Patent application title: Process for Conversion of Natural Gas to Syngas Using a Solid Oxidizing Agent
Inventors:
Kurt M. Vanden Bussche (Lake In The Hills, IL, US)
IPC8 Class: AC10L306FI
USPC Class:
252373
Class name: Compositions gaseous compositions carbon-oxide and hydrogen containing
Publication date: 2009-05-07
Patent application number: 20090114881
ion of natural gas to syngas. The process uses a
solid oxidizing agent in place of an oxidizing gas for the partial
oxidation of the natural gas.Claims:
1. A process for producing a syngas from natural gas comprising:contacting
a natural gas stream with an oxidized solid material in a reaction zone
under reaction conditions, where the reaction temperature is greater than
500.degree. C. thereby generating a syngas and a reduced solid
material;passing the reduced solid material to a regeneration zone;
andcontacting the reduced solid material with an oxidizing gas under
reaction conditions thereby regenerating the oxidized solid material.
2. The process of claim 1 wherein the oxidizing gas comprises air or oxygen.
3. The process of claim 2 wherein the oxidizing gas further comprises steam.
4. The process of claim 1 further comprising feeding steam into the reaction zone.
5. The process of claim 1 wherein the oxidizes solid material is a granular form of a metal oxide, wherein the metal in the metal oxide is selected from the group consisting of alkali metals, alkaline earth metals, transition metals, and mixtures thereof.
6. The process of claim 5 wherein the metal in the metal oxide is selected from the group consisting of manganese (Mn), iron (Fe), nickel (Ni), copper (Cu), zinc (Zn), titanium (Ti), vanadium (V), and mixtures thereof.
7. The process of claim 1 wherein the reaction conditions in the reaction zone include a temperature between 600.degree. C. and 850.degree. C.
8. The process of claim 1 wherein the reaction conditions in the reaction zone include a pressure between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia).
9. The process of claim 1 wherein the natural gas comprises more than 90% methane.
10. The process of claim 1 wherein the syngas comprises H2 and CO.
11. The process of claim 1 wherein the oxidized solid material and the natural gas are carried cocurrently in the reactor.
12. A process for producing a syngas from natural gas comprising:contacting a natural gas stream to an oxidation process in the presence of a solid oxide material and a hydrocarbon activation material under reaction conditions, where the reaction temperature is greater than 500.degree. C. thereby generating a syngas and a reduced solid oxide material, where the natural gas and the solid oxide material are carried cocurrently in a reactor;passing the reduced solid oxide material to a regeneration zone; andoxidizing the reduced solid oxide material with an oxidizing material thereby regenerating the solid oxide material.
13. The process of claim 12 wherein the oxidizing gas comprises air or oxygen.
14. The process of claim 13 wherein the oxidizing gas further comprises steam.
15. The process of claim 12 wherein the oxidizes solid material is a granular form of a metal oxide, and where the metal in the metal oxide is selected from the group consisting of alkali metals, alkaline earth metals, transition metals, and mixtures thereof.
16. The process of claim 15 wherein the metal in the metal oxide is selected from the group consisting of manganese (Mn), iron (Fe), copper (Cu), nickel (Ni), zinc (Zn), titanium (Ti), vanadium (V), and mixtures thereof.
17. The process of claim 12 wherein the hydrocarbon activation material is selected from the group consisting of chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), molybdenum (Mo), technetium (Te), ruthenium (Ru), rhodium (Rh), palladium (Pd), tungsten (W), rhenium (Re), osmium (Os), iridium (Ir), platinum (Pt), and mixtures thereof.
18. The process of claim 12 wherein the reaction conditions in the reaction zone include a temperature between 600.degree. C. and 850.degree. C.
19. The process of claim 12 wherein the reaction conditions in the reaction zone include a pressure between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia).Description:
BACKGROUND OF THE INVENTION
[0001]The present invention relates to a process for converting natural gas into other commercial products. Specifically, the invention relates to the production of syngas from natural gas using a solid oxidizing agent.
[0002]Natural gas generally refers to light gaseous hydrocarbons, and especially comprising methane. Natural gas also contains hydrocarbons such as ethane, propane, butanes, and the like. Natural gas is recovered from underground reservoirs, and is commonly used as an energy source for heating and power generation. Typically, natural gas is recovered at high pressure, processed and fed into a gas pipeline under pressure. Natural gas can comprise undesirable components, such as carbon dioxide, nitrogen and water, which can be removed with technology commonly available. One example is the use of adsorbents for removing non-hydrocarbon components of the natural gas, and or sulfur compounds.
[0003]Natural gas is usually processed to recover heavier hydrocarbon components found in the natural gas, and to increase the relative methane content. Components recovered from natural gas include ethane, propane, butanes, and the like, as well as unsaturated hydrocarbons, leaving methane as the principal component of the processed natural gas.
[0004]Natural gas is most commonly handled in gaseous form, and transported by pipeline to processing plants, and then onto gas pipelines for transmission and distribution. However, there is much natural gas that is located in remote locations, and needs to be transported without the ability to feed the natural gas into a pipeline. In addition natural gas, or more precisely methane, can be processed to produce higher molecular weight hydrocarbon products for use as liquid fuels, lubricants, or monomers for plastics.
[0005]The need for methods of processing methane can improve the recovery and distribution of natural gas, especially when the natural gas is situated in distant and remote locations where the economics depend on how the natural gas is brought to market.
SUMMARY OF THE INVENTION
[0006]The invention is a new process for generating a syngas from natural gas. The process comprises contacting a natural gas stream with an oxidized solid material in a reaction zone, and under reaction conditions to generate a syngas. A reduced solid material is a result of the reaction, and the reduced solid material is regenerated in a regeneration zone. The reduced solid material is regenerated through oxidizing the solid material under reaction conditions thereby generating the oxidized solid material.
[0007]In one embodiment, the process comprises adding steam to the reaction zone to further increase the hydrogen content of the syngas. The formation of syngas is a high temperature reaction with the reaction conditions including a temperature between 400° C. and 900° C. and a pressure between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia).
[0008]Other objects, advantages and applications of the present invention will become apparent to those skilled in the art from the following detailed description and drawing.
BRIEF DESCRIPTION OF THE DRAWING
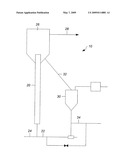
[0009]The drawing is a diagram of a reactor for the process of converting methane to syngas using solid oxidants.
DETAILED DESCRIPTION OF THE INVENTION
[0010]Natural gas is traditionally collected and transported to plants for processing. The primary use of natural gas is for heating, and is processed by removing water, inert gases, and natural gas liquids, or higher molecular weight hydrocarbons found in natural gas. The natural gas is then compressed, or liquefied for transport. However, one new technology is to convert natural gas to methanol for transport as a liquid. This saves on compression costs, and/or liquefaction costs, and provides for a safer material to transport.
[0011]Another process for changing the traditional compression and liquefaction of natural gas, is to convert the natural gas to syngas, or synthesis gas. The first steps will be to remove inert components in the natural gas, such as nitrogen, argon, and carbon dioxide. Natural gas liquids will also be recovered and directed to other processing or transport. The treated natural gas will comprise primarily methane and some ethane with small amounts of higher alkanes, such as propane. Preferably, the natural gas comprises more than 90% methane. Syngas can provide for the generation of liquids from the methane. There are two primary methods of producing syngas from methane. One method is steam reforming where methane and steam react to form carbon monoxide and hydrogen. Steam reforming is energy intensive in that the process consumes over 200 kJ/mole of methane consumed and therefore requires a furnace or other source of continuous heat. A second method is partial oxidation. Partial oxidation comprises burning methane in an oxygen lean environment where the methane is partially oxidized to carbon monoxide along with the production of hydrogen and some steam. Partial oxidation is exothermic and yields a significant amount of heat. Because one reaction is endothermic and the other is exothermic, these reactions are often performed together for efficient energy usage. Combining the steam reforming and partial oxidation yields a third process wherein the heat generated by the partial oxidation is used to drive the steam reforming to yield a syngas. However, the partial oxidation needs a higher concentration of oxygen than is found in air and the energy associated with the separation of air off-sets the advantage of the energy needed for steam reforming.
[0012]Processed for syngas formation are well known and can be found in U.S. Pat. Nos. 7,262,334 and 7,226,548, and are incorporated by reference in their entirety. The resulting syngas comprises carbon monoxide (CO), water (H2O), and hydrogen (H2). The syngas can be catalytically converted to larger hydrocarbons through Fischer-Tropsch synthesis. Fisher-Tropsch synthesis is a known process for the conversion of oxidized carbon to hydrocarbon liquids, as shown in U.S. Pat. No. 4,945,116. Typically the oxidized carbon is carbon monoxide and the source is from the partial combustion of coal.
[0013]The oxidation of hydrocarbons can be carried out with a catalyst such as for the production of butane to maleic anhydride or propylene to acrolein, as shown in U.S. Pat. Nos. 6,437,193 and 6,310,240. These processes are for the insertion of oxygen into a hydrocarbon to produce a desirable oxygenate. The aim of partial combustion of a light hydrocarbon, such as methane, is to strip all of the hydrogen from the hydrocarbon and to produce a gas of CO and H2 for subsequent generation of larger molecules. While the transport mechanism shows that some of the oxygen can come from solids bearing the oxygen, the processes are operated at lower temperatures than partial oxidation for the production of syngas. Indeed, the processes show that at high temperatures the solids are readily reoxidized for regeneration at temperature around 500° C., indicating that the equilibrium of metals with their oxides is unfavorable at higher temperatures.
[0014]However, by controlling the process and by not adding any gaseous oxygen as in the references, and by having the oxygen from the solid oxides taken away with the carbon atoms during the partial combustion, it was found that a favorable control over the production of syngas is achieved through the use of a solid oxidizing agent in a co-current reactor.
[0015]The present invention uses a solid oxidizing agent for the partial oxidation of methane. The advantage with this method is that during the process if there is over oxidation of the methane to produce carbon dioxide (CO2), the process is simultaneously reducing the solid oxidizing agent, and as the product comprising carbon dioxide and reduced solid oxiding agent progress through the reactor, the water gas shift equilibrium will reduce the carbon dioxide to carbon monoxide (CO).
[0016]The process comprises contacting a natural gas stream with an oxidized solid material in a reaction zone, thereby generating a syngas and a reduced solid material. The reduced solid material and syngas are separated, and the reduced solid material is passed to a regeneration zone. In the regeneration zone, the reduced solid material is regenerated through a reaction with an oxidizing gas thereby generating the oxidized solid material.
[0017]The process can be shown with respect to a looping reactor for use in generating the syngas. The reactor 10, as shown in the FIGURE, is a cocurrent flow reactor, and comprises a reaction section 20, and a regeneration section 30. The oxidized solid material is heated and fed to the reaction section 20 through a solid feed conduit 22. Heat is added to the process through the heated solid material. Methane, or natural gas, is fed to the reaction section 20 through a natural gas conduit 24. The methane and the oxidized solid material travel cocurrently up the reaction section 20 where the syngas is formed. The oxidized solid material is reduced to a reduced solid material and the syngas and reduced solid material separate in a separation section 26. The syngas is directed through a product conduit 28 and the reduced solid material is falls down the reactor separation section 26. The reduced solid material is directed through a conduit 32 to the regeneration section 30. In an alternate embodiment, the process can include adding steam to the reaction section 20. The steam can be added with the oxidized solid material through the solid feed conduit 22, thereby facilitating the transport of the oxidized solid material, or the steam can be added with the natural gas through the natural gas conduit 24, or the steam can be added through an independent port (not shown) for more individual control over the amount of steam added to the process. Steam also provides heat that can facilitate the reactions to produce syngas.
[0018]The formation of syngas is a high temperature reaction with the temperature between 500° C. and 900° C., and preferably between 600° C. and 850° C. The reaction conditions include a pressure in the reactor is between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia), and preferably between 1.72 MPa (250 psia) and 4.14 MPa (600 psia).
[0019]In the regeneration section 30, an oxidizing gas is admitted to the section 30 through an oxidizing gas inlet 34. The oxidizing gas can comprise air or oxygen. The oxidizing agent needs to contain oxygen, as the oxygen will be transferred to the syngas during the reaction with natural gas. The oxidizing gas can further include steam. The steam provides several advantages to the regeneration process. The steam provides heat, and increases the volume of gas that facilitates lifting the solid through the regeneration section 30.
[0020]The oxidized solid material is in a granular form and is a metal oxide. The metal oxide comprises a metal selected from the group of alkali metals, alkaline earth metals, transition metals, and mixtures of metals from these groups. A preferred group of metals used are iron (Fe), nickel (Ni), copper (Cu), zinc (Zn), manganese (Mn), cerium (Ce), calcium (Ca), vanadium (V), niobium (Nb), tantalum (Ta), titanium (Ti), zirconium (Zr), hafnium (Hf), yttrium (Y), thorium (Th), lanthanum (La), and neodymium (Nd).
[0021]In another embodiment, the process comprises contacting the natural gas stream with a solid oxide material and a hydrocarbon activation material under reaction conditions, thereby generating a syngas stream and a reduced solid material. The solid oxide, natural gas and hydrocarbon activation material are fed into a reactor and carried co-currently through the reactor. After exiting the reactor the reduced solid and hydrocarbon activation material are separated from the syngas and directed to a regeneration zone for reoxidizing the reduced solid, thereby regenerating the solid oxide for reuse in the reactor.
[0022]The reaction conditions for temperature and pressure for this embodiment are as above.
[0023]The hydrocarbon activation material is a material found to activate the methane and to facilitate the reaction for syngas formation. The activation material is a solid, metal that is present in the reactor and is carried with the solid oxidizing agent. The hydrocarbon activation material is a metal selected from one or more of: chromium (Cr), molybdenum (Mo), tungsten (W), manganese (Mn), technetium (Te), rhenium (Re), iron (Fe), cobalt (Co), nickel (Ni), ruthenium (Ru), platinum (Pt), palladium (Pd), rhodium (Rh), iridium (Ir), and osmium (Os). Preferably, the activation material is selected from one or more of chromium (Cr), molybdenum (Mo), tungsten (W), nickel (Ni), ruthenium (Ru), platinum (Pt), palladium (Pd), rhodium (Rh), and iridium (Ir).
[0024]While the invention has been described with what are presently considered the preferred embodiments, it is to be understood that the invention is not limited to the disclosed embodiments, but it is intended to cover various modifications and equivalent arrangements included within the scope of the appended claims.
Claims:
1. A process for producing a syngas from natural gas comprising:contacting
a natural gas stream with an oxidized solid material in a reaction zone
under reaction conditions, where the reaction temperature is greater than
500.degree. C. thereby generating a syngas and a reduced solid
material;passing the reduced solid material to a regeneration zone;
andcontacting the reduced solid material with an oxidizing gas under
reaction conditions thereby regenerating the oxidized solid material.
2. The process of claim 1 wherein the oxidizing gas comprises air or oxygen.
3. The process of claim 2 wherein the oxidizing gas further comprises steam.
4. The process of claim 1 further comprising feeding steam into the reaction zone.
5. The process of claim 1 wherein the oxidizes solid material is a granular form of a metal oxide, wherein the metal in the metal oxide is selected from the group consisting of alkali metals, alkaline earth metals, transition metals, and mixtures thereof.
6. The process of claim 5 wherein the metal in the metal oxide is selected from the group consisting of manganese (Mn), iron (Fe), nickel (Ni), copper (Cu), zinc (Zn), titanium (Ti), vanadium (V), and mixtures thereof.
7. The process of claim 1 wherein the reaction conditions in the reaction zone include a temperature between 600.degree. C. and 850.degree. C.
8. The process of claim 1 wherein the reaction conditions in the reaction zone include a pressure between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia).
9. The process of claim 1 wherein the natural gas comprises more than 90% methane.
10. The process of claim 1 wherein the syngas comprises H2 and CO.
11. The process of claim 1 wherein the oxidized solid material and the natural gas are carried cocurrently in the reactor.
12. A process for producing a syngas from natural gas comprising:contacting a natural gas stream to an oxidation process in the presence of a solid oxide material and a hydrocarbon activation material under reaction conditions, where the reaction temperature is greater than 500.degree. C. thereby generating a syngas and a reduced solid oxide material, where the natural gas and the solid oxide material are carried cocurrently in a reactor;passing the reduced solid oxide material to a regeneration zone; andoxidizing the reduced solid oxide material with an oxidizing material thereby regenerating the solid oxide material.
13. The process of claim 12 wherein the oxidizing gas comprises air or oxygen.
14. The process of claim 13 wherein the oxidizing gas further comprises steam.
15. The process of claim 12 wherein the oxidizes solid material is a granular form of a metal oxide, and where the metal in the metal oxide is selected from the group consisting of alkali metals, alkaline earth metals, transition metals, and mixtures thereof.
16. The process of claim 15 wherein the metal in the metal oxide is selected from the group consisting of manganese (Mn), iron (Fe), copper (Cu), nickel (Ni), zinc (Zn), titanium (Ti), vanadium (V), and mixtures thereof.
17. The process of claim 12 wherein the hydrocarbon activation material is selected from the group consisting of chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), molybdenum (Mo), technetium (Te), ruthenium (Ru), rhodium (Rh), palladium (Pd), tungsten (W), rhenium (Re), osmium (Os), iridium (Ir), platinum (Pt), and mixtures thereof.
18. The process of claim 12 wherein the reaction conditions in the reaction zone include a temperature between 600.degree. C. and 850.degree. C.
19. The process of claim 12 wherein the reaction conditions in the reaction zone include a pressure between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia).
Description:
BACKGROUND OF THE INVENTION
[0001]The present invention relates to a process for converting natural gas into other commercial products. Specifically, the invention relates to the production of syngas from natural gas using a solid oxidizing agent.
[0002]Natural gas generally refers to light gaseous hydrocarbons, and especially comprising methane. Natural gas also contains hydrocarbons such as ethane, propane, butanes, and the like. Natural gas is recovered from underground reservoirs, and is commonly used as an energy source for heating and power generation. Typically, natural gas is recovered at high pressure, processed and fed into a gas pipeline under pressure. Natural gas can comprise undesirable components, such as carbon dioxide, nitrogen and water, which can be removed with technology commonly available. One example is the use of adsorbents for removing non-hydrocarbon components of the natural gas, and or sulfur compounds.
[0003]Natural gas is usually processed to recover heavier hydrocarbon components found in the natural gas, and to increase the relative methane content. Components recovered from natural gas include ethane, propane, butanes, and the like, as well as unsaturated hydrocarbons, leaving methane as the principal component of the processed natural gas.
[0004]Natural gas is most commonly handled in gaseous form, and transported by pipeline to processing plants, and then onto gas pipelines for transmission and distribution. However, there is much natural gas that is located in remote locations, and needs to be transported without the ability to feed the natural gas into a pipeline. In addition natural gas, or more precisely methane, can be processed to produce higher molecular weight hydrocarbon products for use as liquid fuels, lubricants, or monomers for plastics.
[0005]The need for methods of processing methane can improve the recovery and distribution of natural gas, especially when the natural gas is situated in distant and remote locations where the economics depend on how the natural gas is brought to market.
SUMMARY OF THE INVENTION
[0006]The invention is a new process for generating a syngas from natural gas. The process comprises contacting a natural gas stream with an oxidized solid material in a reaction zone, and under reaction conditions to generate a syngas. A reduced solid material is a result of the reaction, and the reduced solid material is regenerated in a regeneration zone. The reduced solid material is regenerated through oxidizing the solid material under reaction conditions thereby generating the oxidized solid material.
[0007]In one embodiment, the process comprises adding steam to the reaction zone to further increase the hydrogen content of the syngas. The formation of syngas is a high temperature reaction with the reaction conditions including a temperature between 400° C. and 900° C. and a pressure between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia).
[0008]Other objects, advantages and applications of the present invention will become apparent to those skilled in the art from the following detailed description and drawing.
BRIEF DESCRIPTION OF THE DRAWING
[0009]The drawing is a diagram of a reactor for the process of converting methane to syngas using solid oxidants.
DETAILED DESCRIPTION OF THE INVENTION
[0010]Natural gas is traditionally collected and transported to plants for processing. The primary use of natural gas is for heating, and is processed by removing water, inert gases, and natural gas liquids, or higher molecular weight hydrocarbons found in natural gas. The natural gas is then compressed, or liquefied for transport. However, one new technology is to convert natural gas to methanol for transport as a liquid. This saves on compression costs, and/or liquefaction costs, and provides for a safer material to transport.
[0011]Another process for changing the traditional compression and liquefaction of natural gas, is to convert the natural gas to syngas, or synthesis gas. The first steps will be to remove inert components in the natural gas, such as nitrogen, argon, and carbon dioxide. Natural gas liquids will also be recovered and directed to other processing or transport. The treated natural gas will comprise primarily methane and some ethane with small amounts of higher alkanes, such as propane. Preferably, the natural gas comprises more than 90% methane. Syngas can provide for the generation of liquids from the methane. There are two primary methods of producing syngas from methane. One method is steam reforming where methane and steam react to form carbon monoxide and hydrogen. Steam reforming is energy intensive in that the process consumes over 200 kJ/mole of methane consumed and therefore requires a furnace or other source of continuous heat. A second method is partial oxidation. Partial oxidation comprises burning methane in an oxygen lean environment where the methane is partially oxidized to carbon monoxide along with the production of hydrogen and some steam. Partial oxidation is exothermic and yields a significant amount of heat. Because one reaction is endothermic and the other is exothermic, these reactions are often performed together for efficient energy usage. Combining the steam reforming and partial oxidation yields a third process wherein the heat generated by the partial oxidation is used to drive the steam reforming to yield a syngas. However, the partial oxidation needs a higher concentration of oxygen than is found in air and the energy associated with the separation of air off-sets the advantage of the energy needed for steam reforming.
[0012]Processed for syngas formation are well known and can be found in U.S. Pat. Nos. 7,262,334 and 7,226,548, and are incorporated by reference in their entirety. The resulting syngas comprises carbon monoxide (CO), water (H2O), and hydrogen (H2). The syngas can be catalytically converted to larger hydrocarbons through Fischer-Tropsch synthesis. Fisher-Tropsch synthesis is a known process for the conversion of oxidized carbon to hydrocarbon liquids, as shown in U.S. Pat. No. 4,945,116. Typically the oxidized carbon is carbon monoxide and the source is from the partial combustion of coal.
[0013]The oxidation of hydrocarbons can be carried out with a catalyst such as for the production of butane to maleic anhydride or propylene to acrolein, as shown in U.S. Pat. Nos. 6,437,193 and 6,310,240. These processes are for the insertion of oxygen into a hydrocarbon to produce a desirable oxygenate. The aim of partial combustion of a light hydrocarbon, such as methane, is to strip all of the hydrogen from the hydrocarbon and to produce a gas of CO and H2 for subsequent generation of larger molecules. While the transport mechanism shows that some of the oxygen can come from solids bearing the oxygen, the processes are operated at lower temperatures than partial oxidation for the production of syngas. Indeed, the processes show that at high temperatures the solids are readily reoxidized for regeneration at temperature around 500° C., indicating that the equilibrium of metals with their oxides is unfavorable at higher temperatures.
[0014]However, by controlling the process and by not adding any gaseous oxygen as in the references, and by having the oxygen from the solid oxides taken away with the carbon atoms during the partial combustion, it was found that a favorable control over the production of syngas is achieved through the use of a solid oxidizing agent in a co-current reactor.
[0015]The present invention uses a solid oxidizing agent for the partial oxidation of methane. The advantage with this method is that during the process if there is over oxidation of the methane to produce carbon dioxide (CO2), the process is simultaneously reducing the solid oxidizing agent, and as the product comprising carbon dioxide and reduced solid oxiding agent progress through the reactor, the water gas shift equilibrium will reduce the carbon dioxide to carbon monoxide (CO).
[0016]The process comprises contacting a natural gas stream with an oxidized solid material in a reaction zone, thereby generating a syngas and a reduced solid material. The reduced solid material and syngas are separated, and the reduced solid material is passed to a regeneration zone. In the regeneration zone, the reduced solid material is regenerated through a reaction with an oxidizing gas thereby generating the oxidized solid material.
[0017]The process can be shown with respect to a looping reactor for use in generating the syngas. The reactor 10, as shown in the FIGURE, is a cocurrent flow reactor, and comprises a reaction section 20, and a regeneration section 30. The oxidized solid material is heated and fed to the reaction section 20 through a solid feed conduit 22. Heat is added to the process through the heated solid material. Methane, or natural gas, is fed to the reaction section 20 through a natural gas conduit 24. The methane and the oxidized solid material travel cocurrently up the reaction section 20 where the syngas is formed. The oxidized solid material is reduced to a reduced solid material and the syngas and reduced solid material separate in a separation section 26. The syngas is directed through a product conduit 28 and the reduced solid material is falls down the reactor separation section 26. The reduced solid material is directed through a conduit 32 to the regeneration section 30. In an alternate embodiment, the process can include adding steam to the reaction section 20. The steam can be added with the oxidized solid material through the solid feed conduit 22, thereby facilitating the transport of the oxidized solid material, or the steam can be added with the natural gas through the natural gas conduit 24, or the steam can be added through an independent port (not shown) for more individual control over the amount of steam added to the process. Steam also provides heat that can facilitate the reactions to produce syngas.
[0018]The formation of syngas is a high temperature reaction with the temperature between 500° C. and 900° C., and preferably between 600° C. and 850° C. The reaction conditions include a pressure in the reactor is between 0.103 MPa (15 psia) and 6.9 MPa (1000 psia), and preferably between 1.72 MPa (250 psia) and 4.14 MPa (600 psia).
[0019]In the regeneration section 30, an oxidizing gas is admitted to the section 30 through an oxidizing gas inlet 34. The oxidizing gas can comprise air or oxygen. The oxidizing agent needs to contain oxygen, as the oxygen will be transferred to the syngas during the reaction with natural gas. The oxidizing gas can further include steam. The steam provides several advantages to the regeneration process. The steam provides heat, and increases the volume of gas that facilitates lifting the solid through the regeneration section 30.
[0020]The oxidized solid material is in a granular form and is a metal oxide. The metal oxide comprises a metal selected from the group of alkali metals, alkaline earth metals, transition metals, and mixtures of metals from these groups. A preferred group of metals used are iron (Fe), nickel (Ni), copper (Cu), zinc (Zn), manganese (Mn), cerium (Ce), calcium (Ca), vanadium (V), niobium (Nb), tantalum (Ta), titanium (Ti), zirconium (Zr), hafnium (Hf), yttrium (Y), thorium (Th), lanthanum (La), and neodymium (Nd).
[0021]In another embodiment, the process comprises contacting the natural gas stream with a solid oxide material and a hydrocarbon activation material under reaction conditions, thereby generating a syngas stream and a reduced solid material. The solid oxide, natural gas and hydrocarbon activation material are fed into a reactor and carried co-currently through the reactor. After exiting the reactor the reduced solid and hydrocarbon activation material are separated from the syngas and directed to a regeneration zone for reoxidizing the reduced solid, thereby regenerating the solid oxide for reuse in the reactor.
[0022]The reaction conditions for temperature and pressure for this embodiment are as above.
[0023]The hydrocarbon activation material is a material found to activate the methane and to facilitate the reaction for syngas formation. The activation material is a solid, metal that is present in the reactor and is carried with the solid oxidizing agent. The hydrocarbon activation material is a metal selected from one or more of: chromium (Cr), molybdenum (Mo), tungsten (W), manganese (Mn), technetium (Te), rhenium (Re), iron (Fe), cobalt (Co), nickel (Ni), ruthenium (Ru), platinum (Pt), palladium (Pd), rhodium (Rh), iridium (Ir), and osmium (Os). Preferably, the activation material is selected from one or more of chromium (Cr), molybdenum (Mo), tungsten (W), nickel (Ni), ruthenium (Ru), platinum (Pt), palladium (Pd), rhodium (Rh), and iridium (Ir).
[0024]While the invention has been described with what are presently considered the preferred embodiments, it is to be understood that the invention is not limited to the disclosed embodiments, but it is intended to cover various modifications and equivalent arrangements included within the scope of the appended claims.
User Contributions:
Comment about this patent or add new information about this topic: