgacgct caagtcagag 8820gtggcgaaac
ccgacaggac tataaagata ccaggcgttt ccccctggaa gctccctcgt 8880gcgctctcct
gttccgaccc tgccgcttac cggatacctg tccgcctttc tcccttcggg 8940aagcgtggcg
ctttctcata gctcacgctg taggtatctc agttcggtgt aggtcgttcg 9000ctccaagctg
ggctgtgtgc acgaaccccc cgttcagccc gaccgctgcg ccttatccgg 9060taactatcgt
cttgagtcca acccggtaag acacgactta tcgccactgg cagcagccac 9120tggtaacagg
attagcagag cgaggtatgt aggcggtgct acagagttct tgaagtggtg 9180gcctaactac
ggctacacta gaagaacagt atttggtatc tgcgctctgc tgaagccagt 9240taccttcgga
aaaagagttg gtagctcttg atccggcaaa caaaccaccg ctggtagcgg 9300tggttttttt
gtttgcaagc agcagattac gcgcagaaaa aaaggatctc aagaagatcc 9360tttgatcttt
tctacggggt ctgacgctca gtggaacgaa aactcacgtt aagggatttt 9420ggtcatgatc
tatgtcgggt gcggagaaag aggtaatgaa atggcatacg agtaaacttg 9480gtctgacacc
gctgcatgag attatcaaaa aggatcttca cctagatcct tttaaattaa 9540aaatgaagtt
ttaaatcaat ctaaagtata tatgagtaaa cttggtctga cagttaccaa 9600tgcttaatca
gtgaggcacc tatctcagcg atctgtctat ttcgttcatc catagttgcc 9660tgactccccg
tcgtgtagat aactacgata cgggagggct taccatctgg ccccagtgct 9720gcaatgatac
cgcgagaccc acgctcaccg gctccagatt tatcagcaat aaaccagcca 9780gccggaagcg
ccgagcgcag aagtggtcct gcaactttat ccgcctccat ccagtctatt 9840aactgttgcc
gggaagctag agtaagtagt tcgccagtta atagtttgcg gagcgttgtt 9900gccattgcta
caggcatcgt ggtgtcacgc tcgtcgtttg gtatggcttc attcagctcc 9960ggttcccaac
gatcaaggcg agttacatga tcccccatgt tgtgcaaaaa agcggttagc 10020tccttcggtc
ctccgatggt tgtcagaagt aagttggccg cagtgttatc actcatggtt 10080atggcagcac
tgcataattc tcttactgtc atgccatccg taagatgctt ttctgtgact 10140ggtgagtatt
caaccaagtc attctgagaa tagtgtatgc ggcgaccgag ttgctcttgc 10200ccggcgtcaa
tacgggataa taccgcgcca catagcagaa ctttaaaagt gctcatcatt 10260gggaagcgtt
cttcggggcg aaaactctca aggatcttac cgctgttgag atccagttcg 10320atgtaaccca
cacgagcacc caactgatct tcagcatctt ttactttcac cagcgtttct 10380gggtgagcaa
aaacaggaag gcaaaatgcc gcaaaaaagg gaataagggc gacacggaaa 10440tgttgaatac
tcatacgctt cctttttcaa tagtattgaa gcatttatca gggttattgt 10500ctcgggagcg
aatacatatt tgaatgtatt tagaaaaa
105381010361DNAArtificial SequenceSynthetic ubiquitous and hypoxia
inducible gene switch constructexpressing BNP 10taaacaaata
ggggttccgc gcacatttcc ccgaaaagtg ccacctgacg tactgaggac 60gccagggttt
tcccagtcac gacgttgtaa aacgacggcc agtagcagac aagcccgtca 120gggcgcgtca
gcgggtgttg gccctaggac gaaaggaggt cgtgaaatgg ataaaaaaat 180acagcgtttt
tcatgtacaa ctatactagt tgtagtgcct aaataatgct tttaaaactt 240aaaaataatc
aatgtcctca gcggggtgtc ggggatttag gtgacactat aggctgagcg 300ccgcacaggc
atctagaggc tatggcaggg cctgccgccc cgacgttggc tgcgagccct 360gggccttcac
ccgaacttgg ggggtggggt ggggaaaagg aagaaacgcg ggcgtattgg 420ccccaatggg
gtctcggtgg ggtatcgaca gagtgccagc cctgggaccg aaccccgcgt 480ttatgaacaa
acgacccaac accgtgcgtt ttattctgtc tttttattgc cgtcatagcg 540cgggttcctt
ccggtattgt ctccttccgt gtttcaatcg attcaaaaga actcgtccag 600cagacggtaa
aaagcaatgc gttgagaatc cggtgcagca atgccataca gcaccagaaa 660gcgatcagcc
cattcaccgc ccagttcttc agcaatgtca cgggttgcca gtgcgatgtc 720ctgatagcga
tcagccacgc ccaggcgacc gcagtcgata aagccggaga aacggccgtt 780ttccaccata
atgtttggca gacaagcatc gccgtgggtc acaaccaggt cctcgccatc 840tggcatacgt
gctttcaggc gtgcgaacag ttctgccggt gccagaccct gatgttcctc 900gtccaggtca
tcctgatcaa ccaggccagc ttccatgcga gtgcgtgcgc gctcgatacg 960gtgtttagct
tggtgatcga atgggcaagt agctgggtcc agggtatgca gacggcgcat 1020agcatcagcc
atgatggaaa ccttttctgc cggtgccaga tgagaggaca gcagatcctg 1080gcctggaacc
tcgcccagca gcagccagtc gcggccagcc tcggtcacaa catccagcac 1140agctgcgcat
ggaacgccgg tagtagccag ccaggacaga cgagctgctt catcttgcag 1200ttcgttcagt
gcgccggaca gatcggtctt aacaaacagc accggacggc cttgagcgga 1260cagacggaac
acagctgcgt cggagcaacc gatagtctgt tgagcccagt catagccaaa 1320cagacgttcc
acccaagcag ccggagaacc agcgtgcaga ccgtcttgtt caatcatggt 1380ggcaattggg
tgtctgagcg atgtggctcg gctggcgacg caaaagaaga tgcggctgac 1440tgtcgaacag
gaggagcaga gagcgaagcg ggaggctgcg ggctcaattt gcatgcttta 1500gttcctcacc
ttgtcgtatt atactatgcc gatatactat gccgatgatt aattgtcaac 1560gtatacggaa
tagctctgag gccgaggcag cttcggcctc tgcataaata aaaaaaatta 1620gtcagccatg
gggcggagaa tgggcggaac tgggcggagt taggggcggg atgggcggag 1680ttaggggcgg
gactatggtt gctgactaat tgagatgctt gctttgcata cttctgcctg 1740ctggggagcc
tggggacttt ccacacctgg ttgctgacta attgagatgc ttgctttgca 1800tacttctgcc
tgctggggag cctggggact ttccacaccc taacctcgag gccatcgtgg 1860cacgccaggg
ttttcccagt cacgacgttg taaaacgacg gccagtgctc ttctcccccg 1920cgggaggttt
tataaatccg actgtctaga ttgttgttaa atcacacaaa aaaccaacac 1980acagatgtaa
tgaaaataaa gatattttat tatcgattca gctgtcgctg gggctctcca 2040gcatctccat
caggaaggtg tcgatgggca cgtcgccgat cagcctgaag aagaacaggt 2100gctccaggca
cttcaggccg atgctcctca ggctgggcag cctcagcagc agcttggcga 2160atctgccggg
ctcgtcgggg tgggtggtcc tggtgtactc ctccagggcg gcgtacacct 2220tctccctcag
cagctccacc tcctgggcgc ttttcaggcc cctcacctcg gggttgaaca 2280ggatgatggc
cctcaggcag cccagctcgg tcttgtccat cctcatgtcc ctcatcttgc 2340tcaccagctc
ggtcagcacc ctgtcgaaga tggcgcccac tccggcgctg tgggcgctgt 2400tcctatggac
gtgcaggccg gtggccagca ggatgccgtc cctcacgtcg atgctcctgt 2460ggctgaagct
ggcgatcagc agctcgttcc atccggccct cagcaggatc acctggtcgt 2520ccaggggcag
gctgctgaag tggggaatcc tcttggccca ctccaccagg gtgaacagct 2580gcttgtcggc
ggcctggcag atgttggtca cggggtcgtt ggggctgctg ccgctgccgc 2640cggttccgcc
ggggccctcc acgccctggt cgcttttctg ctccacggcg agttcggcct 2700ccagaatcct
gtccacgggc atctccatat ggccgccgta ctcgtcgatg cccagggcgt 2760cggtgaacat
ctgctcgaac tcgaagtcgg ccatgtccag ggcgccgtag ggggcgctgt 2820cgtggggggt
gaagccgggg ccggggctgt cgccgtcgcc cagcatgtcc aggtcgaagt 2880cgtccagggc
gtcggcgtgg gccatggcca cgtcctcgcc gtccaggtgc agctcgtcgc 2940ccaggctcac
gtcggtgggg ggggccacct tccttttctt cttggggccc atggtggcca 3000attgcccccc
cccccccccg catgccgtct aacaaaaaag ccaaaaacgg ccagaattta 3060gcggacaatt
tactagtcta acactgaaaa ttacatattg acccaaatga ttacatttca 3120aaaggtgcct
aaaaaacttc acaaaacaca ctcgccaacc ccgagcgcac gtacccagcc 3180cagcagcccg
ctactcacca agtgacgatc acagcgatcc acaaacaaga accgcgaccc 3240aaatcccggc
tgcgacggaa ctagctgtgc cacacccggc gcgtccttat ataatcatcg 3300gcgttcaccg
ccccacggag atccctccgc agaatcgccg agaagggact acttttcctc 3360gcctgttccg
ctctctggaa agaaaaccag tgccctagag tcacccaagt cccgtcctaa 3420aatgtccttc
tgctgatact ggggttctaa ggccgagtct tatgagcagc gggccgctgt 3480cctgagcgtc
cgggcggaag gatcaggacg ctcgctgcgc ccttcgtctg acgtggcagc 3540gctcgccgtg
aggagggggg cgcccgcggg aggcgccaaa acccggcgcg gaggccctcg 3600agtaggcgag
accaatgggt gcgccatggg ctcttccaaa aatttaggtg acactatagg 3660gcaccgctcg
cacctgcgca caggcccgcg gctacaaact acgaacgatc attctagata 3720ccacatttgt
agaggtttta cttgctttaa aaaacctccc acatctcccc ctgaacctga 3780aacataaaat
gaatgcaaat gttttattaa cttgtttatt gcagcttata atggttacaa 3840attaagcaat
agcatcacaa atttcacaaa ttaagcattt ttttcactgc attctagttg 3900tggtttgtcc
aaactcatca atgtatctta tcatgtctaa tcgattcaca ggttggtggg 3960gctctccagg
atgggggggg gctgggtgtg gctcatgtcg gccacgtccc aaatctcctc 4020caggaagggg
ggcagcttcc tgttcttcag cttcaggctg atacacatat tgctgttctg 4080cattcccagg
gtcctcagct cgctcaggat gctcaggatc ttgccgtaga tcacgctgct 4140cctggcgctg
ccgctcagct ggttcaggat gtagatcctc agggtgttca ggtagtacct 4200ctggatctcc
tccaccagct ggggctgctc caggccgggc ctgtcgctga agatcaccac 4260ggcggtcagc
agggcgtagt ggatgttgtc cagggccatg ctgtacatac atctgcagaa 4320gtgcaggagg
tcctcgatca cctcggccat gccagccttc ctgtagttgt ccctggtgta 4380agcctggttg
ttggcgaaca ggatgctgtc gctggcggcg tcgtacctcc tggccaccct 4440cagcatcatc
acctcgctgc tgcaagcctt cagcagggtg atctggtcgg gctggctgat 4500cttggcgaat
ccgggcaggc ccttggcgaa ctccacgatc agctgcacgg tcaggatggt 4560catctcggtg
atctgcctga agggggtgtc gctctcctcg ttctcgtcgt cggcctgctg 4620ccaggtctgg
gtgatccttt tcaggtcctc gtcgctgggc tgctcgtagc cgtcctgata 4680ccagatcagc
ctggcgatca ggaactgctg gttggcggtc agctggggga tgttcttctg 4740cctgttggtc
accagcagct tgtcgctcag gaacctgggc acgacctcgt gaatcctggc 4800ggcctcgggg
ggggggggct cgcactgcat gatggggggc atgtggtcat cgacggtggt 4860ggtgctcacg
ggcagcttgt ccttctcctt ctgggccttc ttctccttcc ttttcatggc 4920gcactgggtc
tcgggcacca cgcactcggg cctgatcccg ggaaactcgg ggctcacggt 4980cagctgcctc
tggcccttgt tgctgctctc ctcgctgctg ctggtggcgc tgatcctgtg 5040ctgcctcagg
gtcaggggca tgtcggtctc cacgctggcc agcctgtcgg tcacggcgtc 5100cttgttcacg
ttgtcctgca cgaacaggcc ggtcagcagg gccttgatgt cttgcaggct 5160gtccatcttc
aggatcatgt ccaggtcctc cctggggaag atcagcagga acagctgctc 5220cagcctctcc
agcctgctct ccacctcggt caggtgggcc ctggtcaggg ggctcctctt 5280ggtcttgggg
ctgtatctgc actcccagtt gttcttcagg cacttggcgc acttgggctt 5340ctccttgctg
cacttcagct tcttcagcct gcagatgtcg caagcctgct cgatgctgct 5400cagcagcttc
atggtggcca attgcccccc cccccccccg catgcacagg aagatgagct 5460cacacaccag
ctaaggcacc cattatatac cctctaggtc ttcgcggacg tgcgggaacc 5520cacgtggggg
ctccgtcacg tactccgagt cccgcacgca ctcagcgtcg ctctggggcc 5580ccacgtccgg
ccctgacgtg cggcttcgtt tgtcacgtcg cggacgtgcg ggaacccacg 5640tgggggctcc
gtcacgtact ccgagtcccg cacgcactca gcgtcgctct ggggccccac 5700gtccggccct
gacgtgcggc ttcgtttgtc acgtcgcgga cgtgcgggaa cccacgtggg 5760ggctccgtca
cgtactccga gtcccgcacg cactcagcgt cgctctgggg ccccacgtcc 5820ggccctgacg
tgcggcttcg tttgtcacgt cgcggacgtg cgggaaccca cgtgggggct 5880ccgtcacgta
ctccgagtcc cgcacgcact cagcgtcgct ctggggcccc acgtccggcc 5940ctgacgtgcg
gcttcgtttg tcacgtcgcg gacgtgcggg aacccacgtg ggggctccgt 6000cacgtactcc
gagtcccgca cgcactcagc gtcgctctgg ggccccacgt ccggccctga 6060cgtgcggctt
cgtttgtcac gtcgcggacg tgcgggaacc cacgtggggg ctccgtcacg 6120tactccgagt
cccgcacgca ctcagcgtcg ctctggggcc ccacgtccgg ccctgacgtg 6180cggcttcgtt
tgtcacgtcg cggacgtgcg ggaacccacg tgggggctcc gtcacgtact 6240ccgagtcccg
cacgcactca gcgtcgctct ggggccccac gtccggccct gacgtgcggc 6300ttcgtttgtc
acgtcctcga gtggtaatac aatggccggt tcccatggac ctgcatcgtg 6360gtgtaactat
aacggtccta aggtagcgac cgcggagact aggtgtattt atctaagcga 6420tcgcttaatt
aaggccggcc gccgcaataa aatatcttta ttttcattac atctgtgtgt 6480tggttttttg
tgtgaatcca tagtactaac atacgctctc catcaaaaca aaacgaaaca 6540aaacaaacta
gcaaaatagg ctgtccccag tgcaagtcca ggtgccagaa catttctcta 6600tccataatgc
aggggtaccg ggtgatgacg gtgaaaacct ccaattgcgg agtactgtcc 6660tccgagcgga
gtactgtcct ccgagcggag tactgtcctc cgagcggagt actgtcctcc 6720gagcggagta
ctgtcctccg agcggagtac tgtcctccga gcggagagtc cccggggacc 6780tagagggtat
ataatgggtg ccttagctgg tgtgtgacct catcttcctg tacgcccctg 6840caggggcgcg
ccacgcgtcg aagaaggtga gtaatcttaa catgctcttt tttttttttt 6900ttgctaatcc
cttttgtgtg ctgatgttag gatgacattt acaacaaatg tttgttcctg 6960acaggaaaaa
ccttgctggg taccttcgtt gccggacact tcttgtcctc tactttggaa 7020aaaaggaatt
gagagccgct agcgccacca tggaccccca gacagcacct agcagagccc 7080tcctgctcct
gctgttcttg catctggctt tcctgggagg tcgttcccac ccgctgggca 7140gccccggttc
agcctcggac ttggaaacgt ccgggttaca ggagcagcgc aaccatttgc 7200agggcaaact
gtcggagctg caagtggagc agacatccct ggagcccctc caggagagcc 7260cccgtcccac
aggtgtctgg aagtccagag aggtagccac cgagggcatc cgtgggcacc 7320gcaaaatggt
cctctacacc ctgcgggcac cacgaagccc caagatggtg caagggtctg 7380gctgctttgg
gaggaagatg gaccggatca gctccagcag cggcctgggc tgcaaagtgc 7440tgagaaggca
ctaaatcgat tgcgcaaagc tttcgcgata ggcgagacca atgggtgtgt 7500acgtagcggc
cgcgtcgacg atagcttgat gggtggcatc cctgtgaccc ctccccagtg 7560cctctcctgg
ccctggaagt tgccactcca gtgcccacca gccttgtcct aataaaatta 7620agttgcatca
ttttgtctga ctaggtgtcc ttctataata ttatggggtg gaggggggtg 7680gtatggagca
aggggcaagt tgggaagaca acctgtaggg cctgcggggt ctattgggaa 7740ccaagctgga
gtgcagtggc acaatcttgg ctcactgcaa tctccgcctc ctgggttcaa 7800gcgattctcc
tgcctcagcc tcccgagttg ttgggattcc aggcatgcat gaccaggctc 7860agctaatttt
tgtttttttg gtagagacgg ggtttcacca tattggccag gctggtctcc 7920aactcctaat
ctcaggtgat ctacccacct tggcctccca aattgctggg attacaggcg 7980tgaaccactg
ctcccttccc tgtccttctg attttaaaat aactatacca gcaggaggac 8040gtccagacac
agcataggct acctggccat gcccaaccgg tgggacattt gagttgcttg 8100cttggcactg
tcctctcatg cgttgggtcc actcagtaga tgcctgttga attctgattt 8160aaatcggtcc
gcgtacggcg tggtaggtcc gaacgaatcc atggattacc ctgttatccc 8220tactcaagga
catcatccct ttagtgaggg ttaattcacg cagtgggtac ggaactaaag 8280gcagcacaca
tcgtgtaatc atggtcatag ctgtttcctg tgtgaaattg ttatccgcta 8340cgtctctccc
ccgcagtaag ggctagatta actcgtctcg tgaatatccg gaactccctt 8400tagtgagggt
taattgcgtt gcgctcactg cccgctttcc agtcgggaaa cctgtcgtgc 8460cagcttaatc
atggtcatag ctgtttcctg tgtgaaattg ttatccgcta ccggaaacgc 8520ttccttcatg
tgagcaaaag gccagcaaaa ggccaggaac cgtaaaaagg ccgcgttgct 8580ggcgtttttc
cataggctcc gcccccctga cgagcatcac aaaaatcgac gctcaagtca 8640gaggtggcga
aacccgacag gactataaag ataccaggcg tttccccctg gaagctccct 8700cgtgcgctct
cctgttccga ccctgccgct taccggatac ctgtccgcct ttctcccttc 8760gggaagcgtg
gcgctttctc atagctcacg ctgtaggtat ctcagttcgg tgtaggtcgt 8820tcgctccaag
ctgggctgtg tgcacgaacc ccccgttcag cccgaccgct gcgccttatc 8880cggtaactat
cgtcttgagt ccaacccggt aagacacgac ttatcgccac tggcagcagc 8940cactggtaac
aggattagca gagcgaggta tgtaggcggt gctacagagt tcttgaagtg 9000gtggcctaac
tacggctaca ctagaagaac agtatttggt atctgcgctc tgctgaagcc 9060agttaccttc
ggaaaaagag ttggtagctc ttgatccggc aaacaaacca ccgctggtag 9120cggtggtttt
tttgtttgca agcagcagat tacgcgcaga aaaaaaggat ctcaagaaga 9180tcctttgatc
ttttctacgg ggtctgacgc tcagtggaac gaaaactcac gttaagggat 9240tttggtcatg
atctatgtcg ggtgcggaga aagaggtaat gaaatggcat acgagtaaac 9300ttggtctgac
accgctgcat gagattatca aaaaggatct tcacctagat ccttttaaat 9360taaaaatgaa
gttttaaatc aatctaaagt atatatgagt aaacttggtc tgacagttac 9420caatgcttaa
tcagtgaggc acctatctca gcgatctgtc tatttcgttc atccatagtt 9480gcctgactcc
ccgtcgtgta gataactacg atacgggagg gcttaccatc tggccccagt 9540gctgcaatga
taccgcgaga cccacgctca ccggctccag atttatcagc aataaaccag 9600ccagccggaa
gcgccgagcg cagaagtggt cctgcaactt tatccgcctc catccagtct 9660attaactgtt
gccgggaagc tagagtaagt agttcgccag ttaatagttt gcggagcgtt 9720gttgccattg
ctacaggcat cgtggtgtca cgctcgtcgt ttggtatggc ttcattcagc 9780tccggttccc
aacgatcaag gcgagttaca tgatccccca tgttgtgcaa aaaagcggtt 9840agctccttcg
gtcctccgat ggttgtcaga agtaagttgg ccgcagtgtt atcactcatg 9900gttatggcag
cactgcataa ttctcttact gtcatgccat ccgtaagatg cttttctgtg 9960actggtgagt
attcaaccaa gtcattctga gaatagtgta tgcggcgacc gagttgctct 10020tgcccggcgt
caatacggga taataccgcg ccacatagca gaactttaaa agtgctcatc 10080attgggaagc
gttcttcggg gcgaaaactc tcaaggatct taccgctgtt gagatccagt 10140tcgatgtaac
ccacacgagc acccaactga tcttcagcat cttttacttt caccagcgtt 10200tctgggtgag
caaaaacagg aaggcaaaat gccgcaaaaa agggaataag ggcgacacgg 10260aaatgttgaa
tactcatacg cttccttttt caatagtatt gaagcattta tcagggttat 10320tgtctcggga
gcgaatacat atttgaatgt atttagaaaa a
103611111545DNAArtificial SequenceSynthetic ubiquitous and Plexin D1
inducible gene switch expressing tPA 11taaacaaata ggggttccgc
gcacatttcc ccgaaaagtg ccacctgacg tactgaggac 60gccagggttt tcccagtcac
gacgttgtaa aacgacggcc agtagcagac aagcccgtca 120gggcgcgtca gcgggtgttg
gccctaggac gaaaggaggt cgtgaaatgg ataaaaaaat 180acagcgtttt tcatgtacaa
ctatactagt tgtagtgcct aaataatgct tttaaaactt 240aaaaataatc aatgtcctca
gcggggtgtc ggggatttag gtgacactat aggctgagcg 300ccgcacaggc atctagaggc
tatggcaggg cctgccgccc cgacgttggc tgcgagccct 360gggccttcac ccgaacttgg
ggggtggggt ggggaaaagg aagaaacgcg ggcgtattgg 420ccccaatggg gtctcggtgg
ggtatcgaca gagtgccagc cctgggaccg aaccccgcgt 480ttatgaacaa acgacccaac
accgtgcgtt ttattctgtc tttttattgc cgtcatagcg 540cgggttcctt ccggtattgt
ctccttccgt gtttcaatcg attcaaaaga actcgtccag 600cagacggtaa aaagcaatgc
gttgagaatc cggtgcagca atgccataca gcaccagaaa 660gcgatcagcc cattcaccgc
ccagttcttc agcaatgtca cgggttgcca gtgcgatgtc 720ctgatagcga tcagccacgc
ccaggcgacc gcagtcgata aagccggaga aacggccgtt 780ttccaccata atgtttggca
gacaagcatc gccgtgggtc acaaccaggt cctcgccatc 840tggcatacgt gctttcaggc
gtgcgaacag ttctgccggt gccagaccct gatgttcctc 900gtccaggtca tcctgatcaa
ccaggccagc ttccatgcga gtgcgtgcgc gctcgatacg 960gtgtttagct tggtgatcga
atgggcaagt agctgggtcc agggtatgca gacggcgcat 1020agcatcagcc atgatggaaa
ccttttctgc cggtgccaga tgagaggaca gcagatcctg 1080gcctggaacc tcgcccagca
gcagccagtc gcggccagcc tcggtcacaa catccagcac 1140agctgcgcat ggaacgccgg
tagtagccag ccaggacaga cgagctgctt catcttgcag 1200ttcgttcagt gcgccggaca
gatcggtctt aacaaacagc accggacggc cttgagcgga 1260cagacggaac acagctgcgt
cggagcaacc gatagtctgt tgagcccagt catagccaaa 1320cagacgttcc acccaagcag
ccggagaacc agcgtgcaga ccgtcttgtt caatcatggt 1380ggcaattggg tgtctgagcg
atgtggctcg gctggcgacg caaaagaaga tgcggctgac 1440tgtcgaacag gaggagcaga
gagcgaagcg ggaggctgcg ggctcaattt gcatgcttta 1500gttcctcacc ttgtcgtatt
atactatgcc gatatactat gccgatgatt aattgtcaac 1560gtatacggaa tagctctgag
gccgaggcag cttcggcctc tgcataaata aaaaaaatta 1620gtcagccatg gggcggagaa
tgggcggaac tgggcggagt taggggcggg atgggcggag 1680ttaggggcgg gactatggtt
gctgactaat tgagatgctt gctttgcata cttctgcctg 1740ctggggagcc tggggacttt
ccacacctgg ttgctgacta attgagatgc ttgctttgca 1800tacttctgcc tgctggggag
cctggggact ttccacaccc taacctcgag gccatcgtgg 1860cacgccaggg ttttcccagt
cacgacgttg taaaacgacg gccagtgctc ttctcccccg 1920cgggaggttt tataaatccg
actgtctaga ttgttgttaa atcacacaaa aaaccaacac 1980acagatgtaa tgaaaataaa
gatattttat tatcgattca gctgtcgctg gggctctcca 2040gcatctccat caggaaggtg
tcgatgggca cgtcgccgat cagcctgaag aagaacaggt 2100gctccaggca cttcaggccg
atgctcctca ggctgggcag cctcagcagc agcttggcga 2160atctgccggg ctcgtcgggg
tgggtggtcc tggtgtactc ctccagggcg gcgtacacct 2220tctccctcag cagctccacc
tcctgggcgc ttttcaggcc cctcacctcg gggttgaaca 2280ggatgatggc cctcaggcag
cccagctcgg tcttgtccat cctcatgtcc ctcatcttgc 2340tcaccagctc ggtcagcacc
ctgtcgaaga tggcgcccac tccggcgctg tgggcgctgt 2400tcctatggac gtgcaggccg
gtggccagca ggatgccgtc cctcacgtcg atgctcctgt 2460ggctgaagct ggcgatcagc
agctcgttcc atccggccct cagcaggatc acctggtcgt 2520ccaggggcag gctgctgaag
tggggaatcc tcttggccca ctccaccagg gtgaacagct 2580gcttgtcggc ggcctggcag
atgttggtca cggggtcgtt ggggctgctg ccgctgccgc 2640cggttccgcc ggggccctcc
acgccctggt cgcttttctg ctccacggcg agttcggcct 2700ccagaatcct gtccacgggc
atctccatat ggccgccgta ctcgtcgatg cccagggcgt 2760cggtgaacat ctgctcgaac
tcgaagtcgg ccatgtccag ggcgccgtag ggggcgctgt 2820cgtggggggt gaagccgggg
ccggggctgt cgccgtcgcc cagcatgtcc aggtcgaagt 2880cgtccagggc gtcggcgtgg
gccatggcca cgtcctcgcc gtccaggtgc agctcgtcgc 2940ccaggctcac gtcggtgggg
ggggccacct tccttttctt cttggggccc atggtggcca 3000attgcccccc cccccccccg
catgccgtct aacaaaaaag ccaaaaacgg ccagaattta 3060gcggacaatt tactagtcta
acactgaaaa ttacatattg acccaaatga ttacatttca 3120aaaggtgcct aaaaaacttc
acaaaacaca ctcgccaacc ccgagcgcac gtacccagcc 3180cagcagcccg ctactcacca
agtgacgatc acagcgatcc acaaacaaga accgcgaccc 3240aaatcccggc tgcgacggaa
ctagctgtgc cacacccggc gcgtccttat ataatcatcg 3300gcgttcaccg ccccacggag
atccctccgc agaatcgccg agaagggact acttttcctc 3360gcctgttccg ctctctggaa
agaaaaccag tgccctagag tcacccaagt cccgtcctaa 3420aatgtccttc tgctgatact
ggggttctaa ggccgagtct tatgagcagc gggccgctgt 3480cctgagcgtc cgggcggaag
gatcaggacg ctcgctgcgc ccttcgtctg acgtggcagc 3540gctcgccgtg aggagggggg
cgcccgcggg aggcgccaaa acccggcgcg gaggccctcg 3600agtaggcgag accaatgggt
gcgccatggg ctcttccaaa aatttaggtg acactatagg 3660gcaccgctcg cacctgcgca
caggcccgcg gctacaaact acgaacgatc attctagata 3720ccacatttgt agaggtttta
cttgctttaa aaaacctccc acatctcccc ctgaacctga 3780aacataaaat gaatgcaaat
gttttattaa cttgtttatt gcagcttata atggttacaa 3840attaagcaat agcatcacaa
atttcacaaa ttaagcattt ttttcactgc attctagttg 3900tggtttgtcc aaactcatca
atgtatctta tcatgtctaa tcgattcaca ggttggtggg 3960gctctccagg atgggggggg
gctgggtgtg gctcatgtcg gccacgtccc aaatctcctc 4020caggaagggg ggcagcttcc
tgttcttcag cttcaggctg atacacatat tgctgttctg 4080cattcccagg gtcctcagct
cgctcaggat gctcaggatc ttgccgtaga tcacgctgct 4140cctggcgctg ccgctcagct
ggttcaggat gtagatcctc agggtgttca ggtagtacct 4200ctggatctcc tccaccagct
ggggctgctc caggccgggc ctgtcgctga agatcaccac 4260ggcggtcagc agggcgtagt
ggatgttgtc cagggccatg ctgtacatac atctgcagaa 4320gtgcaggagg tcctcgatca
cctcggccat gccagccttc ctgtagttgt ccctggtgta 4380agcctggttg ttggcgaaca
ggatgctgtc gctggcggcg tcgtacctcc tggccaccct 4440cagcatcatc acctcgctgc
tgcaagcctt cagcagggtg atctggtcgg gctggctgat 4500cttggcgaat ccgggcaggc
ccttggcgaa ctccacgatc agctgcacgg tcaggatggt 4560catctcggtg atctgcctga
agggggtgtc gctctcctcg ttctcgtcgt cggcctgctg 4620ccaggtctgg gtgatccttt
tcaggtcctc gtcgctgggc tgctcgtagc cgtcctgata 4680ccagatcagc ctggcgatca
ggaactgctg gttggcggtc agctggggga tgttcttctg 4740cctgttggtc accagcagct
tgtcgctcag gaacctgggc acgacctcgt gaatcctggc 4800ggcctcgggg ggggggggct
cgcactgcat gatggggggc atgtggtcat cgacggtggt 4860ggtgctcacg ggcagcttgt
ccttctcctt ctgggccttc ttctccttcc ttttcatggc 4920gcactgggtc tcgggcacca
cgcactcggg cctgatcccg ggaaactcgg ggctcacggt 4980cagctgcctc tggcccttgt
tgctgctctc ctcgctgctg ctggtggcgc tgatcctgtg 5040ctgcctcagg gtcaggggca
tgtcggtctc cacgctggcc agcctgtcgg tcacggcgtc 5100cttgttcacg ttgtcctgca
cgaacaggcc ggtcagcagg gccttgatgt cttgcaggct 5160gtccatcttc aggatcatgt
ccaggtcctc cctggggaag atcagcagga acagctgctc 5220cagcctctcc agcctgctct
ccacctcggt caggtgggcc ctggtcaggg ggctcctctt 5280ggtcttgggg ctgtatctgc
actcccagtt gttcttcagg cacttggcgc acttgggctt 5340ctccttgctg cacttcagct
tcttcagcct gcagatgtcg caagcctgct cgatgctgct 5400cagcagcttc atggtggcca
attgcccccc cccccccccg catgccccgg aggcggcggg 5460aggcgggggg cgggtccggg
ccacgatgcg cttcctgccc gcgccagccg cccccgaccc 5520cggcgtccgg gcccggatca
gcctcgccgc gcagtccggg ggcggggcgg gggcggctcc 5580gctgcggaca cgccctcact
ccccgccccg gccccgcccg cccccggaac ccccgcctgc 5640ccctggccgc cctgggccac
gccccgccgt ccgcgggtcc cgccgcccgc gccctccagg 5700acccgcccgc ggccccaggg
tccctccccg tagccgccgc cgccgtcgct cgctctcctc 5760gctctttcct cccacttggc
cgcttggcct gcctcgctcg cgcctgtttt ctcttttccg 5820ccctctcccc caccccgcct
ctccctctct ctgtgtctct cggtctctgc gcctctccgc 5880ctctgtcccc accccaaact
ccgcgcgtgt ggcgcttctc cgggttctgc ctctgcttct 5940ccttctccct ccgactccgc
ttcgctctcc agtccctggc agccccgccc ccggcccttt 6000ctagtctcct tctctctcct
gctccgtttt tccgtccctg acgctcgctc cctctctccg 6060tggctccctc tgcctccccc
tcggaccctc cgtccctctc tcggtccctc tgagctcccc 6120tttccttctc cctctgcctt
cccgaaccct gtgtctcccc tccacactca gcctccctcc 6180accacctttc tcaggcactg
tccaggcctt cgctcctcga gtggtaatac aatggccggt 6240tcccatggac ctgcatcgtg
gtgtaactat aacggtccta aggtagcgac cgcggagact 6300aggtgtattt atctaagcga
tcgcttaatt aaggccggcc gccgcaataa aatatcttta 6360ttttcattac atctgtgtgt
tggttttttg tgtgaatcca tagtactaac atacgctctc 6420catcaaaaca aaacgaaaca
aaacaaacta gcaaaatagg ctgtccccag tgcaagtcca 6480ggtgccagaa catttctcta
tccataatgc aggggtaccg ggtgatgacg gtgaaaacct 6540ccaattgcgg agtactgtcc
tccgagcgga gtactgtcct ccgagcggag tactgtcctc 6600cgagcggagt actgtcctcc
gagcggagta ctgtcctccg agcggagtac tgtcctccga 6660gcggagagtc cccggggacc
tagagggtat ataatgggtg ccttagctgg tgtgtgacct 6720catcttcctg tacgcccctg
caggggcgcg ccacgcgtcg aagaaggtga gtaatcttaa 6780catgctcttt tttttttttt
ttgctaatcc cttttgtgtg ctgatgttag gatgacattt 6840acaacaaatg tttgttcctg
acaggaaaaa ccttgctggg taccttcgtt gccggacact 6900tcttgtcctc tactttggaa
aaaaggaatt gagagccgct agcgccacca tggatgcaat 6960gaagcggggg ctctgctgtg
tgctgctgct gtgtggagca gtgttcgttt cgcccagcca 7020ggaaatccat gcccgattca
gaaggggcgc aagaagctac caagtgatct gccgggatga 7080aaaaacgcag atgatatacc
agcaacatca gtcatggctg cgccctgtgc tcagaagcaa 7140ccgggtggag tattgctggt
gcaacagtgg cagggcacag tgccacagcg tccctgtcaa 7200aagttgcagc gagccaaggt
gtttcaacgg gggcacctgt cagcaggccc tgtacttctc 7260agatttcgtg tgccagtgcc
ccgaaggatt tgctgggaag tgctgtgaaa tagataccag 7320ggccacctgt tacgaggacc
agggcatcag ctacaggggc acctggagca cagcggagag 7380tggcgctgag tgtaccaact
ggaacagcag cgcgttggcc cagaagccct acagcgggcg 7440gaggccagat gccatcagac
tcggcctggg gaaccacaac tactgccgga acccagatcg 7500agactcaaag ccctggtgct
acgtctttaa ggcggggaag tacagctcag agttctgctc 7560cacccctgcc tgctctgagg
gaaacagtga ctgctacttt gggaatgggt cagcctaccg 7620tggcacgcac agcctcaccg
agtcgggtgc ctcctgcctc ccgtggaaca gcatgatcct 7680gataggcaat gtttacacag
cacagaaccc cagtgcccag gcactgggcc tgggcaaaca 7740taattactgc cggaatcctg
atggggatgc caagccctgg tgccacgtcc tgaagaaccg 7800caggctgacg tgggagtatt
gcgatgtgcc cagctgctcc acctgtggcc tgagacagta 7860cagccagcct cagtttcgca
tcaaaggcgg cctgttcgcc gacatcgcct cccacccctg 7920gcaggctgcc atctttgcca
agcacaggag gtcgcccgga gagcggttcc tgtgcggggg 7980catactcatc agctcctgct
ggattctctc tgccgcccac tgcttccagg agaggtttcc 8040gccccaccac ctgacggtga
tcttgggcag aacataccgg gtggtccctg gcgaggagga 8100gcagaaattt gaagtcgaaa
aatacattgt ccataaggag ttcgatgatg acacttacga 8160caatgacatt gcgctgctgc
aactgaaatc ggattcgtcc cgctgtgccc aggagagcag 8220cgtggtcaga accgtgtgcc
ttcccccggc ggacctccag ctgccggact ggacggagtg 8280tgagctgtcc ggctacggca
agcatgaggc cctgtctcct ttctattcgg agcggctgaa 8340ggaggctcat gtcagactgt
acccatccag ccgctgcaca tcacaacatt tacttaacag 8400aacagtcacc gacaacatgc
tgtgtgctgg agacactcgg agcggcggcc cccaggcaaa 8460cttgcacgac gcctgccagg
gcgattcggg aggccccctg gtgtgtctga acgatggccg 8520catgactttg gtgggcatca
tcagctgggg cctgggctgt ggacagaagg atgtgcccgg 8580cgtgtacacc aaggttacca
actacctaga ctggattcgt gacaacatgc gaccgtgaat 8640cgattgcgca aagctttcgc
gataggcgag accaatgggt gtgtacgtag cggccgcgtc 8700gacgatagct tgatgggtgg
catccctgtg acccctcccc agtgcctctc ctggccctgg 8760aagttgccac tccagtgccc
accagccttg tcctaataaa attaagttgc atcattttgt 8820ctgactaggt gtccttctat
aatattatgg ggtggagggg ggtggtatgg agcaaggggc 8880aagttgggaa gacaacctgt
agggcctgcg gggtctattg ggaaccaagc tggagtgcag 8940tggcacaatc ttggctcact
gcaatctccg cctcctgggt tcaagcgatt ctcctgcctc 9000agcctcccga gttgttggga
ttccaggcat gcatgaccag gctcagctaa tttttgtttt 9060tttggtagag acggggtttc
accatattgg ccaggctggt ctccaactcc taatctcagg 9120tgatctaccc accttggcct
cccaaattgc tgggattaca ggcgtgaacc actgctccct 9180tccctgtcct tctgatttta
aaataactat accagcagga ggacgtccag acacagcata 9240ggctacctgg ccatgcccaa
ccggtgggac atttgagttg cttgcttggc actgtcctct 9300catgcgttgg gtccactcag
tagatgcctg ttgaattctg atttaaatcg gtccgcgtac 9360ggcgtggtag gtccgaacga
atccatggat taccctgtta tccctactca aggacatcat 9420ccctttagtg agggttaatt
cacgcagtgg gtacggaact aaaggcagca cacatcgtgt 9480aatcatggtc atagctgttt
cctgtgtgaa attgttatcc gctacgtctc tcccccgcag 9540taagggctag attaactcgt
ctcgtgaata tccggaactc cctttagtga gggttaattg 9600cgttgcgctc actgcccgct
ttccagtcgg gaaacctgtc gtgccagctt aatcatggtc 9660atagctgttt cctgtgtgaa
attgttatcc gctaccggaa acgcttcctt catgtgagca 9720aaaggccagc aaaaggccag
gaaccgtaaa aaggccgcgt tgctggcgtt tttccatagg 9780ctccgccccc ctgacgagca
tcacaaaaat cgacgctcaa gtcagaggtg gcgaaacccg 9840acaggactat aaagatacca
ggcgtttccc cctggaagct ccctcgtgcg ctctcctgtt 9900ccgaccctgc cgcttaccgg
atacctgtcc gcctttctcc cttcgggaag cgtggcgctt 9960tctcatagct cacgctgtag
gtatctcagt tcggtgtagg tcgttcgctc caagctgggc 10020tgtgtgcacg aaccccccgt
tcagcccgac cgctgcgcct tatccggtaa ctatcgtctt 10080gagtccaacc cggtaagaca
cgacttatcg ccactggcag cagccactgg taacaggatt 10140agcagagcga ggtatgtagg
cggtgctaca gagttcttga agtggtggcc taactacggc 10200tacactagaa gaacagtatt
tggtatctgc gctctgctga agccagttac cttcggaaaa 10260agagttggta gctcttgatc
cggcaaacaa accaccgctg gtagcggtgg tttttttgtt 10320tgcaagcagc agattacgcg
cagaaaaaaa ggatctcaag aagatccttt gatcttttct 10380acggggtctg acgctcagtg
gaacgaaaac tcacgttaag ggattttggt catgatctat 10440gtcgggtgcg gagaaagagg
taatgaaatg gcatacgagt aaacttggtc tgacaccgct 10500gcatgagatt atcaaaaagg
atcttcacct agatcctttt aaattaaaaa tgaagtttta 10560aatcaatcta aagtatatat
gagtaaactt ggtctgacag ttaccaatgc ttaatcagtg 10620aggcacctat ctcagcgatc
tgtctatttc gttcatccat agttgcctga ctccccgtcg 10680tgtagataac tacgatacgg
gagggcttac catctggccc cagtgctgca atgataccgc 10740gagacccacg ctcaccggct
ccagatttat cagcaataaa ccagccagcc ggaagcgccg 10800agcgcagaag tggtcctgca
actttatccg cctccatcca gtctattaac tgttgccggg 10860aagctagagt aagtagttcg
ccagttaata gtttgcggag cgttgttgcc attgctacag 10920gcatcgtggt gtcacgctcg
tcgtttggta tggcttcatt cagctccggt tcccaacgat 10980caaggcgagt tacatgatcc
cccatgttgt gcaaaaaagc ggttagctcc ttcggtcctc 11040cgatggttgt cagaagtaag
ttggccgcag tgttatcact catggttatg gcagcactgc 11100ataattctct tactgtcatg
ccatccgtaa gatgcttttc tgtgactggt gagtattcaa 11160ccaagtcatt ctgagaatag
tgtatgcggc gaccgagttg ctcttgcccg gcgtcaatac 11220gggataatac cgcgccacat
agcagaactt taaaagtgct catcattggg aagcgttctt 11280cggggcgaaa actctcaagg
atcttaccgc tgttgagatc cagttcgatg taacccacac 11340gagcacccaa ctgatcttca
gcatctttta ctttcaccag cgtttctggg tgagcaaaaa 11400caggaaggca aaatgccgca
aaaaagggaa taagggcgac acggaaatgt tgaatactca 11460tacgcttcct ttttcaatag
tattgaagca tttatcaggg ttattgtctc gggagcgaat 11520acatatttga atgtatttag
aaaaa 115451210207DNAArtificial
SequenceSynthetic NxcI cardomyocyte promoter hypoxia inducible gene
switch expressing EPO 12taaacaaata ggggttccgc gcacatttcc ccgaaaagtg
ccacctgacg tactgaggac 60gccagggttt tcccagtcac gacgttgtaa aacgacggcc
agtagcagac aagcccgtca 120gggcgcgtca gcgggtgttg gccctaggac gaaaggaggt
cgtgaaatgg ataaaaaaat 180acagcgtttt tcatgtacaa ctatactagt tgtagtgcct
aaataatgct tttaaaactt 240aaaaataatc aatgtcctca gcggggtgtc ggggatttag
gtgacactat aggctgagcg 300ccgcacaggc atctagaggc tatggcaggg cctgccgccc
cgacgttggc tgcgagccct 360gggccttcac ccgaacttgg ggggtggggt ggggaaaagg
aagaaacgcg ggcgtattgg 420ccccaatggg gtctcggtgg ggtatcgaca gagtgccagc
cctgggaccg aaccccgcgt 480ttatgaacaa acgacccaac accgtgcgtt ttattctgtc
tttttattgc cgtcatagcg 540cgggttcctt ccggtattgt ctccttccgt gtttcaatcg
attcaaaaga actcgtccag 600cagacggtaa aaagcaatgc gttgagaatc cggtgcagca
atgccataca gcaccagaaa 660gcgatcagcc cattcaccgc ccagttcttc agcaatgtca
cgggttgcca gtgcgatgtc 720ctgatagcga tcagccacgc ccaggcgacc gcagtcgata
aagccggaga aacggccgtt 780ttccaccata atgtttggca gacaagcatc gccgtgggtc
acaaccaggt cctcgccatc 840tggcatacgt gctttcaggc gtgcgaacag ttctgccggt
gccagaccct gatgttcctc 900gtccaggtca tcctgatcaa ccaggccagc ttccatgcga
gtgcgtgcgc gctcgatacg 960gtgtttagct tggtgatcga atgggcaagt agctgggtcc
agggtatgca gacggcgcat 1020agcatcagcc atgatggaaa ccttttctgc cggtgccaga
tgagaggaca gcagatcctg 1080gcctggaacc tcgcccagca gcagccagtc gcggccagcc
tcggtcacaa catccagcac 1140agctgcgcat ggaacgccgg tagtagccag ccaggacaga
cgagctgctt catcttgcag 1200ttcgttcagt gcgccggaca gatcggtctt aacaaacagc
accggacggc cttgagcgga 1260cagacggaac acagctgcgt cggagcaacc gatagtctgt
tgagcccagt catagccaaa 1320cagacgttcc acccaagcag ccggagaacc agcgtgcaga
ccgtcttgtt caatcatggt 1380ggcaattggg tgtctgagcg atgtggctcg gctggcgacg
caaaagaaga tgcggctgac 1440tgtcgaacag gaggagcaga gagcgaagcg ggaggctgcg
ggctcaattt gcatgcttta 1500gttcctcacc ttgtcgtatt atactatgcc gatatactat
gccgatgatt aattgtcaac 1560gtatacggaa tagctctgag gccgaggcag cttcggcctc
tgcataaata aaaaaaatta 1620gtcagccatg gggcggagaa tgggcggaac tgggcggagt
taggggcggg atgggcggag 1680ttaggggcgg gactatggtt gctgactaat tgagatgctt
gctttgcata cttctgcctg 1740ctggggagcc tggggacttt ccacacctgg ttgctgacta
attgagatgc ttgctttgca 1800tacttctgcc tgctggggag cctggggact ttccacaccc
taacctcgag gccatcgtgg 1860cacgccaggg ttttcccagt cacgacgttg taaaacgacg
gccagtgctc ttctcccccg 1920cgggaggttt tataaatccg actgtctaga ttgttgttaa
atcacacaaa aaaccaacac 1980acagatgtaa tgaaaataaa gatattttat tatcgattca
gctgtcgctg gggctctcca 2040gcatctccat caggaaggtg tcgatgggca cgtcgccgat
cagcctgaag aagaacaggt 2100gctccaggca cttcaggccg atgctcctca ggctgggcag
cctcagcagc agcttggcga 2160atctgccggg ctcgtcgggg tgggtggtcc tggtgtactc
ctccagggcg gcgtacacct 2220tctccctcag cagctccacc tcctgggcgc ttttcaggcc
cctcacctcg gggttgaaca 2280ggatgatggc cctcaggcag cccagctcgg tcttgtccat
cctcatgtcc ctcatcttgc 2340tcaccagctc ggtcagcacc ctgtcgaaga tggcgcccac
tccggcgctg tgggcgctgt 2400tcctatggac gtgcaggccg gtggccagca ggatgccgtc
cctcacgtcg atgctcctgt 2460ggctgaagct ggcgatcagc agctcgttcc atccggccct
cagcaggatc acctggtcgt 2520ccaggggcag gctgctgaag tggggaatcc tcttggccca
ctccaccagg gtgaacagct 2580gcttgtcggc ggcctggcag atgttggtca cggggtcgtt
ggggctgctg ccgctgccgc 2640cggttccgcc ggggccctcc acgccctggt cgcttttctg
ctccacggcg agttcggcct 2700ccagaatcct gtccacgggc atctccatat ggccgccgta
ctcgtcgatg cccagggcgt 2760cggtgaacat ctgctcgaac tcgaagtcgg ccatgtccag
ggcgccgtag ggggcgctgt 2820cgtggggggt gaagccgggg ccggggctgt cgccgtcgcc
cagcatgtcc aggtcgaagt 2880cgtccagggc gtcggcgtgg gccatggcca cgtcctcgcc
gtccaggtgc agctcgtcgc 2940ccaggctcac gtcggtgggg ggggccacct tccttttctt
cttggggccc atggtggcca 3000attgcccccc cccccccccg catgccccag tatgatgcct
tctctaaatt taccagaaag 3060aaaggacctg ggatgcttgt cctttaagtg tctctaaaca
tggtttgcat agctggatct 3120cagcttatct gtgcctttcg gctttcgctt ccatacatgg
atctgttccc aacagtaact 3180ccaagctgtc gaaaagaagc tatctggcag ctctctgctt
catccaacac atggatgata 3240aaaatacaca tatagcgatt cttccctcga gtaggcgaga
ccaatgggtg cgccatgggc 3300tcttccaaaa atttaggtga cactataggg caccgctcgc
acctgcgcac aggcccgcgg 3360ctacaaacta cgaacgatca ttctagatac cacatttgta
gaggttttac ttgctttaaa 3420aaacctccca catctccccc tgaacctgaa acataaaatg
aatgcaaatg ttttattaac 3480ttgtttattg cagcttataa tggttacaaa ttaagcaata
gcatcacaaa tttcacaaat 3540taagcatttt tttcactgca ttctagttgt ggtttgtcca
aactcatcaa tgtatcttat 3600catgtctaat cgattcacag gttggtgggg ctctccagga
tggggggggg ctgggtgtgg 3660ctcatgtcgg ccacgtccca aatctcctcc aggaaggggg
gcagcttcct gttcttcagc 3720ttcaggctga tacacatatt gctgttctgc attcccaggg
tcctcagctc gctcaggatg 3780ctcaggatct tgccgtagat cacgctgctc ctggcgctgc
cgctcagctg gttcaggatg 3840tagatcctca gggtgttcag gtagtacctc tggatctcct
ccaccagctg gggctgctcc 3900aggccgggcc tgtcgctgaa gatcaccacg gcggtcagca
gggcgtagtg gatgttgtcc 3960agggccatgc tgtacataca tctgcagaag tgcaggaggt
cctcgatcac ctcggccatg 4020ccagccttcc tgtagttgtc cctggtgtaa gcctggttgt
tggcgaacag gatgctgtcg 4080ctggcggcgt cgtacctcct ggccaccctc agcatcatca
cctcgctgct gcaagccttc 4140agcagggtga tctggtcggg ctggctgatc ttggcgaatc
cgggcaggcc cttggcgaac 4200tccacgatca gctgcacggt caggatggtc atctcggtga
tctgcctgaa gggggtgtcg 4260ctctcctcgt tctcgtcgtc ggcctgctgc caggtctggg
tgatcctttt caggtcctcg 4320tcgctgggct gctcgtagcc gtcctgatac cagatcagcc
tggcgatcag gaactgctgg 4380ttggcggtca gctgggggat gttcttctgc ctgttggtca
ccagcagctt gtcgctcagg 4440aacctgggca cgacctcgtg aatcctggcg gcctcggggg
gggggggctc gcactgcatg 4500atggggggca tgtggtcatc gacggtggtg gtgctcacgg
gcagcttgtc cttctccttc 4560tgggccttct tctccttcct tttcatggcg cactgggtct
cgggcaccac gcactcgggc 4620ctgatcccgg gaaactcggg gctcacggtc agctgcctct
ggcccttgtt gctgctctcc 4680tcgctgctgc tggtggcgct gatcctgtgc tgcctcaggg
tcaggggcat gtcggtctcc 4740acgctggcca gcctgtcggt cacggcgtcc ttgttcacgt
tgtcctgcac gaacaggccg 4800gtcagcaggg ccttgatgtc ttgcaggctg tccatcttca
ggatcatgtc caggtcctcc 4860ctggggaaga tcagcaggaa cagctgctcc agcctctcca
gcctgctctc cacctcggtc 4920aggtgggccc tggtcagggg gctcctcttg gtcttggggc
tgtatctgca ctcccagttg 4980ttcttcaggc acttggcgca cttgggcttc tccttgctgc
acttcagctt cttcagcctg 5040cagatgtcgc aagcctgctc gatgctgctc agcagcttca
tggtggccaa ttgccccccc 5100ccccccccgc atgcacagga agatgagctc acacaccagc
taaggcaccc attatatacc 5160ctctaggtct tcgcggacgt gcgggaaccc acgtgggggc
tccgtcacgt actccgagtc 5220ccgcacgcac tcagcgtcgc tctggggccc cacgtccggc
cctgacgtgc ggcttcgttt 5280gtcacgtcgc ggacgtgcgg gaacccacgt gggggctccg
tcacgtactc cgagtcccgc 5340acgcactcag cgtcgctctg gggccccacg tccggccctg
acgtgcggct tcgtttgtca 5400cgtcgcggac gtgcgggaac ccacgtgggg gctccgtcac
gtactccgag tcccgcacgc 5460actcagcgtc gctctggggc cccacgtccg gccctgacgt
gcggcttcgt ttgtcacgtc 5520gcggacgtgc gggaacccac gtgggggctc cgtcacgtac
tccgagtccc gcacgcactc 5580agcgtcgctc tggggcccca cgtccggccc tgacgtgcgg
cttcgtttgt cacgtcgcgg 5640acgtgcggga acccacgtgg gggctccgtc acgtactccg
agtcccgcac gcactcagcg 5700tcgctctggg gccccacgtc cggccctgac gtgcggcttc
gtttgtcacg tcgcggacgt 5760gcgggaaccc acgtgggggc tccgtcacgt actccgagtc
ccgcacgcac tcagcgtcgc 5820tctggggccc cacgtccggc cctgacgtgc ggcttcgttt
gtcacgtcgc ggacgtgcgg 5880gaacccacgt gggggctccg tcacgtactc cgagtcccgc
acgcactcag cgtcgctctg 5940gggccccacg tccggccctg acgtgcggct tcgtttgtca
cgtcctcgag tggtaataca 6000atggccggtt cccatggacc tgcatcgtgg tgtaactata
acggtcctaa ggtagcgacc 6060gcggagacta ggtgtattta tctaagcgat cgcttaatta
aggccggccg ccgcaataaa 6120atatctttat tttcattaca tctgtgtgtt ggttttttgt
gtgaatccat agtactaaca 6180tacgctctcc atcaaaacaa aacgaaacaa aacaaactag
caaaataggc tgtccccagt 6240gcaagtccag gtgccagaac atttctctat ccataatgca
ggggtaccgg gtgatgacgg 6300tgaaaacctc caattgcgga gtactgtcct ccgagcggag
tactgtcctc cgagcggagt 6360actgtcctcc gagcggagta ctgtcctccg agcggagtac
tgtcctccga gcggagtact 6420gtcctccgag cggagagtcc ccggggacct agagggtata
taatgggtgc cttagctggt 6480gtgtgacctc atcttcctgt acgcccctgc aggggcgcgc
cacgcgtcga agaaggtgag 6540taatcttaac atgctctttt tttttttttt tgctaatccc
ttttgtgtgc tgatgttagg 6600atgacattta caacaaatgt ttgttcctga caggaaaaac
cttgctgggt accttcgttg 6660ccggacactt cttgtcctct actttggaaa aaaggaattg
agagccgcta gcgccaccat 6720gggggtgcat gaatgtcctg cctggctgtg gcttctcctg
tccctgctgt cgctccctct 6780gggcctccca gtcctgggcg ctccaccacg cctcatctgt
gacagccgag tcctggagag 6840atacctgttg gaggccaagg aagccgagaa tatcacgacg
ggctgtgctg aacactgctc 6900cttgaatgag aatatcactg tcccagacac caaagttaat
ttctatgcct ggaagcggat 6960ggaggtcggg cagcaggccg tagaagtctg gcagggcctg
gccctgctgt cggaagctgt 7020cctgcggggc caggccctgt tggtcaacag cagccagccg
tgggagcccc tgcaactgca 7080tgtggataaa gccgtcagtg gccttcgcag cctcaccact
ctgcttcggg ctctgcgagc 7140ccagaaggaa gccatctccc ctccagatgc ggccagcgcc
gctccactcc gaacaatcac 7200tgctgacact ttccgcaaac tgttccgagt ctactccaat
ttcctccggg gaaagctgaa 7260gctgtacaca ggggaggctt gcaggacagg ggacagatga
atcgattgcg caaagctttc 7320gcgataggcg agaccaatgg gtgtgtacgt agcggccgcg
tcgacgatag cttgatgggt 7380ggcatccctg tgacccctcc ccagtgcctc tcctggccct
ggaagttgcc actccagtgc 7440ccaccagcct tgtcctaata aaattaagtt gcatcatttt
gtctgactag gtgtccttct 7500ataatattat ggggtggagg ggggtggtat ggagcaaggg
gcaagttggg aagacaacct 7560gtagggcctg cggggtctat tgggaaccaa gctggagtgc
agtggcacaa tcttggctca 7620ctgcaatctc cgcctcctgg gttcaagcga ttctcctgcc
tcagcctccc gagttgttgg 7680gattccaggc atgcatgacc aggctcagct aatttttgtt
tttttggtag agacggggtt 7740tcaccatatt ggccaggctg gtctccaact cctaatctca
ggtgatctac ccaccttggc 7800ctcccaaatt gctgggatta caggcgtgaa ccactgctcc
cttccctgtc cttctgattt 7860taaaataact ataccagcag gaggacgtcc agacacagca
taggctacct ggccatgccc 7920aaccggtggg acatttgagt tgcttgcttg gcactgtcct
ctcatgcgtt gggtccactc 7980agtagatgcc tgttgaattc tgatttaaat cggtccgcgt
acggcgtggt aggtccgaac 8040gaatccatgg attaccctgt tatccctact caaggacatc
atccctttag tgagggttaa 8100ttcacgcagt gggtacggaa ctaaaggcag cacacatcgt
gtaatcatgg tcatagctgt 8160ttcctgtgtg aaattgttat ccgctacgtc tctcccccgc
agtaagggct agattaactc 8220gtctcgtgaa tatccggaac tccctttagt gagggttaat
tgcgttgcgc tcactgcccg 8280ctttccagtc gggaaacctg tcgtgccagc ttaatcatgg
tcatagctgt ttcctgtgtg 8340aaattgttat ccgctaccgg aaacgcttcc ttcatgtgag
caaaaggcca gcaaaaggcc 8400aggaaccgta aaaaggccgc gttgctggcg tttttccata
ggctccgccc ccctgacgag 8460catcacaaaa atcgacgctc aagtcagagg tggcgaaacc
cgacaggact ataaagatac 8520caggcgtttc cccctggaag ctccctcgtg cgctctcctg
ttccgaccct gccgcttacc 8580ggatacctgt ccgcctttct cccttcggga agcgtggcgc
tttctcatag ctcacgctgt 8640aggtatctca gttcggtgta ggtcgttcgc tccaagctgg
gctgtgtgca cgaacccccc 8700gttcagcccg accgctgcgc cttatccggt aactatcgtc
ttgagtccaa cccggtaaga 8760cacgacttat cgccactggc agcagccact ggtaacagga
ttagcagagc gaggtatgta 8820ggcggtgcta cagagttctt gaagtggtgg cctaactacg
gctacactag aagaacagta 8880tttggtatct gcgctctgct gaagccagtt accttcggaa
aaagagttgg tagctcttga 8940tccggcaaac aaaccaccgc tggtagcggt ggtttttttg
tttgcaagca gcagattacg 9000cgcagaaaaa aaggatctca agaagatcct ttgatctttt
ctacggggtc tgacgctcag 9060tggaacgaaa actcacgtta agggattttg gtcatgatct
atgtcgggtg cggagaaaga 9120ggtaatgaaa tggcatacga gtaaacttgg tctgacaccg
ctgcatgaga ttatcaaaaa 9180ggatcttcac ctagatcctt ttaaattaaa aatgaagttt
taaatcaatc taaagtatat 9240atgagtaaac ttggtctgac agttaccaat gcttaatcag
tgaggcacct atctcagcga 9300tctgtctatt tcgttcatcc atagttgcct gactccccgt
cgtgtagata actacgatac 9360gggagggctt accatctggc cccagtgctg caatgatacc
gcgagaccca cgctcaccgg 9420ctccagattt atcagcaata aaccagccag ccggaagcgc
cgagcgcaga agtggtcctg 9480caactttatc cgcctccatc cagtctatta actgttgccg
ggaagctaga gtaagtagtt 9540cgccagttaa tagtttgcgg agcgttgttg ccattgctac
aggcatcgtg gtgtcacgct 9600cgtcgtttgg tatggcttca ttcagctccg gttcccaacg
atcaaggcga gttacatgat 9660cccccatgtt gtgcaaaaaa gcggttagct ccttcggtcc
tccgatggtt gtcagaagta 9720agttggccgc agtgttatca ctcatggtta tggcagcact
gcataattct cttactgtca 9780tgccatccgt aagatgcttt tctgtgactg gtgagtattc
aaccaagtca ttctgagaat 9840agtgtatgcg gcgaccgagt tgctcttgcc cggcgtcaat
acgggataat accgcgccac 9900atagcagaac tttaaaagtg ctcatcattg ggaagcgttc
ttcggggcga aaactctcaa 9960ggatcttacc gctgttgaga tccagttcga tgtaacccac
acgagcaccc aactgatctt 10020cagcatcttt tactttcacc agcgtttctg ggtgagcaaa
aacaggaagg caaaatgccg 10080caaaaaaggg aataagggcg acacggaaat gttgaatact
catacgcttc ctttttcaat 10140agtattgaag catttatcag ggttattgtc tcgggagcga
atacatattt gaatgtattt 10200agaaaaa
102071316979DNAArtificial SequenceSynthetic
ubiquitous+ hPlexin D1 + hypoxia inducible gene switchconstruct
expressing relaxin and HGF 13taaacaaata ggggttccgc gcacatttcc ccgaaaagtg
ccacctgacg tactgaggac 60gccagggttt tcccagtcac gacgttgtaa aacgacggcc
agtagcagac aagcccgtca 120gggcgcgtca gcgggtgttg gcgggtgtcg gggatttagg
tgacactata ggctgagcgc 180cgcacaggca tctagaggct atggcagggc ctgccgcccc
gacgttggct gcgagccctg 240ggccttcacc cgaacttggg gggtggggtg gggaaaagga
agaaacgcgg gcgtattggc 300cccaatgggg tctcggtggg gtatcgacag agtgccagcc
ctgggaccga accccgcgtt 360tatgaacaaa cgacccaaca ccgtgcgttt tattctgtct
ttttattgcc gtcatagcgc 420gggttccttc cggtattgtc tccttccgtg tttcaatcga
tttagaagaa ctcgtcaaga 480aggcgataga aggcgatgcg ctgcgaatcg ggggcggcga
taccgtaaag gacgaggaag 540cggtcagccc attcgccgcc aagttcttca gcaatatcac
gggtagccaa cgctatgtcc 600tgatagcggt cggccacacc cagccgtcca cagtcgatga
atccagaaaa gcggccattt 660tccaccatga tattcggcaa gcaggcatcg ccgtgggtca
cgacgagatc ctcgccgtcg 720ggcatcctcg ccttgagcct ggcgaacagt tcggctggcg
cgagcccctg atgctcctcg 780tccagatcat cctgatcgac aagaccggct tccatccgag
tacgtgctcg ctcgatgcga 840tgtttcgctt ggtggtcgaa tgggcatgta gccggatcaa
gcgtatgcag ccgccgcatt 900gcatcagcca tgatggatac tttctcggca ggagcaaggt
gagatgacag gagatcctgc 960cccggcactt cgcccaatag cagccagtcc cttcccgctt
cagtgacaac gtcgagcacg 1020gctgcgcaag gaacgcccgt cgtggccagc cacgatagcc
gcgctgcctc gtcctggagt 1080tcattcaggg caccggacag gtcggtcttg acaaaaagaa
ccgggcgccc ctgcgctgac 1140agccggaaca cggcggcatc agagcagccg attgtctgtt
gtgcccagtc atagccgaat 1200agcctctcca cccaagcggc cggagaacca gcgtgcaatc
catcttgttc aatggccgat 1260cccatggtgg ccaattgggt gtctgagcga tgtggctcgg
ctggcgacgc aaaagaagat 1320gcggctgact gtcgaacagg aggagcagag agcgaagcgg
gaggctgcgg gctcaatttg 1380catgcggaat agctctgagg ccgaggcagc ttcggcctct
gcataaataa aaaaaattag 1440tcagccatgg ggcggagaat gggcggaact gggcggagtt
aggggcggga tgggcggagt 1500taggggcggg actatggttg ctgactaatt gagatgcttg
ctttgcatac ttctgcctgc 1560tggggagcct ggggactttc cacacctggt tgctgactaa
ttgagatgct tgctttgcat 1620acttctgcct gctggggagc ctggggactt tccacaccct
aacctcgagg ccatcgtggc 1680acgccagggt tttcccagtc acgacgttgt aaaacgacgg
ccagtgctct tctcccccgc 1740gggaggtttt ataaatccga ctgtctagat tgttgttaaa
tcacacaaaa aaccaacaca 1800cagatgtaat gaaaataaag atattttatt atcgattcag
ctgtcgctgg ggctctccag 1860catctccatc aggaaggtgt cgatgggcac gtcgccgatc
agcctgaaga agaacaggtg 1920ctccaggcac ttcaggccga tgctcctcag gctgggcagc
ctcagcagca gcttggcgaa 1980tctgccgggc tcgtcggggt gggtggtcct ggtgtactcc
tccagggcgg cgtacacctt 2040ctccctcagc agctccacct cctgggcgct tttcaggccc
ctcacctcgg ggttgaacag 2100gatgatggcc ctcaggcagc ccagctcggt cttgtccatc
ctcatgtccc tcatcttgct 2160caccagctcg gtcagcaccc tgtcgaagat ggcgcccact
ccggcgctgt gggcgctgtt 2220cctatggacg tgcaggccgg tggccagcag gatgccgtcc
ctcacgtcga tgctcctgtg 2280gctgaagctg gcgatcagca gctcgttcca tccggccctc
agcaggatca cctggtcgtc 2340caggggcagg ctgctgaagt ggggaatcct cttggcccac
tccaccaggg tgaacagctg 2400cttgtcggcg gcctggcaga tgttggtcac ggggtcgttg
gggctgctgc cgctgccgcc 2460ggttccgccg gggccctcca cgccctggtc gcttttctgc
tccacggcga gttcggcctc 2520cagaatcctg tccacgggca tctccatatg gccgccgtac
tcgtcgatgc ccagggcgtc 2580ggtgaacatc tgctcgaact cgaagtcggc catgtccagg
gcgccgtagg gggcgctgtc 2640gtggggggtg aagccggggc cggggctgtc gccgtcgccc
agcatgtcca ggtcgaagtc 2700gtccagggcg tcggcgtggg ccatggccac gtcctcgccg
tccaggtgca gctcgtcgcc 2760caggctcacg tcggtggggg gggccacctt ccttttcttc
ttggggccca tggtggccaa 2820ttgccccccc ccccccccgc atgccgtcta acaaaaaagc
caaaaacggc cagaatttag 2880cggacaattt actagtctaa cactgaaaat tacatattga
cccaaatgat tacatttcaa 2940aaggtgccta aaaaacttca caaaacacac tcgccaaccc
cgagcgcacg tacccagccc 3000agcagcccgc tactcaccaa gtgacgatca cagcgatcca
caaacaagaa ccgcgaccca 3060aatcccggct gcgacggaac tagctgtgcc acacccggcg
cgtccttata taatcatcgg 3120cgttcaccgc cccacggaga tccctccgca gaatcgccga
gaagggacta cttttcctcg 3180cctgttccgc tctctggaaa gaaaaccagt gccctagagt
cacccaagtc ccgtcctaaa 3240atgtccttct gctgatactg gggttctaag gccgagtctt
atgagcagcg ggccgctgtc 3300ctgagcgtcc gggcggaagg atcaggacgc tcgctgcgcc
cttcgtctga cgtggcagcg 3360ctcgccgtga ggaggggggc gcccgcggga ggcgccaaaa
cccggcgcgg aggccctcga 3420gtaggcgaga ccaatgggtg cgccatgggc tcttccaaaa
atttaggtga cactataggg 3480caccgctcgc acctgcgcac aggcccgcgg ctacaaacta
cgaacgatca ttctagatac 3540cacatttgta gaggttttac ttgctttaaa aaacctccca
catctccccc tgaacctgaa 3600acataaaatg aatgcaaatg ttttattaac ttgtttattg
cagcttataa tggttacaaa 3660ttaagcaata gcatcacaaa tttcacaaat taagcatttt
tttcactgca ttctagttgt 3720ggtttgtcca aactcatcaa tgtatcttat catgtctaat
cgattcacag gttggtgggg 3780ctctccagga tggggggggg ctgggtgtgg ctcatgtcgg
ccacgtccca aatctcctcc 3840aggaaggggg gcagcttcct gttcttcagc ttcaggctga
tacacatatt gctgttctgc 3900attcccaggg tcctcagctc gctcaggatg ctcaggatct
tgccgtagat cacgctgctc 3960ctggcgctgc cgctcagctg gttcaggatg tagatcctca
gggtgttcag gtagtacctc 4020tggatctcct ccaccagctg gggctgctcc aggccgggcc
tgtcgctgaa gatcaccacg 4080gcggtcagca gggcgtagtg gatgttgtcc agggccatgc
tgtacataca tctgcagaag 4140tgcaggaggt cctcgatcac ctcggccatg ccagccttcc
tgtagttgtc cctggtgtaa 4200gcctggttgt tggcgaacag gatgctgtcg ctggcggcgt
cgtacctcct ggccaccctc 4260agcatcatca cctcgctgct gcaagccttc agcagggtga
tctggtcggg ctggctgatc 4320ttggcgaatc cgggcaggcc cttggcgaac tccacgatca
gctgcacggt caggatggtc 4380atctcggtga tctgcctgaa gggggtgtcg ctctcctcgt
tctcgtcgtc ggcctgctgc 4440caggtctggg tgatcctttt caggtcctcg tcgctgggct
gctcgtagcc gtcctgatac 4500cagatcagcc tggcgatcag gaactgctgg ttggcggtca
gctgggggat gttcttctgc 4560ctgttggtca ccagcagctt gtcgctcagg aacctgggca
cgacctcgtg aatcctggcg 4620gcctcggggg gggggggctc gcactgcatg atggggggca
tgtggtcatc gacggtggtg 4680gtgctcacgg gcagcttgtc cttctccttc tgggccttct
tctccttcct tttcatggcg 4740cactgggtct cgggcaccac gcactcgggc ctgatcccgg
gaaactcggg gctcacggtc 4800agctgcctct ggcccttgtt gctgctctcc tcgctgctgc
tggtggcgct gatcctgtgc 4860tgcctcaggg tcaggggcat gtcggtctcc acgctggcca
gcctgtcggt cacggcgtcc 4920ttgttcacgt tgtcctgcac gaacaggccg gtcagcaggg
ccttgatgtc ttgcaggctg 4980tccatcttca ggatcatgtc caggtcctcc ctggggaaga
tcagcaggaa cagctgctcc 5040agcctctcca gcctgctctc cacctcggtc aggtgggccc
tggtcagggg gctcctcttg 5100gtcttggggc tgtatctgca ctcccagttg ttcttcaggc
acttggcgca cttgggcttc 5160tccttgctgc acttcagctt cttcagcctg cagatgtcgc
aagcctgctc gatgctgctc 5220agcagcttca tggtggccaa ttgccccccc ccccccccgc
atgccccgga ggcggcggga 5280ggcggggggc gggtccgggc cacgatgcgc ttcctgcccg
cgccagccgc ccccgacccc 5340ggcgtccggg cccggatcag cctcgccgcg cagtccgggg
gcggggcggg ggcggctccg 5400ctgcggacac gccctcactc cccgccccgg ccccgcccgc
ccccggaacc cccgcctgcc 5460cctggccgcc ctgggccacg ccccgccgtc cgcgggtccc
gccgcccgcg ccctccagga 5520cccgcccgcg gccccagggt ccctccccgt agccgccgcc
gccgtcgctc gctctcctcg 5580ctctttcctc ccacttggcc gcttggcctg cctcgctcgc
gcctgttttc tcttttccgc 5640cctctccccc accccgcctc tccctctctc tgtgtctctc
ggtctctgcg cctctccgcc 5700tctgtcccca ccccaaactc cgcgcgtgtg gcgcttctcc
gggttctgcc tctgcttctc 5760cttctccctc cgactccgct tcgctctcca gtccctggca
gccccgcccc cggccctttc 5820tagtctcctt ctctctcctg ctccgttttt ccgtccctga
cgctcgctcc ctctctccgt 5880ggctccctct gcctccccct cggaccctcc gtccctctct
cggtccctct gagctcccct 5940ttccttctcc ctctgccttc ccgaaccctg tgtctcccct
ccacactcag cctccctcca 6000ccacctttct caggcactgt ccaggccttc gctcctcgag
tggtaataca atggccggtt 6060cccatggacc tgcatcgtgg tgatctatgt cgggtgcgga
gaaagaggta atgaaatggc 6120agaaatggca ccacctaagg tagcgaccgc ggagactagg
tgtatttatc taagcgatcg 6180cttaattaag gccggccgcc gcaataaaat atctttattt
tcattacatc tgtgtgttgg 6240ttttttgtgt gaatccatag tactaacata cgctctccat
caaaacaaaa cgaaacaaaa 6300caaactagca aaataggctg tccccagtgc aagtccaggt
gccagaacat ttctctatcc 6360ataatgcagg ggtaccgggt gatgacggtg aaaacctcca
attgcggagt actgtcctcc 6420gagcggagta ctgtcctccg agcggagtac tgtcctccga
gcggagtact gtcctccgag 6480cggagtactg tcctccgagc ggagtactgt cctccgagcg
gagagtcccc ggggacctag 6540agggtatata atgggtgcct tagctggtgt gtgacctcat
cttcctgtac gcccctgcag 6600gggcgcgcca cgcgtcgaag aaggtgagta atcttaacat
gctctttttt tttttttttg 6660ctaatccctt ttgtgtgctg atgttaggat gacatttaca
acaaatgttt gttcctgaca 6720ggaaaaacct tgctgggtac cttcgttgcc ggacacttct
tgtcctctac tttggaaaaa 6780aggaattgag agccgctagc gccaccatgc ctcgcctgtt
ttttttccac ctgttgggag 6840tctgtttact actgaaccaa ttttccagag cagtcgcgga
ctcatggatg gaggaagtta 6900tcaagttatg cggcagagaa ttagttcgcg cgcagattgc
catttgcggc atgagcacct 6960ggagcaaaag gtctctgagc caggaagatg ctcctcagac
acctagacca gtggcagaaa 7020ttgtgccatc cttcatcaac aaagatacag aaaccataaa
tatgatgtca gaatttgttg 7080ctaatttgcc acaggagctg aagctgaccc tgtctgagat
gcagccagca ttaccacagc 7140tacaacagca cgtccctgtg ctgaaggatt ccagcctgct
gtttgaagaa tttaagaaac 7200ttattcgcaa tagacaaagt gaagccgcag acagcagtcc
ttcagagctg aagtacctgg 7260gcttggatac tcattctcga aaaaagagac aactctacag
tgcattggct aacaagtgtt 7320gccatgttgg ttgtaccaaa agaagccttg ctagattttg
ctgaatcgat tgcgcaaagc 7380tttcgcgata ggcgagacca atgggtgtgt acgtagcggc
cgcgtcgacg atagcttgat 7440gggtggcatc cctgtgaccc ctccccagtg cctctcctgg
ccctggaagt tgccactcca 7500gtgcccacca gccttgtcct aataaaatta agttgcatca
ttttgtctga ctaggtgtcc 7560ttctataata ttatggggtg gaggggggtg gtatggagca
aggggcaagt tgggaagaca 7620acctgtaggg cctgcggggt ctattgggaa ccaagctgga
gtgcagtggc acaatcttgg 7680ctcactgcaa tctccgcctc ctgggttcaa gcgattctcc
tgcctcagcc tcccgagttg 7740ttgggattcc aggcatgcat gaccaggctc agctaatttt
tgtttttttg gtagagacgg 7800ggtttcacca tattggccag gctggtctcc aactcctaat
ctcaggtgat ctacccacct 7860tggcctccca aattgctggg attacaggcg tgaaccactg
ctcccttccc tgtccttctg 7920attttaaaat aactatacca gcaggaggac gtccagacac
agcataggct acctggccat 7980gcccaaccgg tgggacattt gagttgcttg cttggcactg
tcctctcatg cgttgggtcc 8040actcagtaga tgcctgttga attctgattt aaatcggtcc
gcgtacggcg tggtaggtcc 8100gaacgaatcc atggattacc ctgttatgtg gtcctccagg
gttgccgttc cttaactata 8160acggtcctaa ggtagcgacc gcggagacta ggtgtattta
tctaagcgat cgcttaatta 8220aggccggccg ccgcaataaa atatctttat tttcattaca
tctgtgtgtt ggttttttgt 8280gtgaatccat agtactaaca tacgctctcc atcaaaacaa
aacgaaacaa aacaaactag 8340caaaataggc tgtccccagt gcaagtccag gtgccagaac
atttctctat ccataatgca 8400ggggtaccgg gtgatgacgg tgaaaacctc caattgtgct
gtatataaaa ccagtggtta 8460tatgtacagt actgctgtat ataaaaccag tggttatatg
tacagtacgt cgactgctgt 8520atataaaacc agtggttata tgtacagtac tgctgtatat
aaaaccagtg gttatatgta 8580cagtaccccg gggacctaga gggtatataa tgggtgcctt
agctggtgtg tgacctcatc 8640ttcctgtacg cccctgcagg ggcgcgccac gcgtcgaaga
aggtgagtaa tcttaacatg 8700ctcttttttt ttttttttgc taatcccttt tgtgtgctga
tgttaggatg acatttacaa 8760caaatgtttg ttcctgacag gaaaaacctt gctgggtacc
ttcgttgccg gacacttctt 8820gtcctctact ttggaaaaaa ggaattgaga gccgctagcg
ccaccatgtg ggtgaccaaa 8880ctcctgccag ccctgctgct ccagcacgtc ctcctgcatc
tcctcctgct ccccatcgcc 8940atcccctatg cagagggaca aaggaaaaga agaaatacaa
ttcatgagtt caaaaaatca 9000gcaaagacta ccctaatcaa aatagatcca gcactgaaga
taaaaaccaa aaaagtgaat 9060accgccgacc aatgtgctaa tagatgtact aggaacaaag
gacttccatt cacttgcaag 9120gcttttgttt ttgataaagc aagaaaacaa tgcctctggt
tccccttcaa tagcatgtca 9180agtggagtga aaaaagaatt tggccatgaa tttgacctct
atgaaaacaa agactacatt 9240agaaactgca tcattggtaa aggacgcagc tacaagggaa
cagtatctat cactaagagt 9300ggcatcaaat gtcagccctg gagttccatg ataccacacg
aacacagctt tttgccttcg 9360agctatcggg gtaaagacct acaggaaaac tactgtcgaa
atcccagagg cgaagaaggg 9420ggaccctggt gtttcacaag caatccagag gtacgctacg
aagtctgtga cattcctcag 9480tgttcagaag ttgagtgcat gacctgtaat ggggagagtt
atcgaggtct catggatcat 9540acagaatcag gcaagatttg tcagcgctgg gatcatcaga
caccacaccg gcacaaattc 9600ttgcctgaaa gataccccga caagggcttt gatgataatt
attgccgcaa tcccgatggc 9660cagccgaggc cctggtgcta tactcttgac cctcacaccc
gctgggagta ttgcgcaatc 9720aagacatgcg ctgacaatac tatgaatgac actgatgttc
ctttggaaac aactgagtgc 9780atccaaggtc aaggagaagg ctacaggggc actgtcaata
ccatttggaa tggcatcccc 9840tgtcagcgtt gggattctca gtatcctcac gagcatgaca
tgactcctga aaatttcaag 9900tgcaaggacc tacgagaaaa ttactgccga aatccagatg
ggtctgaatc accctggtgt 9960tttaccactg atccaaacat ccgagttggc tactgctccc
aaattccaaa ctgtgatatg 10020tcacatggac aagattgtta tcgtgggaat ggcaaaaatt
atatgggcaa cttatcccaa 10080acaagaagcg gactaacctg ttcaatgtgg gacaagaaca
tggaggactt acatcgtcat 10140atcttctggg aaccagatgc aagtaagctg aatgagaatt
actgccgaaa tccagatgat 10200gatgctcatg gaccctggtg ctacacggga aatccactca
ttccttggga ttattgccct 10260atttctcgtt gtgaaggtga taccacacct acaatagtca
atttagacca tcccgtaata 10320tcttgtgcca aaacgaaaca gctgcgagtt gtaaatggga
ttccaacacg aacaaacata 10380ggatggatgg ttagtttgag atacagaaac aagcatatct
gcggaggatc attgataaag 10440gagagttggg ttcttactgc acgacagtgt ttccctagca
gagacttgaa agattatgag 10500gcttggcttg gcatccacga tgtccacggc agaggcgatg
agaaatgcaa acaggttctc 10560aatgtttccc agctggtata tggccctgaa ggaagcgacc
tggtgctgat gaagctggcc 10620agacccgctg tcctggatga ttttgttagt acgattgatt
tacctaatta tggatgcaca 10680attcctgaaa agaccagttg cagtgtttat ggctggggct
acactggact gattaactat 10740gatggcctat tacgagtggc acatctctat ataatgggaa
atgagaaatg cagccagcat 10800catcgaggga aggtgactct gaatgagtct gaaatatgtg
ctggggctga aaagattgga 10860tcaggaccat gtgaggggga ttatggtggc ccacttgttt
gtgagcaaca taaaatgaga 10920atggttcttg gtgtcattgt tcctggtcgt ggatgtgcca
ttccaaatcg tcctggtatt 10980tttgtccgag tagcctacta cgccaaatgg atacacaaaa
ttattttaac atataaggtg 11040ccccagtcat agatcgattg cgcaaagctt tcgcgatagg
cgagaccaat gggtgtgtac 11100gtagcggccg ctcgagaact tgtttattgc agcttataat
ggttacaaat aaagcaatag 11160catcacaaat ttcacaaata aagcattttt ttcactgcat
tctagttgtg gtttgtccaa 11220actcatcaat gtatcttatc atgtctcgta cggcgtggta
ggtccgaacg aatccatgga 11280ttaccctgtt atccctactc atcttcgtcg gacgcgccgt
taatatttga tcagccattc 11340tctgtatgag tactaacgaa cgtgacgcgg tgttactggt
aactatgact ctcttaaggt 11400agccaaatac atcatccctt tagtgagggt taattcacgc
agtgccgcgg cgtgtagaaa 11460atacctatca gctcgaggac gtgacaaacg aagccgcacg
tcagggccgg acgtggggcc 11520ccagagcgac gctgagtgcg tgcgggactc ggagtacgtg
acggagcccc cacgtgggtt 11580cccgcacgtc cgcgacgtga caaacgaagc cgcacgtcag
ggccggacgt ggggccccag 11640agcgacgctg agtgcgtgcg ggactcggag tacgtgacgg
agcccccacg tgggttcccg 11700cacgtccgcg acgtgacaaa cgaagccgca cgtcagggcc
ggacgtgggg ccccagagcg 11760acgctgagtg cgtgcgggac tcggagtacg tgacggagcc
cccacgtggg ttcccgcacg 11820tccgcgacgt gacaaacgaa gccgcacgtc agggccggac
gtggggcccc agagcgacgc 11880tgagtgcgtg cgggactcgg agtacgtgac ggagccccca
cgtgggttcc cgcacgtccg 11940cgacgtgaca aacgaagccg cacgtcaggg ccggacgtgg
ggccccagag cgacgctgag 12000tgcgtgcggg actcggagta cgtgacggag cccccacgtg
ggttcccgca cgtccgcgac 12060gtgacaaacg aagccgcacg tcagggccgg acgtggggcc
ccagagcgac gctgagtgcg 12120tgcgggactc ggagtacgtg acggagcccc cacgtgggtt
cccgcacgtc cgcgacgtga 12180caaacgaagc cgcacgtcag ggccggacgt ggggccccag
agcgacgctg agtgcgtgcg 12240ggactcggag tacgtgacgg agcccccacg tgggttcccg
cacgtccgcg aagacctaga 12300gggtatataa tgggtgcctt agctggtgtg tgagctcatc
ttcctgtgca tgcggggggg 12360ggggggggca attggccacc atgaaagccc tcaccgctag
acaacaggaa gtgtttgacc 12420tcatcaggga ccatatctcc cagacaggaa tgccccctac
cagggccgaa atcgctcaga 12480ggctgggatt caggagccct aacgctgccg aagaacatct
gaaagccctc gccaggaagg 12540gagtgattga gattgtgtcc ggcgcttcca ggggcattag
actcctgcaa gaggaggaag 12600aaggactccc cctcgtggga agggtcgccg ctggcgaacc
cctcctggct caacaacaca 12660ttgagggaca ctatcaggtc gatcctagcc tctttaaacc
caatgccgat ttcctcctga 12720gggtgtccgg catgagcatg aaggatattg gaatcatgga
cggagacctc ctggctgtgc 12780ataagacaca ggatgtgagg aacggacagg tcgtggtcgc
caggatcgac gacgaagtga 12840cagtgaaaag actcaagaaa cagggaaaca aagtggaact
gctccccgaa aactccgagt 12900ttaagcctat cgtcgtggat ctgaggcagc aatcctttac
cattgaggga ctggctgtgg 12960gagtgattag aaatggcgat tggctcgaat ttcccgggat
tagacctgag tgtgtggtcc 13020ccgaaaccca atgcgctatg aaaagaaaag agaaaaaggc
tcagaaagag aaagacaaac 13080tgcctgtgtc caccacaacc gtcgatgatc acatgccccc
tatcatgcag tgtgagcctc 13140cccctcccga agccgctaga atccacgaag tcgtccccag
gttcctctcc gataagctcc 13200tggaaaccaa tagacaaaag aatatccctc aactcaccgc
taaccaacag tttctgattg 13260ccaggctgat ttggtatcag gatggctatg agcaaccctc
cgacgaagac ctcaagagga 13320tcacacagac atggcaacag gctgacgatg agaatgagga
aagcgatacc cctttcaggc 13380agattaccga aatgacaatc ctcaccgtcc agctcatcgt
cgagtttgcc aaaggactcc 13440ccggattcgc taagattagc caacccgatc agattaccct
cctgaaagcc tgtagctccg 13500aggtcatgat gctgagagtg gctagaaggt acgatgccgc
ttccgatagc gtcctgtttg 13560ccaataacca agcctatacc agggacaatt acaggaaggc
tggcatggcc tatgtgattg 13620aggatgtgct ccacttttgc agatgtatgt actccatggc
tctcgataac attcactatg 13680ccctcctgac agccgtcgtg attttctccg acaggcccgg
actggaacag cctcaactcg 13740tggaagaaat tcagaggtac tatctgaata ccctcagaat
ttacattctg aatcagctct 13800ccggcagcgc tagatccagc gtcatctatg gcaaaatcct
ctccattctg tccgagctga 13860gaacactggg aatgcaaaac tccaatatgt gcattagcct
caagctcaag aatagaaaac 13920tgcctccctt tctggaagaa atttgggatg tggctgacat
gagccatacc caaccccctc 13980ccattctgga aagccctacc aatctgtaaa tcgatagccg
cctggctgag atggggtggg 14040cagggcagag ctgatcaggg ccgagcagaa ccgcactctt
cccaaataaa gcttcctcct 14100tgaaacacaa atgtttctta cttacacccc atcctgattt
cttttcttga gaccagagag 14160tgggaaagct ctctcttgac ctgaggatgg atctgaaaat
tatgagcccc ttgaggacag 14220ggaatgttat tcatctttga attccgcagc atctagcacc
aggtcttgta cagagcaggt 14280gcccaataaa tggttgaatg aatatatgaa aagtagaggc
agagggctgg gcacagtggc 14340tcacgcctgt aatcctagca ctttgggagg ctgaggtggg
tggatcactt gaggtcagga 14400gttcgagacc agcttggcca acatggctaa acctcatctc
tattaaaaat acaaaaatta 14460gctgggctgg tgcctgtaat cccagctact caggaggctg
aggcaggaga atcacttgaa 14520cccaggagga ggagtttgca gtgagccgag atcgcaccat
tgcactccag cctgggcgat 14580aggagcaaaa ctccatctca aaaacaaaaa acaaaacaaa
acaaaaagaa aaaagaaaag 14640taggggcaga gatgtggggc aggagaggtg actcgggctc
agctgcatgg tcctgctctg 14700cttctttttt cttggagttt gtccgttgga tgacaatgat
agtggtgaac aggtatggag 14760ggcttactaa gtaccaggtg tctagacgag gacgctcggg
cttggaccca tggcacatcg 14820tgtaatcatg gtcatagctg tttcctgtgt gaaattgtta
tccgctacgt ctctcccccg 14880cagtaagggc tagattaact cgtctcgtga atatccggaa
ctccctttag tgagggttaa 14940ttgcgttgcg ctcactgccc gctttccagt cgggaaacct
gtcgtgccag cttaatcatg 15000gtcatagctg tttcctgtgt gaaattgtta tccgctaccg
gaaacgcttc cttcatgtga 15060gcaaaaggcc agcaaaaggc caggaaccgt aaaaaggccg
cgttgctggc gtttttccat 15120aggctccgcc cccctgacga gcatcacaaa aatcgacgct
caagtcagag gtggcgaaac 15180ccgacaggac tataaagata ccaggcgttt ccccctggaa
gctccctcgt gcgctctcct 15240gttccgaccc tgccgcttac cggatacctg tccgcctttc
tcccttcggg aagcgtggcg 15300ctttctcata gctcacgctg taggtatctc agttcggtgt
aggtcgttcg ctccaagctg 15360ggctgtgtgc acgaaccccc cgttcagccc gaccgctgcg
ccttatccgg taactatcgt 15420cttgagtcca acccggtaag acacgactta tcgccactgg
cagcagccac tggtaacagg 15480attagcagag cgaggtatgt aggcggtgct acagagttct
tgaagtggtg gcctaactac 15540ggctacacta gaagaacagt atttggtatc tgcgctctgc
tgaagccagt taccttcgga 15600aaaagagttg gtagctcttg atccggcaaa caaaccaccg
ctggtagcgg tggttttttt 15660gtttgcaagc agcagattac gcgcagaaaa aaaggatctc
aagaagatcc tttgatcttt 15720tctacggggt ctgacgctca gtggaacgaa aactcacgtt
aagggatttt ggtcatgcct 15780aggacgaaag gaggtcgtga aatggataaa aaaatacagc
gtttttcatg tacaactata 15840ctagttgtag tgcctaaata atgcttttaa aacttaaaaa
tacgtttaaa ccctcagcga 15900aatggcatac gagtaaactt ggtctgacac cgctgcatga
gattatcaaa aaggatcttc 15960acctagatcc ttttaaatta aaaatgaagt tttaaatcaa
tctaaagtat atatgagtaa 16020acttggtctg acagttacca atgcttaatc agtgaggcac
ctatctcagc gatctgtcta 16080tttcgttcat ccatagttgc ctgactcccc gtcgtgtaga
taactacgat acgggagggc 16140ttaccatctg gccccagtgc tgcaatgata ccgcgagacc
cacgctcacc ggctccagat 16200ttatcagcaa taaaccagcc agccggaagc gccgagcgca
gaagtggtcc tgcaacttta 16260tccgcctcca tccagtctat taactgttgc cgggaagcta
gagtaagtag ttcgccagtt 16320aatagtttgc ggagcgttgt tgccattgct acaggcatcg
tggtgtcacg ctcgtcgttt 16380ggtatggctt cattcagctc cggttcccaa cgatcaaggc
gagttacatg atcccccatg 16440ttgtgcaaaa aagcggttag ctccttcggt cctccgatgg
ttgtcagaag taagttggcc 16500gcagtgttat cactcatggt tatggcagca ctgcataatt
ctcttactgt catgccatcc 16560gtaagatgct tttctgtgac tggtgagtat tcaaccaagt
cattctgaga atagtgtatg 16620cggcgaccga gttgctcttg cccggcgtca atacgggata
ataccgcgcc acatagcaga 16680actttaaaag tgctcatcat tgggaagcgt tcttcggggc
gaaaactctc aaggatctta 16740ccgctgttga gatccagttc gatgtaaccc acacgagcac
ccaactgatc ttcagcatct 16800tttactttca ccagcgtttc tgggtgagca aaaacaggaa
ggcaaaatgc cgcaaaaaag 16860ggaataaggg cgacacggaa atgttgaata ctcatacgct
tcctttttca atagtattga 16920agcatttatc agggttattg tctcgggagc gaatacatat
ttgaatgtat ttagaaaaa 169791413188DNAArtificial SequenceSynthetic
cardiomycyte + hypoxia inducible + hPlexin DI inducible gene switch
constant encoding IGF-1 14taaacaaata ggggttccgc gcacatttcc ccgaaaagtg
ccacctgacg tactgaggac 60gccagggttt tcccagtcac gacgttgtaa aacgacggcc
agtagcagac aagcccgtca 120gggcgcgtca gcgggtgttg gccctaggac gaaaggaggt
cgtgaaatgg ataaaaaaat 180acagcgtttt tcatgtacaa ctatactagt tgtagtgcct
aaataatgct tttaaaactt 240aaaaataatc aatgtcctca gcggggtgtc ggggatttag
gtgacactat aggctgagcg 300ccgcacaggc atctagaggc tatggcaggg cctgccgccc
cgacgttggc tgcgagccct 360gggccttcac ccgaacttgg ggggtggggt ggggaaaagg
aagaaacgcg ggcgtattgg 420ccccaatggg gtctcggtgg ggtatcgaca gagtgccagc
cctgggaccg aaccccgcgt 480ttatgaacaa acgacccaac accgtgcgtt ttattctgtc
tttttattgc cgtcatagcg 540cgggttcctt ccggtattgt ctccttccgt gtttcaatcg
attcaaaaga actcgtccag 600cagacggtaa aaagcaatgc gttgagaatc cggtgcagca
atgccataca gcaccagaaa 660gcgatcagcc cattcaccgc ccagttcttc agcaatgtca
cgggttgcca gtgcgatgtc 720ctgatagcga tcagccacgc ccaggcgacc gcagtcgata
aagccggaga aacggccgtt 780ttccaccata atgtttggca gacaagcatc gccgtgggtc
acaaccaggt cctcgccatc 840tggcatacgt gctttcaggc gtgcgaacag ttctgccggt
gccagaccct gatgttcctc 900gtccaggtca tcctgatcaa ccaggccagc ttccatgcga
gtgcgtgcgc gctcgatacg 960gtgtttagct tggtgatcga atgggcaagt agctgggtcc
agggtatgca gacggcgcat 1020agcatcagcc atgatggaaa ccttttctgc cggtgccaga
tgagaggaca gcagatcctg 1080gcctggaacc tcgcccagca gcagccagtc gcggccagcc
tcggtcacaa catccagcac 1140agctgcgcat ggaacgccgg tagtagccag ccaggacaga
cgagctgctt catcttgcag 1200ttcgttcagt gcgccggaca gatcggtctt aacaaacagc
accggacggc cttgagcgga 1260cagacggaac acagctgcgt cggagcaacc gatagtctgt
tgagcccagt catagccaaa 1320cagacgttcc acccaagcag ccggagaacc agcgtgcaga
ccgtcttgtt caatcatggt 1380ggcaattggg tgtctgagcg atgtggctcg gctggcgacg
caaaagaaga tgcggctgac 1440tgtcgaacag gaggagcaga gagcgaagcg ggaggctgcg
ggctcaattt gcatgcttta 1500gttcctcacc ttgtcgtatt atactatgcc gatatactat
gccgatgatt aattgtcaac 1560gtatacggaa tagctctgag gccgaggcag cttcggcctc
tgcataaata aaaaaaatta 1620gtcagccatg gggcggagaa tgggcggaac tgggcggagt
taggggcggg atgggcggag 1680ttaggggcgg gactatggtt gctgactaat tgagatgctt
gctttgcata cttctgcctg 1740ctggggagcc tggggacttt ccacacctgg ttgctgacta
attgagatgc ttgctttgca 1800tacttctgcc tgctggggag cctggggact ttccacaccc
taacctcgag gccatcgtgg 1860cacgccaggg ttttcccagt cacgacgttg taaaacgacg
gccagtgctc ttctcccccg 1920cgggaggttt tataaatccg actgtctaga ttgttgttaa
atcacacaaa aaaccaacac 1980acagatgtaa tgaaaataaa gatattttat tatcgattca
gctgtcgctg gggctctcca 2040gcatctccat caggaaggtg tcgatgggca cgtcgccgat
cagcctgaag aagaacaggt 2100gctccaggca cttcaggccg atgctcctca ggctgggcag
cctcagcagc agcttggcga 2160atctgccggg ctcgtcgggg tgggtggtcc tggtgtactc
ctccagggcg gcgtacacct 2220tctccctcag cagctccacc tcctgggcgc ttttcaggcc
cctcacctcg gggttgaaca 2280ggatgatggc cctcaggcag cccagctcgg tcttgtccat
cctcatgtcc ctcatcttgc 2340tcaccagctc ggtcagcacc ctgtcgaaga tggcgcccac
tccggcgctg tgggcgctgt 2400tcctatggac gtgcaggccg gtggccagca ggatgccgtc
cctcacgtcg atgctcctgt 2460ggctgaagct ggcgatcagc agctcgttcc atccggccct
cagcaggatc acctggtcgt 2520ccaggggcag gctgctgaag tggggaatcc tcttggccca
ctccaccagg gtgaacagct 2580gcttgtcggc ggcctggcag atgttggtca cggggtcgtt
ggggctgctg ccgctgccgc 2640cggttccgcc ggggccctcc acgccctggt cgcttttctg
ctccacggcg agttcggcct 2700ccagaatcct gtccacgggc atctccatat ggccgccgta
ctcgtcgatg cccagggcgt 2760cggtgaacat ctgctcgaac tcgaagtcgg ccatgtccag
ggcgccgtag ggggcgctgt 2820cgtggggggt gaagccgggg ccggggctgt cgccgtcgcc
cagcatgtcc aggtcgaagt 2880cgtccagggc gtcggcgtgg gccatggcca cgtcctcgcc
gtccaggtgc agctcgtcgc 2940ccaggctcac gtcggtgggg ggggccacct tccttttctt
cttggggccc atggtggcca 3000attgcccccc cccccccccg catgccccag tatgatgcct
tctctaaatt taccagaaag 3060aaaggacctg ggatgcttgt cctttaagtg tctctaaaca
tggtttgcat agctggatct 3120cagcttatct gtgcctttcg gctttcgctt ccatacatgg
atctgttccc aacagtaact 3180ccaagctgtc gaaaagaagc tatctggcag ctctctgctt
catccaacac atggatgata 3240aaaatacaca tatagcgatt cttccctcga gtaggcgaga
ccaatgggtg cgccatgggc 3300tcttccaaaa atttaggtga cactataggg caccgctcgc
acctgcgcac aggcccgcgg 3360ctacaaacta cgaacgatca ttctagatac cacatttgta
gaggttttac ttgctttaaa 3420aaacctccca catctccccc tgaacctgaa acataaaatg
aatgcaaatg ttttattaac 3480ttgtttattg cagcttataa tggttacaaa ttaagcaata
gcatcacaaa tttcacaaat 3540taagcatttt tttcactgca ttctagttgt ggtttgtcca
aactcatcaa tgtatcttat 3600catgtctaat cgattcacag gttggtgggg ctctccagga
tggggggggg ctgggtgtgg 3660ctcatgtcgg ccacgtccca aatctcctcc aggaaggggg
gcagcttcct gttcttcagc 3720ttcaggctga tacacatatt gctgttctgc attcccaggg
tcctcagctc gctcaggatg 3780ctcaggatct tgccgtagat cacgctgctc ctggcgctgc
cgctcagctg gttcaggatg 3840tagatcctca gggtgttcag gtagtacctc tggatctcct
ccaccagctg gggctgctcc 3900aggccgggcc tgtcgctgaa gatcaccacg gcggtcagca
gggcgtagtg gatgttgtcc 3960agggccatgc tgtacataca tctgcagaag tgcaggaggt
cctcgatcac ctcggccatg 4020ccagccttcc tgtagttgtc cctggtgtaa gcctggttgt
tggcgaacag gatgctgtcg 4080ctggcggcgt cgtacctcct ggccaccctc agcatcatca
cctcgctgct gcaagccttc 4140agcagggtga tctggtcggg ctggctgatc ttggcgaatc
cgggcaggcc cttggcgaac 4200tccacgatca gctgcacggt caggatggtc atctcggtga
tctgcctgaa gggggtgtcg 4260ctctcctcgt tctcgtcgtc ggcctgctgc caggtctggg
tgatcctttt caggtcctcg 4320tcgctgggct gctcgtagcc gtcctgatac cagatcagcc
tggcgatcag gaactgctgg 4380ttggcggtca gctgggggat gttcttctgc ctgttggtca
ccagcagctt gtcgctcagg 4440aacctgggca cgacctcgtg aatcctggcg gcctcggggg
gggggggctc gcactgcatg 4500atggggggca tgtggtcatc gacggtggtg gtgctcacgg
gcagcttgtc cttctccttc 4560tgggccttct tctccttcct tttcatggcg cactgggtct
cgggcaccac gcactcgggc 4620ctgatcccgg gaaactcggg gctcacggtc agctgcctct
ggcccttgtt gctgctctcc 4680tcgctgctgc tggtggcgct gatcctgtgc tgcctcaggg
tcaggggcat gtcggtctcc 4740acgctggcca gcctgtcggt cacggcgtcc ttgttcacgt
tgtcctgcac gaacaggccg 4800gtcagcaggg ccttgatgtc ttgcaggctg tccatcttca
ggatcatgtc caggtcctcc 4860ctggggaaga tcagcaggaa cagctgctcc agcctctcca
gcctgctctc cacctcggtc 4920aggtgggccc tggtcagggg gctcctcttg gtcttggggc
tgtatctgca ctcccagttg 4980ttcttcaggc acttggcgca cttgggcttc tccttgctgc
acttcagctt cttcagcctg 5040cagatgtcgc aagcctgctc gatgctgctc agcagcttca
tggtggccaa ttgccccccc 5100ccccccccgc atgcacagga agatgagctc acacaccagc
taaggcaccc attatatacc 5160ctctaggtct tcgcggacgt gcgggaaccc acgtgggggc
tccgtcacgt actccgagtc 5220ccgcacgcac tcagcgtcgc tctggggccc cacgtccggc
cctgacgtgc ggcttcgttt 5280gtcacgtcgc ggacgtgcgg gaacccacgt gggggctccg
tcacgtactc cgagtcccgc 5340acgcactcag cgtcgctctg gggccccacg tccggccctg
acgtgcggct tcgtttgtca 5400cgtcgcggac gtgcgggaac ccacgtgggg gctccgtcac
gtactccgag tcccgcacgc 5460actcagcgtc gctctggggc cccacgtccg gccctgacgt
gcggcttcgt ttgtcacgtc 5520gcggacgtgc gggaacccac gtgggggctc cgtcacgtac
tccgagtccc gcacgcactc 5580agcgtcgctc tggggcccca cgtccggccc tgacgtgcgg
cttcgtttgt cacgtcgcgg 5640acgtgcggga acccacgtgg gggctccgtc acgtactccg
agtcccgcac gcactcagcg 5700tcgctctggg gccccacgtc cggccctgac gtgcggcttc
gtttgtcacg tcgcggacgt 5760gcgggaaccc acgtgggggc tccgtcacgt actccgagtc
ccgcacgcac tcagcgtcgc 5820tctggggccc cacgtccggc cctgacgtgc ggcttcgttt
gtcacgtcgc ggacgtgcgg 5880gaacccacgt gggggctccg tcacgtactc cgagtcccgc
acgcactcag cgtcgctctg 5940gggccccacg tccggccctg acgtgcggct tcgtttgtca
cgtcctcgag tggtaataca 6000atggccggtt cccatggacc tgcatcgtgg tgtaactata
acggtcctaa ggtagcgacc 6060gcggagacta ggtgtattta tctaagcgat cgcttaatta
aggccggccg ccgcaataaa 6120atatctttat tttcattaca tctgtgtgtt ggttttttgt
gtgaatccat agtactaaca 6180tacgctctcc atcaaaacaa aacgaaacaa aacaaactag
caaaataggc tgtccccagt 6240gcaagtccag gtgccagaac atttctctat ccataatgca
ggggtaccgg gtgatgacgg 6300tgaaaacctc caattgcgga gtactgtcct ccgagcggag
tactgtcctc cgagcggagt 6360actgtcctcc gagcggagta ctgtcctccg agcggagtac
tgtcctccga gcggagtact 6420gtcctccgag cggagagtcc ccggggacct agagggtata
taatgggtgc cttagctggt 6480gtgtgacctc atcttcctgt acgcccctgc aggggcgcgc
cacgcgtcga agaaggtgag 6540taatcttaac atgctctttt tttttttttt tgctaatccc
ttttgtgtgc tgatgttagg 6600atgacattta caacaaatgt ttgttcctga caggaaaaac
cttgctgggt accttcgttg 6660ccggacactt cttgtcctct actttggaaa aaaggaattg
agagccgcta gcgccaccat 6720gggaaaaatc agcagcctgc ccacccaatt atttaagtgc
tgcttttgtg atttcttgaa 6780ggtgaagatg cacaccatgt cctcctcgca tctgttctac
ctggcgctgt gcctgctgac 6840cttcaccagc tctgccacgg ctggaccgga gaccctctgc
ggggccgagc tggtggatgc 6900cctgcaattc gtgtgtggag acaggggctt ctacttcaac
aagcccacag ggtatggctc 6960cagcagtcgg agagcccccc agacaggcat cgtggatgag
tgctgcttca gaagctgtga 7020tctaaggagg ctggagatgt attgcgcacc cctcaagcct
gccaagtcag ctcgctctgt 7080ccgtgcccag cgccacaccg acatgcccaa gacccagaag
tatcagcccc catctaccaa 7140caagaacacg aagtctcaga gaaggaaagg aagtacattt
gaagaacgca agtagatcga 7200ttgcgcaaag ctttcgcgat aggcgagacc aatgggtgtg
tacgtagcgg ccgcgtcgac 7260gatagcttga tgggtggcat ccctgtgacc cctccccagt
gcctctcctg gccctggaag 7320ttgccactcc agtgcccacc agccttgtcc taataaaatt
aagttgcatc attttgtctg 7380actaggtgtc cttctataat attatggggt ggaggggggt
ggtatggagc aaggggcaag 7440ttgggaagac aacctgtagg gcctgcgggg tctattggga
accaagctgg agtgcagtgg 7500cacaatcttg gctcactgca atctccgcct cctgggttca
agcgattctc ctgcctcagc 7560ctcccgagtt gttgggattc caggcatgca tgaccaggct
cagctaattt ttgttttttt 7620ggtagagacg gggtttcacc atattggcca ggctggtctc
caactcctaa tctcaggtga 7680tctacccacc ttggcctccc aaattgctgg gattacaggc
gtgaaccact gctcccttcc 7740ctgtccttct gattttaaaa taactatacc agcaggagga
cgtccagaca cagcataggc 7800tacctggcca tgcccaaccg gtgggacatt tgagttgctt
gcttggcact gtcctctcat 7860gcgttgggtc cactcagtag atgcctgttg aattctgatt
taaatcggtc cgcgtacggc 7920gtggtaggtc cgaacgaatc catggattac cctgttatcc
ctactcaagg acatcatccc 7980tttagtgagg gttaattcac gcagtgccgc ggcgtgtaga
aaatacctat cagctcgagg 8040agcgaaggcc tggacagtgc ctgagaaagg tggtggaggg
aggctgagtg tggaggggag 8100acacagggtt cgggaaggca gagggagaag gaaaggggag
ctcagaggga ccgagagagg 8160gacggagggt ccgaggggga ggcagaggga gccacggaga
gagggagcga gcgtcaggga 8220cggaaaaacg gagcaggaga gagaaggaga ctagaaaggg
ccgggggcgg ggctgccagg 8280gactggagag cgaagcggag tcggagggag aaggagaagc
agaggcagaa cccggagaag 8340cgccacacgc gcggagtttg gggtggggac agaggcggag
aggcgcagag accgagagac 8400acagagagag ggagaggcgg ggtgggggag agggcggaaa
agagaaaaca ggcgcgagcg 8460aggcaggcca agcggccaag tgggaggaaa gagcgaggag
agcgagcgac ggcggcggcg 8520gctacgggga gggaccctgg ggccgcgggc gggtcctgga
gggcgcgggc ggcgggaccc 8580gcggacggcg gggcgtggcc cagggcggcc aggggcaggc
gggggttccg ggggcgggcg 8640gggccggggc ggggagtgag ggcgtgtccg cagcggagcc
gcccccgccc cgcccccgga 8700ctgcgcggcg aggctgatcc gggcccggac gccggggtcg
ggggcggctg gcgcgggcag 8760gaagcgcatc gtggcccgga cccgcccccc gcctcccgcc
gcctccgggg catgcggggg 8820gggggggggg caattggcca ccatgaagct cctgtccagc
attgagcaag cctgtgacat 8880ttgcaggctg aaaaagctca agtgtagcaa agagaaaccc
aaatgcgcta agtgtctgaa 8940aaacaactgg gaatgcagat actcccccaa aaccaaaaga
tcccccctca ccagggccca 9000tctgacagag gtcgagtcca gactcgaaag gctggaacag
ctctttctcc tgattttccc 9060tagagaagac ctcgacatga tcctcaagat ggactccctg
caagacatta aggctctgct 9120caccggactg tttgtgcaag acaatgtgaa taaggatgcc
gtcaccgata gactcgcctc 9180cgtggaaacc gatatgcctc tgacactgag gcagcataga
attagcgcta cctccagctc 9240cgaggaaagc tccaacaaag gccaaagaca actgacagtg
tcccccgagt tccccgggat 9300tagacctgag tgtgtggtcc ccgaaaccca atgcgctatg
aaaagaaaag agaaaaaggc 9360tcagaaagag aaagacaaac tgcctgtgtc caccacaacc
gtcgatgatc acatgccccc 9420tatcatgcag tgtgagcctc cccctcccga agccgctaga
atccacgaag tcgtccccag 9480gttcctctcc gataagctcc tggaaaccaa tagacaaaag
aatatccctc aactcaccgc 9540taaccaacag tttctgattg ccaggctgat ttggtatcag
gatggctatg agcaaccctc 9600cgacgaagac ctcaagagga tcacacagac atggcaacag
gctgacgatg agaatgagga 9660aagcgatacc cctttcaggc agattaccga aatgacaatc
ctcaccgtcc agctcatcgt 9720cgagtttgcc aaaggactcc ccggattcgc taagattagc
caacccgatc agattaccct 9780cctgaaagcc tgtagctccg aggtcatgat gctgagagtg
gctagaaggt acgatgccgc 9840ttccgatagc gtcctgtttg ccaataacca agcctatacc
agggacaatt acaggaaggc 9900tggcatggcc tatgtgattg aggatgtgct ccacttttgc
agatgtatgt actccatggc 9960tctcgataac attcactatg ccctcctgac agccgtcgtg
attttctccg acaggcccgg 10020actggaacag cctcaactcg tggaagaaat tcagaggtac
tatctgaata ccctcagaat 10080ttacattctg aatcagctct ccggcagcgc tagatccagc
gtcatctatg gcaaaatcct 10140ctccattctg tccgagctga gaacactggg aatgcaaaac
tccaatatgt gcattagcct 10200caagctcaag aatagaaaac tgcctccctt tctggaagaa
atttgggatg tggctgacat 10260gagccatacc caaccccctc ccattctgga aagccctacc
aatctgtaaa tcgatagccg 10320cctggctgag atggggtggg cagggcagag ctgatcaggg
ccgagcagaa ccgcactctt 10380cccaaataaa gcttcctcct tgaaacacaa atgtttctta
cttacacccc atcctgattt 10440cttttcttga gaccagagag tgggaaagct ctctcttgac
ctgaggatgg atctgaaaat 10500tatgagcccc ttgaggacag ggaatgttat tcatctttga
attccgcagc atctagcacc 10560aggtcttgta cagagcaggt gcccaataaa tggttgaatg
aatatatgaa aagtagaggc 10620agagggctgg gcacagtggc tcacgcctgt aatcctagca
ctttgggagg ctgaggtggg 10680tggatcactt gaggtcagga gttcgagacc agcttggcca
acatggctaa acctcatctc 10740tattaaaaat acaaaaatta gctgggctgg tgcctgtaat
cccagctact caggaggctg 10800aggcaggaga atcacttgaa cccaggagga ggagtttgca
gtgagccgag atcgcaccat 10860tgcactccag cctgggcgat aggagcaaaa ctccatctca
aaaacaaaaa acaaaacaaa 10920acaaaaagaa aaaagaaaag taggggcaga gatgtggggc
aggagaggtg actcgggctc 10980agctgcatgg tcctgctctg cttctttttt cttggagttt
gtccgttgga tgacaatgat 11040agtggtgaac aggtatggag ggcttactaa gtaccaggtg
tctagacgag gacgctcggg 11100cttggaccca tggcacatcg tgtaatcatg gtcatagctg
tttcctgtgt gaaattgtta 11160tccgctacgt ctctcccccg cagtaagggc tagattaact
cgtctcgtga atatccggaa 11220ctccctttag tgagggttaa ttgcgttgcg ctcactgccc
gctttccagt cgggaaacct 11280gtcgtgccag cttaatcatg gtcatagctg tttcctgtgt
gaaattgtta tccgctaccg 11340gaaacgcttc cttcatgtga gcaaaaggcc agcaaaaggc
caggaaccgt aaaaaggccg 11400cgttgctggc gtttttccat aggctccgcc cccctgacga
gcatcacaaa aatcgacgct 11460caagtcagag gtggcgaaac ccgacaggac tataaagata
ccaggcgttt ccccctggaa 11520gctccctcgt gcgctctcct gttccgaccc tgccgcttac
cggatacctg tccgcctttc 11580tcccttcggg aagcgtggcg ctttctcata gctcacgctg
taggtatctc agttcggtgt 11640aggtcgttcg ctccaagctg ggctgtgtgc acgaaccccc
cgttcagccc gaccgctgcg 11700ccttatccgg taactatcgt cttgagtcca acccggtaag
acacgactta tcgccactgg 11760cagcagccac tggtaacagg attagcagag cgaggtatgt
aggcggtgct acagagttct 11820tgaagtggtg gcctaactac ggctacacta gaagaacagt
atttggtatc tgcgctctgc 11880tgaagccagt taccttcgga aaaagagttg gtagctcttg
atccggcaaa caaaccaccg 11940ctggtagcgg tggttttttt gtttgcaagc agcagattac
gcgcagaaaa aaaggatctc 12000aagaagatcc tttgatcttt tctacggggt ctgacgctca
gtggaacgaa aactcacgtt 12060aagggatttt ggtcatgatc tatgtcgggt gcggagaaag
aggtaatgaa atggcatacg 12120agtaaacttg gtctgacacc gctgcatgag attatcaaaa
aggatcttca cctagatcct 12180tttaaattaa aaatgaagtt ttaaatcaat ctaaagtata
tatgagtaaa cttggtctga 12240cagttaccaa tgcttaatca gtgaggcacc tatctcagcg
atctgtctat ttcgttcatc 12300catagttgcc tgactccccg tcgtgtagat aactacgata
cgggagggct taccatctgg 12360ccccagtgct gcaatgatac cgcgagaccc acgctcaccg
gctccagatt tatcagcaat 12420aaaccagcca gccggaagcg ccgagcgcag aagtggtcct
gcaactttat ccgcctccat 12480ccagtctatt aactgttgcc gggaagctag agtaagtagt
tcgccagtta atagtttgcg 12540gagcgttgtt gccattgcta caggcatcgt ggtgtcacgc
tcgtcgtttg gtatggcttc 12600attcagctcc ggttcccaac gatcaaggcg agttacatga
tcccccatgt tgtgcaaaaa 12660agcggttagc tccttcggtc ctccgatggt tgtcagaagt
aagttggccg cagtgttatc 12720actcatggtt atggcagcac tgcataattc tcttactgtc
atgccatccg taagatgctt 12780ttctgtgact ggtgagtatt caaccaagtc attctgagaa
tagtgtatgc ggcgaccgag 12840ttgctcttgc ccggcgtcaa tacgggataa taccgcgcca
catagcagaa ctttaaaagt 12900gctcatcatt gggaagcgtt cttcggggcg aaaactctca
aggatcttac cgctgttgag 12960atccagttcg atgtaaccca cacgagcacc caactgatct
tcagcatctt ttactttcac 13020cagcgtttct gggtgagcaa aaacaggaag gcaaaatgcc
gcaaaaaagg gaataagggc 13080gacacggaaa tgttgaatac tcatacgctt cctttttcaa
tagtattgaa gcatttatca 13140gggttattgt ctcgggagcg aatacatatt tgaatgtatt
tagaaaaa 131881513314DNAArtificial SequenceSynthetic
constitutive + TNF + inducible promoter gene switch encoding
etanercept 15taaacaaata ggggttccgc gcacatttcc ccgaaaagtg ccacctgacg
tactgaggac 60gccagggttt tcccagtcac gacgttgtaa aacgacggcc agtagcagac
aagcccgtca 120gggcgcgtca gcgggtgttg gccctaggac gaaaggaggt cgtgaaatgg
ataaaaaaat 180acagcgtttt tcatgtacaa ctatactagt tgtagtgcct aaataatgct
tttaaaactt 240aaaaataatc aatgtcctca gcggggtgtc ggggatttag gtgacactat
aggctgagcg 300ccgcacaggc atctagaggc tatggcaggg cctgccgccc cgacgttggc
tgcgagccct 360gggccttcac ccgaacttgg ggggtggggt ggggaaaagg aagaaacgcg
ggcgtattgg 420ccccaatggg gtctcggtgg ggtatcgaca gagtgccagc cctgggaccg
aaccccgcgt 480ttatgaacaa acgacccaac accgtgcgtt ttattctgtc tttttattgc
cgtcatagcg 540cgggttcctt ccggtattgt ctccttccgt gtttcaatcg attcaaaaga
actcgtccag 600cagacggtaa aaagcaatgc gttgagaatc cggtgcagca atgccataca
gcaccagaaa 660gcgatcagcc cattcaccgc ccagttcttc agcaatgtca cgggttgcca
gtgcgatgtc 720ctgatagcga tcagccacgc ccaggcgacc gcagtcgata aagccggaga
aacggccgtt 780ttccaccata atgtttggca gacaagcatc gccgtgggtc acaaccaggt
cctcgccatc 840tggcatacgt gctttcaggc gtgcgaacag ttctgccggt gccagaccct
gatgttcctc 900gtccaggtca tcctgatcaa ccaggccagc ttccatgcga gtgcgtgcgc
gctcgatacg 960gtgtttagct tggtgatcga atgggcaagt agctgggtcc agggtatgca
gacggcgcat 1020agcatcagcc atgatggaaa ccttttctgc cggtgccaga tgagaggaca
gcagatcctg 1080gcctggaacc tcgcccagca gcagccagtc gcggccagcc tcggtcacaa
catccagcac 1140agctgcgcat ggaacgccgg tagtagccag ccaggacaga cgagctgctt
catcttgcag 1200ttcgttcagt gcgccggaca gatcggtctt aacaaacagc accggacggc
cttgagcgga 1260cagacggaac acagctgcgt cggagcaacc gatagtctgt tgagcccagt
catagccaaa 1320cagacgttcc acccaagcag ccggagaacc agcgtgcaga ccgtcttgtt
caatcatggt 1380ggcaattggg tgtctgagcg atgtggctcg gctggcgacg caaaagaaga
tgcggctgac 1440tgtcgaacag gaggagcaga gagcgaagcg ggaggctgcg ggctcaattt
gcatgcttta 1500gttcctcacc ttgtcgtatt atactatgcc gatatactat gccgatgatt
aattgtcaac 1560gtatacggaa tagctctgag gccgaggcag cttcggcctc tgcataaata
aaaaaaatta 1620gtcagccatg gggcggagaa tgggcggaac tgggcggagt taggggcggg
atgggcggag 1680ttaggggcgg gactatggtt gctgactaat tgagatgctt gctttgcata
cttctgcctg 1740ctggggagcc tggggacttt ccacacctgg ttgctgacta attgagatgc
ttgctttgca 1800tacttctgcc tgctggggag cctggggact ttccacaccc taacctcgag
gccatcgtgg 1860cacgccaggg ttttcccagt cacgacgttg taaaacgacg gccagtgctc
ttctcccccg 1920cgggaggttt tataaatccg actgtctaga ttgttgttaa atcacacaaa
aaaccaacac 1980acagatgtaa tgaaaataaa gatattttat tatcgattca gctgtcgctg
gggctctcca 2040gcatctccat caggaaggtg tcgatgggca cgtcgccgat cagcctgaag
aagaacaggt 2100gctccaggca cttcaggccg atgctcctca ggctgggcag cctcagcagc
agcttggcga 2160atctgccggg ctcgtcgggg tgggtggtcc tggtgtactc ctccagggcg
gcgtacacct 2220tctccctcag cagctccacc tcctgggcgc ttttcaggcc cctcacctcg
gggttgaaca 2280ggatgatggc cctcaggcag cccagctcgg tcttgtccat cctcatgtcc
ctcatcttgc 2340tcaccagctc ggtcagcacc ctgtcgaaga tggcgcccac tccggcgctg
tgggcgctgt 2400tcctatggac gtgcaggccg gtggccagca ggatgccgtc cctcacgtcg
atgctcctgt 2460ggctgaagct ggcgatcagc agctcgttcc atccggccct cagcaggatc
acctggtcgt 2520ccaggggcag gctgctgaag tggggaatcc tcttggccca ctccaccagg
gtgaacagct 2580gcttgtcggc ggcctggcag atgttggtca cggggtcgtt ggggctgctg
ccgctgccgc 2640cggttccgcc ggggccctcc acgccctggt cgcttttctg ctccacggcg
agttcggcct 2700ccagaatcct gtccacgggc atctccatat ggccgccgta ctcgtcgatg
cccagggcgt 2760cggtgaacat ctgctcgaac tcgaagtcgg ccatgtccag ggcgccgtag
ggggcgctgt 2820cgtggggggt gaagccgggg ccggggctgt cgccgtcgcc cagcatgtcc
aggtcgaagt 2880cgtccagggc gtcggcgtgg gccatggcca cgtcctcgcc gtccaggtgc
agctcgtcgc 2940ccaggctcac gtcggtgggg ggggccacct tccttttctt cttggggccc
atggtggcca 3000attgcccccc cccccccccg catgccgtct aacaaaaaag ccaaaaacgg
ccagaattta 3060gcggacaatt tactagtcta acactgaaaa ttacatattg acccaaatga
ttacatttca 3120aaaggtgcct aaaaaacttc acaaaacaca ctcgccaacc ccgagcgcac
gtacccagcc 3180cagcagcccg ctactcacca agtgacgatc acagcgatcc acaaacaaga
accgcgaccc 3240aaatcccggc tgcgacggaa ctagctgtgc cacacccggc gcgtccttat
ataatcatcg 3300gcgttcaccg ccccacggag atccctccgc agaatcgccg agaagggact
acttttcctc 3360gcctgttccg ctctctggaa agaaaaccag tgccctagag tcacccaagt
cccgtcctaa 3420aatgtccttc tgctgatact ggggttctaa ggccgagtct tatgagcagc
gggccgctgt 3480cctgagcgtc cgggcggaag gatcaggacg ctcgctgcgc ccttcgtctg
acgtggcagc 3540gctcgccgtg aggagggggg cgcccgcggg aggcgccaaa acccggcgcg
gaggccctcg 3600agtaggcgag accaatgggt gcgccatggg ctcttccaaa aatttaggtg
acactatagg 3660gcaccgctcg cacctgcgca caggcccgcg gctacaaact acgaacgatc
attctagata 3720ccacatttgt agaggtttta cttgctttaa aaaacctccc acatctcccc
ctgaacctga 3780aacataaaat gaatgcaaat gttttattaa cttgtttatt gcagcttata
atggttacaa 3840attaagcaat agcatcacaa atttcacaaa ttaagcattt ttttcactgc
attctagttg 3900tggtttgtcc aaactcatca atgtatctta tcatgtctaa tcgattcaca
ggttggtggg 3960gctctccagg atgggggggg gctgggtgtg gctcatgtcg gccacgtccc
aaatctcctc 4020caggaagggg ggcagcttcc tgttcttcag cttcaggctg atacacatat
tgctgttctg 4080cattcccagg gtcctcagct cgctcaggat gctcaggatc ttgccgtaga
tcacgctgct 4140cctggcgctg ccgctcagct ggttcaggat gtagatcctc agggtgttca
ggtagtacct 4200ctggatctcc tccaccagct ggggctgctc caggccgggc ctgtcgctga
agatcaccac 4260ggcggtcagc agggcgtagt ggatgttgtc cagggccatg ctgtacatac
atctgcagaa 4320gtgcaggagg tcctcgatca cctcggccat gccagccttc ctgtagttgt
ccctggtgta 4380agcctggttg ttggcgaaca ggatgctgtc gctggcggcg tcgtacctcc
tggccaccct 4440cagcatcatc acctcgctgc tgcaagcctt cagcagggtg atctggtcgg
gctggctgat 4500cttggcgaat ccgggcaggc ccttggcgaa ctccacgatc agctgcacgg
tcaggatggt 4560catctcggtg atctgcctga agggggtgtc gctctcctcg ttctcgtcgt
cggcctgctg 4620ccaggtctgg gtgatccttt tcaggtcctc gtcgctgggc tgctcgtagc
cgtcctgata 4680ccagatcagc ctggcgatca ggaactgctg gttggcggtc agctggggga
tgttcttctg 4740cctgttggtc accagcagct tgtcgctcag gaacctgggc acgacctcgt
gaatcctggc 4800ggcctcgggg ggggggggct cgcactgcat gatggggggc atgtggtcat
cgacggtggt 4860ggtgctcacg ggcagcttgt ccttctcctt ctgggccttc ttctccttcc
ttttcatggc 4920gcactgggtc tcgggcacca cgcactcggg cctgatcccg ggaaactcgg
ggctcacggt 4980cagctgcctc tggcccttgt tgctgctctc ctcgctgctg ctggtggcgc
tgatcctgtg 5040ctgcctcagg gtcaggggca tgtcggtctc cacgctggcc agcctgtcgg
tcacggcgtc 5100cttgttcacg ttgtcctgca cgaacaggcc ggtcagcagg gccttgatgt
cttgcaggct 5160gtccatcttc aggatcatgt ccaggtcctc cctggggaag atcagcagga
acagctgctc 5220cagcctctcc agcctgctct ccacctcggt caggtgggcc ctggtcaggg
ggctcctctt 5280ggtcttgggg ctgtatctgc actcccagtt gttcttcagg cacttggcgc
acttgggctt 5340ctccttgctg cacttcagct tcttcagcct gcagatgtcg caagcctgct
cgatgctgct 5400cagcagcttc atggtggcca attgcccccc cccccccccg catgcctgtc
tgacgggcaa 5460tgctcctctg agagttgtta cagttcagac atggtaatga ctggttttat
acagctttgc 5520ccctcccaca tggcattttt ttttctccct ctgtcttttg atctgtgtcc
tttgagtgat 5580tcaacatttt actcaggaag acatcattag aatagaaata cttgtttgta
aaggcatggt 5640ttaagaaatt ttgggggagg atgaggtcac tctgaaactt aagaatttgt
caatatcctc 5700gagtggtaat acaatggccg gttcccatgg acctgcatcg tggtgtaact
ataacggtcc 5760taaggtagcg accgcggaga ctaggtgtat ttatctaagc gatcgcttaa
ttaaggccgg 5820ccgccgcaat aaaatatctt tattttcatt acatctgtgt gttggttttt
tgtgtgaatc 5880catagtacta acatacgctc tccatcaaaa caaaacgaaa caaaacaaac
tagcaaaata 5940ggctgtcccc agtgcaagtc caggtgccag aacatttctc tatccataat
gcaggggtac 6000cgggtgatga cggtgaaaac ctccaattgc ggagtactgt cctccgagcg
gagtactgtc 6060ctccgagcgg agtactgtcc tccgagcgga gtactgtcct ccgagcggag
tactgtcctc 6120cgagcggagt actgtcctcc gagcggagag tccccgggga cctagagggt
atataatggg 6180tgccttagct ggtgtgtgac ctcatcttcc tgtacgcccc tgcaggggcg
cgccacgcgt 6240cgaagaaggt gagtaatctt aacatgctct tttttttttt ttttgctaat
cccttttgtg 6300tgctgatgtt aggatgacat ttacaacaaa tgtttgttcc tgacaggaaa
aaccttgctg 6360ggtaccttcg ttgccggaca cttcttgtcc tctactttgg aaaaaaggaa
ttgagagccg 6420ctagcgccac catgctgccc gcccaggtcg ccttcacccc ctacgccccc
gagcccggca 6480gcacctgtag actgagagag tattacgacc agaccgccca gatgtgctgc
tccaagtgca 6540gccccggcca gcacgccaag gtgttctgca ccaagaccag cgacaccgtc
tgcgacagct 6600gcgaggacag cacctacacc cagctgtgga actgggtgcc cgagtgcctg
agctgcggca 6660gcagatgtag cagcgaccag gtggagaccc aggcttgcac cagagagcag
aacagaatct 6720gcacctgtag acccggctgg tactgcgccc tgagcaagca ggagggctgc
cgcctgtgcg 6780cccccctgag aaagtgcaga cccggcttcg gcgtggccag acccggcacc
gagaccagcg 6840acgttgtgtg caagccctgt gcccccggca ccttcagcaa caccaccagc
agcaccgaca 6900tctgccgccc ccaccagatt tgcaacgtgg tggccatccc cggcaacgcc
agcatggacg 6960ccgtgtgtac cagcaccagc cccacccgca gcatggcccc cggcgctgtc
cacctccccc 7020agcccgtgag caccagaagc cagcacaccc agcccacccc cgagcccagc
accgccccca 7080gcaccagctt cctgctgcct atgggaccca gcccccccgc cgagggcagc
accggcgacg 7140agcccaagag ctgcgacaag acccacacct gtcccccctg ccccgccccc
gagctgctgg 7200gcggccccag cgtgttcctg ttccccccca agcccaagga caccctgatg
atctccagaa 7260cccccgaggt gacatgcgtg gtggtggacg tgagccacga ggacccccag
gtgaagttca 7320actggtacgt ggacggcgtg caagtgcata acgccaagac caagcccaga
gagcagcagt 7380acaacagcac ctacagagtg gtgagcgtgc tgaccgtgct gcaccagaac
tggctggacg 7440gcaaggagta caagtgcaag gtgtccaaca aggccctgcc cgcccccatc
gagaaaacca 7500tcagcaaggc caagggccag cccagagagc cccaggtgta caccctgccc
cccagcagag 7560aggagatgac caagaaccag gtgtccctga cctgtctggt gaagggcttc
taccccagcg 7620acatcgccgt ggagtgggag agcaacggcc agcccgagaa caactacaag
accacccccc 7680ccgtgctgga cagcgacggc agcttcttcc tgtacagcaa gctgaccgtg
gacaagagca 7740gatggcagca gggcaacgtg ttcagctgct ccgtgatgca cgaggccctg
cacaaccact 7800acacccagaa gtccctgagc ctgagccccg gcaagtgaat cgattgcgca
aagctttcgc 7860gataggcgag accaatgggt gtgtacgtag cggccgcgtc gacgatagct
tgatgggtgg 7920catccctgtg acccctcccc agtgcctctc ctggccctgg aagttgccac
tccagtgccc 7980accagccttg tcctaataaa attaagttgc atcattttgt ctgactaggt
gtccttctat 8040aatattatgg ggtggagggg ggtggtatgg agcaaggggc aagttgggaa
gacaacctgt 8100agggcctgcg gggtctattg ggaaccaagc tggagtgcag tggcacaatc
ttggctcact 8160gcaatctccg cctcctgggt tcaagcgatt ctcctgcctc agcctcccga
gttgttggga 8220ttccaggcat gcatgaccag gctcagctaa tttttgtttt tttggtagag
acggggtttc 8280accatattgg ccaggctggt ctccaactcc taatctcagg tgatctaccc
accttggcct 8340cccaaattgc tgggattaca ggcgtgaacc actgctccct tccctgtcct
tctgatttta 8400aaataactat accagcagga ggacgtccag acacagcata ggctacctgg
ccatgcccaa 8460ccggtgggac atttgagttg cttgcttggc actgtcctct catgcgttgg
gtccactcag 8520tagatgcctg ttgaattctg atttaaatcg gtccgcgtac ggcgtggtag
gtccgaacga 8580atccatggat taccctgtta tccctactca aggacatcat ccctttagtg
agggttaatt 8640cacgcagtgc cgcggcgtgt agaaaatacc tatcagctcg agcacggggc
tcccactctc 8700aactccgcag cctcagcccc ctcaatgctg aggagcagag ctggtctcct
gccctgacag 8760ctgccaggca catcttgttc cctcaggttg cacaactggg ataaatgacc
cgggatgaag 8820aaaccactgg catccaggaa cttgtcttag accgttttgt aggggaaatg
acctgcaggg 8880actttcccca gggaccacat ccagcttttc ttcgctccca agaaaccagc
agggagcatg 8940cggggggggg ggggggcaat tggccaccat gaagctcctg tccagcattg
agcaagcctg 9000tgacatttgc aggctgaaaa agctcaagtg tagcaaagag aaacccaaat
gcgctaagtg 9060tctgaaaaac aactgggaat gcagatactc ccccaaaacc aaaagatccc
ccctcaccag 9120ggcccatctg acagaggtcg agtccagact cgaaaggctg gaacagctct
ttctcctgat 9180tttccctaga gaagacctcg acatgatcct caagatggac tccctgcaag
acattaaggc 9240tctgctcacc ggactgtttg tgcaagacaa tgtgaataag gatgccgtca
ccgatagact 9300cgcctccgtg gaaaccgata tgcctctgac actgaggcag catagaatta
gcgctacctc 9360cagctccgag gaaagctcca acaaaggcca aagacaactg acagtgtccc
ccgagttccc 9420cgggattaga cctgagtgtg tggtccccga aacccaatgc gctatgaaaa
gaaaagagaa 9480aaaggctcag aaagagaaag acaaactgcc tgtgtccacc acaaccgtcg
atgatcacat 9540gccccctatc atgcagtgtg agcctccccc tcccgaagcc gctagaatcc
acgaagtcgt 9600ccccaggttc ctctccgata agctcctgga aaccaataga caaaagaata
tccctcaact 9660caccgctaac caacagtttc tgattgccag gctgatttgg tatcaggatg
gctatgagca 9720accctccgac gaagacctca agaggatcac acagacatgg caacaggctg
acgatgagaa 9780tgaggaaagc gatacccctt tcaggcagat taccgaaatg acaatcctca
ccgtccagct 9840catcgtcgag tttgccaaag gactccccgg attcgctaag attagccaac
ccgatcagat 9900taccctcctg aaagcctgta gctccgaggt catgatgctg agagtggcta
gaaggtacga 9960tgccgcttcc gatagcgtcc tgtttgccaa taaccaagcc tataccaggg
acaattacag 10020gaaggctggc atggcctatg tgattgagga tgtgctccac ttttgcagat
gtatgtactc 10080catggctctc gataacattc actatgccct cctgacagcc gtcgtgattt
tctccgacag 10140gcccggactg gaacagcctc aactcgtgga agaaattcag aggtactatc
tgaataccct 10200cagaatttac attctgaatc agctctccgg cagcgctaga tccagcgtca
tctatggcaa 10260aatcctctcc attctgtccg agctgagaac actgggaatg caaaactcca
atatgtgcat 10320tagcctcaag ctcaagaata gaaaactgcc tccctttctg gaagaaattt
gggatgtggc 10380tgacatgagc catacccaac cccctcccat tctggaaagc cctaccaatc
tgtaaatcga 10440tagccgcctg gctgagatgg ggtgggcagg gcagagctga tcagggccga
gcagaaccgc 10500actcttccca aataaagctt cctccttgaa acacaaatgt ttcttactta
caccccatcc 10560tgatttcttt tcttgagacc agagagtggg aaagctctct cttgacctga
ggatggatct 10620gaaaattatg agccccttga ggacagggaa tgttattcat ctttgaattc
cgcagcatct 10680agcaccaggt cttgtacaga gcaggtgccc aataaatggt tgaatgaata
tatgaaaagt 10740agaggcagag ggctgggcac agtggctcac gcctgtaatc ctagcacttt
gggaggctga 10800ggtgggtgga tcacttgagg tcaggagttc gagaccagct tggccaacat
ggctaaacct 10860catctctatt aaaaatacaa aaattagctg ggctggtgcc tgtaatccca
gctactcagg 10920aggctgaggc aggagaatca cttgaaccca ggaggaggag tttgcagtga
gccgagatcg 10980caccattgca ctccagcctg ggcgatagga gcaaaactcc atctcaaaaa
caaaaaacaa 11040aacaaaacaa aaagaaaaaa gaaaagtagg ggcagagatg tggggcagga
gaggtgactc 11100gggctcagct gcatggtcct gctctgcttc ttttttcttg gagtttgtcc
gttggatgac 11160aatgatagtg gtgaacaggt atggagggct tactaagtac caggtgtcta
gacgaggacg 11220ctcgggcttg gacccatggc acatcgtgta atcatggtca tagctgtttc
ctgtgtgaaa 11280ttgttatccg ctacgtctct cccccgcagt aagggctaga ttaactcgtc
tcgtgaatat 11340ccggaactcc ctttagtgag ggttaattgc gttgcgctca ctgcccgctt
tccagtcggg 11400aaacctgtcg tgccagctta atcatggtca tagctgtttc ctgtgtgaaa
ttgttatccg 11460ctaccggaaa cgcttccttc atgtgagcaa aaggccagca aaaggccagg
aaccgtaaaa 11520aggccgcgtt gctggcgttt ttccataggc tccgcccccc tgacgagcat
cacaaaaatc 11580gacgctcaag tcagaggtgg cgaaacccga caggactata aagataccag
gcgtttcccc 11640ctggaagctc cctcgtgcgc tctcctgttc cgaccctgcc gcttaccgga
tacctgtccg 11700cctttctccc ttcgggaagc gtggcgcttt ctcatagctc acgctgtagg
tatctcagtt 11760cggtgtaggt cgttcgctcc aagctgggct gtgtgcacga accccccgtt
cagcccgacc 11820gctgcgcctt atccggtaac tatcgtcttg agtccaaccc ggtaagacac
gacttatcgc 11880cactggcagc agccactggt aacaggatta gcagagcgag gtatgtaggc
ggtgctacag 11940agttcttgaa gtggtggcct aactacggct acactagaag aacagtattt
ggtatctgcg 12000ctctgctgaa gccagttacc ttcggaaaaa gagttggtag ctcttgatcc
ggcaaacaaa 12060ccaccgctgg tagcggtggt ttttttgttt gcaagcagca gattacgcgc
agaaaaaaag 12120gatctcaaga agatcctttg atcttttcta cggggtctga cgctcagtgg
aacgaaaact 12180cacgttaagg gattttggtc atgatctatg tcgggtgcgg agaaagaggt
aatgaaatgg 12240catacgagta aacttggtct gacaccgctg catgagatta tcaaaaagga
tcttcaccta 12300gatcctttta aattaaaaat gaagttttaa atcaatctaa agtatatatg
agtaaacttg 12360gtctgacagt taccaatgct taatcagtga ggcacctatc tcagcgatct
gtctatttcg 12420ttcatccata gttgcctgac tccccgtcgt gtagataact acgatacggg
agggcttacc 12480atctggcccc agtgctgcaa tgataccgcg agacccacgc tcaccggctc
cagatttatc 12540agcaataaac cagccagccg gaagcgccga gcgcagaagt ggtcctgcaa
ctttatccgc 12600ctccatccag tctattaact gttgccggga agctagagta agtagttcgc
cagttaatag 12660tttgcggagc gttgttgcca ttgctacagg catcgtggtg tcacgctcgt
cgtttggtat 12720ggcttcattc agctccggtt cccaacgatc aaggcgagtt acatgatccc
ccatgttgtg 12780caaaaaagcg gttagctcct tcggtcctcc gatggttgtc agaagtaagt
tggccgcagt 12840gttatcactc atggttatgg cagcactgca taattctctt actgtcatgc
catccgtaag 12900atgcttttct gtgactggtg agtattcaac caagtcattc tgagaatagt
gtatgcggcg 12960accgagttgc tcttgcccgg cgtcaatacg ggataatacc gcgccacata
gcagaacttt 13020aaaagtgctc atcattggga agcgttcttc ggggcgaaaa ctctcaagga
tcttaccgct 13080gttgagatcc agttcgatgt aacccacacg agcacccaac tgatcttcag
catcttttac 13140tttcaccagc gtttctgggt gagcaaaaac aggaaggcaa aatgccgcaa
aaaagggaat 13200aagggcgaca cggaaatgtt gaatactcat acgcttcctt tttcaatagt
attgaagcat 13260ttatcagggt tattgtctcg ggagcgaata catatttgaa tgtatttaga
aaaa 133141611013DNAArtificial SequenceSynthetic TNF inducible
promoter gene switch construct expressing etanercept 16taaacaaata
ggggttccgc gcacatttcc ccgaaaagtg ccacctgacg tactgaggac 60gccagggttt
tcccagtcac gacgttgtaa aacgacggcc agtagcagac aagcccgtca 120gggcgcgtca
gcgggtgttg gcgggtgtcg gggatttagg tgacactata ggctgagcgc 180cgcacaggca
tctagaggct atggcagggc ctgccgcccc gacgttggct gcgagccctg 240ggccttcacc
cgaacttggg gggtggggtg gggaaaagga agaaacgcgg gcgtattggc 300cccaatgggg
tctcggtggg gtatcgacag agtgccagcc ctgggaccga accccgcgtt 360tatgaacaaa
cgacccaaca ccgtgcgttt tattctgtct ttttattgcc gtcatagcgc 420gggttccttc
cggtattgtc tccttccgtg tttcaatcga ttcaaaagaa ctcgtccagc 480agacggtaaa
aagcaatgcg ttgagaatcc ggtgcagcaa tgccatacag caccagaaag 540cgatcagccc
attcaccgcc cagttcttca gcaatgtcac gggttgccag tgcgatgtcc 600tgatagcgat
cagccacgcc caggcgaccg cagtcgataa agccggagaa acggccgttt 660tccaccataa
tgtttggcag acaagcatcg ccgtgggtca caaccaggtc ctcgccatct 720ggcatacgtg
ctttcaggcg tgcgaacagt tctgccggtg ccagaccctg atgttcctcg 780tccaggtcat
cctgatcaac caggccagct tccatgcgag tgcgtgcgcg ctcgatacgg 840tgtttagctt
ggtgatcgaa tgggcaagta gctgggtcca gggtatgcag acggcgcata 900gcatcagcca
tgatggaaac cttttctgcc ggtgccagat gagaggacag cagatcctgg 960cctggaacct
cgcccagcag cagccagtcg cggccagcct cggtcacaac atccagcaca 1020gctgcgcatg
gaacgccggt agtagccagc caggacagac gagctgcttc atcttgcagt 1080tcgttcagtg
cgccggacag atcggtctta acaaacagca ccggacggcc ttgagcggac 1140agacggaaca
cagctgcgtc ggagcaaccg atagtctgtt gagcccagtc atagccaaac 1200agacgttcca
cccaagcagc cggagaacca gcgtgcagac cgtcttgttc aatcatggtg 1260gcaattgggt
gtctgagcga tgtggctcgg ctggcgacgc aaaagaagat gcggctgact 1320gtcgaacagg
aggagcagag agcgaagcgg gaggctgcgg gctcaatttg catgctttag 1380ttcctcacct
tgtcgtatta tactatgccg atatactatg ccgatgatta attgtcaacg 1440tatacggaat
agctctgagg ccgaggcagc ttcggcctct gcataaataa aaaaaattag 1500tcagccatgg
ggcggagaat gggcggaact gggcggagtt aggggcggga tgggcggagt 1560taggggcggg
actatggttg ctgactaatt gagatgcttg ctttgcatac ttctgcctgc 1620tggggagcct
ggggactttc cacacctggt tgctgactaa ttgagatgct tgctttgcat 1680acttctgcct
gctggggagc ctggggactt tccacaccct aacctcgagg ccatcgtggc 1740acgccagggt
tttcccagtc acgacgttgt aaaacgacgg ccagtgctct tctcccccgc 1800gggaggtttt
ataaatccga ctgtctagat accacatttg tagaggtttt acttgcttta 1860aaaaacctcc
cacatctccc cctgaacctg aaacataaaa tgaatgcaaa tgttttatta 1920acttgtttat
tgcagcttat aatggttaca aattaagcaa tagcatcaca aatttcacaa 1980attaagcatt
tttttcactg cattctagtt gtggtttgtc caaactcatc aatgtatctt 2040atcatgtcta
atcgattcac aggttggtgg ggctctccag gatggggggg ggctgggtgt 2100ggctcatgtc
ggccacgtcc caaatctcct ccaggaaggg gggcagcttc ctgttcttca 2160gcttcaggct
gatacacata ttgctgttct gcattcccag ggtcctcagc tcgctcagga 2220tgctcaggat
cttgccgtag atcacgctgc tcctggcgct gccgctcagc tggttcagga 2280tgtagatcct
cagggtgttc aggtagtacc tctggatctc ctccaccagc tggggctgct 2340ccaggccggg
cctgtcgctg aagatcacca cggcggtcag cagggcgtag tggatgttgt 2400ccagggccat
gctgtacata catctgcaga agtgcaggag gtcctcgatc acctcggcca 2460tgccagcctt
cctgtagttg tccctggtgt aagcctggtt gttggcgaac aggatgctgt 2520cgctggcggc
gtcgtacctc ctggccaccc tcagcatcat cacctcgctg ctgcaagcct 2580tcagcagggt
gatctggtcg ggctggctga tcttggcgaa tccgggcagg cccttggcga 2640actccacgat
cagctgcacg gtcaggatgg tcatctcggt gatctgcctg aagggggtgt 2700cgctctcctc
gttctcgtcg tcggcctgct gccaggtctg ggtgatcctt ttcaggtcct 2760cgtcgctggg
ctgctcgtag ccgtcctgat accagatcag cctggcgatc aggaactgct 2820ggttggcggt
cagctggggg atgttcttct gcctgttggt caccagcagc ttgtcgctca 2880ggaacctggg
cacgacctcg tgaatcctgg cggcctcggg gggggggggc tcgcactgca 2940tgatgggggg
catgtggtca tcgacggtgg tggtgctcac gggcagcttg tccttctcct 3000tctgggcctt
cttctccttc cttttcatgg cgcactgggt ctcgggcacc acgcactcgg 3060gcctgatccc
gggaaactcg gggctcacgg tcagctgcct ctggcccttg ttgctgctct 3120cctcgctgct
gctggtggcg ctgatcctgt gctgcctcag ggtcaggggc atgtcggtct 3180ccacgctggc
cagcctgtcg gtcacggcgt ccttgttcac gttgtcctgc acgaacaggc 3240cggtcagcag
ggccttgatg tcttgcaggc tgtccatctt caggatcatg tccaggtcct 3300ccctggggaa
gatcagcagg aacagctgct ccagcctctc cagcctgctc tccacctcgg 3360tcaggtgggc
cctggtcagg gggctcctct tggtcttggg gctgtatctg cactcccagt 3420tgttcttcag
gcacttggcg cacttgggct tctccttgct gcacttcagc ttcttcagcc 3480tgcagatgtc
gcaagcctgc tcgatgctgc tcagcagctt catggtggca gatctggtcg 3540cgggaggctg
ctggttttcc actacccgaa aaaaatccag cgtctaagca gctgcaagga 3600gagcctttca
gagaagcggg tcctggcagc ggcggggaag tgtccccaaa tgggcagaat 3660agcctccccg
cgtcgggaga gtcgcgtcct tgctcgggtg ttgtaagttc cagtgcaaag 3720tgcccgcccg
ctgctatggg caaagtttcg tggatgcggc tagggttgcg caccgctggc 3780tgggggatca
gcgggagggc tgggccagag gcgaagcccc ctattcgctc cggatctccc 3840ttcccaggac
gcccgcagcg cagctctgct cgccgggctc ctccacccta gcccgccgcc 3900cgctcgctcc
ctctgcctct cgctggaatt actacagcga gttgccggct cagctgtcgc 3960tggggctctc
cagcatctcc atcaggaagg tgtcgatggg cacgtcgccg atcagcctga 4020agaagaacag
gtgctccagg cacttcaggc cgatgctcct caggctgggc agcctcagca 4080gcagcttggc
gaatctgccg ggctcgtcgg ggtgggtggt cctggtgtac tcctccaggg 4140cggcgtacac
cttctccctc agcagctcca cctcctgggc gcttttcagg cccctcacct 4200cggggttgaa
caggatgatg gccctcaggc agcccagctc ggtcttgtcc atcctcatgt 4260ccctcatctt
gctcaccagc tcggtcagca ccctgtcgaa gatggcgccc actccggcgc 4320tgtgggcgct
gttcctatgg acgtgcaggc cggtggccag caggatgccg tccctcacgt 4380cgatgctcct
gtggctgaag ctggcgatca gcagctcgtt ccatccggcc ctcagcagga 4440tcacctggtc
gtccaggggc aggctgctga agtggggaat cctcttggcc cactccacca 4500gggtgaacag
ctgcttgtcg gcggcctggc agatgttggt cacggggtcg ttggggctgc 4560tgccgctgcc
gccggttccg ccggggccct ccacgccctg gtcgcttttc tgctccacgg 4620cgagttcggc
ctccagaatc ctgtccacgg gcatctccat atggccgccg tactcgtcga 4680tgcccagggc
gtcggtgaac atctgctcga actcgaagtc ggccatgtcc agggcgccgt 4740agggggcgct
gtcgtggggg gtgaagccgg ggccggggct gtcgccgtcg cccagcatgt 4800ccaggtcgaa
gtcgtccagg gcgtcggcgt gggccatggc cacgtcctcg ccgtccaggt 4860gcagctcgtc
gcccaggctc acgtcggtgg ggggggccac cttccttttc ttcttggggc 4920ccatggtggc
caattgcccc cccccccccc cgcatgcagc tcctgaagcc agtgaggccc 4980gatgcagata
ccgcggagtg aaatagaaag tctgtgcttt ataaagggtc ttgttgcaga 5040ggcggaggga
aatcccttca aggggaaacc cagggcagag ccagggaaaa aagtttaaca 5100gacacccagc
caagttccac tattaacccc ttcagttgct ctcactgctg aagctcctct 5160ctctgtcctg
gcaaaagaag acacttccaa gactataaaa tacaggcttt tcctcatctt 5220cgactccaaa
aggctatctt tactggaaag ataaaggaca atgctgattg cagaatgaag 5280ctttctgaat
ccaatgtggg ttaagggggt ggggagagaa aaaatggaga cagagtagta 5340aattctactc
tggtttttga actggataaa taacgactat gccatgtgaa ttgattttct 5400ccatcaagga
aatatgtttg caattctaaa agagattaat ttttttctct tacaagagaa 5460aggaaaaagt
tatttctttt gatgagtcat tttttaaaaa agggacacca taacttctta 5520gttttatatt
tattcataaa aatagttctt ttagcaacaa acatcaactt tttgaaacta 5580ttttcttgag
tagaaagtaa aggactgtaa ctgaaattgc tgccaaaaca agggaaacaa 5640tttttttttg
ttttagaaaa gttaccaata atttggttaa attgctggat aattggaatt 5700tttttgcata
cttaaatgca agtgtatgga atccatctga agtggcaaat ctcttggctc 5760gagtaggcga
gaccaatggg tgcgccatgg gctcttccaa aaatttaggt gacactatag 5820ggcaccgctc
gcacctgcgc acaggcataa gccaaatgga actacgagac ctgcatcgtg 5880gtgtaactat
aacggtccta aggtagcgac cgcggagact aggtgtattt atctaagcga 5940tcgcttaatt
aaggccggcc gccgcaataa aatatcttta ttttcattac atctgtgtgt 6000tggttttttg
tgtgaatcca tagtactaac atacgctctc catcaaaaca aaacgaaaca 6060aaacaaacta
gcaaaatagg ctgtccccag tgcaagtcca ggtgccagaa catttctcta 6120tccataatgc
aggggtaccg ggtgatgacg gtgaaaacct ccaattgcgg agtactgtcc 6180tccgagcgga
gtactgtcct ccgagcggag tactgtcctc cgagcggagt actgtcctcc 6240gagcggagta
ctgtcctccg agcggagtac tgtcctccga gcggagagtc cccggggacc 6300tagagggtat
ataatgggtg ccttagctgg tgtgtgacct catcttcctg tacgcccctg 6360caggggcgcg
ccacgcgtcg aagaaggtga gtaatcttaa catgctcttt tttttttttt 6420ttgctaatcc
cttttgtgtg ctgatgttag gatgacattt acaacaaatg tttgttcctg 6480acaggaaaaa
ccttgctggg taccttcgtt gccggacact tcttgtcctc tactttggaa 6540aaaaggaatt
gagagccgct agcgccacca tgctgcccgc ccaggtcgcc ttcaccccct 6600acgcccccga
gcccggcagc acctgtagac tgagagagta ttacgaccag accgcccaga 6660tgtgctgctc
caagtgcagc cccggccagc acgccaaggt gttctgcacc aagaccagcg 6720acaccgtctg
cgacagctgc gaggacagca cctacaccca gctgtggaac tgggtgcccg 6780agtgcctgag
ctgcggcagc agatgtagca gcgaccaggt ggagacccag gcttgcacca 6840gagagcagaa
cagaatctgc acctgtagac ccggctggta ctgcgccctg agcaagcagg 6900agggctgccg
cctgtgcgcc cccctgagaa agtgcagacc cggcttcggc gtggccagac 6960ccggcaccga
gaccagcgac gttgtgtgca agccctgtgc ccccggcacc ttcagcaaca 7020ccaccagcag
caccgacatc tgccgccccc accagatttg caacgtggtg gccatccccg 7080gcaacgccag
catggacgcc gtgtgtacca gcaccagccc cacccgcagc atggcccccg 7140gcgctgtcca
cctcccccag cccgtgagca ccagaagcca gcacacccag cccacccccg 7200agcccagcac
cgcccccagc accagcttcc tgctgcctat gggacccagc ccccccgccg 7260agggcagcac
cggcgacgag cccaagagct gcgacaagac ccacacctgt cccccctgcc 7320ccgcccccga
gctgctgggc ggccccagcg tgttcctgtt cccccccaag cccaaggaca 7380ccctgatgat
ctccagaacc cccgaggtga catgcgtggt ggtggacgtg agccacgagg 7440acccccaggt
gaagttcaac tggtacgtgg acggcgtgca agtgcataac gccaagacca 7500agcccagaga
gcagcagtac aacagcacct acagagtggt gagcgtgctg accgtgctgc 7560accagaactg
gctggacggc aaggagtaca agtgcaaggt gtccaacaag gccctgcccg 7620cccccatcga
gaaaaccatc agcaaggcca agggccagcc cagagagccc caggtgtaca 7680ccctgccccc
cagcagagag gagatgacca agaaccaggt gtccctgacc tgtctggtga 7740agggcttcta
ccccagcgac atcgccgtgg agtgggagag caacggccag cccgagaaca 7800actacaagac
cacccccccc gtgctggaca gcgacggcag cttcttcctg tacagcaagc 7860tgaccgtgga
caagagcaga tggcagcagg gcaacgtgtt cagctgctcc gtgatgcacg 7920aggccctgca
caaccactac acccagaagt ccctgagcct gagccccggc aagtgaatcg 7980attgcgcaaa
gctttcgcga taggcgagac caatgggtgt gtacgtagcg gccgcgtcga 8040cgatagcttg
atgggtggca tccctgtgac ccctccccag tgcctctcct ggccctggaa 8100gttgccactc
cagtgcccac cagccttgtc ctaataaaat taagttgcat cattttgtct 8160gactaggtgt
ccttctataa tattatgggg tggagggggg tggtatggag caaggggcaa 8220gttgggaaga
caacctgtag ggcctgcggg gtctattggg aaccaagctg gagtgcagtg 8280gcacaatctt
ggctcactgc aatctccgcc tcctgggttc aagcgattct cctgcctcag 8340cctcccgagt
tgttgggatt ccaggcatgc atgaccaggc tcagctaatt tttgtttttt 8400tggtagagac
ggggtttcac catattggcc aggctggtct ccaactccta atctcaggtg 8460atctacccac
cttggcctcc caaattgctg ggattacagg cgtgaaccac tgctcccttc 8520cctgtccttc
tgattttaaa ataactatac cagcaggagg acgtccagac acagcatagg 8580ctacctggcc
atgcccaacc ggtgggacat ttgagttgct tgcttggcac tgtcctctca 8640tgcgttgggt
ccactcagta gatgcctgtt gaattctgat ttaaatcggt ccgcgtacgg 8700cgtggtaggt
ccgaacgaat ccatggatta ccctgttatc cctactcaag gacatcatcc 8760ctttagtgag
ggttaattca cgcagtgggt acggaactaa aggcagcaca catcgtgtaa 8820tcatggtcat
agctgtttcc tgtgtgaaat tgttatccgc tacgtctctc ccccgcagta 8880agggctagat
taactcgtct cgtgaatatc cggaactccc tttagtgagg gttaattgcg 8940ttgcgctcac
tgcccgcttt ccagtcggga aacctgtcgt gccagcttaa tcatggtcat 9000agctgtttcc
tgtgtgaaat tgttatccgc taccggaaac gcttccttca tgtgagcaaa 9060aggccagcaa
aaggccagga accgtaaaaa ggccgcgttg ctggcgtttt tccataggct 9120ccgcccccct
gacgagcatc acaaaaatcg acgctcaagt cagaggtggc gaaacccgac 9180aggactataa
agataccagg cgtttccccc tggaagctcc ctcgtgcgct ctcctgttcc 9240gaccctgccg
cttaccggat acctgtccgc ctttctccct tcgggaagcg tggcgctttc 9300tcatagctca
cgctgtaggt atctcagttc ggtgtaggtc gttcgctcca agctgggctg 9360tgtgcacgaa
ccccccgttc agcccgaccg ctgcgcctta tccggtaact atcgtcttga 9420gtccaacccg
gtaagacacg acttatcgcc actggcagca gccactggta acaggattag 9480cagagcgagg
tatgtaggcg gtgctacaga gttcttgaag tggtggccta actacggcta 9540cactagaaga
acagtatttg gtatctgcgc tctgctgaag ccagttacct tcggaaaaag 9600agttggtagc
tcttgatccg gcaaacaaac caccgctggt agcggtggtt tttttgtttg 9660caagcagcag
attacgcgca gaaaaaaagg atctcaagaa gatcctttga tcttttctac 9720ggggtctgac
gctcagtgga acgaaaactc acgttaaggg attttggtca tgcctaggac 9780gaaaggaggt
cgtgaaatgg ataaaaaaat acagcgtttt tcatgtacaa ctatactagt 9840tgtagtgcct
aaataatgct tttaaaactt aaaaataatc tatgtcgggt gcggagaaag 9900aggtaatgaa
atggcaaatc aatgtcctca gcgaaatggc atacgagtaa acttggtctg 9960acaccgctgc
atgagattat caaaaaggat cttcacctag atccttttaa attaaaaatg 10020aagttttaaa
tcaatctaaa gtatatatga gtaaacttgg tctgacagtt accaatgctt 10080aatcagtgag
gcacctatct cagcgatctg tctatttcgt tcatccatag ttgcctgact 10140ccccgtcgtg
tagataacta cgatacggga gggcttacca tctggcccca gtgctgcaat 10200gataccgcga
gacccacgct caccggctcc agatttatca gcaataaacc agccagccgg 10260aagcgccgag
cgcagaagtg gtcctgcaac tttatccgcc tccatccagt ctattaactg 10320ttgccgggaa
gctagagtaa gtagttcgcc agttaatagt ttgcggagcg ttgttgccat 10380tgctacaggc
atcgtggtgt cacgctcgtc gtttggtatg gcttcattca gctccggttc 10440ccaacgatca
aggcgagtta catgatcccc catgttgtgc aaaaaagcgg ttagctcctt 10500cggtcctccg
atggttgtca gaagtaagtt ggccgcagtg ttatcactca tggttatggc 10560agcactgcat
aattctctta ctgtcatgcc atccgtaaga tgcttttctg tgactggtga 10620gtattcaacc
aagtcattct gagaatagtg tatgcggcga ccgagttgct cttgcccggc 10680gtcaatacgg
gataataccg cgccacatag cagaacttta aaagtgctca tcattgggaa 10740gcgttcttcg
gggcgaaaac tctcaaggat cttaccgctg ttgagatcca gttcgatgta 10800acccacacga
gcacccaact gatcttcagc atcttttact ttcaccagcg tttctgggtg 10860agcaaaaaca
ggaaggcaaa atgccgcaaa aaagggaata agggcgacac ggaaatgttg 10920aatactcata
cgcttccttt ttcaatagta ttgaagcatt tatcagggtt attgtctcgg 10980gagcgaatac
atatttgaat gtatttagaa aaa
110131715706DNAArtificial SequenceSynthetic dual promoter inflammation +
hypoxia inducible gene switch construct encoding etanercept and
EPO 17taaacaaata ggggttccgc gcacatttcc ccgaaaagtg ccacctgacg tactgaggac
60gccagggttt tcccagtcac gacgttgtaa aacgacggcc agtagcagac aagcccgtca
120gggcgcgtca gcgggtgttg gcgggtgtcg gggatttagg tgacactata ggctgagcgc
180cgcacaggca tctagaggct atggcagggc ctgccgcccc gacgttggct gcgagccctg
240ggccttcacc cgaacttggg gggtggggtg gggaaaagga agaaacgcgg gcgtattggc
300cccaatgggg tctcggtggg gtatcgacag agtgccagcc ctgggaccga accccgcgtt
360tatgaacaaa cgacccaaca ccgtgcgttt tattctgtct ttttattgcc gtcatagcgc
420gggttccttc cggtattgtc tccttccgtg tttcaatcga tttagaagaa ctcgtcaaga
480aggcgataga aggcgatgcg ctgcgaatcg ggggcggcga taccgtaaag gacgaggaag
540cggtcagccc attcgccgcc aagttcttca gcaatatcac gggtagccaa cgctatgtcc
600tgatagcggt cggccacacc cagccgtcca cagtcgatga atccagaaaa gcggccattt
660tccaccatga tattcggcaa gcaggcatcg ccgtgggtca cgacgagatc ctcgccgtcg
720ggcatcctcg ccttgagcct ggcgaacagt tcggctggcg cgagcccctg atgctcctcg
780tccagatcat cctgatcgac aagaccggct tccatccgag tacgtgctcg ctcgatgcga
840tgtttcgctt ggtggtcgaa tgggcatgta gccggatcaa gcgtatgcag ccgccgcatt
900gcatcagcca tgatggatac tttctcggca ggagcaaggt gagatgacag gagatcctgc
960cccggcactt cgcccaatag cagccagtcc cttcccgctt cagtgacaac gtcgagcacg
1020gctgcgcaag gaacgcccgt cgtggccagc cacgatagcc gcgctgcctc gtcctggagt
1080tcattcaggg caccggacag gtcggtcttg acaaaaagaa ccgggcgccc ctgcgctgac
1140agccggaaca cggcggcatc agagcagccg attgtctgtt gtgcccagtc atagccgaat
1200agcctctcca cccaagcggc cggagaacca gcgtgcaatc catcttgttc aatggccgat
1260cccatggtgg ccaattgggt gtctgagcga tgtggctcgg ctggcgacgc aaaagaagat
1320gcggctgact gtcgaacagg aggagcagag agcgaagcgg gaggctgcgg gctcaatttg
1380catgcggaat agctctgagg ccgaggcagc ttcggcctct gcataaataa aaaaaattag
1440tcagccatgg ggcggagaat gggcggaact gggcggagtt aggggcggga tgggcggagt
1500taggggcggg actatggttg ctgactaatt gagatgcttg ctttgcatac ttctgcctgc
1560tggggagcct ggggactttc cacacctggt tgctgactaa ttgagatgct tgctttgcat
1620acttctgcct gctggggagc ctggggactt tccacaccct aacctcgagg ccatcgtggc
1680acgccagggt tttcccagtc acgacgttgt aaaacgacgg ccagtgctct tctcccccgc
1740gggaggtttt ataaatccga ctgtctagat tgttgttaaa tcacacaaaa aaccaacaca
1800cagatgtaat gaaaataaag atattttatt atcgattcag ctgtcgctgg ggctctccag
1860catctccatc aggaaggtgt cgatgggcac gtcgccgatc agcctgaaga agaacaggtg
1920ctccaggcac ttcaggccga tgctcctcag gctgggcagc ctcagcagca gcttggcgaa
1980tctgccgggc tcgtcggggt gggtggtcct ggtgtactcc tccagggcgg cgtacacctt
2040ctccctcagc agctccacct cctgggcgct tttcaggccc ctcacctcgg ggttgaacag
2100gatgatggcc ctcaggcagc ccagctcggt cttgtccatc ctcatgtccc tcatcttgct
2160caccagctcg gtcagcaccc tgtcgaagat ggcgcccact ccggcgctgt gggcgctgtt
2220cctatggacg tgcaggccgg tggccagcag gatgccgtcc ctcacgtcga tgctcctgtg
2280gctgaagctg gcgatcagca gctcgttcca tccggccctc agcaggatca cctggtcgtc
2340caggggcagg ctgctgaagt ggggaatcct cttggcccac tccaccaggg tgaacagctg
2400cttgtcggcg gcctggcaga tgttggtcac ggggtcgttg gggctgctgc cgctgccgcc
2460ggttccgccg gggccctcca cgccctggtc gcttttctgc tccacggcga gttcggcctc
2520cagaatcctg tccacgggca tctccatatg gccgccgtac tcgtcgatgc ccagggcgtc
2580ggtgaacatc tgctcgaact cgaagtcggc catgtccagg gcgccgtagg gggcgctgtc
2640gtggggggtg aagccggggc cggggctgtc gccgtcgccc agcatgtcca ggtcgaagtc
2700gtccagggcg tcggcgtggg ccatggccac gtcctcgccg tccaggtgca gctcgtcgcc
2760caggctcacg tcggtggggg gggccacctt ccttttcttc ttggggccca tggtggccaa
2820ttgccccccc ccccccccgc atgccgtcta acaaaaaagc caaaaacggc cagaatttag
2880cggacaattt actagtctaa cactgaaaat tacatattga cccaaatgat tacatttcaa
2940aaggtgccta aaaaacttca caaaacacac tcgccaaccc cgagcgcacg tacccagccc
3000agcagcccgc tactcaccaa gtgacgatca cagcgatcca caaacaagaa ccgcgaccca
3060aatcccggct gcgacggaac tagctgtgcc acacccggcg cgtccttata taatcatcgg
3120cgttcaccgc cccacggaga tccctccgca gaatcgccga gaagggacta cttttcctcg
3180cctgttccgc tctctggaaa gaaaaccagt gccctagagt cacccaagtc ccgtcctaaa
3240atgtccttct gctgatactg gggttctaag gccgagtctt atgagcagcg ggccgctgtc
3300ctgagcgtcc gggcggaagg atcaggacgc tcgctgcgcc cttcgtctga cgtggcagcg
3360ctcgccgtga ggaggggggc gcccgcggga ggcgccaaaa cccggcgcgg aggccctcga
3420gtaggcgaga ccaatgggtg cgccatgggc tcttccaaaa atttaggtga cactataggg
3480caccgctcgc acctgcgcac aggcccgcgg ctacaaacta cgaacgatca ttctagatac
3540cacatttgta gaggttttac ttgctttaaa aaacctccca catctccccc tgaacctgaa
3600acataaaatg aatgcaaatg ttttattaac ttgtttattg cagcttataa tggttacaaa
3660ttaagcaata gcatcacaaa tttcacaaat taagcatttt tttcactgca ttctagttgt
3720ggtttgtcca aactcatcaa tgtatcttat catgtctaat cgattcacag gttggtgggg
3780ctctccagga tggggggggg ctgggtgtgg ctcatgtcgg ccacgtccca aatctcctcc
3840aggaaggggg gcagcttcct gttcttcagc ttcaggctga tacacatatt gctgttctgc
3900attcccaggg tcctcagctc gctcaggatg ctcaggatct tgccgtagat cacgctgctc
3960ctggcgctgc cgctcagctg gttcaggatg tagatcctca gggtgttcag gtagtacctc
4020tggatctcct ccaccagctg gggctgctcc aggccgggcc tgtcgctgaa gatcaccacg
4080gcggtcagca gggcgtagtg gatgttgtcc agggccatgc tgtacataca tctgcagaag
4140tgcaggaggt cctcgatcac ctcggccatg ccagccttcc tgtagttgtc cctggtgtaa
4200gcctggttgt tggcgaacag gatgctgtcg ctggcggcgt cgtacctcct ggccaccctc
4260agcatcatca cctcgctgct gcaagccttc agcagggtga tctggtcggg ctggctgatc
4320ttggcgaatc cgggcaggcc cttggcgaac tccacgatca gctgcacggt caggatggtc
4380atctcggtga tctgcctgaa gggggtgtcg ctctcctcgt tctcgtcgtc ggcctgctgc
4440caggtctggg tgatcctttt caggtcctcg tcgctgggct gctcgtagcc gtcctgatac
4500cagatcagcc tggcgatcag gaactgctgg ttggcggtca gctgggggat gttcttctgc
4560ctgttggtca ccagcagctt gtcgctcagg aacctgggca cgacctcgtg aatcctggcg
4620gcctcggggg gggggggctc gcactgcatg atggggggca tgtggtcatc gacggtggtg
4680gtgctcacgg gcagcttgtc cttctccttc tgggccttct tctccttcct tttcatggcg
4740cactgggtct cgggcaccac gcactcgggc ctgatcccgg gaaactcggg gctcacggtc
4800agctgcctct ggcccttgtt gctgctctcc tcgctgctgc tggtggcgct gatcctgtgc
4860tgcctcaggg tcaggggcat gtcggtctcc acgctggcca gcctgtcggt cacggcgtcc
4920ttgttcacgt tgtcctgcac gaacaggccg gtcagcaggg ccttgatgtc ttgcaggctg
4980tccatcttca ggatcatgtc caggtcctcc ctggggaaga tcagcaggaa cagctgctcc
5040agcctctcca gcctgctctc cacctcggtc aggtgggccc tggtcagggg gctcctcttg
5100gtcttggggc tgtatctgca ctcccagttg ttcttcaggc acttggcgca cttgggcttc
5160tccttgctgc acttcagctt cttcagcctg cagatgtcgc aagcctgctc gatgctgctc
5220agcagcttca tggtggccaa ttgccccccc ccccccccgc atgctccctg ctggtttctt
5280gggagcgaag aaaagctgga tgtggtccct ggggaaagtc cctgcaggtc atttccccta
5340caaaacggtc taagacaagt tcctggatgc cagtggtttc ttcatcccgg gtcatttatc
5400ccagttgtgc aacctgaggg aacaagatgt gcctggcagc tgtcagggca ggagaccagc
5460tctgctcctc agcattgagg gggctgaggc tgcggagttg agagtgggag ccccgtgctc
5520gagtggtaat acaatggccg gttcccatgg acctgcatcg tggtgatcta tgtcgggtgc
5580ggagaaagag gtaatgaaat ggcagaaatg gcaccaccta aggtagcgac cgcggagact
5640aggtgtattt atctaagcga tcgcttaatt aaggccggcc gccgcaataa aatatcttta
5700ttttcattac atctgtgtgt tggttttttg tgtgaatcca tagtactaac atacgctctc
5760catcaaaaca aaacgaaaca aaacaaacta gcaaaatagg ctgtccccag tgcaagtcca
5820ggtgccagaa catttctcta tccataatgc aggggtaccg ggtgatgacg gtgaaaacct
5880ccaattgcgg agtactgtcc tccgagcgga gtactgtcct ccgagcggag tactgtcctc
5940cgagcggagt actgtcctcc gagcggagta ctgtcctccg agcggagtac tgtcctccga
6000gcggagagtc cccggggacc tagagggtat ataatgggtg ccttagctgg tgtgtgacct
6060catcttcctg tacgcccctg caggggcgcg ccacgcgtcg aagaaggtga gtaatcttaa
6120catgctcttt tttttttttt ttgctaatcc cttttgtgtg ctgatgttag gatgacattt
6180acaacaaatg tttgttcctg acaggaaaaa ccttgctggg taccttcgtt gccggacact
6240tcttgtcctc tactttggaa aaaaggaatt gagagccgct agcgccacca tgctgcccgc
6300ccaggtcgcc ttcaccccct acgcccccga gcccggcagc acctgtagac tgagagagta
6360ttacgaccag accgcccaga tgtgctgctc caagtgcagc cccggccagc acgccaaggt
6420gttctgcacc aagaccagcg acaccgtctg cgacagctgc gaggacagca cctacaccca
6480gctgtggaac tgggtgcccg agtgcctgag ctgcggcagc agatgtagca gcgaccaggt
6540ggagacccag gcttgcacca gagagcagaa cagaatctgc acctgtagac ccggctggta
6600ctgcgccctg agcaagcagg agggctgccg cctgtgcgcc cccctgagaa agtgcagacc
6660cggcttcggc gtggccagac ccggcaccga gaccagcgac gttgtgtgca agccctgtgc
6720ccccggcacc ttcagcaaca ccaccagcag caccgacatc tgccgccccc accagatttg
6780caacgtggtg gccatccccg gcaacgccag catggacgcc gtgtgtacca gcaccagccc
6840cacccgcagc atggcccccg gcgctgtcca cctcccccag cccgtgagca ccagaagcca
6900gcacacccag cccacccccg agcccagcac cgcccccagc accagcttcc tgctgcctat
6960gggacccagc ccccccgccg agggcagcac cggcgacgag cccaagagct gcgacaagac
7020ccacacctgt cccccctgcc ccgcccccga gctgctgggc ggccccagcg tgttcctgtt
7080cccccccaag cccaaggaca ccctgatgat ctccagaacc cccgaggtga catgcgtggt
7140ggtggacgtg agccacgagg acccccaggt gaagttcaac tggtacgtgg acggcgtgca
7200agtgcataac gccaagacca agcccagaga gcagcagtac aacagcacct acagagtggt
7260gagcgtgctg accgtgctgc accagaactg gctggacggc aaggagtaca agtgcaaggt
7320gtccaacaag gccctgcccg cccccatcga gaaaaccatc agcaaggcca agggccagcc
7380cagagagccc caggtgtaca ccctgccccc cagcagagag gagatgacca agaaccaggt
7440gtccctgacc tgtctggtga agggcttcta ccccagcgac atcgccgtgg agtgggagag
7500caacggccag cccgagaaca actacaagac cacccccccc gtgctggaca gcgacggcag
7560cttcttcctg tacagcaagc tgaccgtgga caagagcaga tggcagcagg gcaacgtgtt
7620cagctgctcc gtgatgcacg aggccctgca caaccactac acccagaagt ccctgagcct
7680gagccccggc aagtgaatcg attgcgcaaa gctttcgcga taggcgagac caatgggtgt
7740gtacgtagcg gccgcgtcga cgatagcttg atgggtggca tccctgtgac ccctccccag
7800tgcctctcct ggccctggaa gttgccactc cagtgcccac cagccttgtc ctaataaaat
7860taagttgcat cattttgtct gactaggtgt ccttctataa tattatgggg tggagggggg
7920tggtatggag caaggggcaa gttgggaaga caacctgtag ggcctgcggg gtctattggg
7980aaccaagctg gagtgcagtg gcacaatctt ggctcactgc aatctccgcc tcctgggttc
8040aagcgattct cctgcctcag cctcccgagt tgttgggatt ccaggcatgc atgaccaggc
8100tcagctaatt tttgtttttt tggtagagac ggggtttcac catattggcc aggctggtct
8160ccaactccta atctcaggtg atctacccac cttggcctcc caaattgctg ggattacagg
8220cgtgaaccac tgctcccttc cctgtccttc tgattttaaa ataactatac cagcaggagg
8280acgtccagac acagcatagg ctacctggcc atgcccaacc ggtgggacat ttgagttgct
8340tgcttggcac tgtcctctca tgcgttgggt ccactcagta gatgcctgtt gaattctgat
8400ttaaatcggt ccgcgtacgg cgtggtaggt ccgaacgaat ccatggatta ccctgttatg
8460tggtcctcca gggttgccgt tccttaacta taacggtcct aaggtagcga ccgcggagac
8520taggtgtatt tatctaagcg atcgcttaat taaggccggc cgccgcaata aaatatcttt
8580attttcatta catctgtgtg ttggtttttt gtgtgaatcc atagtactaa catacgctct
8640ccatcaaaac aaaacgaaac aaaacaaact agcaaaatag gctgtcccca gtgcaagtcc
8700aggtgccaga acatttctct atccataatg caggggtacc gggtgatgac ggtgaaaacc
8760tccaattgtg ctgtatataa aaccagtggt tatatgtaca gtactgctgt atataaaacc
8820agtggttata tgtacagtac gtcgactgct gtatataaaa ccagtggtta tatgtacagt
8880actgctgtat ataaaaccag tggttatatg tacagtaccc cggggaccta gagggtatat
8940aatgggtgcc ttagctggtg tgtgacctca tcttcctgta cgcccctgca ggggcgcgcc
9000acgcgtcgaa gaaggtgagt aatcttaaca tgctcttttt tttttttttt gctaatccct
9060tttgtgtgct gatgttagga tgacatttac aacaaatgtt tgttcctgac aggaaaaacc
9120ttgctgggta ccttcgttgc cggacacttc ttgtcctcta ctttggaaaa aaggaattga
9180gagccgctag cgccaccatg ggcgtccacg aatgccctgc ctggctgtgg ctgctcctgt
9240ccctgctgtc tctccccctc ggcctccccg tcctgggagc ccctcccagg ctgatttgcg
9300atagcagggt gctggagaga tacctgttgg aagccaagga agccgagaat atcacaaccg
9360gatgcgccga gcactgctcc ctgaatgaga atatcacagt gcctgacaca aaggtcaact
9420tttacgcttg gaaaagaatg gaggtcggcc aacaggctgt ggaagtgtgg cagggactgg
9480ctctgctgtc cgaagccgtc ctgaggggcc aagccctcct ggtcaactcc agccaaccct
9540gggagcctct gcaactgcat gtggataagg ctgtgtccgg cctcagatcc ctgacaaccc
9600tcctgagagc cctgggcgct cagaaagagg ctatctcccc ccctgacgct gcctccgccg
9660ctcccctcag aacaatcaca gccgatacct ttagaaaact gtttagagtc tactccaact
9720ttctgagggg caaactgaaa ctgtataccg gagaggcttg caggaccgga gacaggtaaa
9780tcgattgcgc aaagctttcg cgataggcga gaccaatggg tgtgtacgta gcggccgctc
9840gagaacttgt ttattgcagc ttataatggt tacaaataaa gcaatagcat cacaaatttc
9900acaaataaag catttttttc actgcattct agttgtggtt tgtccaaact catcaatgta
9960tcttatcatg tctcgtacgg cgtggtaggt ccgaacgaat ccatggatta ccctgttatc
10020cctactcatc ttcgtcggac gcgccgttaa tatttgatca gccattctct gtatgagtac
10080taacgaacgt gacgcggtgt tactggtaac tatgactctc ttaaggtagc caaatacatc
10140atccctttag tgagggttaa ttcacgcagt gccgcggcgt gtagaaaata cctatcagct
10200cgaggacgtg acaaacgaag ccgcacgtca gggccggacg tggggcccca gagcgacgct
10260gagtgcgtgc gggactcgga gtacgtgacg gagcccccac gtgggttccc gcacgtccgc
10320gacgtgacaa acgaagccgc acgtcagggc cggacgtggg gccccagagc gacgctgagt
10380gcgtgcggga ctcggagtac gtgacggagc ccccacgtgg gttcccgcac gtccgcgacg
10440tgacaaacga agccgcacgt cagggccgga cgtggggccc cagagcgacg ctgagtgcgt
10500gcgggactcg gagtacgtga cggagccccc acgtgggttc ccgcacgtcc gcgacgtgac
10560aaacgaagcc gcacgtcagg gccggacgtg gggccccaga gcgacgctga gtgcgtgcgg
10620gactcggagt acgtgacgga gcccccacgt gggttcccgc acgtccgcga cgtgacaaac
10680gaagccgcac gtcagggccg gacgtggggc cccagagcga cgctgagtgc gtgcgggact
10740cggagtacgt gacggagccc ccacgtgggt tcccgcacgt ccgcgacgtg acaaacgaag
10800ccgcacgtca gggccggacg tggggcccca gagcgacgct gagtgcgtgc gggactcgga
10860gtacgtgacg gagcccccac gtgggttccc gcacgtccgc gacgtgacaa acgaagccgc
10920acgtcagggc cggacgtggg gccccagagc gacgctgagt gcgtgcggga ctcggagtac
10980gtgacggagc ccccacgtgg gttcccgcac gtccgcgaag acctagaggg tatataatgg
11040gtgccttagc tggtgtgtga gctcatcttc ctgtgcatgc gggggggggg gggggcaatt
11100ggccaccatg aaagccctca ccgctagaca acaggaagtg tttgacctca tcagggacca
11160tatctcccag acaggaatgc cccctaccag ggccgaaatc gctcagaggc tgggattcag
11220gagccctaac gctgccgaag aacatctgaa agccctcgcc aggaagggag tgattgagat
11280tgtgtccggc gcttccaggg gcattagact cctgcaagag gaggaagaag gactccccct
11340cgtgggaagg gtcgccgctg gcgaacccct cctggctcaa caacacattg agggacacta
11400tcaggtcgat cctagcctct ttaaacccaa tgccgatttc ctcctgaggg tgtccggcat
11460gagcatgaag gatattggaa tcatggacgg agacctcctg gctgtgcata agacacagga
11520tgtgaggaac ggacaggtcg tggtcgccag gatcgacgac gaagtgacag tgaaaagact
11580caagaaacag ggaaacaaag tggaactgct ccccgaaaac tccgagttta agcctatcgt
11640cgtggatctg aggcagcaat cctttaccat tgagggactg gctgtgggag tgattagaaa
11700tggcgattgg ctcgaatttc ccgggattag acctgagtgt gtggtccccg aaacccaatg
11760cgctatgaaa agaaaagaga aaaaggctca gaaagagaaa gacaaactgc ctgtgtccac
11820cacaaccgtc gatgatcaca tgccccctat catgcagtgt gagcctcccc ctcccgaagc
11880cgctagaatc cacgaagtcg tccccaggtt cctctccgat aagctcctgg aaaccaatag
11940acaaaagaat atccctcaac tcaccgctaa ccaacagttt ctgattgcca ggctgatttg
12000gtatcaggat ggctatgagc aaccctccga cgaagacctc aagaggatca cacagacatg
12060gcaacaggct gacgatgaga atgaggaaag cgatacccct ttcaggcaga ttaccgaaat
12120gacaatcctc accgtccagc tcatcgtcga gtttgccaaa ggactccccg gattcgctaa
12180gattagccaa cccgatcaga ttaccctcct gaaagcctgt agctccgagg tcatgatgct
12240gagagtggct agaaggtacg atgccgcttc cgatagcgtc ctgtttgcca ataaccaagc
12300ctataccagg gacaattaca ggaaggctgg catggcctat gtgattgagg atgtgctcca
12360cttttgcaga tgtatgtact ccatggctct cgataacatt cactatgccc tcctgacagc
12420cgtcgtgatt ttctccgaca ggcccggact ggaacagcct caactcgtgg aagaaattca
12480gaggtactat ctgaataccc tcagaattta cattctgaat cagctctccg gcagcgctag
12540atccagcgtc atctatggca aaatcctctc cattctgtcc gagctgagaa cactgggaat
12600gcaaaactcc aatatgtgca ttagcctcaa gctcaagaat agaaaactgc ctccctttct
12660ggaagaaatt tgggatgtgg ctgacatgag ccatacccaa ccccctccca ttctggaaag
12720ccctaccaat ctgtaaatcg atagccgcct ggctgagatg gggtgggcag ggcagagctg
12780atcagggccg agcagaaccg cactcttccc aaataaagct tcctccttga aacacaaatg
12840tttcttactt acaccccatc ctgatttctt ttcttgagac cagagagtgg gaaagctctc
12900tcttgacctg aggatggatc tgaaaattat gagccccttg aggacaggga atgttattca
12960tctttgaatt ccgcagcatc tagcaccagg tcttgtacag agcaggtgcc caataaatgg
13020ttgaatgaat atatgaaaag tagaggcaga gggctgggca cagtggctca cgcctgtaat
13080cctagcactt tgggaggctg aggtgggtgg atcacttgag gtcaggagtt cgagaccagc
13140ttggccaaca tggctaaacc tcatctctat taaaaataca aaaattagct gggctggtgc
13200ctgtaatccc agctactcag gaggctgagg caggagaatc acttgaaccc aggaggagga
13260gtttgcagtg agccgagatc gcaccattgc actccagcct gggcgatagg agcaaaactc
13320catctcaaaa acaaaaaaca aaacaaaaca aaaagaaaaa agaaaagtag gggcagagat
13380gtggggcagg agaggtgact cgggctcagc tgcatggtcc tgctctgctt cttttttctt
13440ggagtttgtc cgttggatga caatgatagt ggtgaacagg tatggagggc ttactaagta
13500ccaggtgtct agacgaggac gctcgggctt ggacccatgg cacatcgtgt aatcatggtc
13560atagctgttt cctgtgtgaa attgttatcc gctacgtctc tcccccgcag taagggctag
13620attaactcgt ctcgtgaata tccggaactc cctttagtga gggttaattg cgttgcgctc
13680actgcccgct ttccagtcgg gaaacctgtc gtgccagctt aatcatggtc atagctgttt
13740cctgtgtgaa attgttatcc gctaccggaa acgcttcctt catgtgagca aaaggccagc
13800aaaaggccag gaaccgtaaa aaggccgcgt tgctggcgtt tttccatagg ctccgccccc
13860ctgacgagca tcacaaaaat cgacgctcaa gtcagaggtg gcgaaacccg acaggactat
13920aaagatacca ggcgtttccc cctggaagct ccctcgtgcg ctctcctgtt ccgaccctgc
13980cgcttaccgg atacctgtcc gcctttctcc cttcgggaag cgtggcgctt tctcatagct
14040cacgctgtag gtatctcagt tcggtgtagg tcgttcgctc caagctgggc tgtgtgcacg
14100aaccccccgt tcagcccgac cgctgcgcct tatccggtaa ctatcgtctt gagtccaacc
14160cggtaagaca cgacttatcg ccactggcag cagccactgg taacaggatt agcagagcga
14220ggtatgtagg cggtgctaca gagttcttga agtggtggcc taactacggc tacactagaa
14280gaacagtatt tggtatctgc gctctgctga agccagttac cttcggaaaa agagttggta
14340gctcttgatc cggcaaacaa accaccgctg gtagcggtgg tttttttgtt tgcaagcagc
14400agattacgcg cagaaaaaaa ggatctcaag aagatccttt gatcttttct acggggtctg
14460acgctcagtg gaacgaaaac tcacgttaag ggattttggt catgcctagg acgaaaggag
14520gtcgtgaaat ggataaaaaa atacagcgtt tttcatgtac aactatacta gttgtagtgc
14580ctaaataatg cttttaaaac ttaaaaatac gtttaaaccc tcagcgaaat ggcatacgag
14640taaacttggt ctgacaccgc tgcatgagat tatcaaaaag gatcttcacc tagatccttt
14700taaattaaaa atgaagtttt aaatcaatct aaagtatata tgagtaaact tggtctgaca
14760gttaccaatg cttaatcagt gaggcaccta tctcagcgat ctgtctattt cgttcatcca
14820tagttgcctg actccccgtc gtgtagataa ctacgatacg ggagggctta ccatctggcc
14880ccagtgctgc aatgataccg cgagacccac gctcaccggc tccagattta tcagcaataa
14940accagccagc cggaagcgcc gagcgcagaa gtggtcctgc aactttatcc gcctccatcc
15000agtctattaa ctgttgccgg gaagctagag taagtagttc gccagttaat agtttgcgga
15060gcgttgttgc cattgctaca ggcatcgtgg tgtcacgctc gtcgtttggt atggcttcat
15120tcagctccgg ttcccaacga tcaaggcgag ttacatgatc ccccatgttg tgcaaaaaag
15180cggttagctc cttcggtcct ccgatggttg tcagaagtaa gttggccgca gtgttatcac
15240tcatggttat ggcagcactg cataattctc ttactgtcat gccatccgta agatgctttt
15300ctgtgactgg tgagtattca accaagtcat tctgagaata gtgtatgcgg cgaccgagtt
15360gctcttgccc ggcgtcaata cgggataata ccgcgccaca tagcagaact ttaaaagtgc
15420tcatcattgg gaagcgttct tcggggcgaa aactctcaag gatcttaccg ctgttgagat
15480ccagttcgat gtaacccaca cgagcaccca actgatcttc agcatctttt actttcacca
15540gcgtttctgg gtgagcaaaa acaggaaggc aaaatgccgc aaaaaaggga ataagggcga
15600cacggaaatg ttgaatactc atacgcttcc tttttcaata gtattgaagc atttatcagg
15660gttattgtct cgggagcgaa tacatatttg aatgtattta gaaaaa
157061817505DNAArtificial SequenceSynthetic dual promoter gene switch
construct encoding human Factor VIIIC 18taaacaaata ggggttccgc
gcacatttcc ccgaaaagtg ccacctgacg tactgaggac 60gccagggttt tcccagtcac
gacgttgtaa aacgacggcc agtagcagac aagcccgtca 120gggcgcgtca gcgggtgttg
gccctaggac gaaaggaggt cgtgaaatgg ataaaaaaat 180acagcgtttt tcatgtacaa
ctatactagt tgtagtgcct aaataatgct tttaaaactt 240aaaaataatc aatgtcctca
gcggggtgtc ggggatttag gtgacactat aggctgagcg 300ccgcacaggc atctagaggc
tatggcaggg cctgccgccc cgacgttggc tgcgagccct 360gggccttcac ccgaacttgg
ggggtggggt ggggaaaagg aagaaacgcg ggcgtattgg 420ccccaatggg gtctcggtgg
ggtatcgaca gagtgccagc cctgggaccg aaccccgcgt 480ttatgaacaa acgacccaac
accgtgcgtt ttattctgtc tttttattgc cgtcatagcg 540cgggttcctt ccggtattgt
ctccttccgt gtttcaatcg attcaaaaga actcgtccag 600cagacggtaa aaagcaatgc
gttgagaatc cggtgcagca atgccataca gcaccagaaa 660gcgatcagcc cattcaccgc
ccagttcttc agcaatgtca cgggttgcca gtgcgatgtc 720ctgatagcga tcagccacgc
ccaggcgacc gcagtcgata aagccggaga aacggccgtt 780ttccaccata atgtttggca
gacaagcatc gccgtgggtc acaaccaggt cctcgccatc 840tggcatacgt gctttcaggc
gtgcgaacag ttctgccggt gccagaccct gatgttcctc 900gtccaggtca tcctgatcaa
ccaggccagc ttccatgcga gtgcgtgcgc gctcgatacg 960gtgtttagct tggtgatcga
atgggcaagt agctgggtcc agggtatgca gacggcgcat 1020agcatcagcc atgatggaaa
ccttttctgc cggtgccaga tgagaggaca gcagatcctg 1080gcctggaacc tcgcccagca
gcagccagtc gcggccagcc tcggtcacaa catccagcac 1140agctgcgcat ggaacgccgg
tagtagccag ccaggacaga cgagctgctt catcttgcag 1200ttcgttcagt gcgccggaca
gatcggtctt aacaaacagc accggacggc cttgagcgga 1260cagacggaac acagctgcgt
cggagcaacc gatagtctgt tgagcccagt catagccaaa 1320cagacgttcc acccaagcag
ccggagaacc agcgtgcaga ccgtcttgtt caatcatggt 1380ggcaattggg tgtctgagcg
atgtggctcg gctggcgacg caaaagaaga tgcggctgac 1440tgtcgaacag gaggagcaga
gagcgaagcg ggaggctgcg ggctcaattt gcatgcttta 1500gttcctcacc ttgtcgtatt
atactatgcc gatatactat gccgatgatt aattgtcaac 1560gtatacggaa tagctctgag
gccgaggcag cttcggcctc tgcataaata aaaaaaatta 1620gtcagccatg gggcggagaa
tgggcggaac tgggcggagt taggggcggg atgggcggag 1680ttaggggcgg gactatggtt
gctgactaat tgagatgctt gctttgcata cttctgcctg 1740ctggggagcc tggggacttt
ccacacctgg ttgctgacta attgagatgc ttgctttgca 1800tacttctgcc tgctggggag
cctggggact ttccacaccc taacctcgag gccatcgtgg 1860cacgccaggg ttttcccagt
cacgacgttg taaaacgacg gccagtgctc ttctcccccg 1920cgggaggttt tataaatccg
actgtctaga ttgttgttaa atcacacaaa aaaccaacac 1980acagatgtaa tgaaaataaa
gatattttat tatcgattca gctgtcgctg gggctctcca 2040gcatctccat caggaaggtg
tcgatgggca cgtcgccgat cagcctgaag aagaacaggt 2100gctccaggca cttcaggccg
atgctcctca ggctgggcag cctcagcagc agcttggcga 2160atctgccggg ctcgtcgggg
tgggtggtcc tggtgtactc ctccagggcg gcgtacacct 2220tctccctcag cagctccacc
tcctgggcgc ttttcaggcc cctcacctcg gggttgaaca 2280ggatgatggc cctcaggcag
cccagctcgg tcttgtccat cctcatgtcc ctcatcttgc 2340tcaccagctc ggtcagcacc
ctgtcgaaga tggcgcccac tccggcgctg tgggcgctgt 2400tcctatggac gtgcaggccg
gtggccagca ggatgccgtc cctcacgtcg atgctcctgt 2460ggctgaagct ggcgatcagc
agctcgttcc atccggccct cagcaggatc acctggtcgt 2520ccaggggcag gctgctgaag
tggggaatcc tcttggccca ctccaccagg gtgaacagct 2580gcttgtcggc ggcctggcag
atgttggtca cggggtcgtt ggggctgctg ccgctgccgc 2640cggttccgcc ggggccctcc
acgccctggt cgcttttctg ctccacggcg agttcggcct 2700ccagaatcct gtccacgggc
atctccatat ggccgccgta ctcgtcgatg cccagggcgt 2760cggtgaacat ctgctcgaac
tcgaagtcgg ccatgtccag ggcgccgtag ggggcgctgt 2820cgtggggggt gaagccgggg
ccggggctgt cgccgtcgcc cagcatgtcc aggtcgaagt 2880cgtccagggc gtcggcgtgg
gccatggcca cgtcctcgcc gtccaggtgc agctcgtcgc 2940ccaggctcac gtcggtgggg
ggggccacct tccttttctt cttggggccc atggtggcca 3000attgcccccc cccccccccg
catgccgtct aacaaaaaag ccaaaaacgg ccagaattta 3060gcggacaatt tactagtcta
acactgaaaa ttacatattg acccaaatga ttacatttca 3120aaaggtgcct aaaaaacttc
acaaaacaca ctcgccaacc ccgagcgcac gtacccagcc 3180cagcagcccg ctactcacca
agtgacgatc acagcgatcc acaaacaaga accgcgaccc 3240aaatcccggc tgcgacggaa
ctagctgtgc cacacccggc gcgtccttat ataatcatcg 3300gcgttcaccg ccccacggag
atccctccgc agaatcgccg agaagggact acttttcctc 3360gcctgttccg ctctctggaa
agaaaaccag tgccctagag tcacccaagt cccgtcctaa 3420aatgtccttc tgctgatact
ggggttctaa ggccgagtct tatgagcagc gggccgctgt 3480cctgagcgtc cgggcggaag
gatcaggacg ctcgctgcgc ccttcgtctg acgtggcagc 3540gctcgccgtg aggagggggg
cgcccgcggg aggcgccaaa acccggcgcg gaggccctcg 3600agtaggcgag accaatgggt
gcgccatggg ctcttccaaa aatttaggtg acactatagg 3660gcaccgctcg cacctgcgca
caggcccgcg gctacaaact acgaacgatc attctagata 3720ccacatttgt agaggtttta
cttgctttaa aaaacctccc acatctcccc ctgaacctga 3780aacataaaat gaatgcaaat
gttttattaa cttgtttatt gcagcttata atggttacaa 3840attaagcaat agcatcacaa
atttcacaaa ttaagcattt ttttcactgc attctagttg 3900tggtttgtcc aaactcatca
atgtatctta tcatgtctaa tcgattcaca ggttggtggg 3960gctctccagg atgggggggg
gctgggtgtg gctcatgtcg gccacgtccc aaatctcctc 4020caggaagggg ggcagcttcc
tgttcttcag cttcaggctg atacacatat tgctgttctg 4080cattcccagg gtcctcagct
cgctcaggat gctcaggatc ttgccgtaga tcacgctgct 4140cctggcgctg ccgctcagct
ggttcaggat gtagatcctc agggtgttca ggtagtacct 4200ctggatctcc tccaccagct
ggggctgctc caggccgggc ctgtcgctga agatcaccac 4260ggcggtcagc agggcgtagt
ggatgttgtc cagggccatg ctgtacatac atctgcagaa 4320gtgcaggagg tcctcgatca
cctcggccat gccagccttc ctgtagttgt ccctggtgta 4380agcctggttg ttggcgaaca
ggatgctgtc gctggcggcg tcgtacctcc tggccaccct 4440cagcatcatc acctcgctgc
tgcaagcctt cagcagggtg atctggtcgg gctggctgat 4500cttggcgaat ccgggcaggc
ccttggcgaa ctccacgatc agctgcacgg tcaggatggt 4560catctcggtg atctgcctga
agggggtgtc gctctcctcg ttctcgtcgt cggcctgctg 4620ccaggtctgg gtgatccttt
tcaggtcctc gtcgctgggc tgctcgtagc cgtcctgata 4680ccagatcagc ctggcgatca
ggaactgctg gttggcggtc agctggggga tgttcttctg 4740cctgttggtc accagcagct
tgtcgctcag gaacctgggc acgacctcgt gaatcctggc 4800ggcctcgggg ggggggggct
cgcactgcat gatggggggc atgtggtcat cgacggtggt 4860ggtgctcacg ggcagcttgt
ccttctcctt ctgggccttc ttctccttcc ttttcatggc 4920gcactgggtc tcgggcacca
cgcactcggg cctgatcccg ggaaactcgg ggctcacggt 4980cagctgcctc tggcccttgt
tgctgctctc ctcgctgctg ctggtggcgc tgatcctgtg 5040ctgcctcagg gtcaggggca
tgtcggtctc cacgctggcc agcctgtcgg tcacggcgtc 5100cttgttcacg ttgtcctgca
cgaacaggcc ggtcagcagg gccttgatgt cttgcaggct 5160gtccatcttc aggatcatgt
ccaggtcctc cctggggaag atcagcagga acagctgctc 5220cagcctctcc agcctgctct
ccacctcggt caggtgggcc ctggtcaggg ggctcctctt 5280ggtcttgggg ctgtatctgc
actcccagtt gttcttcagg cacttggcgc acttgggctt 5340ctccttgctg cacttcagct
tcttcagcct gcagatgtcg caagcctgct cgatgctgct 5400cagcagcttc atggtggcca
attgcccccc cccccccccg catgccacta agatctgcat 5460tttgacctag tttaaaagta
aaacataagc gtgtcacctc aggatcattg tatttcttgc 5520ttttacacct aatattcttc
aaaattagca actattcctt caaaatgcct taaatacgtt 5580aatattccgc aagcctcccc
tcaattttac aaggctaagt cacttaatac gaaattcgtc 5640attcaaggtt tcaacgtcaa
cagcggaaaa tccagagcgc tctcttacca agcgtccaca 5700gctttcccca ctaactgttc
cggatattca aactcccccg accgaactcc aatctgcctc 5760aaacctaagc acggtgttag
gcaaactgaa aacaaaacca aacccacaag actcgcgaaa 5820taaaaatccc attgaaacac
aagaaggggc acctttctca cactgggatt ccaaagtaaa 5880ggcttccaga aaccacacaa
actacctctt ttccgccttc cccgcgaagt aaactcgaca 5940gaatcgccga tcggttaggc
gtccgttagt ttccgtcaac ccgcgcgcgc caaccgccct 6000cgcccccctc aggtcaacaa
ggcccactgt cccggaaagg cccagcgccg cgtcacctcg 6060ccctcccctc ccccacgccc
tcccctcagc ggcccaccca accccaagaa gcctccacct 6120tgacctcacc cattcccccc
tctcacaccc ccccaaggtc gttacggctg cgggccagta 6180cctgttagcg gataccagga
tcctgccaat caccaaccac gtccacccac agggacacaa 6240acaagctcac ccaacaaagc
caaccgcccc taaatgctcc gggctggcgg aggcaaattt 6300atgcaacgag tgttgcgtca
cttatcaccc ctcacgtaga cgccgtctcc gtgcgccgct 6360gaaagtagtt cgccgcgccc
aacctcccga gaggcagttt aggcttttca actgagcccc 6420aaattcctca tagccgtaag
aaaggctcct aaaaaattat ttcccttctc ctctatgcgc 6480ttctcttcct tctttaggaa
aatataagaa aattatcagg cggatgaatt tgagttgaag 6540aaaccttcct cgaaagctct
ggaaacttcc ttgcagcgcc ggcactgatt ggttaggcct 6600gcgaaggcga ggtcggcgct
gattggtggg ggacgcggtg gctctgttgt tgctgggctc 6660cacctggggt ggcttagcgc
cgaggttgct aagtaactga gcgcgcgtcg ccgcacaggt 6720ctttcctttt ttttcttgag
acggatttcg ctcttgtttc ccaggctgga gtgcaatggc 6780gcgatctcgg ctcatcgcaa
cctccgcctc ccgggttcaa gcgattctcc tgcctcagcc 6840tcccaagtag ctgggattac
agctcgagtg gtaatacaat ggccggttcc catggacctg 6900catcgtggtg taactataac
ggtcctaagg tagcgaccgc ggagactagg tgtatttatc 6960taagcgatcg cttaattaag
gccggccgcc gcaataaaat atctttattt tcattacatc 7020tgtgtgttgg ttttttgtgt
gaatccatag tactaacata cgctctccat caaaacaaaa 7080cgaaacaaaa caaactagca
aaataggctg tccccagtgc aagtccaggt gccagaacat 7140ttctctatcc ataatgcagg
ggtaccgggt gatgacggtg aaaacctcca attgcggagt 7200actgtcctcc gagcggagta
ctgtcctccg agcggagtac tgtcctccga gcggagtact 7260gtcctccgag cggagtactg
tcctccgagc ggagtactgt cctccgagcg gagagtcccc 7320ggggacctag agggtatata
atgggtgcct tagctggtgt gtgacctcat cttcctgtac 7380gcccctgcag gggcgcgcca
cgcgtcgaag aaggtgagta atcttaacat gctctttttt 7440tttttttttg ctaatccctt
ttgtgtgctg atgttaggat gacatttaca acaaatgttt 7500gttcctgaca ggaaaaacct
tgctgggtac cttcgttgcc ggacacttct tgtcctctac 7560tttggaaaaa aggaattgag
agccgctagc gccaccatgg ctaccaggag atattacctc 7620ggcgctgtgg aactgtcctg
ggattacatg cagtccgacc tcggcgaact gcctgtggat 7680gccaggttcc ctcccagggt
gcctaagtcc ttccctttca atacctccgt ggtctacaaa 7740aagacactgt ttgtggaatt
tacagaccat ctgtttaaca ttgccaaacc caggccccct 7800tggatgggcc tcctgggacc
cacaatccaa gccgaagtgt atgacacagt ggtcatcaca 7860ctgaaaaaca tggcctccca
ccccgttagc ctgcacgctg tcggcgtcag ctattggaaa 7920gcctccgagg gagccgaata
cgatgaccaa acctcccaga gggagaaaga ggatgacaaa 7980gtgtttcccg gaggctccca
cacatacgtc tggcaagtgc tcaaggaaaa cggacctatg 8040gcctccgacc ctctgtgtct
gacatactcc tacctgagcc atgtggatct ggtcaaggat 8100ctgaatagcg gactgattgg
cgctctgctc gtgtgtagag aaggctccct ggctaaggaa 8160aagacacaga cactgcataa
gtttatcctc ctgtttgccg tttttgatga gggaaagtcc 8220tggcatagcg aaaccaaaaa
ctccctgatg caggatagag atgccgccag tgctagagct 8280tggcctaaga tgcacaccgt
caatggctat gtgaatagat ccctgcctgg cctcatcgga 8340tgccatagaa aaagcgtcta
ctggcacgtc atcggaatgg gaaccacacc cgaagtgcat 8400agcattttcc ttgaaggaca
cacattcctc gtgaggaacc atagacaagc ctccctggaa 8460atctccccca ttacctttct
gacagcccaa accctcctga tggacctcgg ccaattcctc 8520ctgttttgcc atatctccag
ccatcagcat gacggaatgg aagcctatgt gaaagtggat 8580agctgtcccg aggagcccca
gctcagaatg aagaataacg aagaagccga ggactacgat 8640gacgatctga cagactccga
gatggatgtc gtgaggttcg atgacgataa ctccccctcc 8700ttcattcaga ttagatccgt
ggctaagaaa caccctaaga catgggtcca ctatatcgct 8760gccgaggaag aagattggga
ctatgcccct ctggtcctgg ctcccgatga caggagctat 8820aagtcccagt atctgaataa
cggaccccag agaatcggca ggaagtataa gaaagtgagg 8880ttcatggcct ataccgatga
gacattcaaa accagggagg ctatccaaca cgaaagcgga 8940atcctcggcc ctctgctcta
cggagaggtc ggcgataccc tcctgattat ctttaagaat 9000caggccagta ggccctataa
catttaccct cacggaatca cagatgtgag acctctgtat 9060agcaggagac tccccaaagg
cgtcaagcat ctgaaagact ttcccattct gcctggcgaa 9120atctttaagt ataagtggac
cgtcaccgtc gaggatggcc ctaccaaaag cgatcccagg 9180tgcctcacca ggtactatag
ctccttcgtc aacatggaga gggacctcgc ctccggcctc 9240atcggacccc tcctgatttg
ctataaggaa agcgtcgatc aaagaggaaa ccaaatcatg 9300agcgataaga ggaacgtcat
cttgttctcc gtgtttgacg aaaacaggag ctggtacttg 9360accgaaaaca ttcagaggtt
cctccccaat cccgctggcg tccagcttga ggaccctgag 9420tttcaagcct ccaacattat
gcacagcatt aacggatacg ttttcgatag cctccagctg 9480tccgtctgcc tccacgaggt
cgcttactgg tacattctgt ccatcggagc ccaaaccgat 9540ttcctgagcg tcttttttag
cggatacaca ttcaaacaca aaatggtcta cgaggataca 9600ctgacactgt ttccctttag
cggagagaca gtgtttatga gcatggagaa ccctggcctc 9660tggattctgg gatgccataa
ctccgacttt agaaatagag gaatgacagc cctcctgaaa 9720gtgtccagct gtgacaaaaa
cacaggcgat tactatgagg atagctatga ggacatttcc 9780gcctatctgc tgtcaaaaaa
caatgccatt gagcctagat ccttctccca gaatagcagg 9840caccctagca caagacaaaa
gcaattcaat gccacaacca ttcccgaaaa cgacatcgaa 9900aagacagacc cttggtttgc
ccatagaaca cccatgccca aaatccaaaa cgtcagctcc 9960agcgatctgc tcatgctcct
gaggcagtcc cccacacccc acggcctgag cctgtccgat 10020ctgcaagagg ctaagtatga
gacattctcc gacgatccct cccccggagc cattgactcc 10080aacaatagcc tgagcgaaat
gacacacttt agaccccagc tgcaccatag cggagacatg 10140gtgtttaccc ctgagtccgg
cctccagctc agactcaacg aaaagctcgg cacaaccgct 10200gccacagagc tgaagaaact
ggatttcaaa gtgtccagca caagcaataa cctcatctcc 10260accattccct ccgacaatct
ggctgccgga accgataaca caagctccct gggaccccct 10320tccatgcccg tccactatga
ctcccagctc gacacaaccc tttttggaaa gaaaagctcc 10380cccctcaccg aaagcggagg
ccctctgtcc ctgtccgagg aaaacaatga cagcaagctg 10440ctggagagcg gactgatgaa
ctcccaggaa agctcctggg gaaagaatgt gtccagcaca 10500gagtccggca ggctgtttaa
gggaaagaga gcccacggcc ctgccctcct gacaaaggat 10560aacgctctgt ttaaggtcag
cattagcctc ctgaaaacca ataagacaag caataactcc 10620gccacaaaca gaaagaccca
cattgacgga ccctccctgc tcatcgaaaa ctccccctcc 10680gtgtggcaga atatcctcga
atccgacaca gagtttaaga aagtgacacc cctcatccat 10740gacaggatgc tcatggataa
gaatgccaca gccctcagac tcaaccacat gagcaacaaa 10800accacaagca gcaagaacat
ggagatggtc cagcaaaaga aagagggacc cattccccct 10860gacgctcaga atcccgatat
gtccttcttt aagatgctgt ttctgcctga gtccgccagg 10920tggattcaga ggacccacgg
caaaaactcc ctgaatagcg gacagggacc ctcccccaaa 10980cagctcgtgt ccctgggacc
cgaaaagtcc gtggaaggcc aaaactttct gtccgagaaa 11040aacaaagtgg tcgtgggaaa
gggagagttt accaaggacg ttggcctgaa ggaaatggtg 11100tttcctagct ccagaaatct
gtttctgaca aacctcgaca atctgcatga gaataacaca 11160cacaatcagg aaaagaaaat
ccaagaggaa atcgaaaaga aagagacact gattcaggaa 11220aacgtcgtgc tcccccaaat
ccataccgtc accggaacca aaaactttat gaaaaacctt 11280ttcctcctgt ccaccaggca
gaatgtggaa ggctcctacg atggcgctta cgctcccgtc 11340ctgcaagact ttagatccct
gaatgactcc accaatagaa caaagaaaca cacagcccat 11400ttctccaaga aaggcgagga
ggaaaacctg gaaggactgg gaaaccaaac caaacagatt 11460gtggaaaagt atgcctgtac
cacaagaatt agccctaaca caagccaaca gaatttcgtc 11520acccaaagat ccaagagagc
cctgaagcaa ttcaggctgc ctctggaaga aacagagctg 11580gagaaaagaa ttatcgtgga
tgatacctcc acccaatggt ccaagaatat gaaacacctc 11640acccctagca cactgacaca
gattgactat aacgaaaagg aaaagggagc cattacccaa 11700agccctctgt ccgactgtct
gacaagatcc cactccatcc ctcaggctaa caggagccct 11760ctgcctatcg ctaaggtcag
ctccttccct agcattagac ctatctatct gacaagagtc 11820ctgtttcagg ataactccag
ccatctgcct gccgcctctt atagaaaaaa ggatagcgga 11880gtgcaagagt ccagccattt
cctccaggga gccaaaaaga ataacctgag cctcgccatt 11940ctgacactgg aaatgacagg
cgatcagagg gaggtcggct ccctgggaac ctccgccaca 12000aactccgtga catacaaaaa
ggtcgagaat accgtcctgc ctaagcctga cctccccaaa 12060acctccggca aagtggaact
gctccccaaa gtgcatatct atcagaaaga cctctttcct 12120accgaaacct ccaacggaag
ccccggccac ctggatctgg tcgagggaag cctcctgcaa 12180ggcacagagg gagccattaa
gtggaacgaa gccaatagac ctggcaaagt gcctttcctc 12240agagtcgcca cagagtccag
cgctaagaca cccagcaagc tgctggaccc cctcgcctgg 12300gacaatcact atggcacaca
gattcccaaa gaggaatgga aaagccaaga gaaaagccct 12360gagaaaaccg ctttcaaaaa
gaaagacaca atcctgagcc tcaacgcttg cgaaagcaat 12420cacgctatcg ctgccattaa
cgaaggccaa aacaaacccg aaatcgaagt gacatgggcc 12480aagcagggca ggaccgaaag
actctgctcc cagaatcccc ctgtgctcaa gaggcaccaa 12540agagaaatca caagaacaac
cctccagtcc gaccaagagg aaatcgacta cgatgacaca 12600atctccgtgg aaatgaaaaa
ggaggacttt gacatttacg atgaggatga gaatcagtcc 12660cccaggagct ttcagaaaaa
gacaagacat tactttatcg ctgccgtcga gaggctgtgg 12720gactatggca tgagcagcag
ccctcacgtc ctgaggaaca gagcccagag cggaagcgtc 12780ccccaattca aaaaggtcgt
gtttcaggag ttcacagacg gcagcttcac ccaacccctc 12840tacaggggcg aactgaatga
gcatctggga ctgctcggcc cttacattag agccgaggtg 12900gaggataaca ttatggtcac
ctttagaaat caggccagca ggccctatag cttttactcc 12960agcctcatct cctacgagga
agatcagagg cagggagccg aacccaggaa gaatttcgtc 13020aagcctaacg aaaccaaaac
ctatttctgg aaggtccagc atcacatggc ccctaccaaa 13080gacgaatttg attgcaaagc
ctgggcctat ttctccgacg ttgacctgga gaaagacgtg 13140cattccggcc tcatcggacc
cctcctggtc tgccatacca ataccctcaa ccctgcccac 13200ggcagacagg tgaccgtcca
ggagtttgct ctgtttttca caatctttga cgaaaccaaa 13260agctggtact ttaccgaaaa
catggagagg aactgtagag ccccctgtaa cattcagatg 13320gaggacccca cattcaaaga
gaattacagg ttccatgcca ttaacggata cattatggat 13380accctccccg gactggtcat
ggctcaggat cagaggatca ggtggtatct gctgtctatg 13440ggctccaacg aaaacattca
ctccatccat ttctccggcc atgtgtttac cgtcagaaaa 13500aaggaggagt ataagatggc
cctctacaat ctgtatcccg gagtgtttga gacagtggaa 13560atgctcccct ccaaggctgg
catttggagg gtggaatgcc tcatcggaga gcatctgcac 13620gccggaatgt ccaccctgtt
tctcgtgtat agcaataagt gtcagacacc cctcggcatg 13680gcctccggcc atatcaggga
ctttcagatt accgccagcg gacagtatgg ccaatgggct 13740cccaaactgg ctagactcca
ctatagcgga agcattaacg cttggtccac caaagagcct 13800ttctcctgga ttaaggtgga
cctcctggct cccatgatta tccacggaat caaaacccaa 13860ggcgctagac aaaagtttag
ctccctgtat atctcccagt ttatcattat gtatagcctc 13920gacggaaaga aatggcaaac
ctatagagga aactccaccg gaaccctcat ggtgttcttt 13980ggcaatgtgg atagctccgg
cattaagcat aacattttca atccccctat cattgccagg 14040tacattagac tccaccctac
ccattactcc atcaggagca cactgaggat ggaactgatg 14100ggctgtgacc tcaactcctg
ctccatgccc ctcggcatgg aaagcaaagc cattagcgat 14160gcccaaatca cagcctccag
ctatttcaca aatatgttcg ctacctggag ccctagcaaa 14220gccaggctgc atctgcaagg
caggagcaat gcctggagac ctcaggtcaa caatcccaaa 14280gagtggctgc aagtggattt
ccaaaagaca atgaaagtga caggcgtcac cacacaggga 14340gtgaaaagcc tcctgacaag
catgtacgtc aaggagttcc tcatctccag ctcccaggat 14400ggccatcagt ggaccctgtt
ctttcagaat ggcaaagtga aagtgtttca gggaaaccaa 14460gactccttca cacccgtcgt
gaatagcctc gaccctcccc tcctgacaag atacctgaga 14520atccaccccc aaagctgggt
gcatcagatt gccctcagaa tggaggtcct gggatgcgaa 14580gcccaagacc tctactaaat
cgattgcgca aagctttcgc gataggcgag accaatgggt 14640gtgtacgtag cggccgcgtc
gacgatagct tgatgggtgg catccctgtg acccctcccc 14700agtgcctctc ctggccctgg
aagttgccac tccagtgccc accagccttg tcctaataaa 14760attaagttgc atcattttgt
ctgactaggt gtccttctat aatattatgg ggtggagggg 14820ggtggtatgg agcaaggggc
aagttgggaa gacaacctgt agggcctgcg gggtctattg 14880ggaaccaagc tggagtgcag
tggcacaatc ttggctcact gcaatctccg cctcctgggt 14940tcaagcgatt ctcctgcctc
agcctcccga gttgttggga ttccaggcat gcatgaccag 15000gctcagctaa tttttgtttt
tttggtagag acggggtttc accatattgg ccaggctggt 15060ctccaactcc taatctcagg
tgatctaccc accttggcct cccaaattgc tgggattaca 15120ggcgtgaacc actgctccct
tccctgtcct tctgatttta aaataactat accagcagga 15180ggacgtccag acacagcata
ggctacctgg ccatgcccaa ccggtgggac atttgagttg 15240cttgcttggc actgtcctct
catgcgttgg gtccactcag tagatgcctg ttgaattctg 15300atttaaatcg gtccgcgtac
ggcgtggtag gtccgaacga atccatggat taccctgtta 15360tccctactca aggacatcat
ccctttagtg agggttaatt cacgcagtgg gtacggaact 15420aaaggcagca cacatcgtgt
aatcatggtc atagctgttt cctgtgtgaa attgttatcc 15480gctacgtctc tcccccgcag
taagggctag attaactcgt ctcgtgaata tccggaactc 15540cctttagtga gggttaattg
cgttgcgctc actgcccgct ttccagtcgg gaaacctgtc 15600gtgccagctt aatcatggtc
atagctgttt cctgtgtgaa attgttatcc gctaccggaa 15660acgcttcctt catgtgagca
aaaggccagc aaaaggccag gaaccgtaaa aaggccgcgt 15720tgctggcgtt tttccatagg
ctccgccccc ctgacgagca tcacaaaaat cgacgctcaa 15780gtcagaggtg gcgaaacccg
acaggactat aaagatacca ggcgtttccc cctggaagct 15840ccctcgtgcg ctctcctgtt
ccgaccctgc cgcttaccgg atacctgtcc gcctttctcc 15900cttcgggaag cgtggcgctt
tctcatagct cacgctgtag gtatctcagt tcggtgtagg 15960tcgttcgctc caagctgggc
tgtgtgcacg aaccccccgt tcagcccgac cgctgcgcct 16020tatccggtaa ctatcgtctt
gagtccaacc cggtaagaca cgacttatcg ccactggcag 16080cagccactgg taacaggatt
agcagagcga ggtatgtagg cggtgctaca gagttcttga 16140agtggtggcc taactacggc
tacactagaa gaacagtatt tggtatctgc gctctgctga 16200agccagttac cttcggaaaa
agagttggta gctcttgatc cggcaaacaa accaccgctg 16260gtagcggtgg tttttttgtt
tgcaagcagc agattacgcg cagaaaaaaa ggatctcaag 16320aagatccttt gatcttttct
acggggtctg acgctcagtg gaacgaaaac tcacgttaag 16380ggattttggt catgatctat
gtcgggtgcg gagaaagagg taatgaaatg gcatacgagt 16440aaacttggtc tgacaccgct
gcatgagatt atcaaaaagg atcttcacct agatcctttt 16500aaattaaaaa tgaagtttta
aatcaatcta aagtatatat gagtaaactt ggtctgacag 16560ttaccaatgc ttaatcagtg
aggcacctat ctcagcgatc tgtctatttc gttcatccat 16620agttgcctga ctccccgtcg
tgtagataac tacgatacgg gagggcttac catctggccc 16680cagtgctgca atgataccgc
gagacccacg ctcaccggct ccagatttat cagcaataaa 16740ccagccagcc ggaagcgccg
agcgcagaag tggtcctgca actttatccg cctccatcca 16800gtctattaac tgttgccggg
aagctagagt aagtagttcg ccagttaata gtttgcggag 16860cgttgttgcc attgctacag
gcatcgtggt gtcacgctcg tcgtttggta tggcttcatt 16920cagctccggt tcccaacgat
caaggcgagt tacatgatcc cccatgttgt gcaaaaaagc 16980ggttagctcc ttcggtcctc
cgatggttgt cagaagtaag ttggccgcag tgttatcact 17040catggttatg gcagcactgc
ataattctct tactgtcatg ccatccgtaa gatgcttttc 17100tgtgactggt gagtattcaa
ccaagtcatt ctgagaatag tgtatgcggc gaccgagttg 17160ctcttgcccg gcgtcaatac
gggataatac cgcgccacat agcagaactt taaaagtgct 17220catcattggg aagcgttctt
cggggcgaaa actctcaagg atcttaccgc tgttgagatc 17280cagttcgatg taacccacac
gagcacccaa ctgatcttca gcatctttta ctttcaccag 17340cgtttctggg tgagcaaaaa
caggaaggca aaatgccgca aaaaagggaa taagggcgac 17400acggaaatgt tgaatactca
tacgcttcct ttttcaatag tattgaagca tttatcaggg 17460ttattgtctc gggagcgaat
acatatttga atgtatttag aaaaa 175051916379DNAArtificial
SequenceSynthetic single promoter gene switch construct encoding
human factor VIIIC 19taaacaaata ggggttccgc gcacatttcc ccgaaaagtg
ccacctgacg tactgaggac 60gccagggttt tcccagtcac gacgttgtaa aacgacggcc
agtagcagac aagcccgtca 120gggcgcgtca gcgggtgttg gcgggtgtcg gggatttagg
tgacactata ggctgagcgc 180cgcacaggca tctagaggct atggcagggc ctgccgcccc
gacgttggct gcgagccctg 240ggccttcacc cgaacttggg gggtggggtg gggaaaagga
agaaacgcgg gcgtattggc 300cccaatgggg tctcggtggg gtatcgacag agtgccagcc
ctgggaccga accccgcgtt 360tatgaacaaa cgacccaaca ccgtgcgttt tattctgtct
ttttattgcc gtcatagcgc 420gggttccttc cggtattgtc tccttccgtg tttcaatcga
ttcaaaagaa ctcgtccagc 480agacggtaaa aagcaatgcg ttgagaatcc ggtgcagcaa
tgccatacag caccagaaag 540cgatcagccc attcaccgcc cagttcttca gcaatgtcac
gggttgccag tgcgatgtcc 600tgatagcgat cagccacgcc caggcgaccg cagtcgataa
agccggagaa acggccgttt 660tccaccataa tgtttggcag acaagcatcg ccgtgggtca
caaccaggtc ctcgccatct 720ggcatacgtg ctttcaggcg tgcgaacagt tctgccggtg
ccagaccctg atgttcctcg 780tccaggtcat cctgatcaac caggccagct tccatgcgag
tgcgtgcgcg ctcgatacgg 840tgtttagctt ggtgatcgaa tgggcaagta gctgggtcca
gggtatgcag acggcgcata 900gcatcagcca tgatggaaac cttttctgcc ggtgccagat
gagaggacag cagatcctgg 960cctggaacct cgcccagcag cagccagtcg cggccagcct
cggtcacaac atccagcaca 1020gctgcgcatg gaacgccggt agtagccagc caggacagac
gagctgcttc atcttgcagt 1080tcgttcagtg cgccggacag atcggtctta acaaacagca
ccggacggcc ttgagcggac 1140agacggaaca cagctgcgtc ggagcaaccg atagtctgtt
gagcccagtc atagccaaac 1200agacgttcca cccaagcagc cggagaacca gcgtgcagac
cgtcttgttc aatcatggtg 1260gcaattgggt gtctgagcga tgtggctcgg ctggcgacgc
aaaagaagat gcggctgact 1320gtcgaacagg aggagcagag agcgaagcgg gaggctgcgg
gctcaatttg catgctttag 1380ttcctcacct tgtcgtatta tactatgccg atatactatg
ccgatgatta attgtcaacg 1440tatacggaat agctctgagg ccgaggcagc ttcggcctct
gcataaataa aaaaaattag 1500tcagccatgg ggcggagaat gggcggaact gggcggagtt
aggggcggga tgggcggagt 1560taggggcggg actatggttg ctgactaatt gagatgcttg
ctttgcatac ttctgcctgc 1620tggggagcct ggggactttc cacacctggt tgctgactaa
ttgagatgct tgctttgcat 1680acttctgcct gctggggagc ctggggactt tccacaccct
aacctcgagg ccatcgtggc 1740acgccagggt tttcccagtc acgacgttgt aaaacgacgg
ccagtgctct tctcccccgc 1800gggaggtttt ataaatccga ctgtctagat accacatttg
tagaggtttt acttgcttta 1860aaaaacctcc cacatctccc cctgaacctg aaacataaaa
tgaatgcaaa tgttttatta 1920acttgtttat tgcagcttat aatggttaca aattaagcaa
tagcatcaca aatttcacaa 1980attaagcatt tttttcactg cattctagtt gtggtttgtc
caaactcatc aatgtatctt 2040atcatgtcta atcgattcac aggttggtgg ggctctccag
gatggggggg ggctgggtgt 2100ggctcatgtc ggccacgtcc caaatctcct ccaggaaggg
gggcagcttc ctgttcttca 2160gcttcaggct gatacacata ttgctgttct gcattcccag
ggtcctcagc tcgctcagga 2220tgctcaggat cttgccgtag atcacgctgc tcctggcgct
gccgctcagc tggttcagga 2280tgtagatcct cagggtgttc aggtagtacc tctggatctc
ctccaccagc tggggctgct 2340ccaggccggg cctgtcgctg aagatcacca cggcggtcag
cagggcgtag tggatgttgt 2400ccagggccat gctgtacata catctgcaga agtgcaggag
gtcctcgatc acctcggcca 2460tgccagcctt cctgtagttg tccctggtgt aagcctggtt
gttggcgaac aggatgctgt 2520cgctggcggc gtcgtacctc ctggccaccc tcagcatcat
cacctcgctg ctgcaagcct 2580tcagcagggt gatctggtcg ggctggctga tcttggcgaa
tccgggcagg cccttggcga 2640actccacgat cagctgcacg gtcaggatgg tcatctcggt
gatctgcctg aagggggtgt 2700cgctctcctc gttctcgtcg tcggcctgct gccaggtctg
ggtgatcctt ttcaggtcct 2760cgtcgctggg ctgctcgtag ccgtcctgat accagatcag
cctggcgatc aggaactgct 2820ggttggcggt cagctggggg atgttcttct gcctgttggt
caccagcagc ttgtcgctca 2880ggaacctggg cacgacctcg tgaatcctgg cggcctcggg
gggggggggc tcgcactgca 2940tgatgggggg catgtggtca tcgacggtgg tggtgctcac
gggcagcttg tccttctcct 3000tctgggcctt cttctccttc cttttcatgg cgcactgggt
ctcgggcacc acgcactcgg 3060gcctgatccc gggaaactcg gggctcacgg tcagctgcct
ctggcccttg ttgctgctct 3120cctcgctgct gctggtggcg ctgatcctgt gctgcctcag
ggtcaggggc atgtcggtct 3180ccacgctggc cagcctgtcg gtcacggcgt ccttgttcac
gttgtcctgc acgaacaggc 3240cggtcagcag ggccttgatg tcttgcaggc tgtccatctt
caggatcatg tccaggtcct 3300ccctggggaa gatcagcagg aacagctgct ccagcctctc
cagcctgctc tccacctcgg 3360tcaggtgggc cctggtcagg gggctcctct tggtcttggg
gctgtatctg cactcccagt 3420tgttcttcag gcacttggcg cacttgggct tctccttgct
gcacttcagc ttcttcagcc 3480tgcagatgtc gcaagcctgc tcgatgctgc tcagcagctt
catggtggca gatctggtcg 3540cgggaggctg ctggttttcc actacccgaa aaaaatccag
cgtctaagca gctgcaagga 3600gagcctttca gagaagcggg tcctggcagc ggcggggaag
tgtccccaaa tgggcagaat 3660agcctccccg cgtcgggaga gtcgcgtcct tgctcgggtg
ttgtaagttc cagtgcaaag 3720tgcccgcccg ctgctatggg caaagtttcg tggatgcggc
tagggttgcg caccgctggc 3780tgggggatca gcgggagggc tgggccagag gcgaagcccc
ctattcgctc cggatctccc 3840ttcccaggac gcccgcagcg cagctctgct cgccgggctc
ctccacccta gcccgccgcc 3900cgctcgctcc ctctgcctct cgctggaatt actacagcga
gttgccggct cagctgtcgc 3960tggggctctc cagcatctcc atcaggaagg tgtcgatggg
cacgtcgccg atcagcctga 4020agaagaacag gtgctccagg cacttcaggc cgatgctcct
caggctgggc agcctcagca 4080gcagcttggc gaatctgccg ggctcgtcgg ggtgggtggt
cctggtgtac tcctccaggg 4140cggcgtacac cttctccctc agcagctcca cctcctgggc
gcttttcagg cccctcacct 4200cggggttgaa caggatgatg gccctcaggc agcccagctc
ggtcttgtcc atcctcatgt 4260ccctcatctt gctcaccagc tcggtcagca ccctgtcgaa
gatggcgccc actccggcgc 4320tgtgggcgct gttcctatgg acgtgcaggc cggtggccag
caggatgccg tccctcacgt 4380cgatgctcct gtggctgaag ctggcgatca gcagctcgtt
ccatccggcc ctcagcagga 4440tcacctggtc gtccaggggc aggctgctga agtggggaat
cctcttggcc cactccacca 4500gggtgaacag ctgcttgtcg gcggcctggc agatgttggt
cacggggtcg ttggggctgc 4560tgccgctgcc gccggttccg ccggggccct ccacgccctg
gtcgcttttc tgctccacgg 4620cgagttcggc ctccagaatc ctgtccacgg gcatctccat
atggccgccg tactcgtcga 4680tgcccagggc gtcggtgaac atctgctcga actcgaagtc
ggccatgtcc agggcgccgt 4740agggggcgct gtcgtggggg gtgaagccgg ggccggggct
gtcgccgtcg cccagcatgt 4800ccaggtcgaa gtcgtccagg gcgtcggcgt gggccatggc
cacgtcctcg ccgtccaggt 4860gcagctcgtc gcccaggctc acgtcggtgg ggggggccac
cttccttttc ttcttggggc 4920ccatggtggc caattgcccc cccccccccc cgcatgccgt
ctaacaaaaa agccaaaaac 4980ggccagaatt tagcggacaa tttactagtc taacactgaa
aattacatat tgacccaaat 5040gattacattt caaaaggtgc ctaaaaaact tcacaaaaca
cactcgccaa ccccgagcgc 5100acgtacccag cccagcagcc cgctactcac caagtgacga
tcacagcgat ccacaaacaa 5160gaaccgcgac ccaaatcccg gctgcgacgg aactagctgt
gccacacccg gcgcgtcctt 5220atataatcat cggcgttcac cgccccacgg agatccctcc
gcagaatcgc cgagaaggga 5280ctacttttcc tcgcctgttc cgctctctgg aaagaaaacc
agtgccctag agtcacccaa 5340gtcccgtcct aaaatgtcct tctgctgata ctggggttct
aaggccgagt cttatgagca 5400gcgggccgct gtcctgagcg tccgggcgga aggatcagga
cgctcgctgc gcccttcgtc 5460tgacgtggca gcgctcgccg tgaggagggg ggcgcccgcg
ggaggcgcca aaacccggcg 5520cggaggccct cgagtaggcg agaccaatgg gtgcgccatg
ggctcttcca aaaatttagg 5580tgacactata gggcaccgct cgcacctgcg cacaggcata
agccaaatgg aactacgaga 5640cctgcatcgt ggtgtaacta taacggtcct aaggtagcga
ccgcggagac taggtgtatt 5700tatctaagcg atcgcttaat taaggccggc cgccgcaata
aaatatcttt attttcatta 5760catctgtgtg ttggtttttt gtgtgaatcc atagtactaa
catacgctct ccatcaaaac 5820aaaacgaaac aaaacaaact agcaaaatag gctgtcccca
gtgcaagtcc aggtgccaga 5880acatttctct atccataatg caggggtacc gggtgatgac
ggtgaaaacc tccaattgcg 5940gagtactgtc ctccgagcgg agtactgtcc tccgagcgga
gtactgtcct ccgagcggag 6000tactgtcctc cgagcggagt actgtcctcc gagcggagta
ctgtcctccg agcggagagt 6060ccccggggac ctagagggta tataatgggt gccttagctg
gtgtgtgacc tcatcttcct 6120gtacgcccct gcaggggcgc gccacgcgtc gaagaaggtg
agtaatctta acatgctctt 6180tttttttttt tttgctaatc ccttttgtgt gctgatgtta
ggatgacatt tacaacaaat 6240gtttgttcct gacaggaaaa accttgctgg gtaccttcgt
tgccggacac ttcttgtcct 6300ctactttgga aaaaaggaat tgagagccgc tagcgccacc
atggctacca ggagatatta 6360cctcggcgct gtggaactgt cctgggatta catgcagtcc
gacctcggcg aactgcctgt 6420ggatgccagg ttccctccca gggtgcctaa gtccttccct
ttcaatacct ccgtggtcta 6480caaaaagaca ctgtttgtgg aatttacaga ccatctgttt
aacattgcca aacccaggcc 6540cccttggatg ggcctcctgg gacccacaat ccaagccgaa
gtgtatgaca cagtggtcat 6600cacactgaaa aacatggcct cccaccccgt tagcctgcac
gctgtcggcg tcagctattg 6660gaaagcctcc gagggagccg aatacgatga ccaaacctcc
cagagggaga aagaggatga 6720caaagtgttt cccggaggct cccacacata cgtctggcaa
gtgctcaagg aaaacggacc 6780tatggcctcc gaccctctgt gtctgacata ctcctacctg
agccatgtgg atctggtcaa 6840ggatctgaat agcggactga ttggcgctct gctcgtgtgt
agagaaggct ccctggctaa 6900ggaaaagaca cagacactgc ataagtttat cctcctgttt
gccgtttttg atgagggaaa 6960gtcctggcat agcgaaacca aaaactccct gatgcaggat
agagatgccg ccagtgctag 7020agcttggcct aagatgcaca ccgtcaatgg ctatgtgaat
agatccctgc ctggcctcat 7080cggatgccat agaaaaagcg tctactggca cgtcatcgga
atgggaacca cacccgaagt 7140gcatagcatt ttccttgaag gacacacatt cctcgtgagg
aaccatagac aagcctccct 7200ggaaatctcc cccattacct ttctgacagc ccaaaccctc
ctgatggacc tcggccaatt 7260cctcctgttt tgccatatct ccagccatca gcatgacgga
atggaagcct atgtgaaagt 7320ggatagctgt cccgaggagc cccagctcag aatgaagaat
aacgaagaag ccgaggacta 7380cgatgacgat ctgacagact ccgagatgga tgtcgtgagg
ttcgatgacg ataactcccc 7440ctccttcatt cagattagat ccgtggctaa gaaacaccct
aagacatggg tccactatat 7500cgctgccgag gaagaagatt gggactatgc ccctctggtc
ctggctcccg atgacaggag 7560ctataagtcc cagtatctga ataacggacc ccagagaatc
ggcaggaagt ataagaaagt 7620gaggttcatg gcctataccg atgagacatt caaaaccagg
gaggctatcc aacacgaaag 7680cggaatcctc ggccctctgc tctacggaga ggtcggcgat
accctcctga ttatctttaa 7740gaatcaggcc agtaggccct ataacattta ccctcacgga
atcacagatg tgagacctct 7800gtatagcagg agactcccca aaggcgtcaa gcatctgaaa
gactttccca ttctgcctgg 7860cgaaatcttt aagtataagt ggaccgtcac cgtcgaggat
ggccctacca aaagcgatcc 7920caggtgcctc accaggtact atagctcctt cgtcaacatg
gagagggacc tcgcctccgg 7980cctcatcgga cccctcctga tttgctataa ggaaagcgtc
gatcaaagag gaaaccaaat 8040catgagcgat aagaggaacg tcatcttgtt ctccgtgttt
gacgaaaaca ggagctggta 8100cttgaccgaa aacattcaga ggttcctccc caatcccgct
ggcgtccagc ttgaggaccc 8160tgagtttcaa gcctccaaca ttatgcacag cattaacgga
tacgttttcg atagcctcca 8220gctgtccgtc tgcctccacg aggtcgctta ctggtacatt
ctgtccatcg gagcccaaac 8280cgatttcctg agcgtctttt ttagcggata cacattcaaa
cacaaaatgg tctacgagga 8340tacactgaca ctgtttccct ttagcggaga gacagtgttt
atgagcatgg agaaccctgg 8400cctctggatt ctgggatgcc ataactccga ctttagaaat
agaggaatga cagccctcct 8460gaaagtgtcc agctgtgaca aaaacacagg cgattactat
gaggatagct atgaggacat 8520ttccgcctat ctgctgtcaa aaaacaatgc cattgagcct
agatccttct cccagaatag 8580caggcaccct agcacaagac aaaagcaatt caatgccaca
accattcccg aaaacgacat 8640cgaaaagaca gacccttggt ttgcccatag aacacccatg
cccaaaatcc aaaacgtcag 8700ctccagcgat ctgctcatgc tcctgaggca gtcccccaca
ccccacggcc tgagcctgtc 8760cgatctgcaa gaggctaagt atgagacatt ctccgacgat
ccctcccccg gagccattga 8820ctccaacaat agcctgagcg aaatgacaca ctttagaccc
cagctgcacc atagcggaga 8880catggtgttt acccctgagt ccggcctcca gctcagactc
aacgaaaagc tcggcacaac 8940cgctgccaca gagctgaaga aactggattt caaagtgtcc
agcacaagca ataacctcat 9000ctccaccatt ccctccgaca atctggctgc cggaaccgat
aacacaagct ccctgggacc 9060cccttccatg cccgtccact atgactccca gctcgacaca
accctttttg gaaagaaaag 9120ctcccccctc accgaaagcg gaggccctct gtccctgtcc
gaggaaaaca atgacagcaa 9180gctgctggag agcggactga tgaactccca ggaaagctcc
tggggaaaga atgtgtccag 9240cacagagtcc ggcaggctgt ttaagggaaa gagagcccac
ggccctgccc tcctgacaaa 9300ggataacgct ctgtttaagg tcagcattag cctcctgaaa
accaataaga caagcaataa 9360ctccgccaca aacagaaaga cccacattga cggaccctcc
ctgctcatcg aaaactcccc 9420ctccgtgtgg cagaatatcc tcgaatccga cacagagttt
aagaaagtga cacccctcat 9480ccatgacagg atgctcatgg ataagaatgc cacagccctc
agactcaacc acatgagcaa 9540caaaaccaca agcagcaaga acatggagat ggtccagcaa
aagaaagagg gacccattcc 9600ccctgacgct cagaatcccg atatgtcctt ctttaagatg
ctgtttctgc ctgagtccgc 9660caggtggatt cagaggaccc acggcaaaaa ctccctgaat
agcggacagg gaccctcccc 9720caaacagctc gtgtccctgg gacccgaaaa gtccgtggaa
ggccaaaact ttctgtccga 9780gaaaaacaaa gtggtcgtgg gaaagggaga gtttaccaag
gacgttggcc tgaaggaaat 9840ggtgtttcct agctccagaa atctgtttct gacaaacctc
gacaatctgc atgagaataa 9900cacacacaat caggaaaaga aaatccaaga ggaaatcgaa
aagaaagaga cactgattca 9960ggaaaacgtc gtgctccccc aaatccatac cgtcaccgga
accaaaaact ttatgaaaaa 10020ccttttcctc ctgtccacca ggcagaatgt ggaaggctcc
tacgatggcg cttacgctcc 10080cgtcctgcaa gactttagat ccctgaatga ctccaccaat
agaacaaaga aacacacagc 10140ccatttctcc aagaaaggcg aggaggaaaa cctggaagga
ctgggaaacc aaaccaaaca 10200gattgtggaa aagtatgcct gtaccacaag aattagccct
aacacaagcc aacagaattt 10260cgtcacccaa agatccaaga gagccctgaa gcaattcagg
ctgcctctgg aagaaacaga 10320gctggagaaa agaattatcg tggatgatac ctccacccaa
tggtccaaga atatgaaaca 10380cctcacccct agcacactga cacagattga ctataacgaa
aaggaaaagg gagccattac 10440ccaaagccct ctgtccgact gtctgacaag atcccactcc
atccctcagg ctaacaggag 10500ccctctgcct atcgctaagg tcagctcctt ccctagcatt
agacctatct atctgacaag 10560agtcctgttt caggataact ccagccatct gcctgccgcc
tcttatagaa aaaaggatag 10620cggagtgcaa gagtccagcc atttcctcca gggagccaaa
aagaataacc tgagcctcgc 10680cattctgaca ctggaaatga caggcgatca gagggaggtc
ggctccctgg gaacctccgc 10740cacaaactcc gtgacataca aaaaggtcga gaataccgtc
ctgcctaagc ctgacctccc 10800caaaacctcc ggcaaagtgg aactgctccc caaagtgcat
atctatcaga aagacctctt 10860tcctaccgaa acctccaacg gaagccccgg ccacctggat
ctggtcgagg gaagcctcct 10920gcaaggcaca gagggagcca ttaagtggaa cgaagccaat
agacctggca aagtgccttt 10980cctcagagtc gccacagagt ccagcgctaa gacacccagc
aagctgctgg accccctcgc 11040ctgggacaat cactatggca cacagattcc caaagaggaa
tggaaaagcc aagagaaaag 11100ccctgagaaa accgctttca aaaagaaaga cacaatcctg
agcctcaacg cttgcgaaag 11160caatcacgct atcgctgcca ttaacgaagg ccaaaacaaa
cccgaaatcg aagtgacatg 11220ggccaagcag ggcaggaccg aaagactctg ctcccagaat
ccccctgtgc tcaagaggca 11280ccaaagagaa atcacaagaa caaccctcca gtccgaccaa
gaggaaatcg actacgatga 11340cacaatctcc gtggaaatga aaaaggagga ctttgacatt
tacgatgagg atgagaatca 11400gtcccccagg agctttcaga aaaagacaag acattacttt
atcgctgccg tcgagaggct 11460gtgggactat ggcatgagca gcagccctca cgtcctgagg
aacagagccc agagcggaag 11520cgtcccccaa ttcaaaaagg tcgtgtttca ggagttcaca
gacggcagct tcacccaacc 11580cctctacagg ggcgaactga atgagcatct gggactgctc
ggcccttaca ttagagccga 11640ggtggaggat aacattatgg tcacctttag aaatcaggcc
agcaggccct atagctttta 11700ctccagcctc atctcctacg aggaagatca gaggcaggga
gccgaaccca ggaagaattt 11760cgtcaagcct aacgaaacca aaacctattt ctggaaggtc
cagcatcaca tggcccctac 11820caaagacgaa tttgattgca aagcctgggc ctatttctcc
gacgttgacc tggagaaaga 11880cgtgcattcc ggcctcatcg gacccctcct ggtctgccat
accaataccc tcaaccctgc 11940ccacggcaga caggtgaccg tccaggagtt tgctctgttt
ttcacaatct ttgacgaaac 12000caaaagctgg tactttaccg aaaacatgga gaggaactgt
agagccccct gtaacattca 12060gatggaggac cccacattca aagagaatta caggttccat
gccattaacg gatacattat 12120ggataccctc cccggactgg tcatggctca ggatcagagg
atcaggtggt atctgctgtc 12180tatgggctcc aacgaaaaca ttcactccat ccatttctcc
ggccatgtgt ttaccgtcag 12240aaaaaaggag gagtataaga tggccctcta caatctgtat
cccggagtgt ttgagacagt 12300ggaaatgctc ccctccaagg ctggcatttg gagggtggaa
tgcctcatcg gagagcatct 12360gcacgccgga atgtccaccc tgtttctcgt gtatagcaat
aagtgtcaga cacccctcgg 12420catggcctcc ggccatatca gggactttca gattaccgcc
agcggacagt atggccaatg 12480ggctcccaaa ctggctagac tccactatag cggaagcatt
aacgcttggt ccaccaaaga 12540gcctttctcc tggattaagg tggacctcct ggctcccatg
attatccacg gaatcaaaac 12600ccaaggcgct agacaaaagt ttagctccct gtatatctcc
cagtttatca ttatgtatag 12660cctcgacgga aagaaatggc aaacctatag aggaaactcc
accggaaccc tcatggtgtt 12720ctttggcaat gtggatagct ccggcattaa gcataacatt
ttcaatcccc ctatcattgc 12780caggtacatt agactccacc ctacccatta ctccatcagg
agcacactga ggatggaact 12840gatgggctgt gacctcaact cctgctccat gcccctcggc
atggaaagca aagccattag 12900cgatgcccaa atcacagcct ccagctattt cacaaatatg
ttcgctacct ggagccctag 12960caaagccagg ctgcatctgc aaggcaggag caatgcctgg
agacctcagg tcaacaatcc 13020caaagagtgg ctgcaagtgg atttccaaaa gacaatgaaa
gtgacaggcg tcaccacaca 13080gggagtgaaa agcctcctga caagcatgta cgtcaaggag
ttcctcatct ccagctccca 13140ggatggccat cagtggaccc tgttctttca gaatggcaaa
gtgaaagtgt ttcagggaaa 13200ccaagactcc ttcacacccg tcgtgaatag cctcgaccct
cccctcctga caagatacct 13260gagaatccac ccccaaagct gggtgcatca gattgccctc
agaatggagg tcctgggatg 13320cgaagcccaa gacctctact aaatcgattg cgcaaagctt
tcgcgatagg cgagaccaat 13380gggtgtgtac gtagcggccg cgtcgacgat agcttgatgg
gtggcatccc tgtgacccct 13440ccccagtgcc tctcctggcc ctggaagttg ccactccagt
gcccaccagc cttgtcctaa 13500taaaattaag ttgcatcatt ttgtctgact aggtgtcctt
ctataatatt atggggtgga 13560ggggggtggt atggagcaag gggcaagttg ggaagacaac
ctgtagggcc tgcggggtct 13620attgggaacc aagctggagt gcagtggcac aatcttggct
cactgcaatc tccgcctcct 13680gggttcaagc gattctcctg cctcagcctc ccgagttgtt
gggattccag gcatgcatga 13740ccaggctcag ctaatttttg tttttttggt agagacgggg
tttcaccata ttggccaggc 13800tggtctccaa ctcctaatct caggtgatct acccaccttg
gcctcccaaa ttgctgggat 13860tacaggcgtg aaccactgct cccttccctg tccttctgat
tttaaaataa ctataccagc 13920aggaggacgt ccagacacag cataggctac ctggccatgc
ccaaccggtg ggacatttga 13980gttgcttgct tggcactgtc ctctcatgcg ttgggtccac
tcagtagatg cctgttgaat 14040tctgatttaa atcggtccgc gtacggcgtg gtaggtccga
acgaatccat ggattaccct 14100gttatcccta ctcaaggaca tcatcccttt agtgagggtt
aattcacgca gtgggtacgg 14160aactaaaggc agcacacatc gtgtaatcat ggtcatagct
gtttcctgtg tgaaattgtt 14220atccgctacg tctctccccc gcagtaaggg ctagattaac
tcgtctcgtg aatatccgga 14280actcccttta gtgagggtta attgcgttgc gctcactgcc
cgctttccag tcgggaaacc 14340tgtcgtgcca gcttaatcat ggtcatagct gtttcctgtg
tgaaattgtt atccgctacc 14400ggaaacgctt ccttcatgtg agcaaaaggc cagcaaaagg
ccaggaaccg taaaaaggcc 14460gcgttgctgg cgtttttcca taggctccgc ccccctgacg
agcatcacaa aaatcgacgc 14520tcaagtcaga ggtggcgaaa cccgacagga ctataaagat
accaggcgtt tccccctgga 14580agctccctcg tgcgctctcc tgttccgacc ctgccgctta
ccggatacct gtccgccttt 14640ctcccttcgg gaagcgtggc gctttctcat agctcacgct
gtaggtatct cagttcggtg 14700taggtcgttc gctccaagct gggctgtgtg cacgaacccc
ccgttcagcc cgaccgctgc 14760gccttatccg gtaactatcg tcttgagtcc aacccggtaa
gacacgactt atcgccactg 14820gcagcagcca ctggtaacag gattagcaga gcgaggtatg
taggcggtgc tacagagttc 14880ttgaagtggt ggcctaacta cggctacact agaagaacag
tatttggtat ctgcgctctg 14940ctgaagccag ttaccttcgg aaaaagagtt ggtagctctt
gatccggcaa acaaaccacc 15000gctggtagcg gtggtttttt tgtttgcaag cagcagatta
cgcgcagaaa aaaaggatct 15060caagaagatc ctttgatctt ttctacgggg tctgacgctc
agtggaacga aaactcacgt 15120taagggattt tggtcatgcc taggacgaaa ggaggtcgtg
aaatggataa aaaaatacag 15180cgtttttcat gtacaactat actagttgta gtgcctaaat
aatgctttta aaacttaaaa 15240ataatctatg tcgggtgcgg agaaagaggt aatgaaatgg
caaatcaatg tcctcagcga 15300aatggcatac gagtaaactt ggtctgacac cgctgcatga
gattatcaaa aaggatcttc 15360acctagatcc ttttaaatta aaaatgaagt tttaaatcaa
tctaaagtat atatgagtaa 15420acttggtctg acagttacca atgcttaatc agtgaggcac
ctatctcagc gatctgtcta 15480tttcgttcat ccatagttgc ctgactcccc gtcgtgtaga
taactacgat acgggagggc 15540ttaccatctg gccccagtgc tgcaatgata ccgcgagacc
cacgctcacc ggctccagat 15600ttatcagcaa taaaccagcc agccggaagc gccgagcgca
gaagtggtcc tgcaacttta 15660tccgcctcca tccagtctat taactgttgc cgggaagcta
gagtaagtag ttcgccagtt 15720aatagtttgc ggagcgttgt tgccattgct acaggcatcg
tggtgtcacg ctcgtcgttt 15780ggtatggctt cattcagctc cggttcccaa cgatcaaggc
gagttacatg atcccccatg 15840ttgtgcaaaa aagcggttag ctccttcggt cctccgatgg
ttgtcagaag taagttggcc 15900gcagtgttat cactcatggt tatggcagca ctgcataatt
ctcttactgt catgccatcc 15960gtaagatgct tttctgtgac tggtgagtat tcaaccaagt
cattctgaga atagtgtatg 16020cggcgaccga gttgctcttg cccggcgtca atacgggata
ataccgcgcc acatagcaga 16080actttaaaag tgctcatcat tgggaagcgt tcttcggggc
gaaaactctc aaggatctta 16140ccgctgttga gatccagttc gatgtaaccc acacgagcac
ccaactgatc ttcagcatct 16200tttactttca ccagcgtttc tgggtgagca aaaacaggaa
ggcaaaatgc cgcaaaaaag 16260ggaataaggg cgacacggaa atgttgaata ctcatacgct
tcctttttca atagtattga 16320agcatttatc agggttattg tctcgggagc gaatacatat
ttgaatgtat ttagaaaaa 1637920158PRTHomo sapiensHuman Insulin like growth
factor (IGF-1) 20Met Gly Lys Ile Ser Ser Leu Pro Thr Gln Leu Phe Lys Cys
Cys Phe1 5 10 15Cys Asp
Phe Leu Lys Val Lys Met His Thr Met Ser Ser Ser His Leu20
25 30Phe Tyr Leu Ala Leu Cys Leu Leu Thr Phe Thr Ser
Ser Ala Thr Ala35 40 45Gly Pro Glu Thr
Leu Cys Gly Ala Glu Leu Val Asp Ala Leu Gln Phe50 55
60Val Cys Gly Asp Arg Gly Phe Tyr Phe Asn Lys Pro Thr Gly
Tyr Gly65 70 75 80Ser
Ser Ser Arg Arg Ala Pro Gln Thr Gly Ile Val Asp Glu Cys Cys85
90 95Phe Arg Ser Cys Asp Leu Arg Arg Leu Glu Met
Tyr Cys Ala Pro Leu100 105 110Lys Pro Ala
Lys Ser Ala Arg Ser Val Arg Ala Gln Arg His Thr Asp115
120 125Met Pro Lys Thr Gln Lys Tyr Gln Pro Pro Ser Thr
Asn Lys Asn Thr130 135 140Lys Ser Gln Arg
Arg Lys Gly Ser Thr Phe Glu Glu Arg Lys145 150
15521288PRTHomo sapiensHuman basic fibroblast growth factor (bFGF)
21Met Val Gly Val Gly Gly Gly Asp Val Glu Asp Val Thr Pro Arg Pro1
5 10 15Gly Gly Cys Gln Ile Ser
Gly Arg Gly Ala Arg Gly Cys Asn Gly Ile20 25
30Pro Gly Ala Ala Ala Trp Glu Ala Ala Leu Pro Arg Arg Arg Pro Arg35
40 45Arg His Pro Ser Val Asn Pro Arg Ser
Arg Ala Ala Gly Ser Pro Arg50 55 60Thr
Arg Gly Arg Arg Thr Glu Glu Arg Pro Ser Gly Ser Arg Leu Gly65
70 75 80Asp Arg Gly Arg Gly Arg
Ala Leu Pro Gly Gly Arg Leu Gly Gly Arg85 90
95Gly Arg Gly Arg Ala Pro Glu Arg Val Gly Gly Arg Gly Arg Gly Arg100
105 110Gly Thr Ala Ala Pro Arg Ala Ala
Pro Ala Ala Arg Gly Ser Arg Pro115 120
125Gly Pro Ala Gly Thr Met Ala Ala Gly Ser Ile Thr Thr Leu Pro Ala130
135 140Leu Pro Glu Asp Gly Gly Ser Gly Ala
Phe Pro Pro Gly His Phe Lys145 150 155
160Asp Pro Lys Arg Leu Tyr Cys Lys Asn Gly Gly Phe Phe Leu
Arg Ile165 170 175His Pro Asp Gly Arg Val
Asp Gly Val Arg Glu Lys Ser Asp Pro His180 185
190Ile Lys Leu Gln Leu Gln Ala Glu Glu Arg Gly Val Val Ser Ile
Lys195 200 205Gly Val Cys Ala Asn Arg Tyr
Leu Ala Met Lys Glu Asp Gly Arg Leu210 215
220Leu Ala Ser Lys Cys Val Thr Asp Glu Cys Phe Phe Phe Glu Arg Leu225
230 235 240Glu Ser Asn Asn
Tyr Asn Thr Tyr Arg Ser Arg Lys Tyr Thr Ser Trp245 250
255Tyr Val Ala Leu Lys Arg Thr Gly Gln Tyr Lys Leu Gly Ser
Lys Thr260 265 270Gly Pro Gly Gln Lys Ala
Ile Leu Phe Leu Pro Met Ser Ala Lys Ser275 280
28522193PRTHomo sapiensHuman erythropoietin 22Met Gly Val His Glu
Cys Pro Ala Trp Leu Trp Leu Leu Leu Ser Leu1 5
10 15Leu Ser Leu Pro Leu Gly Leu Pro Val Leu Gly
Ala Pro Pro Arg Leu20 25 30Ile Cys Asp
Ser Arg Val Leu Gln Arg Tyr Leu Leu Glu Ala Lys Glu35 40
45Ala Glu Asn Ile Thr Thr Gly Cys Ala Glu His Cys Ser
Leu Asn Glu50 55 60Asn Ile Thr Val Pro
Asp Thr Lys Val Asn Phe Tyr Ala Trp Lys Arg65 70
75 80Met Glu Val Gly Gln Gln Ala Val Glu Val
Trp Gln Gly Leu Ala Leu85 90 95Leu Ser
Glu Ala Val Leu Arg Gly Gln Ala Leu Leu Val Asn Ser Ser100
105 110Gln Pro Trp Glu Pro Leu Gln Leu His Val Asp Lys
Ala Val Ser Gly115 120 125Leu Arg Ser Leu
Thr Thr Leu Leu Arg Ala Leu Gly Ala Gln Lys Glu130 135
140Ala Ile Ser Pro Pro Asp Ala Ala Ser Ala Ala Pro Leu Arg
Thr Ile145 150 155 160Thr
Ala Asp Thr Phe Arg Lys Leu Phe Arg Val Tyr Ser Asn Phe Leu165
170 175Arg Gly Lys Leu Lys Leu Tyr Thr Gly Glu Ala
Cys Arg Thr Gly Asp180 185
190Arg23134PRTHomo sapiensHuman BNP 23Met Asp Pro Gln Thr Ala Pro Ser Arg
Ala Leu Leu Leu Leu Leu Phe1 5 10
15Leu His Leu Ala Phe Leu Gly Gly Arg Ser His Pro Leu Gly Ser
Pro20 25 30Gly Ser Ala Ser Asp Leu Glu
Thr Ser Gly Leu Gln Glu Gln Arg Asn35 40
45His Leu Gln Gly Lys Leu Ser Glu Leu Gln Val Glu Gln Thr Ser Leu50
55 60Glu Pro Leu Gln Glu Ser Pro Arg Pro Thr
Gly Val Trp Lys Ser Arg65 70 75
80Glu Val Ala Thr Glu Gly Ile Arg Gly His Arg Lys Met Val Leu
Tyr85 90 95Thr Leu Arg Ala Pro Arg Ser
Pro Lys Met Val Gln Gly Ser Gly Cys100 105
110Phe Gly Arg Lys Met Asp Arg Ile Ser Ser Ser Ser Gly Leu Gly Cys115
120 125Lys Val Leu Arg Arg
His13024562PRTHomo sapiensHuman tPA 24Met Asp Ala Met Lys Arg Gly Leu Cys
Cys Val Leu Leu Leu Cys Gly1 5 10
15Ala Val Phe Val Ser Pro Ser Gln Glu Ile His Ala Arg Phe Arg
Arg20 25 30Gly Ala Arg Ser Tyr Gln Val
Ile Cys Arg Asp Glu Lys Thr Gln Met35 40
45Ile Tyr Gln Gln His Gln Ser Trp Leu Arg Pro Val Leu Arg Ser Asn50
55 60Arg Val Glu Tyr Cys Trp Cys Asn Ser Gly
Arg Ala Gln Cys His Ser65 70 75
80Val Pro Val Lys Ser Cys Ser Glu Pro Arg Cys Phe Asn Gly Gly
Thr85 90 95Cys Gln Gln Ala Leu Tyr Phe
Ser Asp Phe Val Cys Gln Cys Pro Glu100 105
110Gly Phe Ala Gly Lys Cys Cys Glu Ile Asp Thr Arg Ala Thr Cys Tyr115
120 125Glu Asp Gln Gly Ile Ser Tyr Arg Gly
Thr Trp Ser Thr Ala Glu Ser130 135 140Gly
Ala Glu Cys Thr Asn Trp Asn Ser Ser Ala Leu Ala Gln Lys Pro145
150 155 160Tyr Ser Gly Arg Arg Pro
Asp Ala Ile Arg Leu Gly Leu Gly Asn His165 170
175Asn Tyr Cys Arg Asn Pro Asp Arg Asp Ser Lys Pro Trp Cys Tyr
Val180 185 190Phe Lys Ala Gly Lys Tyr Ser
Ser Glu Phe Cys Ser Thr Pro Ala Cys195 200
205Ser Glu Gly Asn Ser Asp Cys Tyr Phe Gly Asn Gly Ser Ala Tyr Arg210
215 220Gly Thr His Ser Leu Thr Glu Ser Gly
Ala Ser Cys Leu Pro Trp Asn225 230 235
240Ser Met Ile Leu Ile Gly Asn Val Tyr Thr Ala Gln Asn Pro
Ser Ala245 250 255Gln Ala Leu Gly Leu Gly
Lys His Asn Tyr Cys Arg Asn Pro Asp Gly260 265
270Asp Ala Lys Pro Trp Cys His Val Leu Lys Asn Arg Arg Leu Thr
Trp275 280 285Glu Tyr Cys Asp Val Pro Ser
Cys Ser Thr Cys Gly Leu Arg Gln Tyr290 295
300Ser Gln Pro Gln Phe Arg Ile Lys Gly Gly Leu Phe Ala Asp Ile Ala305
310 315 320Ser His Pro Trp
Gln Ala Ala Ile Phe Ala Lys His Arg Arg Ser Pro325 330
335Gly Glu Arg Phe Leu Cys Gly Gly Ile Leu Ile Ser Ser Cys
Trp Ile340 345 350Leu Ser Ala Ala His Cys
Phe Gln Glu Arg Phe Pro Pro His His Leu355 360
365Thr Val Ile Leu Gly Arg Thr Tyr Arg Val Val Pro Gly Glu Glu
Glu370 375 380Gln Lys Phe Glu Val Glu Lys
Tyr Ile Val His Lys Glu Phe Asp Asp385 390
395 400Asp Thr Tyr Asp Asn Asp Ile Ala Leu Leu Gln Leu
Lys Ser Asp Ser405 410 415Ser Arg Cys Ala
Gln Glu Ser Ser Val Val Arg Thr Val Cys Leu Pro420 425
430Pro Ala Asp Leu Gln Leu Pro Asp Trp Thr Glu Cys Glu Leu
Ser Gly435 440 445Tyr Gly Lys His Glu Ala
Leu Ser Pro Phe Tyr Ser Glu Arg Leu Lys450 455
460Glu Ala His Val Arg Leu Tyr Pro Ser Ser Arg Cys Thr Ser Gln
His465 470 475 480Leu Leu
Asn Arg Thr Val Thr Asp Asn Met Leu Cys Ala Gly Asp Thr485
490 495Arg Ser Gly Gly Pro Gln Ala Asn Leu His Asp Ala
Cys Gln Gly Asp500 505 510Ser Gly Gly Pro
Leu Val Cys Leu Asn Asp Gly Arg Met Thr Leu Val515 520
525Gly Ile Ile Ser Trp Gly Leu Gly Cys Gly Gln Lys Asp Val
Pro Gly530 535 540Val Tyr Thr Lys Val Thr
Asn Tyr Leu Asp Trp Ile Arg Asp Asn Met545 550
555 560Arg Pro25185PRTHomo sapiensHuman relaxin
25Met Pro Arg Leu Phe Phe Phe His Leu Leu Gly Val Cys Leu Leu Leu1
5 10 15Asn Gln Phe Ser Arg Ala
Val Ala Asp Ser Trp Met Glu Glu Val Ile20 25
30Lys Leu Cys Gly Arg Glu Leu Val Arg Ala Gln Ile Ala Ile Cys Gly35
40 45Met Ser Thr Trp Ser Lys Arg Ser Leu
Ser Gln Glu Asp Ala Pro Gln50 55 60Thr
Pro Arg Pro Val Ala Glu Ile Val Pro Ser Phe Ile Asn Lys Asp65
70 75 80Thr Glu Thr Ile Asn Met
Met Ser Glu Phe Val Ala Asn Leu Pro Gln85 90
95Glu Leu Lys Leu Thr Leu Ser Glu Met Gln Pro Ala Leu Pro Gln Leu100
105 110Gln Gln His Val Pro Val Leu Lys
Asp Ser Ser Leu Leu Phe Glu Glu115 120
125Phe Lys Lys Leu Ile Arg Asn Arg Gln Ser Glu Ala Ala Asp Ser Ser130
135 140Pro Ser Glu Leu Lys Tyr Leu Gly Leu
Asp Thr His Ser Arg Lys Lys145 150 155
160Arg Gln Leu Tyr Ser Ala Leu Ala Asn Lys Cys Cys His Val
Gly Cys165 170 175Thr Lys Arg Ser Leu Ala
Arg Phe Cys180 18526728PRTHomo sapiensHuman hepatocyte
growth factor (HGF) 26Met Trp Val Thr Lys Leu Leu Pro Ala Leu Leu Leu Gln
His Val Leu1 5 10 15Leu
His Leu Leu Leu Leu Pro Ile Ala Ile Pro Tyr Ala Glu Gly Gln20
25 30Arg Lys Arg Arg Asn Thr Ile His Glu Phe Lys
Lys Ser Ala Lys Thr35 40 45Thr Leu Ile
Lys Ile Asp Pro Ala Leu Lys Ile Lys Thr Lys Lys Val50 55
60Asn Thr Ala Asp Gln Cys Ala Asn Arg Cys Thr Arg Asn
Lys Gly Leu65 70 75
80Pro Phe Thr Cys Lys Ala Phe Val Phe Asp Lys Ala Arg Lys Gln Cys85
90 95Leu Trp Phe Pro Phe Asn Ser Met Ser Ser
Gly Val Lys Lys Glu Phe100 105 110Gly His
Glu Phe Asp Leu Tyr Glu Asn Lys Asp Tyr Ile Arg Asn Cys115
120 125Ile Ile Gly Lys Gly Arg Ser Tyr Lys Gly Thr Val
Ser Ile Thr Lys130 135 140Ser Gly Ile Lys
Cys Gln Pro Trp Ser Ser Met Ile Pro His Glu His145 150
155 160Ser Phe Leu Pro Ser Ser Tyr Arg Gly
Lys Asp Leu Gln Glu Asn Tyr165 170 175Cys
Arg Asn Pro Arg Gly Glu Glu Gly Gly Pro Trp Cys Phe Thr Ser180
185 190Asn Pro Glu Val Arg Tyr Glu Val Cys Asp Ile
Pro Gln Cys Ser Glu195 200 205Val Glu Cys
Met Thr Cys Asn Gly Glu Ser Tyr Arg Gly Leu Met Asp210
215 220His Thr Glu Ser Gly Lys Ile Cys Gln Arg Trp Asp
His Gln Thr Pro225 230 235
240His Arg His Lys Phe Leu Pro Glu Arg Tyr Pro Asp Lys Gly Phe Asp245
250 255Asp Asn Tyr Cys Arg Asn Pro Asp Gly
Gln Pro Arg Pro Trp Cys Tyr260 265 270Thr
Leu Asp Pro His Thr Arg Trp Glu Tyr Cys Ala Ile Lys Thr Cys275
280 285Ala Asp Asn Thr Met Asn Asp Thr Asp Val Pro
Leu Glu Thr Thr Glu290 295 300Cys Ile Gln
Gly Gln Gly Glu Gly Tyr Arg Gly Thr Val Asn Thr Ile305
310 315 320Trp Asn Gly Ile Pro Cys Gln
Arg Trp Asp Ser Gln Tyr Pro His Glu325 330
335His Asp Met Thr Pro Glu Asn Phe Lys Cys Lys Asp Leu Arg Glu Asn340
345 350Tyr Cys Arg Asn Pro Asp Gly Ser Glu
Ser Pro Trp Cys Phe Thr Thr355 360 365Asp
Pro Asn Ile Arg Val Gly Tyr Cys Ser Gln Ile Pro Asn Cys Asp370
375 380Met Ser His Gly Gln Asp Cys Tyr Arg Gly Asn
Gly Lys Asn Tyr Met385 390 395
400Gly Asn Leu Ser Gln Thr Arg Ser Gly Leu Thr Cys Ser Met Trp
Asp405 410 415Lys Asn Met Glu Asp Leu His
Arg His Ile Phe Trp Glu Pro Asp Ala420 425
430Ser Lys Leu Asn Glu Asn Tyr Cys Arg Asn Pro Asp Asp Asp Ala His435
440 445Gly Pro Trp Cys Tyr Thr Gly Asn Pro
Leu Ile Pro Trp Asp Tyr Cys450 455 460Pro
Ile Ser Arg Cys Glu Gly Asp Thr Thr Pro Thr Ile Val Asn Leu465
470 475 480Asp His Pro Val Ile Ser
Cys Ala Lys Thr Lys Gln Leu Arg Val Val485 490
495Asn Gly Ile Pro Thr Arg Thr Asn Ile Gly Trp Met Val Ser Leu
Arg500 505 510Tyr Arg Asn Lys His Ile Cys
Gly Gly Ser Leu Ile Lys Glu Ser Trp515 520
525Val Leu Thr Ala Arg Gln Cys Phe Pro Ser Arg Asp Leu Lys Asp Tyr530
535 540Glu Ala Trp Leu Gly Ile His Asp Val
His Gly Arg Gly Asp Glu Lys545 550 555
560Cys Lys Gln Val Leu Asn Val Ser Gln Leu Val Tyr Gly Pro
Glu Gly565 570 575Ser Asp Leu Val Leu Met
Lys Leu Ala Arg Pro Ala Val Leu Asp Asp580 585
590Phe Val Ser Thr Ile Asp Leu Pro Asn Tyr Gly Cys Thr Ile Pro
Glu595 600 605Lys Thr Ser Cys Ser Val Tyr
Gly Trp Gly Tyr Thr Gly Leu Ile Asn610 615
620Tyr Asp Gly Leu Leu Arg Val Ala His Leu Tyr Ile Met Gly Asn Glu625
630 635 640Lys Cys Ser Gln
His His Arg Gly Lys Val Thr Leu Asn Glu Ser Glu645 650
655Ile Cys Ala Gly Ala Glu Lys Ile Gly Ser Gly Pro Cys Glu
Gly Asp660 665 670Tyr Gly Gly Pro Leu Val
Cys Glu Gln His Lys Met Arg Met Val Leu675 680
685Gly Val Ile Val Pro Gly Arg Gly Cys Ala Ile Pro Asn Arg Pro
Gly690 695 700Ile Phe Val Arg Val Ala Tyr
Tyr Ala Lys Trp Ile His Lys Ile Ile705 710
715 720Leu Thr Tyr Lys Val Pro Gln Ser725
User Contributions:
Comment about this patent or add new information about this topic:
| People who visited this patent also read: | |
| Patent application number | Title |
|---|---|
| 20120147267 | VIDEO PROCESSOR TIMING GENERATION |
| 20120147266 | SHARED-PLL AUDIO CLOCK RECOVERY IN MULTIMEDIA INTERFACES |
| 20120147265 | GENERATION AND PROVISION OF MEDIA METADATA |
| 20120147264 | METHOD FOR THE SEMI-AUTOMATIC EDITING OF TIMED AND ANNOTATED DATA |
| 20120147263 | METHOD AND APPARATUS FOR MOTION-COMPENSATED INTERPOLATION (MCI) WITH CONSERVATIVE MOTION MODEL |