Patent application title: Detection/Measurement Of Malaria Infection Disease Utilizing Natural Immunity By Hemozoin Induction, Screening Of Preventative Or Therapeutic Medicine For Malaria Infection Disease, And Regulation Of Natural Immunity Induction
Inventors:
Shizuo Akira (Osaka, JP)
Shizuo Akira (Osaka, JP)
Ken Ishii (Osaka, JP)
Ken Ishii (Osaka, JP)
Cevayir Coban (Osaka, JP)
Assignees:
Osaka University
IPC8 Class: AA61K3900FI
USPC Class:
4242721
Class name: Parasitic organism or component thereof or substance produced by said parasitic organism (e.g., schistosoma, dirofilaria, trichinella, fasciola, ancylostoma, ascaris, etc.) parasitic protozoan (e.g., trypanosoma, trichomonas, leishmania, entamoeba, etc.) plasmodium
Publication date: 2009-02-12
Patent application number: 20090041808
Claims:
1. A method for detecting and measuring malaria infection, wherein a
TLR9-mediated, MyD88-dependent innate immune activity induced by hemozoin
that is hydrophobic heme polymers produced as a hemoglobin metabolite by
malaria parasites, is detected and measured.
2. The method for detecting and measuring malaria infection according to claim 1, wherein the detection and measurement of an innate immune activity is a detection and measurement of an immune-cell activation.
3. The method for detecting and measuring malaria infection according to claim 2, wherein the detection and measurement of an immune-cell activation is a detection and measurement of a cytokine production by immune cells.
4. The method for detecting and measuring malaria infection according to claim 3, wherein the cytokine is one or more cytokines selected from TNF-.alpha., IL-12p40, MCP-1, IL-6, and IFNα.
5. The method for detecting and measuring malaria infection according to claim 2, wherein the immune-cell is a spleen-cell or dendritic-cell.
6. A method for screening or testing the quality of a vaccine for malaria infection, wherein a method for detecting and measuring malaria infection according to claim 1 is used as a method for evaluating a test vaccine.
7. A method for screening a preventative or therapeutic agent for malaria infection, wherein a TLR9-mediated, MyD88-dependent innate immune activity induced by hemozoin that is hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, synthetic hemozoin, or derivatives thereof, is detected and measured in the presence of a test substance.
8. A method for screening a preventative or therapeutic agent for malaria infection, wherein a method for detecting and measuring an innate immune activity is a method for detecting and measuring an innate immune activity according to claim 2.
9. An adjuvant for regulating an induction of TLR9-mediated, MyD88-dependent innate immunity, which consists of hemozoin that is hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, synthetic hemozoin, or derivatives thereof.
10. An immunostimulant for inducing TLR9-mediated, MyD88-dependent innate immunity, comprising as an active ingredient hemozoin that is hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, synthetic hemozoin, or derivatives thereof.
11. A method for screening a preventative or therapeutic agent for malaria infection, wherein a cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ nonhuman animal and a cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- nonhuman animal, are measured and evaluated.
12. The method for screening a preventative or therapeutic agent for malaria infection according to claim 11, wherein the cytokine production level is measured as a serum cytokine level by ELISA.
13. The method for screening according to claim 11, wherein the nonhuman animal is a mouse.
14. A method for screening a preventative or therapeutic agent for malaria infection, wherein a cytokine production level in cells in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ gene-expressing cells and a cytokine production level in cells in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- gene-expressing cells, are compared and evaluated.
15. The method for screening a preventative or therapeutic agent for malaria infection according to claim 14, wherein the cells are spleen cells or dendritic cells.
16. The method for screening a preventative or therapeutic agent for malaria infection according to claim 14, wherein the cytokine production level in cells is measured as a cytokine level in a cell-culture supernatant by ELISA.
17. The method for screening a preventative or therapeutic agent for malaria infection according to claim 11, wherein the measurement of the cytokine production level is a measurement of the production level of any one or more cytokines selected from TNF-.alpha., IL-12p40, MCP-1, IL-6, and IFNα.
18. A method for using protein (a) or (b) as defined below as a receptor for a ligand consisting of hemozoin, synthetic hemozoin, or derivatives thereof:(a) a protein consisting of the amino acid sequence shown by SEQ ID No: 2;(b) a protein consisting of an amino acid sequence in which one or a few amino acids are deleted, substituted, or added in the amino acid sequence shown by SEQ ID No:2, the protein being responsive to hemozoin, synthetic hemozoin, or derivatives thereof.
19. The method for using according to claim 18, wherein the protein is encoded by a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof.
20. The method for using according to claim 18, wherein the protein is encoded by a DNA which hybridizes under a stringent condition with a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof.
21. A method for using hemozoin, synthetic hemozoin, or derivatives thereof as a ligand for receptor protein (a) or (b) as defined below:(a) a protein consisting of the amino acid sequence shown by SEQ ID No: 2;(b) a protein consisting of an amino acid sequence in which one or a few amino acids are deleted, substituted, or added in the amino acid sequence shown by SEQ ID No: 2, the protein being responsive to hemozoin, synthetic hemozoin, or derivatives thereof.
22. The method for using according to claim 21, wherein the protein is encoded by a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof.
23. The method for using according to claim 21, wherein the protein is encoded by a DNA which hybridizes under a stringent condition with a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof, the protein being responsive to hemozoin.
24. A TLR9 agonist which contains hemozoin, synthetic hemozoin, or derivatives thereof as an active ingredient.
25. The TLR9 agonist according to claim 24, which activates MyD88.
26. The TLR9 agonist according to claim 25, which induces an immune response through the TLR-MyD88-dependent pathway.
27. The TLR9 agonist according to claim 26, wherein the immune response through the TLR-MyD88-dependent pathway is a production of any one or more cytokines selected from TNF-.alpha., IL-12p40, MCP-1, IL-6, and IFNα.
28. The TLR9 agonist according to claim 24, which activates TLR9 selectively.
29. The TLR9 agonist according to claim 28, which does not activate TLR2, TLR4, TLR7, or TRIF.
30. The TLR9 agonist according to claim 24, which is used as an agent for controlling innate immunity.
31. A method for using hemozoin, synthetic hemozoin, or derivatives thereof as a ligand for TLR9.
32. The method for using according to claim 31, which comprises a method for inducing an immune response through the TLR9-MyD88-dependent pathway.
33. The method for using according to claim 32, wherein the immune response through the TLR9-MyD88-dependent pathway is a production of any one or more cytokines selected from TNF-.alpha., IL-12p40, MCP-1, IL-6, and IFNα.
34. The method for using according to claim 31, which comprises a method for controlling innate immunity.
Description:
TECHNICAL FIELD
[0001]The instant invention relates to a method for detecting and measuring malaria infection by detecting and measuring TLR9-mediated, MyD88-dependent innate immune activity induced by hemozoin, hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites; screening of a vaccine for malaria infection or a preventative or therapeutic agent for malaria infection using the method for detection and measurement; regulation of the induction of innate immunity using the hemozoin or synthetic hemozoin as an adjuvant or immunostimulant; a TLR9 agonist containing hemozoin, synthetic hemozoin or derivatives thereof as an active ingredient; and the like.
BACKGROUND ART
[0002]Malaria infection is the major cause of disease and mortality of humans, especially in the tropical regions of the world. Due to a complex life cycle and rapid polymorphism of malaria parasites, host-parasite interactions and resulting innate immune responses to malaria parasites are still poorly understood despite the necessity of effective immunotherapies against malaria (Nat. Med. 4:520-524, 1998; Nat. Rev. Immunol. 4: 169-180, 2004). Robust innate immune activation, including proinflammatory cytokine production in response to malaria parasites and/or a metabolite released from ruptured infected red blood cells, has been linked to the major symptoms such as high fever (Ann. Trop. Med. Parasitol. 91: 533-542, 1997). Recent evidence suggests that toll-like receptors (TLRs) are involved in innate immune responses to a variety of pathogens including Plasmodium (Annu. Rev. Immunol. 20: 197-216, 2002; Nat. Rev. Immunol. 4:499-511, 2004).
[0003]In murine malaria infection, myeloid differentiation factor 88 (MyD88), an essential adaptor molecule for TLR-mediated cytokine production, was shown to be critical for IL-12 induction by Plasmodium berghei parasites that cause liver damage (J. Immunol. 167: 5928-5934, 2001). A recent study has reported that P. falciparum blood-stage parasites activate human plasmacytoid dendritic cells (DCs) and murine DCs through MyD88-dependent and TLR9-dependent pathways. Although the responsible molecule is yet unidentified, it is suggested that the molecule is a protein contained in schizont lysate or a complex thereof (J. Immunol. 172: 4926-4933, 2004).
[0004]Hemozoin (HZ), known as a malaria pigment, is a detoxification product of heme molecules persisting in food vacuoles of Plasmodium parasites (Int. J. Parasitol. 32: 1645-1653, 2002; Ann. Trop. Med. Parasitol. 91: 501-516, 1997). Intercellular HZ is released into blood during schizont rupture and phagocytosed by myeloid cells, which results in the concentration of HZ in the reticulo-endothelial system (Ann. Trop. Med. Parasitol. 91:501-516, 1997). Specifically, malaria parasites in the red blood cells digest host hemoglobin to hydrophobic heme polymers known as HZ. Then the polymers are released into the blood stream, captured in the reticulo-endotherial system, and accumulate in the system. It has been reported that HZ produced from P. falciparum activates macrophages to produce proinflammatory cytokines, chemokines and nitrogen, and enhances the maturation of human myeloid dendritic cells (DCs) (J. Inflamm. 45: 85-96, 1995; Infect. Immun. 70: 3939-3943, 2002). These studies promoted the need for studying the molecular mechanism through which HZ activates innate immunity and for better understanding the malaria parasite-host interactions. [0005]Non-patent document 1: Nat. Med. 4: 520-524, 1998 [0006]Non-patent document 2: Nat. Rev. Immunol. 4: 169-180, 2004 [0007]Non-patent document 3: Ann. Trop. Med. Parasitol. 91: 533-542, 1997 [0008]Non-patent document 4: Annu. Rev. Immunol. 20: 197-216, 2002 [0009]Non-patent document 5: Nat. Rev. Immunol. 4: 499-511, 2004 [0010]Non-patent document 6: J. Immunol. 167: 5928-5934, 2001 [0011]Non-patent document 7: J. Immunol. 172: 4926-4933, 2004 [0012]Non-patent document 8: Int. J. Parasitol. 32: 1645-1653, 2002 [0013]Non-patent document 9: Ann. Trop. Med. Parasitol. 91: 501-516, [0014]Non-patent document 10: J. Inflamm. 45: 85-96, 1995 [0015]Non-patent document 11: Infect. Immun. 70: 3939-3943, 2002
DISCLOSURE OF THE INVENTION
Object to be Solved by the Invention
[0016]An object of the instant invention is to elucidate the malaria parasite-host interactions in malaria infection, and to provide, based on the findings, a method for detecting and measuring malaria infection utilizing an induction by hemozoin (HZ), hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, for the diagnosis of malaria infection; screening of a vaccine for malaria infection and of a preventative or therapeutic agent for malaria infection using the method for detection and measurement; and a means for regulating the induction of innate immunity using the HZ, synthetic HZ or derivatives thereof as an adjuvant or immunostimulant; and the like.
Means to Solve the Object
[0017]Malaria parasites in red blood cells digest host hemoglobin to hydrophobic heme polymers known as hemozoin (HZ). The polymers are then released into the blood stream, captured in the reticulo-endothelial system and accumulated in the system. While studying about malaria parasite-host interactions to elucidate the molecular-level mechanism of the immune responses in which HZ activates innate immunity, the present inventors found that HZ, the heme metabolite produced by human falciparum malaria parasites, is a novel ligand that strongly stimulates toll-like receptor 9 (TLR9), a receptor for innate immunity. More specifically, the present inventors found that HZ purified from P. falciparum activates MyD88-dependent murine immune cells through TLR9 both in vitro and in vivo, and confirmed that this activation is inhibited by chloroquine, a known antimalarial agent.
[0018]Based on these findings, the present inventors found that it is possible to detect and measure malaria infection by detecting and measuring the HZ-induced, TLR9-mediated, and MyD88-dependent innate immune activity, and that the detection and measurement of malaria infection can be used for the diagnosis of malaria infection, and thus the instant invention has been completed. The present inventors also found that it is possible to screen a vaccine for malaria infection or of a preventative or therapeutic agent for malaria infection using the method for detecting and measuring malaria infection by detecting and measuring the HZ-induced innate immune activity, and thus the instant invention has been accomplished. Further, based on the above findings, the present inventors found that it is possible to regulate HZ-induced, TLR-9 mediated, and MyD88-dependent innate immune induction using HZ, synthetic HZ, or derivatives thereof as an adjuvant or immunostimulant. Specifically, the present inventors became the first to demonstrate that hemozoin is a ligand with a selective immune-response control and activation effect which induces Th1 via TLR9-MyD88 dependent pathway but does not activate other TLR families or TRIF dependent pathway which is a MyD88-independent pathway. The instant invention has been accomplished as a result of the above findings.
[0019]More specifically, the instant invention relates to a method for detecting and measuring malaria infection, wherein a TLR9-mediated, MyD88-dependent innate immune activity induced by hemozoin that is hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, is detected and measured ("1"); the method for detecting and measuring malaria infection according to "1", wherein the detection and measurement of an innate immune activity is a detection and measurement of an immune-cell activation ("2"); the method for detecting and measuring malaria infection according to "2", wherein the detection and measurement of an immune-cell activation is a detection and measurement of a cytokine production by immune cells ("3"); the method for detecting and measuring malaria infection according to "3", wherein the cytokine is one or more cytokines selected from TNF-α, IL-12p40, MCP-1, IL-6, and IFNα ("4"); the method for detecting and measuring malaria infection according to any one of "2" to "4", wherein the immune-cell is a spleen-cell or dendritic-cell ("5"); a method for screening or testing the quality of a vaccine for malaria infection, wherein a method for detecting and measuring malaria infection according to any one of "1" to "5" is used as a method for evaluating a test vaccine ("6"); a method for screening a preventative or therapeutic agent for malaria infection, wherein a TLR9-mediated, MyD88-dependent innate immune activity induced by hemozoin that is hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, synthetic hemozoin, or derivatives thereof, is detected and measured in the presence of a test substance ("7"); and a method for screening a preventative or therapeutic agent for malaria infection, wherein a method for detecting and measuring an innate immune activity is a method for detecting and measuring an innate immune activity according to any one of "2" to "5" ("8").
[0020]The instant invention is also comprised of an adjuvant for regulating an induction of TLR9-mediated, MyD88-dependent innate immunity, which consists of hemozoin that is hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, synthetic hemozoin, or derivatives thereof ("9"); and an immunostimulant for inducing TLR9-mediated, MyD88-dependent innate immunity, comprising as an active ingredient hemozoin that is hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites, synthetic hemozoin, or derivatives thereof ("10").
[0021]The instant invention further relates to a method for screening a preventative or therapeutic agent for malaria infection, wherein a cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ nonhuman animal and a cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- nonhuman animal, are measured and evaluated ("11"); the method for screening a preventative or therapeutic agent for malaria infection according to "11", wherein the cytokine production level is measured as a serum cytokine level by ELISA ("12"); the method for screening according to "11" or "12," wherein the nonhuman animal is a mouse ("13"); a method for screening a preventative or therapeutic agent for malaria infection, wherein a cytokine production level in cells in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ gene-expressing cells and a cytokine production level in cells in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- gene-expressing cells, are compared and evaluated ("14"); the method for screening a preventative or therapeutic agent for malaria infection according to "14", wherein the cells are spleen cells or dendritic cells ("15"); the method for screening a preventative or therapeutic agent for malaria infection according to "14" or "15", wherein the cytokine production level in cells is measured as a cytokine level in a cell-culture supernatant by ELISA ("16"); and the method for screening a preventative or therapeutic agent for malaria infection according to any one of "11" to "16", wherein the measurement of the cytokine production level is a measurement of the production of any one or more cytokines selected from TNF-α, IL-12p40, MCP-1, IL-6, and IFNα ("17").
[0022]The instant invention furthermore relates to a method for using protein (a) or (b) as defined below as a receptor for a ligand consisting of hemozoin, synthetic hemozoin, or derivatives thereof: (a) a protein consisting of the amino acid sequence shown by SEQ ID No: 2; (b) a protein consisting of an amino acid sequence in which one or a few amino acids are deleted, substituted, or added in the amino acid sequence shown by SEQ ID No: 2, the protein being responsive to hemozoin, synthetic hemozoin, or derivatives thereof ("18"); the method for using according to "18", wherein the protein is encoded by a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof ("19"); the method for using according to "18", wherein the protein is encoded by a DNA which hybridizes under a stringent condition with a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof ("20"); a method for using hemozoin, synthetic hemozoin, or derivatives thereof as a ligand for receptor protein (a) or (b) as defined below: (a) a protein consisting of the amino acid sequence shown by SEQ ID No: 2; (b) a protein consisting of an amino acid sequence in which one or a few amino acids are deleted, substituted, or added in the amino acid sequence shown by SEQ ID No: 2, the protein being responsive to hemozoin, synthetic hemozoin, or derivatives thereof ("21"); the method for using according to "21", wherein the protein is encoded by a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof ("22"); and the method for using according to "21", wherein the protein is encoded by a DNA which hybridizes under a stringent condition with a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof, the protein being responsive to hemozoin ("23").
[0023]The instant invention still further relates to a TLR9 agonist which contains hemozoin, synthetic hemozoin, or derivatives thereof as an active ingredient ("24"); the TLR9 agonist according to "24", which activates MyD88 ("25"); the TLR9 agonist according to "25", which induces an immune response through the TLR-MyD88-dependent pathway ("26"); the TLR9 agonist according to "26", wherein the immune response through the TLR-MyD88-dependent pathway is a production of any one or more cytokines selected from TNF-α, IL-12p40, MCP-1, IL-6, and IFNα ("27"); the TLR9 agonist according to any one of "24" to "27", which activates TLR9 selectively ("28"); the TLR9 agonist according to "28", which does not activate TLR2, TLR4, TLR7, or TRIF ("29"); and the TLR9 agonist according to any one of "24" to "29", which is used as an agent for controlling innate immunity ("30").
[0024]The instant invention furthermore relates to a method for using hemozoin, synthetic hemozoin, or derivatives thereof as a ligand for TLR9 ("31"), the method for using according to "31", which comprises a method for inducing an immune response through the TLR9-MyD88-dependent pathway ("32"); the method for using according to "32", wherein the immune response through the TLR9-MyD88-dependent pathway is a production of any one or more cytokines selected from TNF-α, IL-12p40, MCP-1, IL-6, and IFNα ("33"); and the method for using according to any one of "31" to "33", which comprises a method for controlling innate immunity ("34").
BRIEF DESCRIPTION OF DRAWINGS
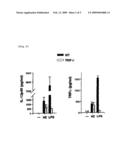
[0025][FIG. 1] It is a series of figures showing the results of the measurement of proinflammatory cytokine productions in the culture supernatant by ELISA after stimulating spleen cells and DCs (human myeloid dendritic cells) with purified HZ in vitro, to study whether purified HZ from P. falciparum activates the murine immune system in an example of the instant invention.
[0026][FIG. 2] It is a set of figures showing the results of the analysis and determination of TNFα or IL-12p40 production level by ELISA using mice lacking TRIF (Toll/IL-1 receptor (TIR)-region-containing adaptor), an essential adaptor molecule for the MyD88-independent pathway, to confirm that HZ-induced innate immune activation is solely dependent on MyD88 in an example of the instant invention.
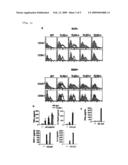
[0027][FIG. 3] It is a series of figures showing (b) the results of flow cytometric analysis for CD40 and CD86 expressions and (c) the results of ELISA analysis for IFNα production by FL-DCs, using spleen cells and DCs to study whether HZ-induced innate immune activation is impaired or altered in an example of the instant invention.
[0028][FIG. 4] It is a series of figures showing the results of the measurement of IL-6 and MCP-1 production levels in the sera by ELISA, after injecting synthetic HZ to wild type mice or TLR9.sup.-/- mice to study whether synthetic HZ activates the innate immune system in a TLR9-dependent manner in an example of the instant invention.
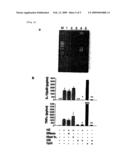
[0029][FIG. 5] It is a set of figures showing the results of the analysis to determine the purity of HZ to exclude any possible contaminants in P. falciparum culture or during HZ preparation from P. falciparum culture, in an example of the instant invention. (a) shows the results of ethidium-bromide staining of HZ that was run on an agarose gel for the detection of DNA contamination and (b) shows the results of the measurement of TNFα and IL-12p40 by ELISA after FL-DCs from C57/B6 mice were incubated with either HZ or DNase treatment, heat-inactivation, and/or CQ (10 μM).
BEST MODE OF CARRYING OUT THE INVENTION
[0030]The instant invention is comprised of a method wherein malaria infection is detected and measured by detecting and measuring TLR9-mediated, MyD88-dependent innate immune activity induced by HZ, hydrophobic heme polymers produced as a hemoglobin metabolite by malaria parasites. In the instant invention, HZ-induced innate immune activity can be detected and measured by detecting and measuring the state of TLR9-mediated, MyD88-dependent activation by HZ in immune cells such as spleen cells or dendritic cells. TLR9-mediated, MyD88-dependent activation by HZ can be detected and measured by detecting and measuring HZ-induced cytokine productions in immune cells. Cytokine production can be detected and measured by detecting and measuring the production of any one or more cytokines selected from TNF-α, IL-12p40, MCP-1, IL-6, and IFNα. Common cytokine detection and measurement methods such as cytokine ELISA method can be used for the detection and measurement of the cytokine production. The diagnosis of malaria infection is enabled by the method for detecting and measuring malaria infection of the instant invention.
[0031]The instant invention is further comprised of using the method for detecting and measuring malaria infection by HZ induction of the instant invention as a method for evaluating a test vaccine thus as a method for screening or testing a vaccine for malaria infection. The method for screening or testing a vaccine can be used as a method for evaluating test vaccines in developing a vaccine, in tests involved in the manufacture of the developed vaccine, and the like. The instant invention is furthermore comprised of using the method for detecting and measuring malaria infection of the instant invention as a method for screening a preventative or therapeutic agent for malaria infection. The method for screening can be performed by the method of the instant invention wherein TLR9-mediated, MyD88-dependent innate immune activity induced by HZ, synthetic HZ, or derivatives thereof is detected and measured in the presence of a test substance.
[0032]The instant invention is still further comprised of using HZ, synthetic HZ, or derivatives thereof that has been found out to be a novel ligand for TLR9 in the instant invention, as an adjuvant for regulating the induction of innate immunity or as an immunostimulant for inducing innate immunity. An induction of TLR9-mediated, MyD88-dependent innate immunity can be regulated by using HZ, synthetic HZ, or derivatives thereof as an adjuvant for regulating the induction of innate immunity or as an immunostimulant for inducing innate immunity. In the instant invention, HZ (insoluble crystalloid structure) can be prepared by the method for purifying from red blood cells that are infected with P. falciparum (3D7 strain) (Infect. Immun. 70: 3939-3943, 2002: Proc. Natl. Acad. Sci. U.S.A 93: 11865-11870, 1996; Proc. Natl. Acad. Sci. U.S.A 88: 325-329, 1991). Further, synthetic HZ (β-hematin) can be manufactured by the purification employing the protocol of Jaramillo et al. (J. Immunol. 172: 3101-3110) based on acetic acid treatment and alkaline bicarbonate wash. Both the adjuvant for regulating the induction of innate immunity and the immunostimulant for inducing innate immunity can be applied according to commonly known application forms.
[0033]The instant invention further relates to a method for screening a preventative or therapeutic agent for malaria infection, wherein the cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ nonhuman animals such as wild type mice, and the cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- nonhuman animal, are measured and evaluated; and a method for screening a preventative or therapeutic agent for malaria infection, wherein the cytokine production level in cells in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ gene-expressing cells such as spleen cells, dendritic cells and the like, and the cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin, or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- gene-expressing cells, are compared and evaluated. The above cytokines can be exemplified by TNF-α, IL-12p40, MCP-1, IL-6, IFNα. Further, the cytokine production level in the above nonhuman animals can be measured as a serum cytokine level in the nonhuman animals by ELISA, and the cytokine production level in cells can be measured as the cytokine level in the cell-culture supernatant by ELISA. Further, MyD88.sup.-/- mice and MyD88.sup.-/- gene-expressing cells can be prepared by the method described in the publication of WO00/41561, and TLR9.sup.-/- mice and TLR9.sup.-/- gene-expressing cells by the method described in Japanese Laid-Open Patent Application No. 2002-34565.
[0034]The instant invention furthermore relates to a method for using (a) a protein consisting of the amino acid sequence shown by SEQ ID No: 2; or (b) a protein consisting of an amino acid sequence in which one or a few amino acids are deleted, substituted, or added in the amino acid sequence shown by SEQ ID No: 2, and is responsive to hemozoin, synthetic hemozoin, or derivatives thereof, as a receptor for a ligand consisting of hemozoin, synthetic hemozoin, or derivatives thereof. As above proteins, a protein encoded by a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof; or a protein encoded by a DNA which hybridizes under a stringent condition with a DNA comprising the nucleotide sequence shown by SEQ ID No: 1 or a complementary sequence thereof, and is responsive to hemozoin, synthetic hemozoin, or derivatives thereof, can be used. Herein, "under a stringent condition" means a condition under which so-called specific hybrids are formed and nonspecific hybrids are not formed. More specifically, such conditions include a condition wherein DNAs with 50-70% or more homology are hybridized and DNAs with less homology are not hybridized, or a hybridization condition with a salt concentration corresponding to 1×SSC, 0.1% SDS or 0.1×SSC, 0.1% SDS at 65° C., which is an usual washing condition in southern hybridization.
[0035]Specific aspects of the above method for using the instant invention include: a method for detecting and measuring malaria infection, wherein TLR9-mediated, MyD88-dependent innate immune activity induced by hemozoin, synthetic hemozoin, or derivatives thereof is detected and measured; a method for screening or testing the quality of a vaccine for malaria infection; a method for screening a preventative or therapeutic agent for malaria infection, wherein TLR9-mediated, MyD88-dependent innate immune activity induced by hemozoin, synthetic hemozoin, or derivatives thereof is detected and measured; a method for screening a preventative or therapeutic agent for malaria infection, wherein the cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ nonhuman animals and the cytokine production level in the case of administering a test substance and hemozoin, synthetic hemozoin or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- nonhuman animal, are measured and evaluated; a method for screening a preventative or therapeutic agent for malaria infection, wherein the cytokine production level in cells in the case of administering a test substance and hemozoin, synthetic hemozoin or derivatives thereof to MyD88.sup.+/+ and TLR9.sup.+/+ gene-expressing cells and the cytokine production level in cells in the case of administering a test substance and hemozoin, synthetic hemozoin or derivatives thereof to MyD88.sup.-/- and/or TLR9.sup.-/- gene-expressing cells, are compared and evaluated; and a method for elucidating a mechanism of immune-response induction through the TLR9-MyD88-dependent pathway.
[0036]The instant invention still further relates to a TLR9 agonist, a substance comprising hemozoin, synthetic hemozoin, or derivatives thereof as an active ingredient, and interacting with TLR9 to induce fully activated intracellular signaling. The TLR9 agonist is capable of activating MyD88; inducing immune responses involving the production and the like of any one or more cytokines selected from TNF-α, IL-12p40, MCP-1, IL-6, and IFNα through the TLR-MyD88 dependent pathway; and selectively activating TLR9 without activating TLR2, TLR4, TLR7, or TRIF, and thus the TLR9 agonist can be used as an agent for regulating innate immunity.
[0037]The instant invention furthermore relates to a method for using hemozoin, synthetic hemozoin, or derivatives thereof as a ligand for TLR9. Such method includes a method for inducing immune responses involving the production and the like of any one or more cytokines selected from TNF-α, IL-12p40, MCP-1, IL-6, and IFNα through the TLR9-MyD88 dependent pathway; and a method for controlling innate immunity. Specific aspects of such methods include the methods exemplified above as specific aspects of a method for using the instant invention.
[0038]The instant invention will be described more specifically with reference to the following examples, while the technical scope of the instant invention will not be limited to these exemplifications.
EXAMPLE 1
Activation of Innate Immune Activity by HZ and the Regulation Thereof
(Materials and Methods)
(Mice)
[0039]Mutant mice (MyD88-, TRIF-, TLR2-, TLR4-, TLR7-, and TLR9-deficient mice) either on a 129/Ola×C57/BL6 or C57/BL6 background were generated as described in literatures (Immunity 9:143-150, 1999; Immunity 11: 443-451, 1999; Nature 408: 740-745, 2000; Nat. Immunol. 3: 196-200, 2002; Science 301: 640-643, 2003). Age-matched groups of wild type mice and mutant mice were used for experiments. For in-vivo studies, 1500 μg HZ purified from P. falciparum culture and synthetic HZ (β-hematin) were injected intraperitoneally into wild-type mice, MyD88.sup.-/- mice, or TLR9.sup.-/- mice (J. Immunol. 172: 3101-3110, 2004). Sera were collected from tails at 1, 2, 4, and 6 hours for cytokine ELISA.
(Reagents)
[0040]Synthetic CpG oligodeoxynucleotides (ODN) D35 were synthesized and purchased from Hokkaido System Science Co., Ltd. Lipopolysaccharides (LPS) from Salmonella minnesota Re-595, hemin chloride, and chloroquine (CQ) were purchased from Sigma-Aldrich. DNase (DNase-I) was purchased from Invitrogen.
(Preparation of HZ and Synthetic HZ (β-Hematin))
[0041]HZ (nonsoluble crystalloid structure) was purified from red blood cells infected with P. falciparum (3D7 strain) (Infect. Immun. 70: 3939-3943, 2002; Proc. Natl. Acad. Sci. U.S.A 93: 11865-11870, 1996; Proc. Natl. Acad. Sci. U.S.A 88: 325-329, 1991). In brief, after saponin lysis of red blood cells, parasites were sonicated and washed seven to eight times in 2% dodecyl sodium sulfate (SDS). Then the pellets were incubated with Proteinase K (2 mg/ml) at 37° C. overnight. The pellets were then washed three times in 2% SDS and incubated in 6 M urea for 3 hours at room temperature on a shaker. After three to five washes in 2% SDS and then in distilled water, the HZ pellets were resuspended in distilled water, and sonicated again before use. In some experiments, as described in a literature (J. Immunol. 167: 2602-2607, 2001), HZ was either heat inactivated at 95° C. for 15 minutes or treated with 100 U/ml DNase-I for 1 hour. The DNase-I treatment was performed by removing genomic DNA completely from P. falciparum crude extract (FIG. 5a).
[0042]Quantification of nucleic acid and protein was performed by using a spectrophotometer or by ethidium bromide staining in agarose gel, BCA method (Biorad), or the pyrogallol red method (Wako Pure Chemical Industries, Ltd.). Total lipid (TP) was measured by TLC (thin-layer chromatography) using Bligh-Dyer method (Toray Research Center) or by an enzymatic method using the Iatron LQ (Mitsubishi Kagaku Iatron, Inc.). Synthetic HZ (β-hematin) was purified using the protocol of Jaramillo et al. (J. Immunol. 172: 3101-3110) based on acetic acid treatment and alkaline bicarbonate wash. To avoid endotoxin contaminations, all solutions were prepared using endotoxin-free PBS or distilled water. Endotoxin levels measured by LAL assays (Bio-Whittaker) were less than 0.001 EU for each nmole HZ used.
(Quantification of HZ or Synthetic HZ (β-Hematin))
[0043]The concentration of HZ or synthetic HZ was determined by depolymerizing heme polymers in 20 mM sodium hydroxide/2% SDS solution for 2 hours at room temperature. The O.D. was read at 400 nm (Proc. Natl. Acad. Sci. U.S.A 93: 11865-11870, 1996). The molar extinction coefficient for heme is 1×105 at 400 nm, and 25 μg P. falciparum HZ is equal to 29 nmole of heme content.
(Cells)
[0044]Single cell suspensions of spleen cells (5×105 cells/well) were cultured in complete RPMI 1640 medium supplemented with 10% FCS for 48 hours. Flt3 ligand-induced bone marrow-derived DCs (FL-DCs) (1×105 cells/well) were generated by culturing bone marrow cells with Flt3 ligand (100 ng/ml; Pepro Tech) for eight to nine days in DMEM medium containing 10% FCS. The cells were stimulated in the presence of the indicated stimuli and supernatants were collected for cytokine ELISA.
(Cytokine ELISA)
[0045]Murine TNF-α, IL-12p40, MCP-1, IL-6 (R & D Systems), and IFNα (PBL Bio. Lab) were measured either from the supernatants or the sera by ELISA according to the manufacturer's instructions.
(Flow Cytometric Analysis of Costimulatory Molecule Expressions)
[0046]Cell surface molecule expressions of the stimulated cells were measured as described in a literature (J. Exp. Med. 196: 269-274, 2002). In brief, the stimulated cells were washed in cooled PBS, fixed, and stained with FITC-labeled antibody, PE-labeled antibody, Cy-Chrome-labeled antibody, and APC-labeled antibody, in the presence of anti-CD 16 antibody for 30 minutes at room temperature. The stained cells were washed, re-suspended in PBS/0.1% BSA/0.1% NaN3, and analyzed by FACSCaliber followed by analysis using CellQuest software (BD). All antibodies were obtained from BD.
(Statistical Analysis)
[0047]Statistically significant differences were analyzed using Student's t-test. P<0.05 was considered significant.
(Test, Results, and Evaluations)
[0048](Activation of Murine Spleen Cells and Dentritic Cells Though the MyD88-Dependent Pathway by Purified HZ from P. falciparum)
[0049]To examine whether purified HZ from P. falciparum activates the murine immune system, spleen cells and DCs (murine myeloid dendritic cells) were stimulated in vitro with purified HZ and proinflammatory cytokine production in the culture supernatant was measured by ELISA. FL-DCs (Fit3 ligand-induced bone marrow-derived DCs) produced large amounts of TNFα, IL-12p40, monocyte chemoattractant factor-1 (MCP-1), and IL-6, in response to HZ in a dose-dependent manner to a similar extent to that of CpG ODN (FIG. 1a). To examine the roles of TLRs in HZ-induced innate immune activation, mice lacking MyD88, an essential adaptor molecule for cytokine inductions mediated by many TLRs, were used (Nat. Rev. Immunol. 4: 499-511, 2004). FL-DCs from MyD88.sup.-/- mice showed significantly impaired levels of TNFα, IL-12p40, MCP-1, and IL-6 productions upon stimulation with HZ (FIG. 1b).
[0050]Similarly, MyD88.sup.-/- spleen cells showed impaired responses to produce MCP-1, IL-6, TNFα, IL-12p40, and INF-inducible protein 10 (IP-10) in response to HZ (FIG. 1c). Additionally, hemozoin stimulated both CD11c.sup.+, B220.sup.+ plasmacytoid DC subset and CD11c.sup.+, B220.sup.- myeloid DC subset of FL-DCs to up-regulate the expressions of CD40 and CD86 that were inhibited in both FL-DC subsets in MyD88.sup.-/- mice (FIG. 1d).
(TRIF-Dependency of HZ Activation of Innate Immune Responses)
[0051]To confirm that HZ-induced innate immune activation is solely dependent on MyD88, mice lacking TRIF (Toll/IL-1 receptor (TIR)-region-containing adaptor), an essential adaptor molecule for the MyD88-independent pathway, were used (Nat. Rev. Immunol. 4: 499-511, 2004). In contrast to MyD88.sup.-/- mice, FL-DCs from TRIF.sup.-/- mice responded to HZ and produced TNFα and IL-12p40 (p<0.05, TRIF.sup.-/- vs. media) comparable to the value of wild type mice (p>0.05) (FIG. 2). LPS-induced TNFα and IL-12p40 were impaired in FL-DCs of TRIF.sup.-/- mice, suggesting that a large amount of HZ purified from P. falciparum cultures was not contaminated with LPS. These data demonstrated that HZ activates a proinflammatory response in mice through MyD88 and that one of the MyD88-dependent TLRs is involved in the recognition of HZ.
(TLR-9-Dependency of HZ Activation of Innate Immune Responses)
[0052]Further experiments were performed using spleen cells and DCs obtained from TLR2.sup.-/- mice, TLR4.sup.-/- mice, TLR7.sup.-/- mice, and TLR9.sup.-/- mice to examine whether HZ-induced innate immune activation was impaired or altered. HZ stimulated FL-DCs in wild type mice, TLR2.sup.-/- mice, TLR4.sup.-/- mice, and TLR7.sup.-/- mice to up-regulate CD40 and CD86 both in CD11c.sup.+, B220+ plasmacytoid DC subset and CD11c.sup.+, B220.sup.- myeloid DC subset (FIG. 3a). In contrast, both FL-DC subsets derived from TLR9.sup.-/- mice failed to up-regulate CD40 and CD86 in response to HZ (FIG. 3a).
[0053]Similarly, FL-DCs derived from wild type mice, TLR2.sup.-/- mice, TLR4.sup.-/- mice, and TLR7.sup.-/- mice, but not from TLR9.sup.-/- mice, produced TNFα, IL-12p40, MCP-1, and IL-6 in response to HZ (FIG. 3b). It is of note that HZ did not induce IFNα by FL-DCs, suggesting that the HZ-induced cytokine profile is similar to that of K-type CpGODN (also known as B-type), but distinct from that of D-type ODN (also know as A-type) or known natural DNA ligands for TLR9 such as bacterial DNA, viral DNA (FIG. 3c) (Nat. Rev. Immunol. 4: 249-258, 2004). Nevertheless, these data clearly demonstrate that TLR9 and MyD88 are critical for HZ-induced activation in murine spleen cells and DCs.
(In Vivo MyD88/TLR9 Dependency of HZ Activation of Proinflammatory Cytokines)
[0054]To confirm that HZ activation is mediated by TLR9 and dependent on MyD88 in vivo, purified P. falciparum HZ was injected intraperitoneally into wild type mice and MyD88.sup.-/- mice or TLR9.sup.-/- mice, and the serum cytokine production levels were monitored. The HZ injection significantly increased serum levels of MCP-1 and IL-6 in wild type mice, which peaked between 1 and 4 hours and declined within 6 hours (FIG. 4a). In contrast, such increases were completely inhibited in MyD88.sup.-/- mice and TLR9.sup.-/- mice. After 6 hours, the cytokine levels declined in wild type mice. These data clearly demonstrate that HZ-induced proinflammatory responses were mediated by TLR9 and MyD88 both in vitro and in vivo.
[0055]Synthetic HZ (β-hematin, synthesized from monomeric heme in laboratory conditions) is structurally similar to HZ formed naturally by parasites (Proc. Natl. Acad. Sci. U.S.A 93: 11865-11870, 1996; Proc. Natl. Acad. Sci. U.S.A 88: 325-329, 1991), and is free of contaminant derived from parasites or cultures. To examine whether synthetic HZ activates the innate immune system in a TLR9-dependent manner, synthetic HZ was injected into wild-type mice or TLR9.sup.-/- mice, and then IL-6 and MCP-1 production levels in sera were monitored. Synthetic HZ injection into wild type mice induced the productions of MCP-1 and IL-6 in sera, which peaked at 1-4 hours (FIG. 4b). In contrast, such responses were inhibited in TLR9.sup.-/- mice (FIG. 4b). These data suggest that synthetic HZ as well as natural HZ purified from P. falciparum stimulates the murine innate immune system, excluding the contribution of other possible contaminants to HZ-induced, TLR9-mediated innate immune activation.
[0056]To exclude any possible contaminants in or during HZ preparation from the P. falciparum culture a series of analyses was performed to determine the purity of HZ in addition to the extensive purification method described above (Infect. Immun. 70: 3939-3943, 2002). DNA or RNA was not detected in HZ or DNase-treated HZ solution (1 mM) either by ethidium bromide-stained agarose gel (FIG. 5) or with a spectrophotometer. DNase treatment or heat-inactivation had no effect on HZ-induced TNFα and IL-12p40 productions by DCs (FIG. 5b). Further, genomic DNA and RNA isolated from P. falciparum did not activate innate immune responses significantly, including the productions of TNFα and IL-12 (unpublished experiment and (J. Immunol. 172:3989-3993, 2004)). No protein or lipid was detected in the HZ solution (1 mM) by any method. Taken together, these data strongly suggest that HZ-induced, TLR9-mediated innate immune activation was not due to other contaminants.
(Chloroquine-Sensitivity of HZ-Induced Innate Immune Activation)
[0057]The anti-malarial formulation, chloroquine (CQ), has been reported to inhibit proinflammatory responses during malaria infection in addition to endosome/lysosome maturations and malarial hemozoin crystal formations, whereas the exact mechanism of anti-malarial effects of CQ is still under debate (Int. J. Parasitol. 32: 1645-1653, 2002; Life Sci. 74: 1957-1972, 2004). Recent evidence suggests that CQ also inhibits TLR9-mediated innate immune activation (J. Immunol. 160: 1122-1131, 1998). To examine the effect of CQ to HZ-induced proinflammatory responses, FL-DCs were stimulated with HZ in the presence of CQ. HZ-induced TNFα and IL-12p40 productions were diminished because of CQ (FIG. 5b).
[0058]These findings suggests that in addition to previously known mechanisms of CQ-inhibition of malaria pathogenesis, CQ may also inhibit HZ-induced, TLR9-mediated innate immune responses during malaria infection, possibly contributing to its therapeutic effects. Although further studies are needed, it is possible that in addition to its ability to inhibit HZ formation, CQ may also interfere with the interaction of HZ with TLR9 or inhibit the maturation of HZ-containing food vacuoles, thereby inhibiting the following innate immune activation.
[0059]It has been reported that TLR9 recognizes CpG motifs in microbial DNA or self-DNA-chromatin complex with specific IgG, whereas a non-DNA molecule as a TLR9 ligand has not been reported (Trends Immunol. 25: 381-386, 2004; Annu. Rev. Immunol. 20: 709-760, 2002). Recent study reported the first evidence of a non-DNA ligand recognized by TLR9. HZ is a crystal form of polymerized heme (ferriprotoporphyrin IX) produced in food vacuoles of parasites during the degradation of hemoglobin from red blood cells. Once captured by phagocytes such as macrophages and DCs, HZ accumulates in phagosomes where TLR9 can be recruited from endoplasmic reticulum via P13 kinase (J. Exp. Med. 196: 269-274, 2002; Nat. Immunol. 5: 190-198, 2004), suggesting that TLR9 can recognize HZ in phagosomes.
[0060]HZ is extremely hydrophobic, which may contribute to its immunostimulatory effect according to the hypothesis proposed recently (Nat. Rev. Immunol. 4:469-478, 2004). It is of interest that HZ is originally derived from hemoglobin in the host red blood cells, but is modified by parasites from toxic heme into HZ, a metabolite which is nontoxic to parasites, so that the parasites may survive, which suggests that inert molecules (heme) become active "non-self" molecules in the innate immune system during malaria infection.
[0061]The biological role of HZ-induced, TLR9-mediated innate immune activation during malaria infection and of the host defense against such activation is currently under investigation. Preliminary results suggest that innate immune responses to malaria parasites are dependent on multiple factors in the host, including TLRs as well as and Plasmodium. Including the recent study, it is important to study whether TLR-mediated innate immune activation during malaria infection contributes to host protective immunity or to malarial immune escape mechanism. Further, it is also important to study the molecular mechanism by which TLR9 discriminates between CpGDNA and HZ. Nevertheless, observations to date clearly demonstrate that HZ, a heme metabolite during malaria infection, activates the innate immune system via TLR9-mediated, MyD88-dependent, and chloroquine-sensitive pathway, which may open a key to further understanding of malaria parasite-host interactions in innate immunity.
DESCRIPTION OF DRAWINGS
[0062]FIG. 1. Purified HZ from P. falciparum activates proinflammatory responses through the MyD88-dependent pathway: (a) FL-DCs from wild-type (WT) mice were stimulated with purified hemozoin (HZ) (30 μM and 100 μM) for 24 hours. TNFα, IL-12p40, MCP-1, or IL-6 production in the supernatant was measured by ELISA. As a control, 3 μM CpGDNA(D35) was used. (b) FL-DCs and (c) spleen cells of wild-type mice (solid bars) and of MyD88.sup.-/- mice (open bars) were stimulated with 30 μM HZ and CpGDNA (D35, 3 μM), or LPS (100 ng/ml) for 24 hours. The production of TNFα, IL-12p40, MCP-1, or IL-6 was measured by ELISA in the supernatant. (d) Myeloid DCs (CD11c.sup.+, B220.sup.-) and plasmacytoid DCs (CD11c.sup.+, B220.sup.+) were analyzed by flow-cytometry for CD40 and CD86 expressions. The shaded areas represent cells not stimulated by HZ, while solid lines represent cells stimulated by HZ. These results show the mean value+S.D. of duplicate cultures, and are representative of at least five independent experiments. "n.d." means "not detected".
[0063]FIG. 2. HZ-induced DC activation is TRIF-independent: FL-DCs from wild-type (WT) mice and TRIF.sup.-/- mice were incubated with 30 μM HZ and LPS (100 ng/ml) for 24 hours, after which supernatants were analyzed by ELISA to determine the production level of TNFα or IL-12p40. The results are the mean value+SEM for four independent experiments (n=4). P>0.05, HZ (WT) vs. HZ (TRIF.sup.-/-). "n.d." means "not detected".
[0064]FIG. 3. TLR9 mediates HZ-induced innate immune activation: (a) Myeloid DCs (CD11c.sup.+, B220.sup.-) and plasmacytoid DCs (CD11c.sup.+, B220.sup.+) from wild type (WT) mice and TLR2.sup.-/- mice, TLR4.sup.-/- mice, TLR7.sup.-/- mice, and TLR9.sup.-/- mice were analyzed by flow-cytometry to determine CD40 and CD88 expressions. The shaded areas represent cells not stimulated by HZ and the solid lines represent cells stimulated by HZ. Wild-type cells and TLR9.sup.-/- cells were incubated with the indicated stimuli and analyzed by ELISA for (b) TNFα, IL-12p40, MCP-1, or IL-6 production by spleen cells and for (c) TNFα production by FL-DCs. The results are representatives of at least five independent experiments. "n.d." means "not detected".
[0065]FIG. 4. Serum cytokine productions by HZ and synthetic HZ (β-hematin) in vivo are dependent on MyD88 and TLR9: Either 1500 μg of (a) purified P. falciparum HZ or (b) synthetic HZ (sHZ) was intraperitoneally injected into MyD88.sup.-/- mice, TLR9.sup.-/- mice, or wild-type (WT) mice. Serum levels of MCP-1 and IL-6 were measured by ELISA at the indicated time points.
[0066]FIG. 5. Hemozoin is free of DNA, and HZ-induced proinflammatory cytokine production is DNase-resistant and heat-resistant, but chloroquine sensitive:
(a) To detect DNA contamination, HZ was run on an agarose gel and stained with ethidium bromide. M, marker; lane 1, HZ solution (1 mM, 5 μl); lane 2, heat-inactivated HZ (1 mM, 5 μl); lane 3, DNase-treated HZ (1 mM, 5 μl); lane 4, P. falciparum crude extract (5 μl from packed 100 ml of culture containing 4.5% parasitemia); DNase-treated crude extract (5 μl). (b) FL-DCs from C57/B6 mice were incubated either with 30 μM HZ or DNase treatment, heat-inactivation, and/or CQ (10 μM) for 24 hours. Then the supernatants were collected and measured for TNFα or IL-12p40 by ELISA. As a control, 3 μM CpGODN (D35) was used. The value of TNFα by D35 is shown in the figure. The results are representatives of one of three independent experiments performed in duplicate (mean+S.D.). *P.<0.05, HZ vs. HZ+CQ; **P.<0.05, CpGODN vs. CpGODN+CQ. "n.d." means "not detected".
INDUSTRIAL APPLICABILITY
[0067]In the instant invention, the inventors have elucidated the molecular level mechanism of immune responses in which HZ activates innate immunity in malaria parasites-host interactions. Based on this finding, in the instant invention, a method for detecting and measuring malaria infection that can be used for a diagnosis of malaria infection; and a method for screening a vaccine for malaria infection or a preventative or therapeutic agent for malaria infection using the method for detecting and measuring malaria infection by HZ induction were developed. Based on the above finding, a means for regulating HZ-induced, TLR9-mediated, and MyD88-dependent innate immune induction by using HZ, synthetic HZ, or derivatives thereof as an adjuvant or immunostimulant, was further developed.
[0068]By means of such developments, the instant invention presents measures to address a diagnosis of malaria infection; a creation of a vaccine or a preventative or therapeutic agent for malaria infection; and further, a regulation of innate immune induction as an immunotherapy for malaria infection, i.e., measures to address a diagnosis of malaria infection by targeting the innate-immune-related molecules including HZ, TLR9 (Toll-like receptor 9), and MyD88 or the signaling pathways thereof; and the development of an anti-malarial agent or an immunotherapy. Thus the instant invention can greatly contribute in these areas of applications. Further, CpGODN known as a TLR9 ligand has been developed as an innate-immune enhancer for combination therapy using CpGODN with a therapeutic agent for cancer or an infection; an anti-allergic agent; and a vaccine adjuvant. Therefore, hemozoin, synthetic hemozoin, or derivatives thereof can be developed in a similar manner as CpGODN as an innate-immune enhancer to be used with a therapeutic agent for cancer or for an infection in a combination therapy; an anti-allergic agent; and a vaccine adjuvant. Further, that hemozoin does not induce INFα in murine PDCs is an important finding for comparisons with various CpGODN activities.
Sequence CWU
1
213257DNAHomo sapiens 1ccgctgctgc ccctgtggga agggacctcg agtgtgaagc
atccttccct gtagctgctg 60tccagtctgc ccgccagacc ctctggagaa gcccctgccc
cccagcatgg gtttctgccg 120cagcgccctg cacccgctgt ctctcctggt gcaggccatc
atgctggcca tgaccctggc 180cctgggtacc ttgcctgcct tcctaccctg tgagctccag
ccccacggcc tggtgaactg 240caactggctg ttcctgaagt ctgtgcccca cttctccatg
gcagcacccc gtggcaatgt 300caccagcctt tccttgtcct ccaaccgcat ccaccacctc
catgattctg actttgccca 360cctgcccagc ctgcggcatc tcaacctcaa gtggaactgc
ccgccggttg gcctcagccc 420catgcacttc ccctgccaca tgaccatcga gcccagcacc
ttcttggctg tgcccaccct 480ggaagagcta aacctgagct acaacaacat catgactgtg
cctgcgctgc ccaaatccct 540catatccctg tccctcagcc ataccaacat cctgatgcta
gactctgcca gcctcgccgg 600cctgcatgcc ctgcgcttcc tattcatgga cggcaactgt
tattacaaga acccctgcag 660gcaggcactg gaggtggccc cgggtgccct ccttggcctg
ggcaacctca cccacctgtc 720actcaagtac aacaacctca ctgtggtgcc ccgcaacctg
ccttccagcc tggagtatct 780gctgttgtcc tacaaccgca tcgtcaaact ggcgcctgag
gacctggcca atctgaccgc 840cctgcgtgtg ctcgatgtgg gcggaaattg ccgccgctgc
gaccacgctc ccaacccctg 900catggagtgc cctcgtcact tcccccagct acatcccgat
accttcagcc acctgagccg 960tcttgaaggc ctggtgttga aggacagttc tctctcctgg
ctgaatgcca gttggttccg 1020tgggctggga aacctccgag tgctggacct gagtgagaac
ttcctctaca aatgcatcac 1080taaaaccaag gccttccagg gcctaacaca gctgcgcaag
cttaacctgt ccttcaatta 1140ccaaaagagg gtgtcctttg cccacctgtc tctggcccct
tccttcggga gcctggtcgc 1200cctgaaggag ctggacatgc acggcatctt cttccgctca
ctcgatgaga ccacgctccg 1260gccactggcc cgcctgccca tgctccagac tctgcgtctg
cagatgaact tcatcaacca 1320ggcccagctc ggcatcttca gggccttccc tggcctgcgc
tacgtggacc tgtcggacaa 1380ccgcatcagc ggagcttcgg agctgacagc caccatgggg
gaggcagatg gaggggagaa 1440ggtctggctg cagcctgggg accttgctcc ggccccagtg
gacactccca gctctgaaga 1500cttcaggccc aactgcagca ccctcaactt caccttggat
ctgtcacgga acaacctggt 1560gaccgtgcag ccggagatgt ttgcccagct ctcgcacctg
cagtgcctgc gcctgagcca 1620caactgcatc tcgcaggcag tcaatggctc ccagttcctg
ccgctgaccg gtctgcaggt 1680gctagacctg tcccacaata agctggacct ctaccacgag
cactcattca cggagctacc 1740acgactggag gccctggacc tcagctacaa cagccagccc
tttggcatgc agggcgtggg 1800ccacaacttc agcttcgtgg ctcacctgcg caccctgcgc
cacctcagcc tggcccacaa 1860caacatccac agccaagtgt cccagcagct ctgcagtacg
tcgctgcggg ccctggactt 1920cagcggcaat gcactgggcc atatgtgggc cgagggagac
ctctatctgc acttcttcca 1980aggcctgagc ggtttgatct ggctggactt gtcccagaac
cgcctgcaca ccctcctgcc 2040ccaaaccctg cgcaacctcc ccaagagcct acaggtgctg
cgtctccgtg acaattacct 2100ggccttcttt aagtggtgga gcctccactt cctgcccaaa
ctggaagtcc tcgacctggc 2160aggaaaccag ctgaaggccc tgaccaatgg cagcctgcct
gctggcaccc ggctccggag 2220gctggatgtc agctgcaaca gcatcagctt cgtggccccc
ggcttctttt ccaaggccaa 2280ggagctgcga gagctcaacc ttagcgccaa cgccctcaag
acagtggacc actcctggtt 2340tgggcccctg gcgagtgccc tgcaaatact agatgtaagc
gccaaccctc tgcactgcgc 2400ctgtggggcg gcctttatgg acttcctgct ggaggtgcag
gctgccgtgc ccggtctgcc 2460cagccgggtg aagtgtggca gtccgggcca gctccagggc
ctcagcatct ttgcacagga 2520cctgcgcctc tgcctggatg aggccctctc ctgggactgt
ttcgccctct cgctgctggc 2580tgtggctctg ggcctgggtg tgcccatgct gcatcacctc
tgtggctggg acctctggta 2640ctgcttccac ctgtgcctgg cctggcttcc ctggcggggg
cggcaaagtg ggcgagatga 2700ggatgccctg ccctacgatg ccttcgtggt cttcgacaaa
acgcagagcg cagtggcaga 2760ctgggtgtac aacgagcttc gggggcagct ggaggagtgc
cgtgggcgct gggcactccg 2820cctgtgcctg gaggaacgcg actggctgcc tggcaaaacc
ctctttgaga acctgtgggc 2880ctcggtctat ggcagccgca agacgctgtt tgtgctggcc
cacacggacc gggtcagtgg 2940tctcttgcgc gccagcttcc tgctggccca gcagcgcctg
ctggaggacc gcaaggacgt 3000cgtggtgctg gtgatcctga gccctgacgg ccgccgctcc
cgctacgtgc ggctgcgcca 3060gcgcctctgc cgccagagtg tcctcctctg gccccaccag
cccagtggtc agcgcagctt 3120ctgggcccag ctgggcatgg ccctgaccag ggacaaccac
cacttctata accggaactt 3180ctgccaggga cccacggccg aatagccgtg agccggaatc
ctgcacggtg ccacctccac 3240actcacctca cctctgc
325721032PRTHomo sapiens 2Met Gly Phe Cys Arg Ser
Ala Leu His Pro Leu Ser Leu Leu Val Gln1 5
10 15Ala Ile Met Leu Ala Met Thr Leu Ala Leu Gly Thr
Leu Pro Ala Phe 20 25 30Leu
Pro Cys Glu Leu Gln Pro His Gly Leu Val Asn Cys Asn Trp Leu 35
40 45Phe Leu Lys Ser Val Pro His Phe Ser
Met Ala Ala Pro Arg Gly Asn 50 55
60Val Thr Ser Leu Ser Leu Ser Ser Asn Arg Ile His His Leu His Asp65
70 75 80Ser Asp Phe Ala His
Leu Pro Ser Leu Arg His Leu Asn Leu Lys Trp 85
90 95Asn Cys Pro Pro Val Gly Leu Ser Pro Met His
Phe Pro Cys His Met 100 105
110Thr Ile Glu Pro Ser Thr Phe Leu Ala Val Pro Thr Leu Glu Glu Leu
115 120 125Asn Leu Ser Tyr Asn Asn Ile
Met Thr Val Pro Ala Leu Pro Lys Ser 130 135
140Leu Ile Ser Leu Ser Leu Ser His Thr Asn Ile Leu Met Leu Asp
Ser145 150 155 160Ala Ser
Leu Ala Gly Leu His Ala Leu Arg Phe Leu Phe Met Asp Gly
165 170 175Asn Cys Tyr Tyr Lys Asn Pro
Cys Arg Gln Ala Leu Glu Val Ala Pro 180 185
190Gly Ala Leu Leu Gly Leu Gly Asn Leu Thr His Leu Ser Leu
Lys Tyr 195 200 205Asn Asn Leu Thr
Val Val Pro Arg Asn Leu Pro Ser Ser Leu Glu Tyr 210
215 220Leu Leu Leu Ser Tyr Asn Arg Ile Val Lys Leu Ala
Pro Glu Asp Leu225 230 235
240Ala Asn Leu Thr Ala Leu Arg Val Leu Asp Val Gly Gly Asn Cys Arg
245 250 255Arg Cys Asp His Ala
Pro Asn Pro Cys Met Glu Cys Pro Arg His Phe 260
265 270Pro Gln Leu His Pro Asp Thr Phe Ser His Leu Ser
Arg Leu Glu Gly 275 280 285Leu Val
Leu Lys Asp Ser Ser Leu Ser Trp Leu Asn Ala Ser Trp Phe 290
295 300Arg Gly Leu Gly Asn Leu Arg Val Leu Asp Leu
Ser Glu Asn Phe Leu305 310 315
320Tyr Lys Cys Ile Thr Lys Thr Lys Ala Phe Gln Gly Leu Thr Gln Leu
325 330 335Arg Lys Leu Asn
Leu Ser Phe Asn Tyr Gln Lys Arg Val Ser Phe Ala 340
345 350His Leu Ser Leu Ala Pro Ser Phe Gly Ser Leu
Val Ala Leu Lys Glu 355 360 365Leu
Asp Met His Gly Ile Phe Phe Arg Ser Leu Asp Glu Thr Thr Leu 370
375 380Arg Pro Leu Ala Arg Leu Pro Met Leu Gln
Thr Leu Arg Leu Gln Met385 390 395
400Asn Phe Ile Asn Gln Ala Gln Leu Gly Ile Phe Arg Ala Phe Pro
Gly 405 410 415Leu Arg Tyr
Val Asp Leu Ser Asp Asn Arg Ile Ser Gly Ala Ser Glu 420
425 430Leu Thr Ala Thr Met Gly Glu Ala Asp Gly
Gly Glu Lys Val Trp Leu 435 440
445Gln Pro Gly Asp Leu Ala Pro Ala Pro Val Asp Thr Pro Ser Ser Glu 450
455 460Asp Phe Arg Pro Asn Cys Ser Thr
Leu Asn Phe Thr Leu Asp Leu Ser465 470
475 480Arg Asn Asn Leu Val Thr Val Gln Pro Glu Met Phe
Ala Gln Leu Ser 485 490
495His Leu Gln Cys Leu Arg Leu Ser His Asn Cys Ile Ser Gln Ala Val
500 505 510Asn Gly Ser Gln Phe Leu
Pro Leu Thr Gly Leu Gln Val Leu Asp Leu 515 520
525Ser His Asn Lys Leu Asp Leu Tyr His Glu His Ser Phe Thr
Glu Leu 530 535 540Pro Arg Leu Glu Ala
Leu Asp Leu Ser Tyr Asn Ser Gln Pro Phe Gly545 550
555 560Met Gln Gly Val Gly His Asn Phe Ser Phe
Val Ala His Leu Arg Thr 565 570
575Leu Arg His Leu Ser Leu Ala His Asn Asn Ile His Ser Gln Val Ser
580 585 590Gln Gln Leu Cys Ser
Thr Ser Leu Arg Ala Leu Asp Phe Ser Gly Asn 595
600 605Ala Leu Gly His Met Trp Ala Glu Gly Asp Leu Tyr
Leu His Phe Phe 610 615 620Gln Gly Leu
Ser Gly Leu Ile Trp Leu Asp Leu Ser Gln Asn Arg Leu625
630 635 640His Thr Leu Leu Pro Gln Thr
Leu Arg Asn Leu Pro Lys Ser Leu Gln 645
650 655Val Leu Arg Leu Arg Asp Asn Tyr Leu Ala Phe Phe
Lys Trp Trp Ser 660 665 670Leu
His Phe Leu Pro Lys Leu Glu Val Leu Asp Leu Ala Gly Asn Gln 675
680 685Leu Lys Ala Leu Thr Asn Gly Ser Leu
Pro Ala Gly Thr Arg Leu Arg 690 695
700Arg Leu Asp Val Ser Cys Asn Ser Ile Ser Phe Val Ala Pro Gly Phe705
710 715 720Phe Ser Lys Ala
Lys Glu Leu Arg Glu Leu Asn Leu Ser Ala Asn Ala 725
730 735Leu Lys Thr Val Asp His Ser Trp Phe Gly
Pro Leu Ala Ser Ala Leu 740 745
750Gln Ile Leu Asp Val Ser Ala Asn Pro Leu His Cys Ala Cys Gly Ala
755 760 765Ala Phe Met Asp Phe Leu Leu
Glu Val Gln Ala Ala Val Pro Gly Leu 770 775
780Pro Ser Arg Val Lys Cys Gly Ser Pro Gly Gln Leu Gln Gly Leu
Ser785 790 795 800Ile Phe
Ala Gln Asp Leu Arg Leu Cys Leu Asp Glu Ala Leu Ser Trp
805 810 815Asp Cys Phe Ala Leu Ser Leu
Leu Ala Val Ala Leu Gly Leu Gly Val 820 825
830Pro Met Leu His His Leu Cys Gly Trp Asp Leu Trp Tyr Cys
Phe His 835 840 845Leu Cys Leu Ala
Trp Leu Pro Trp Arg Gly Arg Gln Ser Gly Arg Asp 850
855 860Glu Asp Ala Leu Pro Tyr Asp Ala Phe Val Val Phe
Asp Lys Thr Gln865 870 875
880Ser Ala Val Ala Asp Trp Val Tyr Asn Glu Leu Arg Gly Gln Leu Glu
885 890 895Glu Cys Arg Gly Arg
Trp Ala Leu Arg Leu Cys Leu Glu Glu Arg Asp 900
905 910Trp Leu Pro Gly Lys Thr Leu Phe Glu Asn Leu Trp
Ala Ser Val Tyr 915 920 925Gly Ser
Arg Lys Thr Leu Phe Val Leu Ala His Thr Asp Arg Val Ser 930
935 940Gly Leu Leu Arg Ala Ser Phe Leu Leu Ala Gln
Gln Arg Leu Leu Glu945 950 955
960Asp Arg Lys Asp Val Val Val Leu Val Ile Leu Ser Pro Asp Gly Arg
965 970 975Arg Ser Arg Tyr
Val Arg Leu Arg Gln Arg Leu Cys Arg Gln Ser Val 980
985 990Leu Leu Trp Pro His Gln Pro Ser Gly Gln Arg
Ser Phe Trp Ala Gln 995 1000
1005Leu Gly Met Ala Leu Thr Arg Asp Asn His His Phe Tyr Asn Arg
1010 1015 1020Asn Phe Cys Gln Gly Pro
Thr Ala Glu1025 1030
User Contributions:
Comment about this patent or add new information about this topic: